Abstract
Rice aroma, one of the most important qualities of rice, was the comprehensive result of volatiles in rice and human sense. In this study, the main volatile compounds in rice were analyzed by using gas chromatography-mass spectrometry and gas chromatography-olfactometry, and their correlations with sensory score were investigated. A total of eighty-five volatiles were found in rice samples. By combining odor activity value and correlation analysis, nine volatiles were considered as potential characteristic volatiles in rice aroma, namely hexanal, 2-pentylfuran, octanal, 2-acetyl-1-pyrroline (2-AP), 1-octen-3-ol, trans-2-octenal, decanal, trans-2-nonenal and trans, trans-2,4-decadienal. It was found that the volatiles negatively correlated with sensory scores were positively correlated with hexanal. It indicated that hexanal might be a representative of the negative volatiles of rice aroma. The effects of the nine potential characteristic volatiles on rice aroma were investigated by using sensory analysis. The results showed that the odor intensity and preference level of 2-AP, hexanal, and 1-octen-3-ol were significantly affected by the content. Furthermore, the aroma of cooked rice was significantly different after adding 2-AP, hexanal or trans, trans-2,4-decadienal. Rice aroma was increased by adding 2-AP and deteriorated by adding hexanal or trans, trans-2,4-decadienal, indicating that 2-AP contributed positively to rice aroma while hexanal and trans, trans-2,4-decadienal contributed negatively to rice aroma. Hexanal, 2-AP, and trans, trans-2,4-decadienal were suggested to be the key characteristic volatiles for future aroma evaluation.
Keywords: Rice, Aroma, GC-MS, Characteristic volatiles, Sensory analysis
Graphical abstract
Highlights
-
•
Eighty-five volatiles were identified by using GC-MS.
-
•
Characteristic volatiles were explored using OAV, GC-O, and correlation analysis.
-
•
Nine volatiles were identified as potential characteristic volatiles.
-
•
Effect of potential key volatiles on rice aroma was estimated by sensory analysis.
-
•
Hexanal, 2-AP and trans, trans-2,4-decadienal were highly contributed to rice aroma.
1. introduction
Rice aroma is generated by the interaction between volatiles in rice and olfactory receptors. It's one of the vital attributes that influenced the popularity of rice, and affected consumer preference to a certain extent (Akhoundzadeh et al., 2018; Yang et al., 2008). Therefore, aromatic rice with good appearance, texture and fragrance is more popular with consumer in the market, and more expensive than non-aromatic rice.
Currently, more than 500 volatile compounds have been detected in aromatic and non-aromatic rice, including aldehydes, ketones, alcohols, phenols, esters and heterocyclics and other compounds. Although many volatiles had been identified, only a few of them were considered to have important contributions to rice aroma (Ramtekey et al., 2021; Verma and Srivastav, 2020). The characteristic volatiles in rice samples were investigated in many works (Zheng et al., 2022; Choi and Lee, 2021; Wei et al., 2021). Since the 20th century, 2-acetyl-1-pyrroline (2-AP) has been reported to be a key aroma compound in rice in multiple studies, providing the flavor of popcorn (Kasote et al., 2021; Wei et al., 2021; Park et al., 2010; Maraval et al., 2008). It was considered as the most important discriminator between aromatic and non-aromatic rice. However, rice samples with similar 2-AP content might have different aroma quality, suggesting that some volatiles other than 2-AP also had important contribution to rice aroma. And different characteristic volatiles were obtained for different rice samples.
Heptanal, octanal, trans-2-decenal, 1-heptanol, trans-2-decen-1-ol, 3,7,11-trimethyl-3-dodecanol, 3-octene-2-one, and 2-AP were considered as biomarkers for distinguishing Wuchang rice from other rice (Hu et al., 2023b). Zhao et al. (2022) considered 22 volatile compounds (including benzaldehyde, 2-pentylfuran, trans-2-nonenal, 3-octen-2-one, 1-octanol, nonanal, 2-methoxy-4-vinylphenol, trans-2-heptenal, 2-octen-1-ol and so on) as key volatiles in cooked rice form different regions in China. 1-Octen-3-ol, 1-ethyl-3,5-dimethylbenzene, 2,6,11-trimethyldodecane, 3-ethyloctane, 2,7,10-trimethyldodecane, methyl salicylate, 2-octanone, and heptanal were selected as important compounds to discriminate different japonica rice cultivars (Lee et al., 2022). However, there was no conclusion as to which volatiles played a key role in the overall aroma of rice and could be used as key aroma compounds for evaluating rice aroma, making it difficult to make a breakthrough in the method of evaluating rice aroma quality.
Meanwhile, the aroma system of rice is very complex and not all volatile compounds have positive effects on rice aroma. Some compounds such as α-pyrrolidone, pyridine, guaiacol, indole and p-xylene were reported to possess fruity and floral odors and be beneficial to rice aroma, but lipid oxidation products such as hexanal, trans-2-octenal, octanal, and decanal were reported to possess undesirable odors and have negatively effect on rice aroma (Griglione et al., 2015; Ma et al., 2020; Nadaf et al., 2016). Therefore, the evaluation of rice aroma needs to be combined with other volatile compounds rather than using by 2-AP alone to evaluate rice aroma.
Gas chromatography-mass spectrometry (GC-MS) was widely used for qualitive and quantitative volatile compounds in rice. Since GC-MS can't directly explain the aroma of volatile compounds, it is often used in combination with gas chromatography-olfactometry (GC-O) and odor activity value (OAV) to evaluate the importance of volatile compounds to the overall flavor. However, the interactions between different volatile compounds would influence the final perceived. High levels of 1-propanol and 2-phenylethanol were reported to significantly inhibit the volatilization of 3-methylbutyric acid from liquor (Niu et al., 2020). In cheese, δ-dodecalactone promoted the expression of lactone fruity flavor, but γ-dodecalactone had an inhibitory effect on the expression of lactone fruity flavor (Chen et al., 2022). GC-O and OAV analysis ignore the interaction between volatile compounds. Hence, the GC-O and OVA results need further validation.
In this paper, multiple analysis techniques including GC-MS, GC-O, OAV analysis and sensory analysis were applied to analyze the characteristic volatiles in rice and their influence on cooked rice aroma. The volatiles in rice were first analyzed and quantified by using GC-MS. Then, GC-O analysis, correlation analysis between sensory scores and volatile contents, and OAV analysis were carried out to screen the main potential characteristic volatiles. Finally, the effects of the potential characteristic volatiles on the aroma quality of rice were investigated by sensory methods including sensory ranking and triangle test.
2. materials and methods
2.1. samples and chemicals
Thirty-one rice varieties (Suyunuo, Daohuaxiang, Meixiangzhan, Yuzhenxiang, Della, Basmati 370, Xiangjingnuo, XiangjingR109, Suxiangjing1hao, Xiangjing 111, Baimaoxiangnuo, Kajinuo, Zhongxiang1hao, Wuxiangjing 14, Dahuaxiangnuo, Yixiang B, Luxiang 90, Songxiang 06–317, Longxiang 04, Wuyou A, Chuanxiang 29B, Longfeng 06, Nongxiang 99, Yuzhuxiang, Meiguoxiangdao, Jasmine 85, Zhongjia 17, Zhonghua 11, Koshihikari, Zhong 2B, D50) were harvested in 2021 and 2022. Thirty-one samples harvested in 2021 were used for volatile profile analysis by GC-MS. Nine of the 31 samples planted and harvested in 2022 were used for GC-O analysis. After dehulled by a sheller (Satake, Tokyo, Japan) and milled by rice a polisher (LTJM-2008, Jing Ao), the rice samples were stored at the temperature of 4 °C, and analyzed within half a month.
2-Methyl-3-heptanedone used as internal standards, 2-pentylfuran, octanal, trans-2-octenal, 1-octen-3-ol, decanal and trans-2-nonenal were purchased from Tokyo Chemical Industry (Shanghai, China). Hexanal, isopropanol and trans, trans-2.4-decadienal were obtained from Shanghai Macklin Biochemical Co., Ltd (Shanghai, China), and 2-AP (10% w/w in toluene) was purchased from Toronto Research Chemicals (Toronto, Ontario, Canada).
2.2. preparation of cooked rice
The rice sample was cooked according the method in Chinese Agricultural Industry Standard NY/T 3837-2021 with some modifications. Briefly, 30g of milled rice was weighed into an aluminum box and washed with deionized water for twice. After adding appropriate deionized water (30 g for glutinous rice, 37.5 g for non-glutinous rice), the sample was sealed and soaked for 30 min. Then, the rice sample was steamed for 40min and simmered for 20min, and ready for the following analysis.
2.3. gas chromatography-mass spectrometry analysis
After 5g of cooked rice and 10 μL of 1 μg/mL 2-methyl-3-heptanone were added into a 40 mL brown extraction vial, the vial was sealed. The solid phase microextraction (SPME) fiber ((DVB/CAR/PDMS, 50/30 μm, 1 cm), Anpel, Shanghai, China) was exposed to the headspace of the vial at a temperature of 80 °C for 30 min. Then, the SPME fiber was inserted into the injection port of GC-MS (7200, Agilent, California, USA), and desorbed at 250 °C for 5 min. Spilt mode (5:1) was applied during injection. The volatiles were separated and evaluated by using a DB-WAX column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies Co.) with high-purity helium (purity >99.999%) as carrier gas at a flow rate of 1 mL/min. The oven temperature was set as 40 °C for 5 min, then programmed to 230 °C at 5 °C/min and maintained for 10 min. The mass selective detector was operated in electronic impact ionization mode (70 eV) with a scan range of m/z 40–500. The ion source temperature was 230 °C. All experiments were performed in triplicate.
The volatiles were identified first by comparing the mass spectra with those in the NIST 14 spectral database and self-established rice volatile compounds database, and then by comparing the Kovates’ retention indices (RIs) calculated from the retention times of a series of n-alkanes (C6–C24) (Equation 1) with reference values provided by NIST14. The relative content of volatiles were calculated by using Equation 2.
| (1) |
Where RTR(X), RTR(Z), RTR(Z+1) represent the retention time of tested compound x and n-alkanes with carbon numbers of Z, Z+1, respectively, and RTR(Z) < RTR(X) < RTR(Z+1).
| (2) |
where C is the relative content of tested volatile (ng/g); c is the concentration of 2-methyl-3-heptanone (μg/mL); A and A1 are the peak area of 2-methyl-3-heptanone and tested volatiles, respectively; v is volume of 2-methyl-3-heptanone (μL); m is mass of cooked rice (g).
2.4. gas chromatography-olfactometry analysis
An olfactory detector (9100, Brechbühler, Steinwiesenstrasse 3, Schlieren, Switzerland) was coupled to GC for the identification of odor-active compounds. The extraction procedure and instrument conditions for GC were basically the same as those described in section 2.3, except that the split mode was set to 2.5:1. Sensory panelists sniffed and recorded the odor characteristics, intensity and duration of the stimuli as well as their retention time. A 5-point scale was used for intensity ratings. All experiments were repeated five times.
2.5. sensory analysis
The sensory analysis was carried out in the sensory laboratory of Rice Product Quality Supervision and Inspection Centre, Ministry of Agriculture and Rural Affairs. Twelve sensory panelists (5 males and 7 females) were selected from the sensory laboratory of Rice Product Quality Supervision and Inspection Centre, Ministry of Agriculture and Rural Affairs, according to the GB/T 16291.1-2012 (Chinese National Standard). One week prior to the sensory experiment, the sensory panelists were trained once a day for half an hour on the purpose and methodology of the experiment, including the knowledge and description of the samples. During sensory analysis, at least 1 min was allowed to elapse between the evaluation of two samples, and no more than 7 samples were evaluated at one time, in order to avoid a "carry-over" effect.
Each panelist was authorized to conduct sensory analysis, had at least three years of sensory experience and had participated in sensory evaluation tests for rice flavor and eating quality. All samples used in the sensory analysis were non-toxic and no side effects on the body. And the sensory panelists in this study gave informed consent via the statement “I am aware that my responses are confidential, and I agree to participate in this study” where an affirmative reply was required to enter the study. They can withdraw from the study at any time without any reason.
2.5.1. sensory score evaluation
The sensory score evaluation of cooked rice was performed according to NY/T 596–2002 (Chinese Agricultural Industry Standard). The rice sample was first cooked as mentioned in section 2.2 and then scored by five sensory panelists with respect to the intensity of rice popcorn aroma. Very strong: 9–10 points; strong: 7–8 points; medium: 5–6 points; weak: 3–4 points; recognized or around the threshold: 1–2 points; no popcorn aroma: 0 point. The average score of 5 panelists was used as the final sensory score of rice aroma.
2.5.2. sensory ranking
The sensory ranking was performed with reference to GB/T 12318-2008 (Chinese National Standard). In order to simulate the aroma of volatiles in rice, five volatile solutions (10 μL, in isopropanol solution) with five concentrations were added to aluminum boxes, respectively. The simulated contents covered the contents of test volatiles in rice samples. After sealed and randomly numbered, the aluminum boxes were ranked by seven panelists according to odor intensity and preference level. The concentration of standard solution added was calculated by using Equation (3). Since relative contents of volatiles were obtained in section 2.3, correction factors (f) were determined by using the method in Chinese Light Industry standards (QB/T 4850-2015), and facilitated the calculation of volatile contents.
| (3) |
Where C1 is the content of volatile in standard solutions (μg/mL) and C is the relative content of volatile in rice (ng/g); f is the correction factor; m1 is mass of cooked rice (g); v1 is volume of standard solution added (μL). The volatile contents in standard solutions and corresponding simulated contents in the rice samples were shown in Table S1.
To assess whether there were significant differences between samples, Ftest was determined according to Equation 4. There were significant differences among samples (p ≤ 0.05) if Ftest > F (9.11); otherwise, there were no significant differences. In order to explore which samples were significantly different from others, the least significant difference (LSD) was further calculated by using Equation 5. There were significant differences between two samples (p ≤ 0.05) if the difference in rank between two samples was equal to or greater than LSD; otherwise, there was no significant difference.
| (4) |
| (5) |
where j and p are the numbers of panelists and samples, respectively; Ri is the rank sum of the ith sample and z value is 1.96 (p ≤ 0.05).
2.5.3. triangle test
Triangle test was carried out according to ISO 4120-2021. During the test, panelists was given a set of three cooked rice samples and informed that two of the samples were the same and the other was different. The set of rice samples contained the same cooked rice and a standard solution of one volatile had been added to one or two of the samples. Panelists reported which sample they thought was different and described the aroma differences. The test was repeated 24 times for each volatile. There was a perceptible difference between the samples with and without adding volatile, if the number of correct responses was greater than or equal to the number given in the standard (13/24, p ≤ 0.05).
3. results and discussion
3.1. characteristic volatile compounds in rice
Eighty-five volatile compounds were detected by GC-MS in rice samples (Table 1), including 2-AP, acids (3), alcohols (11), aldehydes (17), alkanes (4), aromatics (12), esters (7), furans (5), ketones (16) and others (9). Among the volatiles, only 4 volatiles were detected in all rice samples, namely hexanal, 2-pentylfuran, nonanal and trans-2-octenal. Aldehydes were the most abundant in rice, accounting for 37.28–86.82% of total volatiles (Fig. 1). Among the aldehydes, nonanal (4.52–106.92 ng/g) and hexanal (1.63–48.38 ng/g) were more abundant than other aldehydes (Table 1). In addition, a high relative content of furans (3.63–21.26%) was obtained. And 2-pentylfuran was the most abundant furan, whose relative content was 2.54–30.31 ng/g. It was considered as one of the important compounds to distinguish aromatic rice from non-aromatic rice (Setyaningsih et al., 2019). The proportions of ketones and alcohols ranged from 2.32 to 16.35% and 2.37–11.22%, respectively. Among the ketones and alcohols, 3-nonen-2-one, 6-methyl-5-hepten-2-one and 1-octen-3-ol played an important role in rice aroma due to their high contents and low odor thresholds. However, esters, acids and alkanes had low relative content proportions, accounting for 0–14.52%, 0–3.86% and 0–1.33%, respectively. In that they had high thresholds, only few esters, acids and alkanes produced unique aromas in rice. Their contribution to rice aroma was limited. The content of 2-AP in test rice samples were 0–5.18 ng/g, accounting for 0–7.48% of total volatiles. Owing to the low threshold (0.053 ng/g), 2-AP made an important contribution to rice aroma and was used to distinguish rice varieties (Hu et al., 2020). Besides, rice also contained a large number of other volatiles, such as indole, 4-ethylphenol, pyridine and p-cymene etc., which accounted for 0–49.92% of total volatiles. Among these, indole possessed the highest content (0–47.75 ng/g). It was reported to be one of the characteristic volatiles of the unique aroma of black rice, was higher in freshly cooked rice and decreased slightly with prolonged storage (Dong et al., 2008; Shinoda et al., 2020).
Table 1.
The relative contents of volatiles in rice samples.
| NO. | Classified | Compounds | Odora | Frequencyb | Thresholdc (ng/g) | Content (ng/g) | OAV | RId |
|---|---|---|---|---|---|---|---|---|
| Comp1 | Aldehydes | Hexanal | green tomato, green and grass-like | 31 | 5 | 1.63-48.38 | 0.33-9.68 | 1071/1078 |
| Comp2 | Aldehydes | Heptanal | fruity, fatty and rancid-like | 28 | 2.8 | 0–3.69 | 0–1.32 | 1174/1185 |
| Comp3 | Aldehydes | Octanal | citrus, fruity, floral, and fatty | 25 | 0 0.587 | 0–16.62 | 0–28.31 | 1277/1286 |
| Comp4 | Aldehydes | trans-2-Heptenal | fruity, green, fatty | 30 | 40 | 0–4.84 | <1 | 1308/1334 |
| Comp5 | Aldehydes | Nonanal | fat, citrus, green | 31 | 1.1 | 4.52–106.92 | 4.11–97.20 | 1381/1395 |
| Comp6 | Aldehydes | trans-2-Octenal | green and fatty-like | 31 | 3 | 1.04–7.32 | 0.35-2.44 | 1414/1428 |
| Comp7 | Aldehydes | trans, trans-2,4-Heptadienal | fatty, sweet, fruity citrus | 13 | 15.4 | 0–0.46 | <1 | 1479/1490 |
| Comp8 | Aldehydes | Decanal | soap, orange peel, tallow | 17 | 3 | 0–22.06 | 0–7.35 | 1482/1500 |
| Comp9 | Aldehydes | Benzaldehyde | nutty and bitter-like | 29 | 750.89 | 0–8.10 | <1 | 1504/1508 |
| Comp10 | Aldehydes | trans-2-Nonenal | fatty, tallow, beany, cucumber and woody-like | 30 | 0.19 | 0–5.96 | 0–31.37 | 1523/1532 |
| Comp11 | Aldehydes | Benzeneacetaldehyde | floral, herbal | 6 | 6.3 | 0–0.76 | <1 | 1619/1636 |
| Comp12 | Aldehydes | trans-2-Decenal | fatty and waxy-like | 24 | 17–250 | 0–1.25 | <1 | 1629/1634 |
| Comp13 | Aldehydes | 2-Butyl-2-octenal | green, vegetable, cucumber, fatty | 17 | 20 | 0–4.86 | <1 | 1655/1659 |
| Comp14 | Aldehydes | trans, trans- 2,4-Nonadienal | fatty, waxy and nutty-like | 2 | 0.1 | 0–0.57 | 0–5.7 | 1681/1686 |
| Comp15 | Aldehydes | 2-Undecenal | sweet | 7 | – | 0–0.90 | – | 1737/1755 |
| Comp16 | Aldehydes | trans, trans-2,4-Decadienal | chicken, fatty | 30 | 0.077 | 0–6.83 | 0–88.70 | 1793/1805 |
| Comp17 | Aldehydes | 2,6,6-Trimethyl-1-cyclohexene-1-carboxaldehyde | – | 2 | – | 0–0.08 | – | 1604/1590 |
| Comp18 | 2-AP | 2-Acetyl-1-pyrroline | popcorn, sweet | 27 | 0.053 | 0–5.18 | 0–97.74 | 1321/1331 |
| Comp19 | Acids | Nonanoic acid | waxy, dirty | 1 | 4600–9000 | 0–0.05 | <1 | 2155/2174 |
| Comp20 | Acids | Tetradecanoic acid | – | 14 | 10000 | 0–1.29 | <1 | 2669/2685 |
| Comp21 | Acids | n-Hexadecanoic acid | waxy, fatty | 26 | 20000 | 0–5.50 | <1 | 2879/2875 |
| Comp22 | Alcohols | 1-Octen-3-ol | mushroom | 30 | 1.5 | 0–9.17 | 0–6.11 | 1445/1459 |
| Comp23 | Alcohols | 2-Methyl-6-hepten-1-ol | – | 1 | – | 0–0.43 | – | 1459/1480 |
| Comp24 | Alcohols | 2-Ethyl-1-hexanol | citrus, floral, oily, sweet | 2 | 25482.2 | 0–1.65 | <1 | 1485/1494 |
| Comp25 | Alcohols | Linalol | lemon; orange; citrus; floral; sweet | 3 | 0.22 | 0–0.47 | 0–2.14 | 1541/1554 |
| Comp26 | Alcohols | 1-Octanol | citrus, fruity and floral-like | 2 | 125.8 | 0–4.47 | <1 | 1552/1558 |
| Comp27 | Alcohols | (Z)- 5-Octen-1-ol | green, melon, mushroom | 7 | 6 | 0–1.96 | <1 | 1606/1608 |
| Comp28 | Alcohols | 1-Nonanol | floral and citrus-like | 4 | 45.5 | 0–0.63 | <1 | 1653/1663 |
| Comp29 | Alcohols | α, α-Dimethylbenzenemethanol | – | 6 | – | 0–0.21 | – | 1744/1759 |
| Comp30 | Alcohols | trans-3,7,11-Trimethyl-1,6,10-dodecatrien-3-ol | – | 3 | – | 0–0.40 | – | 2029/2028 |
| Comp31 | Alcohols | Cedrol | cedarwood | 28 | – | 0–2.90 | – | 2101/2016 |
| Comp32 | Alcohols | 2-Phenoxyethanol | – | 5 | – | 0–0.20 | – | 2121/2107 |
| Comp33 | Alkanes | Heptane | – | 2 | 5000 | 0–0.08 | <1 | 704/705 |
| Comp34 | Alkanes | Tricyclo[2.2.1.0(2,6)]heptane, 1,7-dimethyl-7-(4-methyl-3-pentenyl) | – | 9 | – | 0–0.95 | – | 1560/1555 |
| Comp35 | Alkanes | β-Copaene | – | 2 | – | 0–0.44 | – | 1579/1562 |
| Comp36 | Alkanes | Diphenylmethane | sweet, green, wet, plastic | 2 | – | 0–0.14 | – | 1990/1994 |
| Comp37 | Aromatics | Toluene | ethereal-like | 29 | 527 | 0–0.59 | <1 | 1027/1036 |
| Comp38 | Aromatics | Ethylbenzene | – | 6 | 2205 | 0–0.12 | <1 | 11112/1123 |
| Comp39 | Aromatics | 1,3-Dimethylbenzene | – | 5 | – | 0–0.22 | – | 1126/1140 |
| NO. | Classified | Compounds | Odora | Frequencyb | Thresholdc (ng/g) | Content (ng/g) | OAV | RId | |
|---|---|---|---|---|---|---|---|---|---|
| Comp40 | Aromatics | Styrene | balsamic, gasoline | 29 | 65 | 0–1.55 | <1 | 1241/1250 | |
| Comp41 | Aromatics | Naphthalene | tar | 30 | 6 | 0–1.07 | <1 | 1714/1712 | |
| Comp42 | Aromatics | 1,3-Dimethoxybenzene | – | 1 | – | 0–0.60 | – | 1727/1730 | |
| Comp43 | Aromatics | 1,2,3,5,6,8a-Hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)- Naphthalene | – | 9 | 6 | 0–0.28 | <1 | 1742/1759 | |
| Comp44 | Aromatics | (R)-1-Methyl-4-(1,2,2-trimethylcyclopentyl)-benzene | – | 4 | – | 0–0.49 | – | 1802/1825 | |
| Comp45 | Aromatics | Butylated hydroxytoluene | phenolic, camphoreous | 4 | – | 0–0.78 | – | 1897/1911 | |
| Comp46 | Aromatics | 4-Isopropyl-6-methyl-1-methylene-1,2,3,4-tetrahydronaphthalene | – | 2 | – | 0–0.03 | – | 1938/1954 | |
| Comp47 | Aromatics | Biphenyl | pungent, green, geranium | 3 | 0. 5 | 0–0.11 | <1 | 1963/1967 | |
| Comp48 | Aromatics | 1,6-Dimethyl-4-(1-methylethyl)- naphthalene | – | 30 | – | 0–0.26 | – | 2198/2200 | |
| Comp49 | Esters | 1,6-Octadien-3-ol, 3,7-dimethyl-formate | – | 2 | – | 0–0.24 | – | 1541/1579 | |
| Comp50 | Esters | Hexadecanoic acid, methyl ester | – | 3 | – | 0–0.14 | – | 2206/2223 | |
| Comp51 | Esters | Hexadecanoic acid, ethyl ester | – | 3 | – | 0–2.40 | – | 2246/2270 | |
| Comp52 | Esters | Diethyl phthalate | – | 11 | – | 0–1.19 | – | 2350/2359 | |
| Comp53 | Esters | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester | – | 26 | – | 0–6.30 | – | 2520/2526 | |
| NO. | Classified | Compounds | Odora | Frequencyb | Thresholdc (ng/g) | Content (ng/g) | OAV | RId | |
|---|---|---|---|---|---|---|---|---|---|
| Comp54 | Esters | Dibutyl phthalate | – | 29 | – | 0–3.56 | – | 2660/2678 | |
| Comp55 | Esters | Methyl salicylate | Wintergreen, minty | 7 | 40 | 0–0.36 | <1 | 1752/1753 | |
| Comp56 | Furans | 2-Propylfuran | – | 2 | – | 0–0.04 | – | 1023/1011 | |
| Comp57 | Furans | 2-n-Butylfuran | nutty, roasted3 | 27 | – | 0–1.79 | – | 1125/1123 | |
| Comp58 | Furans | 2-Pentylfuran | floral, fruity, nutty, bean | 31 | 5.8 | 2.54-30.31 | 0.44-5.23 | 1222/1229 | |
| Comp59 | Furans | 2-Hexylfuran | – | 1 | – | 0–0.03 | – | 1318/1323 | |
| Comp60 | Furans | 2-n-Heptylfuran | faint, fruity, sweet, wine-like | 1 | – | 0–0.22 | – | 1423/1429 | |
| Comp61 | Ketones | 2-Heptanone | fruity and floral-like | 22 | 140 | 0–4.12 | <1 | 1172/1184 | |
| Comp62 | Ketones | 3-Octanone | nut | 12 | 1.3 | 0.0.29 | <1 | 1244/1261 | |
| Comp63 | Ketones | 2-Octanone | fruity and floral-like | 18 | 50.2 | 0–8.34 | <1 | 1274/1287 | |
| Comp64 | Ketones | 6-Methyl-5-hepten-2-one | banana-like | 30 | 68 | 0–3.17 | <1 | 1325/1338 | |
| Comp65 | Ketones | 2-Nonanone | fruity and herbaceous-like | 12 | 200 | 0–0.62 | <1 | 1377/1390 | |
| Comp66 | Ketones | 2-Decanone | orange-like floral | 7 | 8.3–41 | 0–0.90 | <1 | 1485/1495 | |
| Comp67 | Ketones | 3-Nonen-2-one | pleasant fruity | 14 | 800 | 0–0.71 | <1 | 1501/1506 | |
| Comp68 | Ketones | 6-Undecanone | – | 8 | 85–410 | 0–0.43 | <1 | 1519/1527 | |
| Comp69 | Ketones | Isophorone | camphoreous, fruity, musty | 24 | – | 0–4.93 | – | 1574/1577 | |
| Comp70 | Ketones | 6-Methyl-3,5-heptadien-2-one | – | 2 | – | 0–1.66 | – | 1576/1582 | |
| Comp71 | Ketones | 2-Undecanone | fruity, fatty | 10 | 5.5 | 0–0.61 | <1 | 1587/1599 | |
| Comp72 | Ketones | 2-Tridecanone | fatty, waxy, mushroom | 22 | – | 0–0.88 | – | 1798/1814 | |
| Comp73 | Ketones | 2-Pentadecanone | fatty, spicy, floral | 29 | – | 0–7.62 | – | 2009/2023 | |
| Comp74 | Ketones | 1-(2-Hydroxy-5-methylphenyl)- ethanone | – | 6 | – | 0–2.28 | – | 2172/2178 | |
| NO. | Classified | Compounds | Odora | Frequencyb | Thresholdc (ng/g) | Content (ng/g) | OAV | RId |
|---|---|---|---|---|---|---|---|---|
| Comp75 | Ketones | Benzophenone | – | 7 | – | 0–0.96 | – | 2449/2457 |
| Comp76 | Ketones | (E)-6,10-dimethylundeca-5,9-dien-2-one | Rose, floral, fruity | 30 | 60 | 0–5.44 | <1 | 1841/1856 |
| Comp77 | Others | 2-Methoxyphenol | – | 1 | – | 0–0.41 | – | 1838/1836 |
| Comp78 | Others | Pyridine | Pungent-like | 8 | 2000 | 0–0.21 | <1 | 1169/1176 |
| Comp79 | Others | p-Cymene | solvent, gasoline, citrus | 4 | 5.01 | 0–0.05 | <1 | 1255/1272 |
| Comp80 | Others | 2-Pentylthiophene | fruity, slightly fatty, cranberry | 21 | – | 0–0.82 | – | 1444/1452 |
| Comp81 | Others | Longifolene | Sweet, woody | 2 | – | 0–3.00 | – | 1552/1565 |
| Comp82 | Others | γ-Cadinene | wood | 2 | – | 0–0.13 | – | 1743/1745 |
| Comp83 | Others | α-Calacorene | wood | 14 | – | 0–0.14 | – | 1896/1916 |
| Comp84 | Others | 4-Ethylphenol | smoke, phenolic, creosote | 1 | 21 | 0–0.17 | <1 | 2156/2174 |
| Comp85 | Others | Indole | mothball, burnt | 28 | 40 | 0–47.75 | 0–1.19 | 2413/2414 |
Notes.
brepresent the number of times the compound was detected in 31 rice samples.
odor descriptions were obtained from http://www.thegoodscentscompany.com/,https://www.flavornet.org/and Verma, D. K and Srivastav, P. P. (Verma and Srivastav, 2020).
odor thresholds were obtained from Gemert, L. J. V. (Gemert, 2003).
before “/”: RIs calculated using n-alkanes C6 to C24 as external standards on a DB-Wax column; after “/”: reference RIs obtained from https://webbook.nist.gov/.
Fig. 1.
The profile of chemical group proportion of volatiles in rice samples.
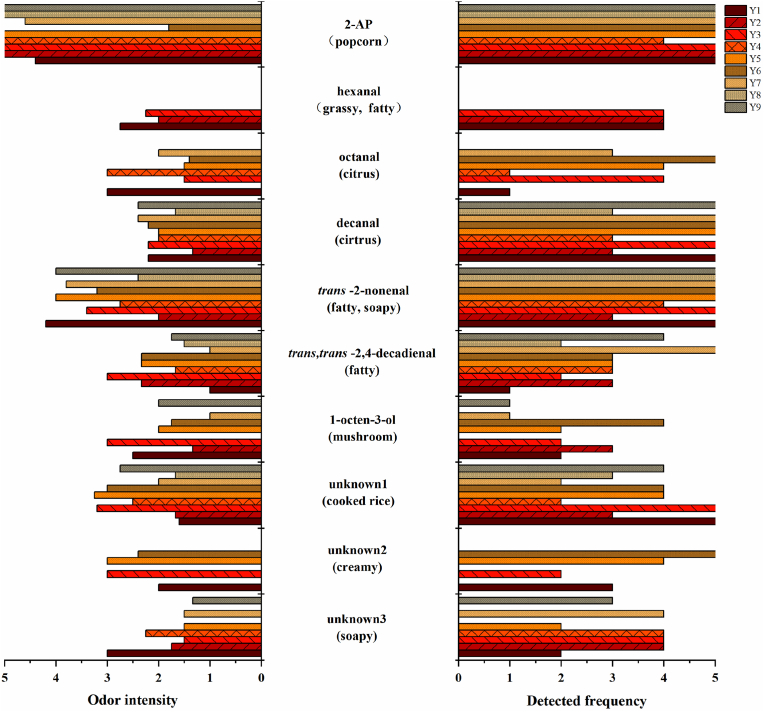
GC-O was used for the analysis of odor characteristic compounds. It could effectively explore active-odor compounds from varieties of volatiles. Nine rice samples were analyzed by GC-O, including 3 glutinous rice (Suyunuo; Xiangjingnuo; kajinuo), 3 japonica rice (Suxiangjing1hao; XiangjingR109; Koshihikari) and 3 indica rice (Chuanxiang29B; Yuzhenxiang; Yixiang B). Koshihikari was a non-aromatic rice, and the others were aromatic rice. Ten volatiles were sniffed through GC-O analysis, including 2-AP, 5 aldehydes (hexanal, octanal, trans-2-nonenal, decanal and tans, trans-2,4-decadienal), one alcohol (1-octen-3-ol) and 3 unknown volatiles (unknown1-3) (Fig. 2).
Fig. 2.
The odor intensity and detection frequency of odor-active volatiles sniffed during GC-O analysis. Y1: Suyunuo; Y2: Xiangjingnuo; Y3: kajinuo; Y4: Suxiangjing1hao; Y5: XiangjingR109; Y6: Koshihikari; Y7: Chuanxiang 29B; Y8: Yuzhenxiang; Y9: Yixiang B.
The odors of hexanal, octanal, trans-2-nonenal, decanal and trans, trans-2,4-decadienal were described as grassy and fatty, citrus, fatty and soap, citrus, fatty, respectively. Hexanal was only detected in glutinous rice, with odor intensity of 2.25–2.75 and detection frequency of 4 times. As it was an oxidation product of lipid whose content was higher in glutinous rice than non-glutinous rice (Yang et al., 2010), it was easier to be smelled in glutinous rice. Octanal was perceived 5 times in Y6, 4 times in Y3 and Y5, 3 times in Y7, with odor intensity varying from 0 to 3. Decanal, trans-2-nonenal and trans, trans-2,4-decadienal were perceived in all samples. The odor intensity of decanal and trans, trans-2,4-decadienal was 1-3, while that of trans-2-nonenal was 2-4.2. Among them, decanal and trans-2-nonenal were perceived in all repeats in six and seven samples, respectively. Trans, trans-2,4-decadienal was perceived in all repeats in one sample (Y7). Meanwhile, 2-AP was perceived in all samples and the odor intensity was 1.8-5 with a popcorn aroma. It was found that the odor intensity of 2-AP in aromatic rice (4-5) were greater than that in non-aromatic rice (1.8, Y6). It was consisted with previous study that higher odor intensity of 2-AP was obtained for aromatic rice than non-aromatic rice (Wei et al., 2021). The odor of 1-octen-3-ol was perceived in 7 samples and described as mushroom flavor with the odor intensity ranged from 1 to 3. It was reported to contribute greatly to rice flavor due to its high content and low threshold (Wang et al., 2019). In addition, 3 unknown volatiles, which were described as cooked rice, creamy and soapy, respectively, were also sniffed during GC-O analysis.
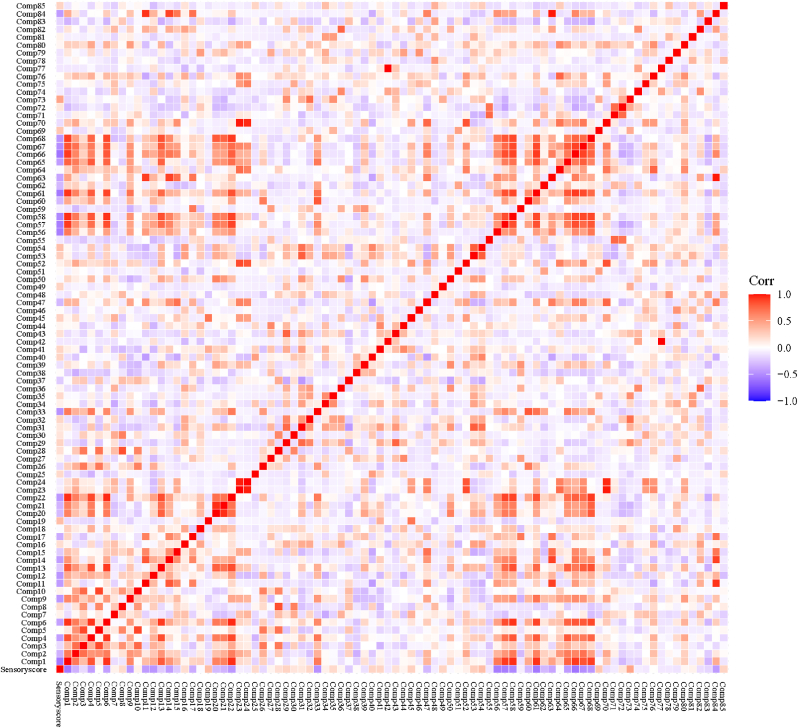
The rice aroma was produced by comprehensive result of volatiles. The correlations between volatile content and the sensory score were investigated (Fig. 3). As seen from Fig. 3, hexanal (Comp1) had a negative correlation with sensory score (r = -0.58) and positive correlations with trans-2-heptenal (Comp4, r = 0.83), trans-2-octenal (Comp6, r = 0.93), 2-butyl-2-octenal (Comp13, r = 0.8), 1-octen-3-ol (Comp22, r = 0.94), 2-n-butylfuran (Comp57, r = 0.91), 2-pentylfuran (Comp58, r = 0.94),2-heptanone (Comp61, r = 0.93), 2-decanone (Comp66, r = 0.91), 3-nonen-2-one (Comp67, r = 0.83), 6-undecanone (Comp68, r = 0.9). All of them had a negative correlation with sensory score (r < -0.4), except for 2-buty-2-octenal (r = -0.23). Additionally, benzaldehyde (Comp9), benzeneacetaldehyde (Comp11), trans-2-decenal (Comp12), trans, trans-2,4-nonadienal (Comp14), heptane (Com33), 2-Nonanone (Comp65) and 4-ethylphenol (Comp84) also had a negative correlation with sensory score (r < -0.4), and positively correlated with hexanal (r ≥ 0.35). It revealed that hexanal could be a representative of negative volatiles for rice aroma.
Fig. 3.
Correlation analysis between volatiles and sensory score. The compound numbers in this were the same as those in Table 1.
Octanal (Comp3) was considered as an early oxidation marker and increased during storage (Choi et al., 2019). It had positive correlations with decanal (Comp8, r = 0.56) and trans-2-nonenal (Comp10, r = 0.75). They had low correlations with sensory score (octanal, r = −0.16; decanal, r = 0.19, trans-2-nonenal, r = 0.07). Nevertheless, GC-O analysis showed that they were active-odor compounds in rice and had an influence on rice aroma. Nonanal (Comp5), an abundant volatile in rice, was one of the most important volatiles, contributing to the aroma profile of different rice varieties (Chen et al., 2023; Hu et al., 2023a). It had a positive correlation (r = 0.88) with trans-2-nonenal and also had a low correlation (r = 0.01) with sensory score. Trans, trans-2,4-decadienal (Comp16) had positive correlation (r = 0.46) with 2-AP (Comp18). However, both trans, trans-2,4-decadienal and 2-AP were weakly correlated with sensory score (r = 0.18, −0.02, respectively). Besides, trans, trans-2,4-decadienal, 2-AP also had positive correlations with trans-2-heptenal (Comp4) and p-cymene (Comp79) (r = 0.41, 0.51 respectively). The low correlation between 2-AP and sensory score might be caused by the influence of other volatiles. Correlation analysis was implemented by using rice samples with hexanal, 2-pentylfuran, trans-2-octenal and 1-octen-3-ol content around the odor thresholds, respectively. The results showed that the correlation between 2-AP and sensory score was significantly improved by using rice samples with low contents of hexanal, 2-pentylfuran, trans-2-octenal and 1-octen-3-ol, which increased to 0.38, 0.31, 0.46 and 0.41, respectively. It was indicted that the perception sensitivity of 2-AP was reduced due to the high content of negative volatiles.
1-Octen-3-ol was a degradation product of linoleic acid, and was an odor-active alcohol with a mushroom flavor. It was considered as a source of unpleasant odor in rice bran and increased with time during storage (Dias et al., 2021; Gao et al., 2021; Splivallo et al., 2011). It was positively correlated with hexanal (r = 0.94), trans-2-octenal (r = 0.95), and 2-pentylfuran (r = 0.89). Trans-2-octenal was reported to be associated with the nutty and roasty flavors of rice (Griglione et al., 2015; Zhao et al., 2020). 2-Pentylfuran was an important odor active in wild rice (Cho and Kays, 2013), whose flavor was described as bean, green and almond. It was reported that hexanal, trans-2-octanal, 2-pentylfuran, and 1-octen-3-ol were often used as markers of rice ageing in previous studies (Griglione et al., 2015; Zhou et al., 2015). It was consistent with the fact that they were negatively correlated with the sensory score. Hexanal, 2-pentylfuran, trans-2-octenal and 1-octen-3-ol were positively correlated (r ≥ 0.89) with each other. Thus, they might have an additive or synergistic effect with each other and adversely contribute to rice aroma. Ketones contributed fruity, nutty, floral flavor to rice aroma. A positive correlation (r = 0.47) was found between 2-pentadecanone (Comp 61) and sensory score.
OAV analysis is an important method to evaluate the contribution of volatiles to food aroma. It was implemented by evaluating the ratio of volatile content to odor threshold. In this paper, the relative contents of volatiles were obtained, and the relative OAVs were calculated (Table 1). Fourteen volatiles in rice were found to have relative OAVs greater than 1, namely hexanal, heptanal, 2-pentylfuran, octanal, 2-AP, nonanal, trans-2-octenal, 1-octen-3-ol, decanal, trans-2-nonenal, linalool, trans, trans-2,4-nonadienal, trans, trans-2,4-decadienal and indole (Table 1). Among them, 2-AP, nonanal, trans, trans-2,4-decadienal, trans2-nonenal and octanal had relative OAVs higher than 10, which could reach to 97.74, 97.20, 88.70, 31.37 and 28.31, respectively. Hence, 2-AP, nonanal, trans, trans-2,4-decadienal, trans2-nonenal and octanal were considered to have great contributions to rice aroma due to their high relative OVAs. The relative OAVs of hexanal, heptanal, 2-pentylfuran, trans-2-octenal, 1-octen-3-ol, decanal, linalool, trans, trans-2,4-nonadienal, and indole could reach to 9.68, 1.32, 5.23, 2.44, 6.11, 7.35, 2.14, 5.7 and 1.19, respectively. They were also supposed to contribute considerably to the aroma of rice. Among them, 1-octen-3-ol, hexanal, trans-2-octenal, 2-pentylfuran, and trans, trans-2,4-nonadienal showed a negative correlation (r < −0.4) with sensory score. Trans, trans-2,4-nonadienal was detected in only two rice samples, more data were needed to verify the result that trans, trans-2,4-nonadienal was a characteristic volatile in rice.
Combined the results of correlation analysis, OAV analysis and GC-O analysis, hexanal, 2-pentylfuran, octanal, 2-AP, 1-octen-3-ol, trans-2-octenal, decanal, trans-2-nonenal and trans, trans-2,4-decadienal were screened preliminarily as the potential characteristic volatiles. To further confirm the result, the absolutely OAVs of these compounds were obtained through their correlation factors which were determined according to Chinese Light Industry Standards (QB/T 4850-2015). It was found that the OAVs of hexanal, 2-pentylfuruan, octanal, 2-AP, trans-2-octenal, 1-octen-3-ol, decanal, trans-2-nonenal and trans, trans-2,4-decadienal were greater than 1, and reached to 139.82, 2.35, 136.10, 10770.57, 11.56, 53.53, 9.42, 64.47 and 32.86, respectively. Hence, hexanal, 2-pentylfuran, octanal, 2-AP, 1-octen-3-ol, trans-2-octenal, decanal, trans-2-nonenal and trans, trans-2,4-decadienal were considered as the potential characteristic volatiles of rice odor, and analyzed in the following sensory analysis.
3.2. The effect of characteristic volatiles on the aroma of rice
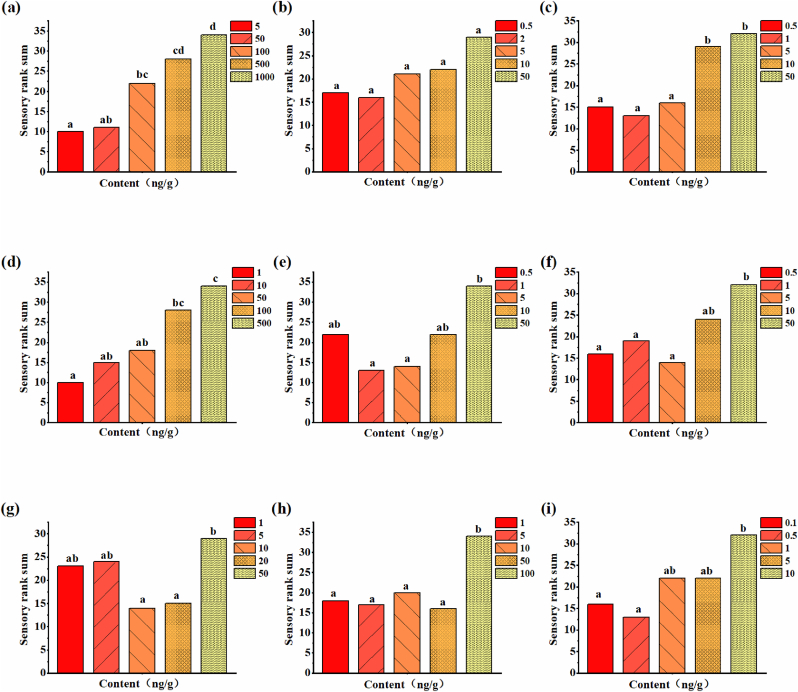
The odor of volatiles often varied with contents (Kaeppler and Mueller, 2013). In order to elucidate the odor perception of characteristic volatiles and to explore the changes in volatile aroma caused by different contents, sensory ranking of odor intensity and preference level was performed at different contents. The range of volatile content in rice samples was obtained by multiplying the relative content range by a correction factor (Table 2). Significant sensory differences in the odor intensity at different levels of content were found for all test volatiles except for 2-pentylfuran (Table 2). For hexanal, octanal, 2-AP, and 1-octen-3-ol, there were significant differences in the odor intensity perceived within the content ranges of rice samples. The odor intensity of hexanal had significant differences among the content ranges of 23.56–50 ng/g, 50–100 ng/g, 100–500 ng/g and 500–699.09 ng/g (Fig. 4). And significant differences in odor intensity between 0.5 and 5 ng/g and 10–79.78 ng/g, 1–100 ng/g and 50–570.84 ng/g, 0.5–10 ng/g and 50–80.53 ng/g were obtained for octanal, 2-AP, and 1-octen-3-ol, respectively. However, no significant differences in odor intensity were observed for trans-2-octenal, decanal and trans-2-nonenal and trans, trans-2,4-decadienal within the content range of rice samples.
Table 2.
Ftest values, odor descriptions and content range of volatiles.
| volatiles | F1test | F2test | contenta (ng/g) | Odor description |
|---|---|---|---|---|
| hexanal | 25.14 | 29.71 | 23.56–699.09 | grassy, aged rice flavor |
| 2-pentylfuran | 6.06 | 3.20 | 1.14-13.64 | green |
| octanal | 17.71 | 6.06 | 0–79.78 | citrus |
| 2-AP | 20.69 | 9.94 | 0–570.84 | popcorn, cooked rice |
| trans-2-octenal | 16.23 | −6.29 | 4.92-34.69 | unpleasant smell |
| 1-octen-3-ol | 11.89 | 9.49 | 0–80.53 | mushroom, sweet |
| decanal | 9.26 | 7.11 | 0–28.26 | fruity |
| trans-2-nonenal | 12.57 | 9.49 | 0–58.02 | fat, green |
| trans, trans-2.4-decadienal | 12.11 | 16.11 | 0–2.53 | fatty |
Notes: F1test and F2test were the F values of sensory intensity and preference level, respectively.
the content range of volatile in rice samples.
Fig. 4.
Sensory rank sum of odor intensity for volatile compounds at different contents. (a) hexanal; (b) 2-pentylfuran; (c) octanal; (d) 2-AP; (e) trans-octenal; (f) 1-octen-3-ol; (g) decanal; (h) trans-2-nonenal; (i) trans, trans-2,4-decadienal.
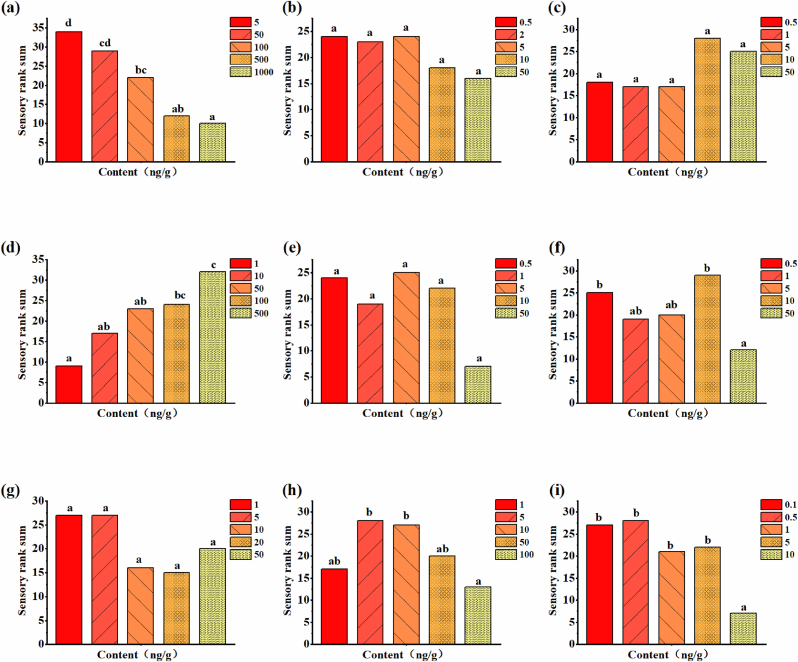
Moreover, the result of sensory ranking suggested that significant differences in preference level were found among the test contents for hexanal, 2-AP, 1-octen-3-ol, trans-2-nonenal and trans, trans-2,4-decadienal (Table 2). However, no significant difference in preference level was observed for trans-2-nonenal and trans, trans-2,4-decadienal within the content range of the rice sample. The preference level decreased with the content of hexanal and 1-octen-3-ol increasing (Fig. 5), indicating that hexanal and 1-octen-3-ol had a negative contribution to rice aroma. Furthermore, the negative contribution was also evidenced by the odor descriptions of hexanal and 1-octen-3-ol during sensory analysis, which were described as unpleasant grassy and aged rice flavor, and mushroom flavor. The preference level of 2-AP increased with the content, implying that 2-AP positively influenced the aroma of rice.
Fig. 5.
Sensory rank sum of preference level for volatile compounds at different contents. (a) hexanal; (b) 2-pentylfuran; (c) octanal; (d) 2-AP; (e) trans-octenal; (f) 1-octen-3-ol; (g) decanal; (h) trans-2-nonenal; (i) trans, trans-2,4-decadienal.
The perception of volatiles in rice matrix might be different from that without matrix, as the rice matrix was quite complex. To verify the influence of the characteristic volatile on rice aroma, each characteristic volatile was added to cooked rice samples, and consequently, triangle test was carried out. Triangle test was usually used to analyze whether there were perceptible differences between two samples. The result showed that the addition of hexanal, 2AP and trans, trans-2,4-decadienal caused significant changes in rice aroma (the number of correct selections were 24, 18 and 17, respectively). Moreover, according to the sensory description, adding 2-AP made the rice aroma stronger, while adding hexanal and trans, trans-2,4-decadienal made rice odor unpleasant. Therefore, an increase in 2-AP content in rice would improve the aroma quality of rice, and an increase in the contents of hexanal and trans, trans-2,4-decadienal would worsen the aroma quality of rice. Meanwhile, there were no significant perceptible differences between samples with and without adding 2-pentylfuran, octanal, trans-2-octenal, 1-octen-3-ol, decanal and trans-2-nonenal (the number of correct selections were 7, 10, 9, 10,11 and 9, respectively). Thus, to some extent, increasing in the contents of 2-pentylfuran, octanal, trans-2-octenal, 1-octen-3-ol, decanal and trans-2-nonenal would not cause significant perceptible changes in rice aroma.
Sensory ranking analysis showed that there were significant differences in the odor intensity of hexanal, 2-AP, octanal, and 1-octen-3-ol in the content range of rice samples. Moreover, significant differences in the preference level were observed for hexanal, 2-AP and 1-octen-3-ol, indicating significant influence of these volatiles on rice aroma. The result of triangle test showed that significant perceptible change in the aroma of cooked rice was observed after adding hexanal or 2-AP, further proving the significant effect of hexanal and 2-AP on rice aroma. Rice aroma increased by adding 2-AP and deteriorated by adding hexanal, indicating that 2-AP contributed positively to rice aroma while hexanal contributed negatively to rice aroma. Meanwhile, trans, trans-2,4-decadienal also had a negative effect on rice aroma as rice aroma was found to deteriorate after adding trans, trans-2,4-decadienal. Therefore, hexanal, trans, trans-2,4-decadienal and 2-AP were important characteristic volatiles for rice aroma. Their contents were supposed to have a great influence on the aroma quality of rice.
4. conclusion
In this study, 85 volatile compounds were found in rice by gas chromatography-mass spectrometry analysis. Correlation analysis revealed that the volatiles negatively correlated with sensory score were positively correlated (r ≥ 0.35) with hexanal, indicating that hexanal could represent compounds negatively correlated with sensory score. GC-O analysis, OAV analysis and correlation analysis indicated that hexanal, 2-pentylfuran, octanal, 2-AP, 1-octen-3-ol, trans-2-octenal, decanal, trans-2-nonenal, and trans, trans-2,4-decadienal were potential characteristic volatiles for rice aroma. Meanwhile, the results of sensory analysis implied that hexanal, 2-AP, 1-octen-3-ol, trans-2-nonenal, and trans, trans-2,4-decadienal had significant effects on the aroma of rice. Among them, 2-AP was found to enhance the rice aroma, while hexanal, 1-octen-3-ol had a negative effect on the rice aroma. Moreover, it was found that addition of 2-AP significantly enhanced the aroma of rice while addition of hexanal and trans, trans-2,4-decadienal significantly deteriorated the aroma of rice. Their contents were supposed to have a great effect on the aroma quality of rice. Hence, hexanal, trans, trans-2,4-decadienal and 2-AP were proposed to be the key volatiles in future aroma evaluation. This study investigated the characteristic volatiles of rice and their effects on rice aroma, providing a reference for the evaluation of aromatic rice and amelioration of rice quality in the future.
CRediT authorship contribution statement
Shuimei Li: Methodology, Investigation, Formal analysis, Validation, Data curation, Writing – original draft, Writing – review & editing. Hongyan Li: Investigation, Visualization, Validation. Lin Lu: Methodology, Validation, Resources. Gaoneng Shao: Resources, Methodology. Zhenling Guo: Investigation, Validation. Yuntao He: Investigation, Validation. Yong Wang: Resources, Supervision. Xiaohui Yang: Resources, Project administration. Mingxue Chen: Resources, Supervision, Project administration, Funding acquisition. Xianqiao Hu: Conceptualization, Methodology, Resources, Data curation, Project administration, Writing – review & editing.
Declaration of competing interest
All the authors of this paper have approved the manuscript that is enclosed and no conflict of interest exists in the submission of this manuscript, and the contents of this manuscript have not copyrighted or published previously and is not under consideration for publication elsewhere.
Acknowledgments
This work was supported by the "Pioneer" and "Leading Goose" R&D Program of Zhejiang (No. 2023C02014), Central Public-interest Scientific Institution Basal Research Fund (No. CPSIBRF-CNRRI-202125, Y2023LM10), the earmarked fund for China Agriculture Research System (CARS-01), and The Agricultural Science and Technology Innovation Program (ASTIP).
Handling Editor: Professor Aiqian Ye
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2024.100794.
Contributor Information
Mingxue Chen, Email: cmingxue@163.com.
Xianqiao Hu, Email: hxhxqiao@aliyun.com.
Appendix A. Supplementary data
The following is/are the supplementary data to this article.
Data availability
Data will be made available on request.
References
- Akhoundzadeh H., Gholami A., Masoum S., Moazeni-Pourasil R.S. Headspace solid-phase microextraction GC-MS for rapid rice aroma analysis using optimization tools. Chromatographia. 2018;81(6):931–945. doi: 10.1007/s10337-018-3517-1. [DOI] [Google Scholar]
- Chen C., Liu Z., Yu H., Lou X., Huang J., Yuan H., Tian H. Characterization of six lactones in cheddar cheese and their sensory interactions studied by odor activity values and feller's additive model. J. Agric. Food Chem. 2022;70(1):301–308. doi: 10.1021/acs.jafc.1c07924. [DOI] [PubMed] [Google Scholar]
- Chen J., Liu Y., Yang M., Shi X., Mei Y., Li J., Wen J. Analysis of the differences in volatile organic compounds in different rice varieties based on GC-IMS Technology combined with multivariate statistical modelling. Molecules. 2023;28(22) doi: 10.3390/molecules28227566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S., Kays S.J. Aroma-active compounds of wild rice (Zizania palustris L.) Food Res. Int. 2013;54(2):1463–1470. [Google Scholar]
- Choi S., Lee J. Volatile and sensory profiles of different black rice (Oryza sativa L.) cultivars varying in milling degree. Food Res. Int. 2021;141 doi: 10.1016/j.foodres.2021.110150. [DOI] [PubMed] [Google Scholar]
- Choi S., Seo H.S., Lee K.R., Lee S., Lee J., Lee J. Effect of milling and long-term storage on volatiles of black rice (Oryza sativa L.) determined by headspace solid-phase microextraction with gas chromatography–mass spectrometry. Food Chem. 2019;276:572–582. doi: 10.1016/j.foodchem.2018.10.052. [DOI] [PubMed] [Google Scholar]
- Dias L.G., Hacke A., Bergara S.F., Villela O.V., Mariutti L.R.B., Bragagnolo N. Identification of volatiles and odor-active compounds of aromatic rice by OSME analysis and SPME/GC-MS. Food Res. Int. 2021;142 doi: 10.1016/j.foodres.2021.110206. [DOI] [PubMed] [Google Scholar]
- Dong S.Y., Kyu S.L., O-Young J., Kee-Jong K., Stanley J.K. Characterization of volatile aroma compounds in cooked black rice. J. Agric. Food Chem. 2008;56:235–240. doi: 10.1021/jf072360c. [DOI] [PubMed] [Google Scholar]
- Gao C., Li Y., Pan Q., Fan M., Wang L., Qian H. Analysis of the key aroma volatile compounds in rice bran during storage and processing via HS-SPME GC/MS. J. Cereal. Sci. 2021;99 doi: 10.1016/j.jcs.2021.103178. [DOI] [Google Scholar]
- Gemert L.J.V. 2003. Compilations of Odour Threshold Values in Air, Water and Other Media. [Google Scholar]
- Griglione A., Liberto E., Cordero C., Bressanello D., Cagliero C., Rubiolo P.…Sgorbini B. High-quality Italian rice cultivars: chemical indices of ageing and aroma quality. Food Chem. 2015;172:305–313. doi: 10.1016/j.foodchem.2014.09.082. [DOI] [PubMed] [Google Scholar]
- Hu X., Fang C., Zhang W., Lu L., Guo Z., Li S., Chen M. Change in volatiles, soluble sugars and fatty acids of glutinous rice, japonica rice and indica rice during storage. LWT. 2023;174 doi: 10.1016/j.lwt.2022.114416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X.Q., Lu L., Guo Z.L., Zhu Z.W. Volatile compounds, affecting factors and evaluation methods for rice aroma: a review. Trends Food Sci. Tech. 2020;97:136–146. doi: 10.1016/j.tifs.2020.01.003. [DOI] [Google Scholar]
- Hu S.Y., Ren H.B., Song Y., Liu F., Qian L.L., Zuo F., Meng L. Analysis of volatile compounds by GCMS reveals their rice cultivars. Sci. Rep. 2023;13(1) doi: 10.1038/s41598-023-34797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeppler K., Mueller F. Odor classification: a review of factors influencing perception-based odor arrangements. Chem. Senses. 2013;38(3):189–209. doi: 10.1093/chemse/bjs141. [DOI] [PubMed] [Google Scholar]
- Kasote D., Singh V.K., Bollinedi H., Singh A.K., Sreenivasulu N., Regina A. Profiling of 2-acetyl-1-pyrroline and other volatile compounds in raw and cooked rice of traditional and improved varieties of India. Foods. 2021;10(8) doi: 10.3390/foods10081917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.K., Jang S., Koh H.J. Identification of volatile organic compounds related to the eating quality of cooked japonica rice. Sci. Rep. 2022;12(1) doi: 10.1038/s41598-022-21863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R., Tian Y., Chen L., Jin Z. Impact of cooling rates on the flavor of cooked rice during storage. Food Biosci. 2020;35 doi: 10.1016/j.fbio.2020.100563. [DOI] [Google Scholar]
- Maraval I., Mestres C., Pernin K., Ribeyre F., Boulanger R., Guichard E., Gunata Z. Odor-active compounds in cooked rice cultivars from Camargue (France) analyzed by GC-O and GC-MS. J. Agric. Food Chem. 2008;56(13):5291–5298. doi: 10.1021/jf7037373. [DOI] [PubMed] [Google Scholar]
- Nadaf A., Jawali N., Mathure S. first ed. 2016. Scented Rice (Oryza Sativa L.) Cultivars of India: A Perspective on Quality and Diversity. [Google Scholar]
- Niu Y., Zhang J., Xiao Z., Zhu J. Evaluation of the perceptual interactions between higher alcohols and off-odor acids in laimao baijiu by σ–τ plot and partition coefficient. J. Agric. Food Chem. 2020;68(50):14938–14949. doi: 10.1021/acs.jafc.0c05676. [DOI] [PubMed] [Google Scholar]
- Park J.S., Kim K.Y., Baek H.H. Potent aroma-active compounds of cooked Korean non-aromatic rice. Food Sci. Biotechnol. 2010;19(5):1403–1407. doi: 10.1007/s10068-010-0200-1. [DOI] [Google Scholar]
- Ramtekey V., Cherukuri S., Modha K.G., Kumar A., Kethineni U.B., Pal G.…Kumar S. Extraction, characterization, quantification, and application of volatile aromatic compounds from Asian rice cultivars. Rev. Anal. Chem. 2021;40(1):272–292. doi: 10.1515/revac-2021-0137. [DOI] [Google Scholar]
- Setyaningsih W., Majchrzak T., Dymerski T., Namiesnik J., Palma M. Key-marker volatile compounds in aromatic rice (oryza sativa) grains: an HS-SPME extraction method combined with GCxGC-TOFMS. Molecules. 2019;24(22) doi: 10.3390/molecules24224180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda R., Takahashi K., Ichikawa S., Wakayama M., Kobayashi A., Miyagawa S., Uchimura T. Using SPME-GC/REMPI-TOFMS to measure the volatile odor-active compounds in freshly cooked rice. ACS Omega. 2020;5(32):20638–20642. doi: 10.1021/acsomega.0c03037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splivallo R., Ottonello S., Mello A., Karlovsky P. Truffle volatiles: from chemical ecology to aroma biosynthesis. New Phytol. 2011;189(3):688–699. doi: 10.1111/j.1469-8137.2010.03523.x. [DOI] [PubMed] [Google Scholar]
- Verma D.K., Srivastav P.P. A paradigm of volatile aroma compounds in rice and their product with extraction and identification methods: a comprehensive review. Food Res. Int. 2020;130 doi: 10.1016/j.foodres.2019.108924. [DOI] [PubMed] [Google Scholar]
- Wang Z., Su H., Bi X., Zhang M. Effect of fragmentation degree on sensory and texture attributes of cooked rice. J. Food Process. Pres. 2019;43(4) [Google Scholar]
- Wei X., Sun Q., Methven L., Elmore J.S. Comparison of the sensory properties of fragrant and non-fragrant rice (Oryza sativa), focusing on the role of the popcorn-like aroma compound 2-acetyl-1-pyrroline. Food Chem. 2021;339 doi: 10.1016/j.foodchem.2020.128077. [DOI] [PubMed] [Google Scholar]
- Yang D.S., Lee K.S., Kays S.J. Characterization and discrimination of premium-quality, waxy, and black-pigmented rice based on odor-active compounds. J. Sci. Food Agric. 2010;90(15):2595–2601. doi: 10.1002/jsfa.4126. [DOI] [PubMed] [Google Scholar]
- Yang D.S., Shewfelt R.L., Lee K.S., Kays S.J. Comparison of odor-active compounds from six distinctly different rice flavor types. J. Agric. Food Chem. 2008;56(8):2780–2787. doi: 10.1021/jf072685t. [DOI] [PubMed] [Google Scholar]
- Zhao Q. Xi, Xu J., Jin D., Wu Y., Tong Q., Yin Y., Xu X. A comparative HS-SPME/GC-MS-based metabolomics approach for discriminating selected japonica rice varieties from different regions of China in raw and cooked form. Food Chem. 2022;385 doi: 10.1016/j.foodchem.2022.132701. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Yousaf L., Xue Y., Shen Q. Changes in flavor of fragrant rice during storage under different conditions. J. Sci. Food Agric. 2020;100(8):3435–3444. doi: 10.1002/jsfa.10379. [DOI] [PubMed] [Google Scholar]
- Zheng Z., Zhang C., Liu K., Liu Q. Volatile organic compounds, evaluation methods and processing properties for cooked rice flavor. Rice. 2022;15(1):53. doi: 10.1186/s12284-022-00602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Wang X., Si X., Blanchard C., Strappe P. The ageing mechanism of stored rice: a concept model from the past to the present. J. Stored Prod. Res. 2015;64:80–87. doi: 10.1016/j.jspr.2015.09.004. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.