Abstract
Butyrate, a short-chain fatty acid fiber fermentation product, induces colonocyte apoptosis in part via a Fas-mediated (extrinsic) pathway. In previous studies, we demonstrated that docosahexaenoic acid (DHA, 22:6Δ4,7,10,13,16,19) enhances the effect of butyrate by increasing mitochondrial lipid oxidation and mitochondrial Ca2+-dependent apoptosis in the colon. In this study, we further examined the mechanism of DHA-butyrate synergism in 1) human colon tumor (HCT-116 isogenic p53+/+ vs. p53−/−) cells and 2) primary cultures of rat colonic crypts. Herein, we show that DHA and butyrate promote apoptosis by enhancing mitochondrial Ca2+ accumulation in both isogenic cell lines. Ca2+ accumulation and apoptosis were inhibited by blockade of mitochondrial uniporter-mediated Ca2+ uptake. In addition, Mito-Q, a mitochondria-targeted antioxidant, also blocked apoptosis induced by DHA and butyrate. In complementary experiments, rats were fed diets supplemented with either corn oil (control, contains no DHA) or fish oil (contains DHA). Colonic crypts were isolated and incubated with or without butyrate, after which the mitochondria-to-cytosol Ca2+ ratio and crypt viability were measured. No significant difference (P > 0.05) in basal mitochondrial Ca2+ levels was observed between fish oil- or corn oil-fed animals. In contrast, when fish oil was the dietary lipid source, crypts incubated with butyrate exhibited a significant increase (3.6-fold, P < 0.001) in mitochondrial Ca2+ compared with corn oil plus butyrate treatment. On the basis of these data, we propose that the combination of DHA and butyrate compared with butyrate alone further enhances colonocyte apoptosis by inducing a p53-independent, oxidation-sensitive, mitochondrial Ca2+-dependent (intrinsic) pathway.
Keywords: n-3 polyunsaturated fatty acids, chemoprevention, fish oil, fermentable fiber
The Frequency of Colon Cancer is increasing among people who migrate from low-incidence to high-incidence regions (26). This variation in geographical incidence of colon cancer indicates that environmental factors, especially modifiable factors like diet and physical activity, play a major role in disease onset (26, 32). Fat and fiber are two of the most widely investigated dietary components in the prevention of colon carcinogenesis. Currently, there is a plethora of experimental, epidemiological, and clinical evidence indicating that diets rich in n-3 polyunsaturated fatty acids (PUFA), e.g., docosahexaenoic acid (DHA, 22:6Δ4,7,10,13,16,19) and eicosapentaenoic acid (EPA, 20:5Δ5,8,11,14,17) found in fish oil, are protective against colon tumorigenesis (3, 5, 12, 13, 16, 19, 22). In contrast, dietary lipids rich in n-6 PUFA (found primarily in vegetable oils), e.g., linoleic acid (LA, 18:2Δ9,12), enhance the development of colon tumors (13, 22, 64). Importantly, the typical Western diet contains 10 to 20 times more n-6 than n-3 PUFA (61). There is also evidence that the consumption of fiber, a luminal source of butyrate, is chemoprotective against colorectal cancers (9, 39). Although there is still debate challenging the premise that dietary fiber reduces colon cancer risk (1, 48, 49), we have demonstrated that the chemoprotective/proapoptotic effect of n-3 PUFA is enhanced when a highly fermentable fiber, pectin (or its fermentation product butyrate), is added to the diet (13, 38, 42, 54). This is significant because the transformation of colonic epithelium to carcinoma is in part associated with a progressive inhibition of apoptosis (13, 28, 58). Hence, chemotherapeutic agents that restore the normal apoptotic pathways have the potential for effectively treating cancers that depend on aberrations of the apoptotic pathway to stay alive (24). Along these lines, we have demonstrated that measurements of apoptosis have greater prognostic value to detect dietary effects on colon tumor incidence than do measurements of cell proliferation (4, 30).
With regard to mechanisms of action, we have recently demonstrated that dietary DHA is incorporated into mitochondrial membrane phospholipids, thereby enhancing oxidative stress induced by butyrate metabolism (14, 29, 54). We subsequently identified a pathway for the induction of apoptosis in colonic epithelium involving the synergistic enhancement of mitochondrial phospholipid hydroperoxides by these two bio-active molecules (42). Our results indicate that the effects of individual chemoprotective nutrients (n-3 PUFA and butyrate) may not be as important as the nutritional combination. This may explain why a diet containing highly fermentable fiber is protective when fish oil is the lipid source (13, 54).
Ca2+ is one of the most versatile universal signaling mediators of cellular apoptosis. Cumulative evidence indicates a central role for mitochondrial Ca2+ accumulation in intrinsically mediated apoptosis (43, 46, 56). Although the importance of the endoplasmic reticulum (ER) as the major storage organelle is indisputable, it has been known for some time that mitochondrial sequestration of large amounts of Ca2+ contributes to cell death via apoptosis or necrosis (46, 47, 53). Several lines of recent evidence suggest that apoptosis is induced in response to alterations in intracellular Ca2+ compartmentalization and enhanced mitochondrial Ca2+ accumulation (43, 46). The mitochondrial uniporter, a Ca2+-selective ion channel present on the outer mitochondrial membrane, mediates rapid mitochondrial uptake of the Ca2+ following release from ER stores (35, 43). Furthermore, activation of the uniporter associated with a rise in mitochondrial Ca2+ stimulates the generation of reactive oxygen species (ROS) and free fatty acids, which promote the opening of the permeability transition pore (PTP) (56, 62). Opening of the PTP causes dissipation of the mitochondrial membrane potential and eventually the release of Ca2+. However, under certain circumstances, mitochondrial Ca2+ accumulation acts as a trigger for release of proapoptotic molecules from the mitochondria that leads to execution of the cells (27).
With accumulating evidence favoring a central role for mitochondrial Ca2+ in cellular apoptosis, we determined the effect of DHA and butyrate on mitochondrial Ca2+ levels. After we recently demonstrated using young adult mouse colonocytes that DHA and butyrate combination synergistically alters intracellular Ca2+ compartmentalization by enhancing mitochondrial Ca2+ accumulation through a store-operated channel-mediated mechanism (36), in this study our primary objective was to corroborate these novel findings utilizing human colonocyte cell lines and an in vivo rat model system. Since mitochondria and the p53 tumor suppressor gene play a critical role in damage-induced apoptosis (52, 57), we postulated that the combination of DHA and butyrate significantly enhances mitochondrial Ca2+ accumulation and apoptosis in a p53-dependent fashion. To address this hypothesis, we used isogenic p53 wild-type and deficient human colon tumor (HCT-116) cell lines to determine the effect of DHA and butyrate on mitochondrial Ca2+ levels. In addition, to establish the relevance of our in vitro observations, parallel experiments were conducted using intact crypts isolated from rats fed fish oil (contains DHA) or corn oil (contains no DHA, control) incubated with butyrate ex vivo. Data from these experiments recapitulated observations in the human cell lines, providing conclusive evidence that DHA (from fish oil) and butyrate enhance mitochondrial Ca2+ accumulation, thereby enhancing the induction of apoptosis.
MATERIALS AND METHODS
Materials
McCoy’s 5A medium, GlutaMAX and Leibovitz medium were purchased from GIBCO BRL (Grand Island, NY). Hanks’ balanced salt solution (HBSS) was from Mediatech (Herndon, VA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT). Fatty acids were obtained from NuChek Prep (Elysian, MN). Fluo-4 AM, rhod-2 AM, MitoTracker Green FM, and LIVE/DEAD viability/cytotoxicity kit were purchased from Molecular Probes (Eugene, OR). RU-360 was purchased from Calbiochem (San Diego, CA). Mito-Q, a mitochondrion-targeted antioxidant, was a gift from Dr. Michael Murphy, Medical Research Council Dunn Human Nutrition Unit, Cambridge, UK. Cell death detection ELISA kit was obtained from Roche Applied Science (Indianapolis, IN). Two-well Lab-Tek chambered coverglass slides were purchased from Nunc (Naperville, IL). Precast 4–20% Tris-glycine gels were from Invitrogen (Carlsbad, CA). Electroblotting polyvinylidene difluoride (PVDF) membranes were obtained from Millipore (Burlington, MA). Super Signal West Femto maximum sensitivity substrate was from Pierce (Rockford, IL). Primary rabbit anti-p53 antibody was obtained from Calbiochem (San Diego, CA) and peroxidase-conjugated goat anti-rabbit IgG secondary antibody was purchased from Kirkegaard & Perry (Gaithersburg, MD). Sodium butyrate, EDTA, and all other reagents were obtained from Sigma (St. Louis, MO).
Stock solutions of fluo-4 AM and rhod-2 AM were prepared in DMSO and diluted with medium to 3.0 and 2.0 μM, respectively (final concentration of the vehicle DMSO was maintained at 0.1–0.3% in culture). RU-360 (1 mM stock) was prepared in degassed water and diluted to a final concentration of 10 μM for cell treatment. Mito-Q stock (50 mM) was prepared in sterile water and a final concentration of 5 μM was used for experiments. Fish oil for dietary experiments was obtained from Omega Protein (Houston, TX) and corn oil was purchased from Dyets (Bethlehem, PA).
Cell culture
Isogenic p53 wild-type (p53+/+) and p53-deficient (p53−/−) cell lines were obtained from Dr. Bert Vogelstein, Johns Hopkins University, Baltimore, MD (11). HCT-116 cells were cultured in McCoy’s 5A medium supplemented with 10% FBS and 2 mM GlutaMAX at 37°C in 5% CO2. For all fluorescence measurements, p53+/+ (passages 3–8) and p53−/− (passages 15–19) cells were seeded onto Lab-Tek two-well chambered coverglass slides at a density of 1.5 × 105 for 72 h to achieve 50–70% confluence. For apoptosis assays, cells were seeded onto 35-mm cell culture dishes or six-well plates at a density of 3.5 × 104. BSA-complexed fatty acids were added to cultures 24 h after cell plating as previously described (23). Select cultures were treated with DHA (22:6, n-3) or LA (18:2, n-6) (50 μM) for 72 h. Cells were coincubated with sodium butyrate (0.5 mM) in McCoy’s 5A medium for the final 6, 12, or 24 h of fatty acid pretreatment.
Analysis of mitochondrial Ca2+
Cells treated with fatty acid and butyrate were washed with Leibovitz medium and coloaded with 3 μM fluo-4 AM and 2 μM rhod-2 AM for 1 h at 37°C. For quantification of fluo-4 and rhod-2 fluorescence, excitation wavelengths of 488 and 550 nm were used and fluorescence emission was monitored at 530 and 580 nm, respectively. Ten-15 areas with 20–25 cells per area and 4–8 wells per treatment were imaged and the ratio of mitochondrial-to-cytosolic Ca2+ level was calculated. Cells or crypts were analyzed with a Zeiss Stallion Dual Detector Imaging workstation (Carl Zeiss Microimaging, Thornwood, NY) equipped with a 300-W xenon fluorescent light source with rapid switching (<2 ms) between excitation wavelengths. Images were collected via a ×20 objective (0.75 numerical aperture) and a ROPER CoolSnap HQ camera. Fluo-4 calibration was performed using the Calcium Calibration buffer kit 2 (Molecular Probes) as previously described (36). In select experiments, MitoTracker Green FM was used to confirm the subcellular localization of rhod-2 fluorescence (10). MitoTracker Green is a green-fluorescent mitochondrial stain that localizes in the mitochondria regardless of membrane potential. This probe has an added advantage in that it is essentially nonfluorescent in aqueous solution, only becoming fluorescent when it accumulates in the lipid environment of the mitochondria. In addition, to confirm that rhod-2 fluorescence corresponded to mitochondrial Ca2+ accumulation, experiments were performed in cultures incubated with RU-360 (10 μM), an inhibitor of the mitochondrial uniporter, for 30 min prior to butyrate cotreatment (2). Cells were subsequently washed and treated with fatty acid and butyrate for the final 6, 12, or 24 h following which the mitochondrial-to-cytosolic Ca2+ ratio was determined by fluorescence microscopy. Mitochondrial-to-cytosolic ratio refers to relative fluorescence intensities of Rhod-2 to Fluo-4.
Apoptosis assay
Apoptosis was measured by cellular fragmentation ELISA (Roche). Floating cells were washed, lysed, and centrifuged to sediment nuclei. Supernatants containing mono- and oligo-nucleosomes were incubated with substrate and subsequently analyzed by ELISA as previously described (23). To determine the association between mitochondrial Ca2+ and apoptosis, select cultures were preincubated with RU-360 (10 μM) for 30 min prior to butyrate exposure. Cells were washed and treated with 5 mM butyrate and apoptosis was measured following a 6-, 12-, or 24-h incubation period. To determine the involvement of oxidative stress in fatty acid-and butyrate-induced apoptosis, select cultures were preincubated with 5 μM Mito-Q for 1 h prior to butyrate treatment as previously described (42). Cells were then treated with fatty acid and 5 mM butyrate for an additional 12 h and apoptosis was quantified.
Western blotting
Cell lysates from p53+/+ or p53−/− cells were immunoblotted with p53 antibody by the method of Davidson et al. (21). Briefly, samples were treated with SDS sample buffer and subjected to electrophoresis in 4–20% precast Tris-glycine mini-gels. After electrophoresis, proteins were electroblotted onto a PVDF membrane by use of a Hoefer Mighty Small Transphor unit (Pharmacia, Piscataway, NJ) at 400 mA for 75 min. Following transfer, the membrane was incubated with rabbit anti-p53 antibody overnight at 4°C, followed by peroxidase-labeled goat anti-rabbit IgG incubation for 1 h at room temperature. Bands were developed by using Super Signal West Femto maximum sensitivity substrate, and blots were scanned with a Bio-Rad Fluor-S Max MultiImager system (Hercules, CA).
Animals and diets
Animal use was approved by the University Animal Care Committee of Texas A&M University and conformed to National Institutes of Health (NIH) guidelines. Male weanling (28-day-old) Sprague-Dawley rats (n = 60) were housed individually in raised wire cages. The animals were maintained in a temperature- and humidity-controlled animal facility with a daily 12-h light and 12-h dark photocycle as previously described (54). Rats were acclimated for 1 wk prior to receiving semipurified experimental diets. The animals were weighed before the start of the diet and were randomly assigned to the experimental diets (n = 15 rats/diet) so that the mean initial body weights did not differ between the two diet groups. Rats were placed on experimental diets for 3 wk ± 3 days. The experimental diet composition is described in Supplemental Tables S1 and S2. The experimental diets differed only in the type of fat (corn oil or fish oil). The major differences among the fatty acid composition of the dietary lipid sources were significantly higher amounts of EPA (20:5n-3) and DHA (22:6n-3) in the fish oil compared with corn oil diet and higher amounts of linoleic acid (LA, 18:2n-6) in the corn oil diet (22, 67). Each diet contained 15% dietary fat by weight and 6% dietary fiber by weight. Cellulose, a poorly fermentable fiber, was chosen as the source of fiber. The fish oil diet contained 3.5 g of corn oil/100 g diet to prevent essential fatty acid deficiency. Food and water were freely available to the animals at all times. To minimize fatty acid oxidation, diets were stored at −80°C and fresh food was provided every 24 h.
Isolation of colonic crypts
Following the feeding period, rats were killed by CO2 asphyxiation and cervical dislocation. The colon was removed and the colonic crypts were isolated as described previously (29, 66) with slight modification. Briefly, the colon was flushed with PBS to remove feces and incubated at 37°C in Ca2+ and Mg2+-free HBSS containing 25 mM dithiothreitol, 0.1% fatty acid-free BSA, 1 mM glutamine, and 30 mM EDTA to dislodge crypts from the colonic extracellular matrix. The incubation buffer was developed to minimize the generation of crypt fragments and to reduce mucin levels, thereby improving image background. Following a 15-min incubation period with gentle shaking, the mucosa was gently removed with a rubber cell scraper and intact crypts were isolated. Subsequently, crypt suspensions were centrifuged at 100 g for 3 min, the supernatant discarded and the pellet gently resuspended in HBSS. The centrifugation step was repeated three times. Crypts were then resuspended in Leibovitz medium and immediately analyzed. The time elapsed from animal termination to image capture did not exceed 70 min. The isolation time period was optimized on the basis of preliminary experiments that demonstrated that crypt viability decreased after 120 min.
Measurement of mitochondrial-to-cytosolic Ca2+ ratio in primary culture
Isolated colonic crypts were incubated with or without 5 mM butyrate in Leibovitz medium for 30 min at 37°C as we have previously described (29). The ex vivo incubation of intact crypts ± butyrate allowed for comparison without the confounding effects of animal-to-animal variability. Crypts were subsequently coloaded with fluo-4 AM and rhod-2 AM for 30 min and imaged, and the ratio of mitochondrial-to-cytosolic Ca2+ was determined as described above. A total of 10–15 areas with intact crypts were imaged for each sample. This ratiometric technique is well suited for studying significant differences between treatments because it minimizes the variability in dye loading and changes in the size or shape of the crypts.
Crypt cell viability was evaluated in each sample following Ca2+ measurement. For this purpose, crypt pellets were coloaded with ethidium homodimer-1 and calcein AM (Invitrogen). Live cells have ubiquitous intracellular esterase activity and convert nonfluorescent calcein AM to a highly fluorescent calcein that is retained inside the live cell to emit a uniform green signal. Ethidium homodimer-1 enters cells with a damaged plasma membrane and is excluded by the intact plasma membrane of viable cells. Therefore, ethidium homodimer-1 enters dead cells with a compromised plasma membrane and undergoes a 40-fold enhancement in fluorescence to produce a bright red fluorescence differentiating it from viable green cells.
Statistical analysis
The effect of independent variables (treatment effects) was assessed by SuperAnova. Differences among means were determined by t/F type tests of contrast. P values <0.05 were considered to be statistically significant. In primary culture experiments, to determine whether the ratios of the mitochondrial-to-cytosolic Ca2+ level varied with diet treatments, the linear mixed effect model (50) was performed using the “nlme” procedure within the statistical software R (51). A subject-level random effect was modeled to account for similarity of measurements collected from the same rat. The main fixed treatment effects considered in the model include oil (corn vs. fish oil), fiber (with and without butyrate) and fat-butyrate interactions. Furthermore, ratios of the mitochondrial-to-cytosolic Ca2+ level were considered at the log-scale to reduce potential effects of outlying observations at the right tail of the data distribution. Several inferential statistical analyses within the mixed effects modeling framework were utilized to examine the effect of diet on the mitochondria-to-cytosolic Ca2+ ratio.
RESULTS
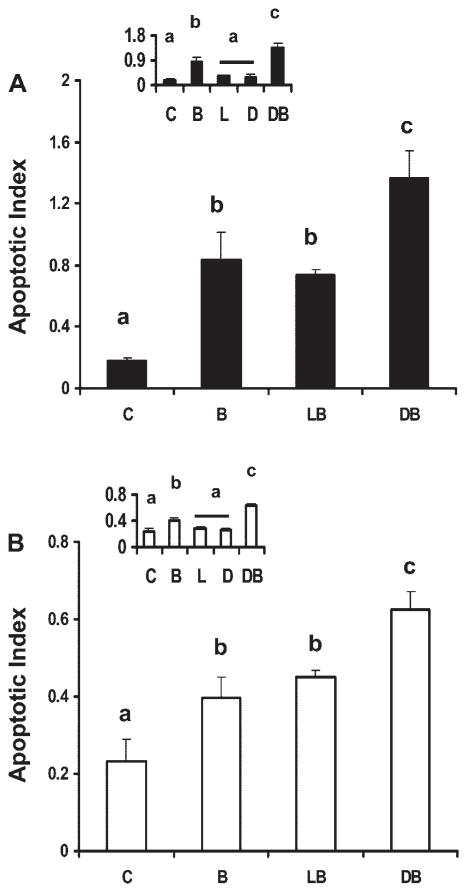
Effect of fatty acid and butyrate cotreatment on induction of apoptosis in p53 wild-type and p53 null colonic cell lines. The role of fatty acid and butyrate cotreatment on cellular apoptosis was initially investigated. For this purpose, p53+/+ and p53−/− cells (Supplemental Fig. S1) were harvested following fatty acid (50 μM) and butyrate (5 mM) cotreatment and analyzed by TUNEL assay. These levels are considered physiologically relevant because they fall well within the ranges of blood and intestinal lumen levels (18, 39). Butyrate coincubation with DHA in p53+/+ cells induced a 655 and 85% increase in apoptotic index compared with untreated control and to LA (control, n-6 PUFA) and butyrate-treated cells, respectively (Fig. 1A). Similarly, isogenic p53−/− cells exhibited a 200% increase in apoptosis compared with untreated control and a 39% increase relative to LA− and butyrate-treated cells (Fig. 1B). In comparison, control cultures containing either DHA or LA alone, in the absence of butyrate, had no effect on apoptosis (Fig. 1, inset).
Fig. 1.
Effect of fatty acid and butyrate cotreatment on apoptosis in p53 wild-type and knockout cells. p53 Wild-type (p53+/+, solid bars; A) and p53 knockout (p53−/−) cells (open bars; B) were treated with fatty acid (50 μM) for a total of 72 h and 0 or 5 mM butyrate for the final 12 h. Insets show fatty acid-only treatment effects compared with control treatment groups, from independent experiments. Nonadherent cells were harvested and apoptosis was measured by the nucleosomal fragmentation release assay. Data are means ± SE from a representative experiment, n = 6–8 wells per treatment. Control cultures (insets) containing no treatment (C), butyrate only (B), linoleic acid (LA) only (L), docosahexaenoic acid (DHA) only (D), LA and butyrate (LB), DHA and butyrate (DB). Data demonstrate that DB significantly enhanced apoptotic index compared with the other treatment groups whereas fatty acid only had no effect. Bars not sharing a common letter are significantly different at P < 0.05.
Effect of DHA and butyrate cotreatment on intracellular Ca2+ levels
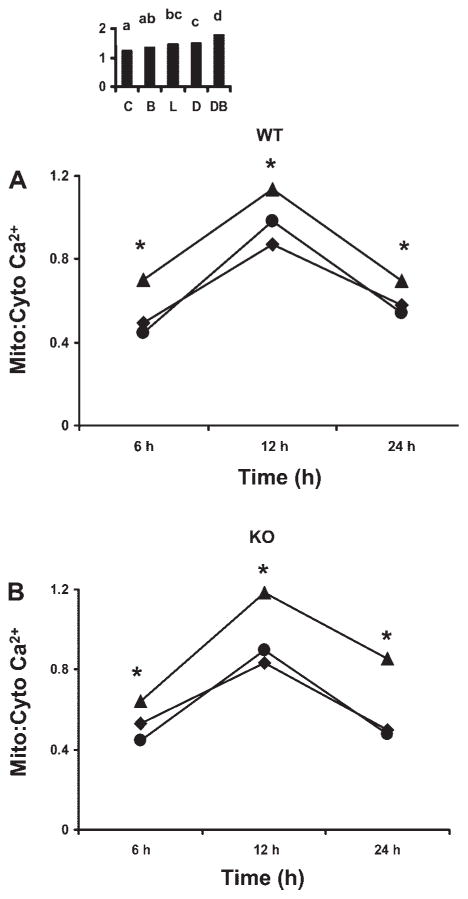
Since mitochondria are key organelles involved in the regulation of cell death and intracellular Ca2+ homeostasis, we measured the ratio of mitochondrial-to-cytosolic Ca2+ ratio in individual cells. DHA-pretreated p53+/+ and p53−/− cells demonstrated an elevated mitochondrial-to-cytosolic Ca2+ ratio compared with LA pretreated and untreated control cells at 6, 12, and 24 h of butyrate cotreatment (Fig. 2). Butyrate cotreatment for 6 h, increased (P < 0.0001) the mitochondrial-to-cytosolic Ca2+ ratio in cells incubated with DHA by 43 and 57% compared with untreated or LA− and butyrate-cotreated p53+/+ cells, respectively (Fig. 2A). Similarly, p53−/− cells treated with DHA + butyrate exhibited a 44 and 22% increase in mitochondrial Ca2+ levels compared with LA + butyrate-treated and untreated cells, respectively (Fig. 2B). In addition, following a 12-h incubation with butyrate, DHA increased (P < 0.0001) mitochondrial Ca2+ by 31% compared with untreated control in p53+/+ cells (Fig. 2A). In contrast to DHA-treated cultures, cells incubated in the presence of LA showed no change in the mitochondrial-to-cytosolic Ca2+ ratio compared with untreated cells. The p53−/− exhibited a similar pattern of response (Fig. 2B). For comparative purposes, 24 h butyrate cotreatment was also examined. Consistent with observations made at earlier time points, DHA enhanced the mitochondrial-to-cytosolic ratio by 30 and 75% compared with LA and butyrate treatment in p53+/+ (Fig. 2A) and p53−/− cells (Fig. 2B), respectively. Control cultures (24 h) containing butyrate in the absence of fatty acid did not exhibit a significant difference in mitochondrial Ca2+ compared with untreated p53+/+ cells (Fig. 2A, inset). The p53+/+ cells treated with DHA in the absence of butyrate did not demonstrate any significant difference in mitochondrial-to-cytosolic Ca2+ ratio compared with LA-pretreated cells (Fig. 2A inset). In contrast, butyrate-treated cells incubated with DHA exhibited a significantly higher (P < 0.0001) mitochondrial-to-cytosolic Ca2+ ratio. Similar trends were observed in p53+/+ cells (data not shown).
Fig. 2.
Effect of fatty acid and butyrate cotreatment on colonocyte mitochondrial Ca2+ levels. The p53+/+ [wild-type (WT); A] and p53−/− cells [knockout (KO); B] were exposed to 50 μM fatty acid for 72 h in the presence of 5 mM butyrate for the final 6, 12, or 24 h. The p53+/+ cells were pretreated with 50 μM fatty acid in the presence or absence (A, inset) of 5 mM butyrate for the final 12 h following which mitochondria-to-cytosolic Ca2+ (Mito:Cyto Ca2+) was measured. Cells were coloaded with fluo-4 AM (3 μM) and rhod-2 AM (2 μM) and the ratio of mitochondria-to-cytosolic Ca2+ was evaluated as described in the MATERIALS AND METHODS. Data represent means from a representative experiment, n = 3 independent experiments, n = 20–35 cells per treatment per experiment. A significant (P < 0.001) difference between the combination of LA with butyrate and DHA with butyrate was observed for both cell lines starting from 6 h. In p53+/+ cells, butyrate-only treatment did not show any difference compared with untreated cells. DHA or LA in the absence of butyrate did not show any significant difference in the ratio of mitochondrial-to-cytosolic Ca2+ ratio. ▲, DHA + butyrate, ●, LA + butyrate, ■, control. *DB > LB and C, P < 0.001. Bars not sharing a common letter are significantly different at P < 0.05.
Effect of a mitochondrial uniporter inhibitor (RU-360) on intracellular Ca2+ levels
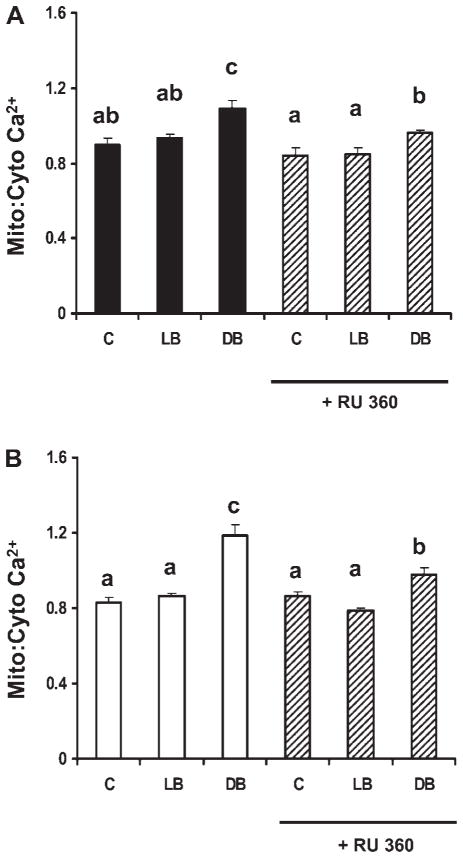
To investigate the role of the mitochondrial uniporter in Ca2+ uptake, cells were treated with RU-360, an inhibitor of the mitochondrial Ca2+ uniporter. Since the combination of DHA and butyrate maximally induced apoptosis and mitochondrial Ca2+ (Figs. 1 and 2), only control vs. LA + butyrate and DHA + butyrate treatments were examined. As expected, RU-360 (10 μM) partially inhibited the butyrate-induced increase in mitochondrial Ca2+ in DHA primed cells by 58 and 62% (P < 0.001) in p53+/+ (Fig. 3A) and p53−/− cells (Fig. 3B), respectively. In comparison, RU-360 had no significant effect on mitochondrial Ca2+ levels in both LA and butyrate-treated and untreated p53+/+ or p53−/− cells.
Fig. 3.
Effect of a mitochondrial uniporter inhibitor (RU-360) on mitochondrial Ca2+ levels following fatty acid and butyrate cotreatment. The p53+/+ cells (A) and p53−/− (B) were pretreated with fatty acid for a total of 72 h and RU-360 (10 μM) (hatched bars) for 30 min prior to butyrate cotreatment for the last 12 h. Cells were coincubated with fluo-4 AM (3 μM) and rhod-2 AM (2 μM) for 1 h and the mitochondrial-to-cytosolic Ca2+ ratio was measured. Data represent means ± SE from a representative experiment, n = 2 independent experiments, n = 20; 40 cells per treatment per experiment. Note that RU-360 partially reversed the effects of the combination treatment (DHA and butyrate) on the intracellular Ca2+ ratio in both cell lines. Bars not sharing a common letter are significantly different at P < 0.05.
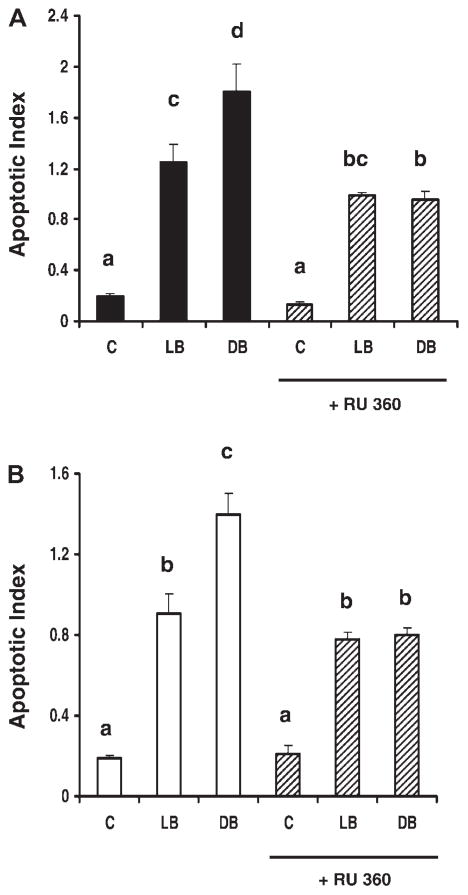
Inhibition of the Ca2+ uniporter partially suppresses the induction of apoptosis following DHA and butyrate cotreatment. The relationship between mitochondrial Ca2+ homeostasis and cellular apoptosis was examined by quantifying apoptosis following the blockade of mitochondrial Ca2+ uptake. For this purpose, select p53+/+ and p53−/− cultures were treated with RU-360. Results obtained in experiments with butyrate (12 h) following DHA or LA cotreatment are shown in Fig. 4. RU-360 significantly (P < 0.0001) reduced apoptosis by 47% in DHA and butyrate-pretreated p53+/+ cells compared with those preincubated with DHA and butyrate only (Fig. 4A). Similarly, p53−/− cells exhibited a 44% decrease (P < 0.0001) in apoptosis in the presence of RU-360 (Fig. 4B). In contrast, inhibition of the uniporter had no significant effect on cells treated with LA + butyrate or untreated cultures in both cell types. Taken together, these results demonstrate that mitochondrial Ca2+ uptake is required for the enhanced apoptosis associated with DHA and butyrate cotreatment.
Fig. 4.
Effect of RU-360 on the induction of apoptosis. The p53+/+ cells (A) and p53−/− cells (B) were treated with DHA or LA (50 μM) for 48 h and subsequently coincubated with 10 μM RU-360 (hatched bars) for 30 min. Following coincubation with fatty acid and butyrate for the final 12 h, the nucleosomal fragmentation assay was used to quantify apoptosis. Data are means ± SE, n = 6 wells per treatment. Bars not sharing a common letter are significantly different at P < 0.05.
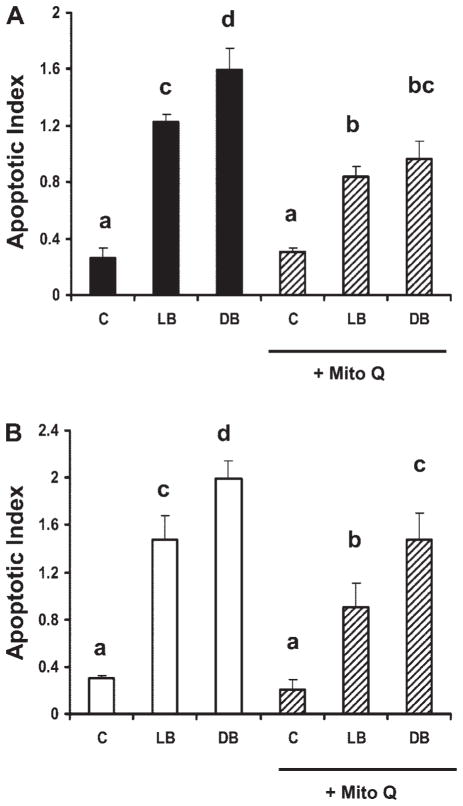
Mitochondria-specific antioxidant, Mito-Q, inhibits apoptosis induced by DHA and butyrate cotreatment. To probe the association of mitochondrial oxidative stress with the induction of apoptosis, select p53+/+ and p53−/− cultures were treated with 5 μM Mito-Q for 12 h in the presence of butyrate and fatty acid. Mito-Q significantly decreased apoptosis (P < 0.0001) in DHA and butyrate-cotreated cultures by 40% in p53+/+ cells and 26% in p53−/− cells (Fig. 5). Mito-Q also inhibited apoptosis in LA and butyrate-treated cultures by 32 and 39% in p53+/+ and p53−/− cultures, respectively (Fig. 5). In contrast, Mito-Q had no effect on untreated cell cultures. Collectively, these results suggest that mitochondrial-derived ROS production may in part mediate fatty acid and butyrate-induced colonocyte apoptosis.
Fig. 5.
Effect of a mitochondrial-targeted antioxidant (Mito-Q) on cellular apoptosis following fatty acid and butyrate cotreatment. The p53+/+ and p53−/− cultures were treated with 50 μM DHA or LA for 72 h and butyrate for the final 12 h. Select cultures were pretreated with 5 μM Mito-Q for 24 h prior to butyrate cotreatment. Nonadherent cells were harvested and apoptosis was measured by the nucleosomal fragmentation release assay. Data are means ± SE, n = 6 wells per treatment. A: p53+/+ cells (solid bars) ± Mito-Q incubation (hatched bars). B: p53−/− cells (open bars) ± Mito-Q incubation (hatched bars). Bars not sharing a common letter are significantly different at P < 0.05.
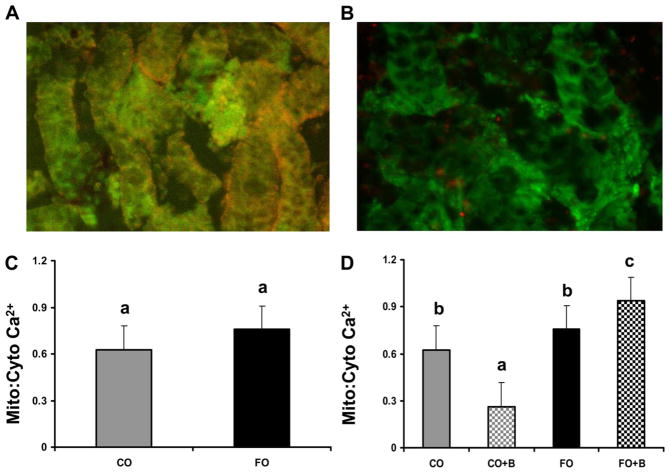
Mitochondrial-to-cytosolic Ca2+ ratio increases in colonic crypts isolated from rats fed fish oil followed by butyrate incubation ex vivo. Intact colonic crypts isolated from rats fed experimental diets containing either LA (corn oil) or DHA (fish oil) were incubated with fluo-4 AM and rhod-2 AM (Fig. 6A) for the purpose of imaging mitochondrial-to-cytosolic Ca2+ responses following incubation with butyrate (5 mM) ex vivo. Only crypts that exhibited higher than 85% cell viability were included in the analysis (Fig. 6B). Dietary fat composition did not significantly (P = 0.52) influence the basal mitochondrial-to-cytosolic Ca2+ ratio (Fig. 6C). In contrast, butyrate treatment and dietary lipid-butyrate interactions in the main model were significant; both P values < 0.0001. A significant decrease in mitochondrial-Ca2+-cytosolic ratio was seen in crypts from animals fed corn oil incubated in the presence of butyrate. However, the colonic crypts from fish oil-fed animals incubated in the presence of 5 mM butyrate for 30 min exhibited a 3.6-fold increase (P < 0.001) in the mitochondrial-to-cytosolic Ca2+ ratio compared with crypts incubated with butyrate isolated from corn oil-fed animals (Fig. 6D). These data demonstrate that DHA + butyrate combination enhances mitochondrial Ca2+ stores in primary rat crypt cultures.
Fig. 6.
Measurement of intracellular Ca2+ in colonic crypt primary cultures. Rats were fed either fish oil (FO, n-3 PUFA) or corn oil (CO, n-6 PUFA)-enriched diets for 3 wk, and colonic crypts were isolated and subsequently incubated with or without 5 mM butyrate (B) for 30 min. Crypts were coin-cubated with fluo-4 AM (3 μM) and rhod-2 AM (2 μM) for 30 min and the mitochondrial-to-cytosolic Ca2+ ratio was determined. A: representative fluorescence image of crypts coloaded with fluo-4 and rhod-2. B: example of select cultures incubated with calcein (green) and ethidium homodimer (orange) to assess cell viability. C: relative mitochondrial-to-cytosol Ca2+ ratios in isolated crypts under basal conditions, no butyrate added to the culture media. D: effect of exogenous butyrate on mitochondrial Ca2+ levels. Data are means ± SE from 15 rats per treatment group with at least 15 images per rat. Bars not sharing a common letter are significantly different, P < 0.001. Bars not sharing a common letter are significantly different at P < 0.05.
DISCUSSION
The in vivo molecular mechanisms underlying n-3 PUFA-induced apoptosis in the colon are just emerging (15). Furthermore, it is unclear whether observations derived from specialized cell lines accurately reflect endogenous cellular mechanisms. In the present study, we compared the effects of dietary fish vs. corn oil, enriched in DHA (22:6n-3) and LA (18:2n-6), respectively, in combination with butyrate, on mitochondrial Ca2+ accumulation in primary colon crypt cultures and in human colonocyte tumor cell lines. Furthermore, our studies examined the relationship between the p53 tumor suppressor gene and mitochondrial Ca2+ signals with regard to the induction of colonocyte apoptosis in HCT-116 cells. The present data reveal that 1) the combination of DHA and butyrate enhance apoptosis by stimulating mitochondrial Ca2+ accumulation in a p53-independent manner; 2) blockade of the mitochondrial uniporter inhibits Ca2+-induced colonocyte apoptosis; 3) suppression of mitochondrial oxidative stress with a mitochondria-targeted antioxidant partially inhibits apoptosis; and 4) butyrate incubation with primary colonic crypts isolated from fish oil-fed animals increases mitochondrial Ca2+ stores compared with cultures from corn oil-fed animals.
We initially investigated whether p53 plays an essential role in DHA and butyrate-induced apoptosis. This is significant because inactivating mutations in this tumor suppressor gene occur in ~50% of all colon tumors (44, 59). Existing data suggest that apoptosis can be mediated by the induction of the p53-regulated modulator PUMA (45, 57, 63), a mitochondrial protein (41, 65). Given the central role of mitochondria in the commitment to apoptosis, we hypothesized that DHA and butyrate interactively promote p53-dependent apoptosis by triggering changes in mitochondrial Ca2+ levels that contribute to caspase activation and colonocyte cell death. We used isogenic p53 wild-type and deficient human colon tumor (HCT-116) cell lines to determine whether chemoprotective nutrients modulate intracellular Ca2+ compartmentalization to induce colonocyte apoptosis. The results confirm and extend our previous observations (36, 42) and demonstrate that DHA and butyrate synergistically enhance mitochondrial Ca2+ accumulation, which serves as a trigger for apoptosis in a p53-independent manner.
Consistent with previous observations (12, 29, 30, 54), our data demonstrate that pleiotropic bioactive components generated by fermentable fiber (butyrate) and fish oil (DHA) act synergistically to enhance apoptosis. This is noteworthy because it has now been clearly established that the transformation of colonic epithelium to carcinoma is in part associated with a progressive inhibition of apoptosis (4, 28, 58). Hence, chemotherapeutic agents in combination that restore the normal apoptotic pathways have the potential for effectively reducing cancer risk (24, 55). Although it has been well documented that butyrate is an inhibitor of histone deacetylases and can activate the Fas receptor-mediated extrinsic death pathway (23, 60, 66), the role of these mechanisms in the induction of colonocyte apoptosis may be a secondary consequence of its ability to promote cellular oxidation (40, 42). For example, serving as the primary energy source for colonic epithelial cells, butyrate induces cellular ROS generation (8, 42). This is relevant because DHA compared with LA is oxidatively susceptible primarily because of its high degree of unsaturation (14, 25, 29). Consistent with this hypothesis, the combined effects of fatty acid and butyrate-induced apoptosis were partially blocked by coincubation with a mitochondrially targeted antioxidant, Mito-Q (34). Mito-Q reduces colonocyte lipid oxidation induced by butyrate in the presence of fatty acids (42). These data are noteworthy because lipid peroxidation can directly trigger the release of proapoptotic factors from mitochondria into the cytosol (7, 33). Current experiments are exploring whether other long-chain fatty acids, e.g., EPA, when combined with butyrate can perturb intracellular Ca2+ and induce apoptosis in colonocytes.
The overloading of mitochondrial Ca2+ can activate the mitochondrial transition (MPT) pore and trigger a series of events culminating in cellular apoptosis (17, 31, 37). Since we have previously observed that butyrate and DHA treatment dissipate the MPT and enhance apoptosis in vitro (42) and in vivo (29), we determined the effect of cotreatment on mitochondrial Ca2+ homeostasis. Interestingly, the combination of DHA and butyrate maximally increased intramitochondrial Ca2+ levels at 6, 12, and 24 h in p53+/+ and p53−/− cells. With regard to the biological relevance of this effect, the magnitude of the changes in intramitochondrial Ca2+ levels are consistent with previous observations in apoptotic cells (6, 43). Butyrate cotreatment (12 h) in DHA- vs. LA-incubated cells also increased apoptosis in both p53+/+ and p53−/− cells. RU-360, a mitochondrial Ca2+ uniporter inhibitor (2), attenuated (P < 0.05) DHA and butyrate-induced mitochondrial Ca2+ accumulation and apoptosis by ~40% in all cell culture models. Along these lines, we have previously demonstrated that butyrate induces colonocyte apoptosis via a Fas receptor-mediated extrinsic pathway (23). In an extension of our findings, we demonstrate for the first time that the combination of DHA and butyrate, compared with LA and butyrate, enhances apoptosis by additionally recruiting a p53-independent, oxidation-sensitive, mitochondrial Ca2+-dependent (intrinsic) apoptotic pathway in colonocytes. This could in part explain why the combination of dietary fish oil (containing DHA) and pectin (fermented to butyrate in the colonic lumen) reduces aberrant crypt and tumor formation in the colon (12, 13, 22, 29, 54; Covert KL, Sanders LM, Hong MY, Taddeo S, Turner ND, Chapkin RS, Lupton JR, unpublished observations).
To address the physiological relevance of our observations, an in vivo proof-of-principle experiment was conducted. For this purpose, intact viable colonic crypts were isolated from rats fed diets containing either DHA or LA. Crypts were labeled with appropriate fluorescent probes to simultaneously measure cytosolic and mitochondrial Ca2+ pools. Having previously demonstrated using this model system that crypts from rats fed fish vs. corn oil incubated with butyrate ex vivo more readily undergo apoptosis (29), we hypothesized that primary crypt cultures enriched in DHA would exhibit an increase in mitochondrial Ca2+ following incubation with butyrate. Although no significant difference in the basal mitochondrial-to-cytosolic Ca2+ ratio in colonic crypts from fish oil or corn oil-fed animals was noted, the addition of butyrate to primary cultures induced mitochondrial Ca2+ accumulation exclusively in animals fed fish oil. In addition, a decrease in the mitochondrial-Ca2+-cytosolic ratio was noted in crypts from animals fed corn oil coincubated with butyrate vs. corn oil only (control) crypts. To our knowledge, this is the first report ever to measure mitochondrial Ca2+ in primary culture. Overall, the mitochondrial Ca2+ data are consistent with previous in vivo results indicating that colon crypts from fish oil or purified DHA ethyl ester-fed rats exposed to butyrate exhibit increased apoptosis, i.e., decreased mitochondria membrane potential, elevated cytochrome c translocation to cytosol, and caspase 3 activation (29).
In summary, our results indicate that DHA and butyrate work coordinately in the colon to trigger a previously unrecognized proapoptotic cycle involving p53-independent, mitochondrial Ca2+ loading. This is noteworthy in view of the search for toxicologically innocuous cancer chemoprevention approaches that are free of safety problems intrinsic to drugs administered over long periods of time.
Acknowledgments
The authors gratefully acknowledge Michael Murphy for generously providing the Mito-Q, Bert Vogelstein for the p53 isogenic cell lines, Richard Weis for donation of the fish oil, and Robert Burghardt and Laurie Davidson for thoughtful advice.
GRANTS
This study was supported by NIH Grants CA59034, CA74552, and P30ES09106.
References
- 1.Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, van Leeuwen JB, Reid ME, Ritenbaugh C, Vargas PA, Bhattacharyya AB, Earnest DL, Sampliner RE. Lack of effect of high-fiber cereal supplement on the recurrence of colorectal adenomas. N Engl J Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 2.An J, Chen Y, Huang ZJ. Critical upstream signals of cytochrome C release induced by a novel Bcl-2 inhibitor. J Biol Chem. 2004;279:19133–19140. doi: 10.1074/jbc.M400295200. [DOI] [PubMed] [Google Scholar]
- 3.Anti M, Armelao F, Marra G, Percesepe A, Bartoli GM, Palozza P, Parrella P, Canetta C, Gentiloni N, De Vitis I, Gasbarrini G. Effects of different doses of fish oil on rectal cell proliferation in patients with sporadic colonic adenomas. Gastroenterology. 1994;107:1709–1718. doi: 10.1016/0016-5085(94)90811-7. [DOI] [PubMed] [Google Scholar]
- 4.Archer SY, Meng S, Shei A, Hodin RA. p21WAF1 is required for butyrate-mediated growth inhibition of human colon cancer cells. Proc Natl Acad Sci USA. 1998;95:6791–6796. doi: 10.1073/pnas.95.12.6791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartram HP, Gostner A, Scheppach W, Reddy BS, Rao CV, Dusel G, Richter F, Richter A, Kasper H. Effects of fish oil on rectal cell proliferation, mucosal fatty acids, and prostaglandin E2 release in healthy subjects. Gastroenterology. 1993;105:1317–1322. doi: 10.1016/0016-5085(93)90135-y. [DOI] [PubMed] [Google Scholar]
- 6.Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bayir H, Fadeel B, Palladino MJ, Witasp E, Kurnikov IV, Tyrurina YY, Tyurin VA, Amoscato AA, Jiang J, Kochanek PM, DeKosky ST, Greenberger JS, Shvedova AA, Kagan VE. Apoptotic interactions of cytochrome c: redox flirting with anionic phospholipids within and outside of mitochondria. Biochim Biophys Acta. 2006;1757:648–659. doi: 10.1016/j.bbabio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Benard O, Balasubramanian KA. Modulation of glutathione level during butyrate-induced differentiation in human colon derived HT-29 cells. Mol Cell Biochem. 1997;170:109–114. doi: 10.1023/a:1006892929652. [DOI] [PubMed] [Google Scholar]
- 9.Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, Tjonneland A, Overvad K, Martinez C, Dorronsoro M, Gonzalez CA, Key TJ, Trichopoulou A, Naska A, Vineis P, Tumino R, Krogh V, Bueno-de-Mesquita HB, Peeters PH, Berglund G, Hallmans G, Lund E, Skeie G, Kaaks R, Riboli E. Dietary fibre in food and protection against colorectal cancer in the European prospective investigation into cancer and nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. doi: 10.1016/s0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- 10.Boitier E, Rea R, Duchen MR. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J Cell Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 12.Chang WC, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced tumorigenesis by increased cell Differentiation and apoptosis rather than decreased cell proliferation. J Nutr. 1998;18:351–357. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- 13.Chang WCL, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;18:721–730. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- 14.Chapkin RS, Hong MY, Fan YY, Davidson LA, Sanders LM, Henderson CE, Barhoumi R, Burghardt RC, Turner ND, Lupton JR. Dietary n-3 PUFA alter colonocyte mitochondrial membrane composition and function. Lipids. 2002;37:193–199. doi: 10.1007/s11745-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 15.Chapkin RS, McMurray DN, Lupton JR. Colon cancer, fatty acids and anti-inflammatory compounds. Curr Opin Gastroenterol. 2007;23:48–54. doi: 10.1097/MOG.0b013e32801145d7. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J, Ogawa K, Kuriki K, Yokoyama Y, Kamiya T, Seno K, Okuyama H, Wang J, Luo C, Fujii T, Ickikawa H, Shirai T, Tokudome S. Increased intake of n-3 polyunsaturated fatty acids elevates the level of apoptosis in the normal sigmoid colon of patients polypectomized for adenomas/tumors. Cancer Lett. 2003;193:17–24. doi: 10.1016/s0304383502007176. [DOI] [PubMed] [Google Scholar]
- 17.Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J. 2006;272:433–450. doi: 10.1111/j.1742-4658.2005.05103.x. [DOI] [PubMed] [Google Scholar]
- 18.Conquer JA, Holub BJ. Effect of supplementation with different doses of DHA on the levels of circulating DHA as nonesterified fatty acid in subjects of Asian Indian background. J Lipid Res. 1998;39:286–292. [PubMed] [Google Scholar]
- 19.Courtney ED, Matthews S, Finlayson C, Di Pierro D, Belluzzi A, Roda E, Kang JY, Leicester RJ. Eicosapentaenoic acid (EPA) reduces crypt cell proliferation and increases apoptosis in normal colonic mucosa in subjects with a history of colorectal adenomas. Int J Colorectal Dis. 2007;22:765–776. doi: 10.1007/s00384-006-0240-4. [DOI] [PubMed] [Google Scholar]
- 21.Davidson LA, Lupton JR, Jiang YH, Chapkin RS. Carcinogen and dietary lipid regulate ras expression and localization in rat colon without affecting farnesylation kinetics. Carcinogenesis. 1999;20:785–791. doi: 10.1093/carcin/20.5.785. [DOI] [PubMed] [Google Scholar]
- 22.Davidson LA, Nguyen DV, Hokanson RM, Callaway ES, Isett RB, Turner ND, Dougherty ER, Wang N, Lupton JR, Carroll RJ, Chapkin RS. Chemopreventive n-3 polyunsaturated fatty acids reprogram genetic signatures during colon cancer initiation and progression in the rat. Cancer Res. 2004;64:6797–6804. doi: 10.1158/0008-5472.CAN-04-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan YY, Zhang J, Barhoumi R, Burghardt RC, Turner ND, Lupton JR, Chapkin RS. Antagonism of CD95 signaling blocks butyrate induction of apoptosis in young adult mouse colonic cells. Am J Physiol Cell Physiol. 1999;277:C310–C319. doi: 10.1152/ajpcell.1999.277.2.C310. [DOI] [PubMed] [Google Scholar]
- 24.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nature Rev Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 25.Gardner HW. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic Biol Med. 1989;7:65–86. doi: 10.1016/0891-5849(89)90102-0. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E, Willet W. Dietary factors and risk of colon cancer. Ann Med. 1994;26:443–452. doi: 10.3109/07853899409148367. [DOI] [PubMed] [Google Scholar]
- 27.Hajnoczky G, Hoek JB. Cell signaling. Mitochondrial longevity pathways. Science. 2007;315:607–609. doi: 10.1126/science.1138825. [DOI] [PubMed] [Google Scholar]
- 28.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2007;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 29.Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, Sanders LM, Fan YY, Davidson LA, Murphy ME, Spinka CM, Carroll J, Lupton JR. Fish oil increases mitochondrial phospholipid unsaturation, upregulating reactive oxygen species and apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- 30.Hong MY, Lupton JR, Morris JS, Wang N, Carroll RJ, Davidson LA, Elder RH, Chapkin RS. Dietary fish oil reduces O6-methylguanine DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol Biomarkers Prev. 2000;9:819–826. [PubMed] [Google Scholar]
- 31.Jacobson J, Duchen MR. Mitochondrial oxidative stress and cell death in astrocytes—requirement for stored Ca2+ and sustained opening of the permeability transition pore. J Cell Sci. 2002;1115:1175–1188. doi: 10.1242/jcs.115.6.1175. [DOI] [PubMed] [Google Scholar]
- 32.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 33.Kagan VE, Tyurin VA, Jiang J, Tyurina YY, Ritov VB, Amoscato AA, Osipov AN, Belikova NA, Kapralov AA, Kini V, Vlasova II, Zhao Q, Zou M, Di P, Svistunenko DA, Kurnikov IV, Borisenko GG. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nature Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 34.Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, Smith RA, Murphy MP. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 35.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 36.Kolar S, Barhoumi R, Lupton JR, Chapkin RS. Docosahexaenoic acid and butyrate synergistically induce colonocyte apoptosis by enhancing mitochondrial Ca2+ accumulation. Cancer Res. 2007;67:5561–5568. doi: 10.1158/0008-5472.CAN-06-4716. [DOI] [PubMed] [Google Scholar]
- 37.Kowaltowski AJ, Castilho RF, Vercesi AE. Mitochondrial permeability transition and oxidative stress. FEBS Lett. 2001;495:12–15. doi: 10.1016/s0014-5793(01)02316-x. [DOI] [PubMed] [Google Scholar]
- 38.Lee DY, Lupton JR, Aukema HM, Chapkin RS. Dietary fat and fiber alter rat colonic mucosal lipid mediators and cell proliferation. J Nutr. 1993;123:1808–1817. doi: 10.1093/jn/123.11.1808. [DOI] [PubMed] [Google Scholar]
- 39.Lupton JR. Microbial degradation products influence colon cancer risk; the butyrate controversy. J Nutr. 2000;134:479–482. doi: 10.1093/jn/134.2.479. [DOI] [PubMed] [Google Scholar]
- 40.Mariadason JM, Corner GA, Augenlicht LH. Genetic reprogramming in pathways of colonic cell maturation induced by short chain fatty acids: comparison with trichostatin A, sulindac, and curcumin and implications for chemoprevention of colon cancer. Cancer Res. 2000;60:4561–4572. [PubMed] [Google Scholar]
- 41.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 42.Ng Y, Barhoumi R, Tjalkens RB, Fan YY, Kolar S, Wang N, Lupton JR, Chapkin RS. The role of docosahexaenoic acid in mediating mitochondrial membrane lipid oxidation and apoptosis in colonocytes. Carcinogenesis. 2005;26:1914–1921. doi: 10.1093/carcin/bgi163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nutt LK, Chandra J, Pataer A, Fang B, Roth JA, Swisher SG, O’Neil RG, McConkey DJ. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J Biol Chem. 2002;277:20301–20308. doi: 10.1074/jbc.M106817200. [DOI] [PubMed] [Google Scholar]
- 44.Olschwang S, Hamelin R, Laurent-Puig P, Thuille B, De Rycke Y, Li YJ, Muzeau F, Girodet J, Salmon RJ, Thomas G. Alternative genetic pathways in colorectal carcinogenesis. Proc Natl Acad Sci USA. 1997;94:12122–12127. doi: 10.1073/pnas.94.22.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oren M. Decision making by p53: life, death and cancer. Cell Death Differ. 2003;10:431–442. doi: 10.1038/sj.cdd.4401183. [DOI] [PubMed] [Google Scholar]
- 46.Orrenius S. Mitochondrial regulation of apoptotic cell death. Toxicol Lett. 2004;149:19–23. doi: 10.1016/j.toxlet.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 47.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 48.Park Y, Hunter DJ, Spiegelman D, Bergkvist L, Berrino F, van den Brandt PA, Buring JE, Colditz GA, Freudenheim JL, Fuchs CS, Giovannucci E, Goldbohm RA, Graham S, Harnack L, Hartma M, Jacob DR, Jr, Kato I, Krogh V, Leitzmann MF, McCullough ML, Miller AB, Pietinen P, Rohan TE, Schatzkin A, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Smith-Warner SA. Dietary fiber intake and risk of colorectal cancer. A pooled analysis of prospective cohort studies. JAMA. 2005;294:2849–2857. doi: 10.1001/jama.294.22.2849. [DOI] [PubMed] [Google Scholar]
- 49.Peters U, Sinha R, Chatterjee N, Subar AF, Ziegler RG, Kulldorff M, Bresalier R, Weissfeld JL, Flood A, Schatzkin A, Hayes RB. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet. 2003;361:1491–1495. doi: 10.1016/S0140-6736(03)13173-X. [DOI] [PubMed] [Google Scholar]
- 50.Pinhero JC, Bates DM. Mixed Effects Models in S and S-PLUS. New York: Springer Verlag; 2000. [Google Scholar]
- 51.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 52.Rahman-Roblick R, Roblick UJ, Hellman U, Conrotto P, Liu T, Becker S, Hirschberg D, Jornvall H, Auer G, Wiman KG. p53 targets identified by protein expression profiling. Proc Natl Acad Sci USA. 2007;104:5401–5406. doi: 10.1073/pnas.0700794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rizzuto R, Pozzan T. Microdomain of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 54.Sanders LM, Henderson CE, Hong MY, Barhoumi R, Burghardt RC, Wang N, Spinka CM, Carroll RJ, Turner ND, Chapkin RS, Lupton JR. Enhancement of reactive oxygen species by dietary fish oil and attenuation of antioxidant defenses by dietary pectin coordinately heightens apoptosis in rat. J Nutr. 2004;134:3233–3238. doi: 10.1093/jn/134.12.3233. [DOI] [PubMed] [Google Scholar]
- 55.Sarkar FH, Li Y. Using chemopreventive agents to enhance the efficacy of cancer therapy. Cancer Res. 2006;66:3347–50. doi: 10.1158/0008-5472.CAN-05-4526. [DOI] [PubMed] [Google Scholar]
- 56.Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha apoptotic signalling. J Biol Chem. 2001;276:12035–12040. doi: 10.1074/jbc.M010603200. [DOI] [PubMed] [Google Scholar]
- 57.Shibue T, Suzuki S, Okamoto H, Yoshida H, Ohba Y, Takaoka A, Taniguchi T. Differential contribution of Puma and Noxa in dual regulation of p53-mediated apoptotic pathways. EMBO J. 2006;25:4952–4962. doi: 10.1038/sj.emboj.7601359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Siniscrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–241. [PubMed] [Google Scholar]
- 59.Smith G, Carey FA, Beattie J, Wilkie MJ, Lightfoot TJ, Coxhead J, Garner RC, Steele RJ, Wolf CR. Mutations in APC, Kirsten-ras, and p53-alternative genetic pathways to colorectal cancer. Proc Natl Acad Sci USA. 2002;99:9433–9438. doi: 10.1073/pnas.122612899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Smith JG, Yokoyama WH, German JB. Butyric acid from the diet: actions at the level of gene expression. Crit Rev Food Sci. 1998;38:259–297. doi: 10.1080/10408699891274200. [DOI] [PubMed] [Google Scholar]
- 61.Spector AA. Essentiality of fatty acids. Lipids. 1999;34:S1–S3. doi: 10.1007/BF02562220. [DOI] [PubMed] [Google Scholar]
- 62.Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. doi: 10.1016/j.ceca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 63.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2001;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 64.Whelan J, McEntee MF. Dietary (n-6) PUFA and intestinal tumorigenesis. J Nutr. 2004;134:3421S–3426S. doi: 10.1093/jn/134.12.3421S. [DOI] [PubMed] [Google Scholar]
- 65.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 66.Zhang J, Wu G, Chapkin RS, Lupton JR. Energy metabolism of rat colonocytes changes during the tumorigenic process and is dependent on diet and carcinogen. J Nutr. 1998;128:1262–1269. doi: 10.1093/jn/128.8.1262. [DOI] [PubMed] [Google Scholar]
- 67.Zhang P, Smith R, Chapkin RS, McMurray DN. Dietary (n-3) poly-unsaturated fatty acids modulate murine Th1/Th2 balance toward the Th2 pole by suppression of Th1 development. J Nutr. 2005;135:1745–1751. doi: 10.1093/jn/135.7.1745. [DOI] [PubMed] [Google Scholar]