Abstract
Ion channels are abundantly expressed in both excitable and non-excitable cells, thereby regulating the Ca2+ influx and downstream signaling pathways of physiological processes. The immune system is specialized in the process of cancer cell recognition and elimination, and is regulated by different ion channels. In comparison with the immune cells, ion channels behave differently in cancer cells by making the tumor cells more hyperpolarized and influence cancer cell proliferation and metastasis. Therefore, ion channels comprise an important therapeutic target in anti-cancer treatment. In this review, we discuss the implication of ion channels in regulation of Ca2+ homeostasis during the crosstalk between immune and cancer cell as well as their role in cancer progression.
Facts
Ion channels regulate Ca2+ influx and downstream signaling pathways in immune and cancer cells.
Altered regulation of ion channels is implicated in carcinogenesis.
Cytotoxicity of immune cells against cancer cells depends highly on Ca2+ signaling
Ion channels comprise an attractive tool for targeted therapy for cancer
Open Questions
Are blockers of K+ and CRAC channels able to inhibit cancer progression?
What is the role of immune cell-specific ion channels in cancer therapy?
What cancer-specific ion channels are involved in neoplastic transformation in vivo?
Physiological processes depend on the continued flow of ions into and out of cells defeating a barrier impermeable to ions such as plasma membrane, which is built in a form of phospholipid bilayer. Thus, the hydrophobic membrane acts as a serious energy barrier for transporting ions. Ions are charged molecules that have low solubility in the hydrocarbon core of lipid bilayer, thereby having low permeability coefficients across the bilayer. There is a large difference in the electric potential between the two sides of a biological membrane. In order to transfer ions across the membrane and equilibrate both sides of the membrane, eukaryotic cells are equipped in the integrally embedded pore-forming membrane proteins (ion channels) and biological pumps. Such structure allows for the passage of ions through the channel. Opening and closing of the ion channel is usually controlled chemically or mechanically. Depending on the type of ion channel, its conformational change may occur because of changes in the membrane potential (voltage-gated channels), ligand binding (chemical activation) or ligand-driven stretching of the membrane (stretch-activated ion channels). Body response to the external stimuli can be linked to the regulation of ion channel activity. Ion channels play a crucial role in various physiological processes including flow of nerve impulses, muscle contraction, cell division and hormone secretion.1 The intracellular concentration of the key signaling ion such as calcium (Ca2+) depends on electrical gradients driven in turn by sodium (Na+) and potassium (K+) channels. The role of ion channels in pathogenesis of various diseases including cancer and its treatment has been extensively studied. The prime function of an immune cell is to remove cancer cells from the body by cytotoxic T lymphocytes (CTL or CD8+ cells) and natural killer (NK) cells through polarized discharge of the contents of cytotoxic granules towards the target cells.2 The effector function of CTL and NK cells as well as their proliferation and apoptosis of cancer cells largely depend on Ca2+ signaling. The role of ion channels in the regulation of intracellular Ca2+ concentration is well described in the literature. Alterations in Ca2+ homeostasis due to ion channel dysfunction contribute to the common traits of neoplastic transformation, which are known as hallmarks of cancer. These hallmarks include different stages of tumor development like unlimited replication, tissue invasion and metastasis, evasion of apoptosis, sustained angiogenesis, self-sufficiency in growth signals and insensitivity to anti-growth signals.3, 4 Additionally, modulation of ion channel-mediated Ca2+ concentration in CTLs regulates their antitumor action.5, 6
Regulation of Intracellular Ca2+ Concentration
Na+ and K+ are the most abundant cations in biological systems. Na+ ions are mainly present at high concentrations outside the cell, unlike K+ ions that are present at high concentrations inside the cell. Gradients for these ions across the cell membrane provide the energy source for action potentials generated by opening of Na+ and K+ channels7, 8 and for transporting solutes and other ions across the cell membrane via coupled transporters. Among several ions, the gradient for Ca2+ ions is the largest. The cytosol is surrounded by two big Ca2+ stores: the extracellular space, where the Ca2+ concentration is ~1.8 mM, and the sarco-endoplasmic reticulum, where the Ca2+ concentration varies from 300 μM to 2 mM.9 In immune cells, the intracellular Ca2+ concentration is ~0.1 μM in the resting state, but it is significantly increased (~10-fold) when the cells are activated.10
Plasma membrane Ca2+ channels and Ca2+ influx are particularly important at different steps of the cell-cycle progression and proliferation of immune cells.11, 12, 13 The molecular features of Ca2+ channels are well defined, which allows for the distinction of four main types of these channels including voltage-activated, receptor-activated, store-operated and second messenger-operated channels. Receptor-activated, store-operated and second messenger-operated channels are ubiquitous, whereas voltage-activated calcium channels are specific for excitable cells. Voltage-activated calcium channels (e.g., L-, T-, N-, P-, Q-type Ca2+ channels) open when the plasma membrane is depolarized. Receptor-activated calcium channels (e.g., P2X purinergic receptors) open when a ligand binds to the channel,14 whereas store-operated calcium channels (e.g., transient receptor potential (TRP)) and archetype calcium release-activated channels (CRAC) are activated when the level of Ca2+ within the lumen of the ER decreases below a threshold level.15, 16 Another type, second messenger-operated channels (e.g., arachidonic acid-regulated Ca2+ current) are activated by intracellular second messengers like arachidonic acid.17 The role of CRAC, TRPM4 and P2X channels are important in case of immune cells in the continuous effort to keep Ca2+ at an optimal level in order to maintain the cellular functions in parallel with ion pumps like Na+/K+ pumps.18, 19 In non-excitable cells including immune cells, the membrane potential plays an important role in setting the electrical driving force for Ca2+ entry. In cells where voltage-independent Ca2+ channels like TRPM4 and two-pore K+ channels (K2P) are present, Ca2+ influx only depends on the electrochemical gradient over the membrane and intensifies when the membrane potential is more negative (hyperpolarized).20
Among different ion channels involved in the regulation of Ca2+ homeostasis, CRAC channels are the most important. CRAC channels have been widely characterized21 and are known because of their high ion selectivity for Ca2+ and low conductance. CRAC channels are activated through the binding of the endoplasmic Ca2+ depletion sensor, known as stromal interaction molecule 1 (STIM1) and STIM2 to the CRAC channel units ORAI1-3 (also known as CRACM1-3).10 ORAI1 is a widely expressed surface glycoprotein with four predicted transmembrane domains, intracellular amino- and carboxyl-termini and no sequence homology to other ion channels except for its homologues ORAI2 and ORAI3.22, 23 The activation of ORAI/CRAC channels involves a complex series of coordinated steps, during which STIM proteins sense the depletion of ER Ca2+ stores and pass on this store depletion to the CRAC channels.24, 25 In resting cells with filled up Ca2+ stores, STIM proteins are diffusely distributed all over the ER membrane. Following the depletion of Ca2+ stores, STIM proteins get activated, oligomerize and redistribute into puncta within junctional ER sites, which are in close proximity to the plasma membrane.26
Role of Ion Channels in Maintaining the Normal Membrane Potential
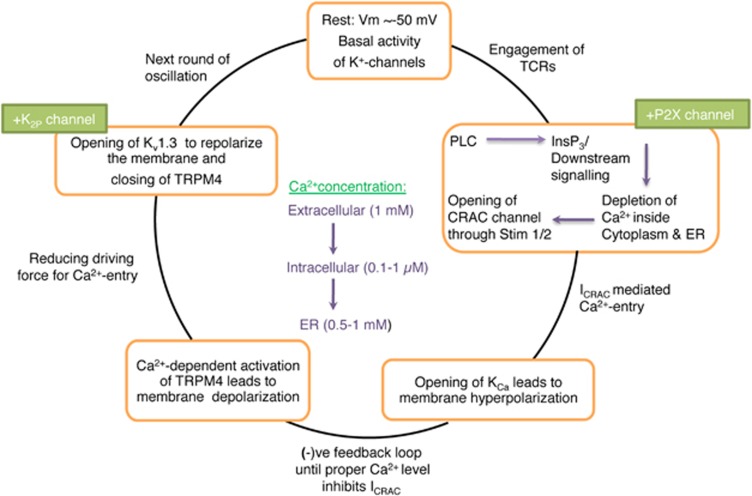
The resting potential of a lymphocyte membrane is ~−50 mV. Membrane potential alterations mainly occur when lymphocytes get activated. TCR engagement activates PLCγ1, which catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol trisphosphate (IP3) and di-acyl glycerol. IP3 stimulates the release of Ca2+ from intracellular ER stores, which triggers the opening of plasma membrane CRAC channels. It is the resulting influx of extracellular Ca2+ that is responsible for the sustained rise in cytoplasmic Ca2+ after TCR stimulation. Ca2+ binds to the cytoplasmic Ca2+-dependent protein calmodulin, which then activates the phosphatase calcineurin. This phosphatase dephosphorylates and activates the nuclear factor of transcription of activated T cells (NFAT), which enters the nucleus and helps to initiate interleukin-2 (IL-2) gene transcription.10 During the activation of immune cells, opening of CRAC channels raises the intracellular Ca2+ level. To maintain the balance in membrane conductance, KCa channels get opened to hyperpolarize the membrane, which results in Ca2+ efflux. A negative feedback loop is established when the level of Ca2+ inside the cell is high enough to inhibit CRAC channels. Beside the Ca2+-dependent activation of TRPM4 channels in T cells, there is also involvement of Kv1.3 channels in order to repolarize the membrane (Figure 1). Along with these conventional ion channels, the K2P TWIK-related acid-sensitive K+ channels 1 and 3 (TASK-1/K2P3.1 and TASK-3/K2P9.1) are known to regulate immune cell effector functions by hyperpolarizing the membrane.27
Figure 1.
Fluctuations of membrane potential during activation of immune cells. Ca2+ influx in lymphocytes depends on the gradient between the extracellular Ca2+ concentration (~1 mM) and the intracellular Ca2+ concentration (~0.1 μM) as well as the electrochemical gradient established by K+ channels (specifically, Kv1.3, Kca3.1 and partially by K2P channels) and the Na+-permeable channel TRPM4. CRAC channels are activated upon the engagement of antigen receptors (i.e., TCRs, BCRs). This is mediated through the activation of PLCγ, the production of IP3 and the release of Ca2+ from ER Ca2+ stores. The subsequent activation of STIM1 and STIM2 results in the opening of ORAI1 CRAC channels and SOCE. Sustained Ca2+ entry through CRAC channels leads to the activation of Ca2+-dependent enzymes and transcription factors, including calcineurin and NFAT.28 Additionally, P2X receptors (e.g., P2X4 and P2X7) are non-selective Ca2+ channels activated by extracellular ATP mediating Ca2+ influx in order to augment SOCE-mediated activation of signaling molecules (according to Launay P, 2004; Feske S, 2012). Abbreviations: TCR, T cell receptor; PLCγ1, phospholipase Cγ1; NFAT, nuclear factor of activated T cells; CRAC, calcium release-activated channels; STIM1/2, stromal interaction molecule 1/2; SOCE, store-operated calcium entry; P2X, purinergic receptor 2X
Ion Channels in Immune Cells
Activation and the effector role of immune cells is dependent on Ca2+ influx, which is regulated by a group of ion channels located in the plasma membrane of the cell. The detailed characteristics of certain ion channels and their implication in the cellular functions became possible with the help of ‘gold standard' patch-clamp technique. The role of individual types of ion channels in the physiology of immune cells is briefly presented.
K+ channels
K+ channels comprise the major ion channel family expressed in immune cells that regulate important cellular processes including Ca2+-mediated cellular proliferation, migration and finally controlling cell volume.28 They regulate membrane potential by driving K+ efflux resulting in membrane hyperpolarization. From the superfamily of K+ channels, immune cells express voltage-gated (Kv1.3), calcium-activated (KCa3.1), inwardly rectifying potassium channels (Kir) and two-pore gated channels (K2P).29 In regard to the structural diversity of the channels, there are several types like six transmembrane one pore (Kv) or transmembrane two pore (K2P).29 Kv channels are further subdivided into three conserved gene families: Kv (shaker-like), Ether-a-go-go (EAG) and KCNQ (Kv7).30 In addition, KCa channels are grouped into big-conductance calcium-activated channels (BKCa (KCa1.1)), intermediate-conductance calcium-activated channels (IKCa (KCa3.1)) and small-conductance calcium-activated channels (SKCa (KCa2.1, KCa2.2, KCa2.3)).30
The role of Kv1.3 and KCa3.1 in mediating the efflux of K+ in order to maintain the hyperpolarization of the cell membrane (Figure 1) is well explained in the literature.27 K+ channels are differently expressed in various subsets of lymphocytes followed by their activation. For example, naïve and regulatory human T cells mainly express Kv1.3, whereas the expression of KCa3.1 is upregulated upon activation by cognate antigen.31, 32, 33 Interestingly, a recent study has shown that Kv1.3 channels are indispensable for the differentiation of CD8+ T cells into effector cells with cytotoxic ability.34 Moreover, Kv1.3 channels accumulate specifically at the immune synapse (IS) between cytotoxic and target cells in order to modulate the killing process mediated by CTL and NK cells.35, 36 In addition, blocking of KCa3.1 in NK cells increases their tumor cell killing ability and comprises an excellent target for cancer immunotherapy.37
Kir channels are responsible for stabilization of the resting membrane potential near to the K+ equilibrium potential by passing positive charge mostly into the cell (inward direction) rather than in the opposite direction.38 This type of channels is present in a significant amount in macrophages, dendritic cells and microglia.39 Studies have shown that Kir2.0 and Kir4.0 family members interact with NIL-16, neuronal variant of interleukin 16 (IL-16).40 As the cytokine IL-16 has been characterized mostly in the immune system, the identification of NIL-16 emphasizes the connection of Kir channels with the immune and nervous system. On the basis of the observation that memantine inhibits the amplitude of inwardly rectifying K+ current though the Kir channels in macrophages and microglial cells, it is postulated that blocking the Kir channels may influence the functional activity of macrophages.41 Kir4.1 channel has been lately also found to be a target of the autoantibody response in a subgroup of persons with multiple sclerosis, which suggests that autoreactive T cells are key to the pathogenesis of this disease.42
K2P (KCNK), better known as 'leak channels' are important for setting the resting membrane potential. Furthermore, their action is mainly voltage-independent and can be regulated via various stimuli including mechanical stimulation, lipids, Gq proteins or muscarine.27, 43 TASK-1/K2P3.1 and TASK-3/K2P9.1, the two functional members of the K2P family are expressed in T lymphocytes and contribute to the modulation of T-cell effector function including interferon-γ (IFN-γ) and IL-2 secretion as well as T-cell proliferation. Selective blockade of TASK channels present on T lymphocytes leads to improvement of the experimental autoimmune encephalomyelitis course, a model of multiple sclerosis.27
Transient receptor potential (TRP) channel
Among the superfamily of 28 TRP cation channels,44 immune cells mainly express TRPMC and TRPM subfamilies like TRPC-1, 3, 5 and TRPM-2, 4, 7.45 These channels have biophysical properties to be non-selective and permeable to several cations like Ca2+ and Na+ 45. Regulation of intracellular Ca2+ concentration is indispensable for lymphocyte activation, and TRP channels may both increase Ca2+ influx (TRPC3) or decrease Ca2+ influx through membrane depolarization (TRPM4). The function of TRPM4 channel is well documented in maintaining the normal membrane potential of an immune cell and controlling the Ca2+ flux mechanism.10 Interestingly, TRPM4 channel mainly conducts Na+ and K+ cations.46 Activation of TRPM4 channels occurs in response to the increase in intracellular Ca2+ concentration resulting in Na+ influx, membrane depolarization and a reduction in electrical driving force for Ca2+ influx (Figure 1). Therefore, TRPM4 channel acts as a negative feedback mechanism for the regulation of store-operated Ca2+ entry by CRAC-ORAI as thereby preventing the cellular Ca2+ overload.47
Purinergic receptors
P2X receptors are membrane ion channels with the ability to influx several non-selective cations like Na+ and Ca2+, and are activated by extracellular adenosine 5'-triphosphate (ATP).48 P2X receptors belong to the class of ligand-activated ion channels and there are three P2X receptors expressed in human T cells: P2X-1, 4, 7.49 Among these three, principally P2X7 is abundantly expressed in immune cells and regulates Ca2+ influx process resulting in the activation of downstream signaling mediators and T-cell proliferation.50, 51, 52
Store-operated calcium channels (SOCs)
CRAC is the major store-operated Ca2+ channel of immune cells with the biophysical properties of higher Ca2+ dependence and low conductivity in the range of 0.024–0.4 pS.16 CRAC channels get opened with the signal of depleting endoplasmic reticulum (ER) Ca2+ pool. This signal in ER is mainly mediated by ER Ca2+ sensors stromal interaction molecule (STIM) 1 and STIM2 and transferred to the pore-forming subunits of the CRAC channel, mainly ORAI1–3. This results in the activation of the CRAC channel. Lymphocytes express two STIM isoforms, STIM1 and STIM2, which mediate store-operated Ca2+ entry in B and T cells.53, 54 CD4+ and CD8+ T cells from ORAI1- and STIM1-deficient patients exhibit defective production of various cytokines, including IL-2, IL-17, IFN-γ and tumor necrosis factor (TNF).55 Furthermore, store-operated calcium entry is indispensable for the cytotoxic action of CTLs. STIM1- and STIM2-mediated store-operated calcium entry in CD8+ T cells is crucial for anti-tumor immunity.5
Anti-tumor Action of Immune Cells
Human immune system has the great potential to destroy cancer cells either by CTL or NK cells without being toxic to the healthy tissue and organs. These distinct immune cells are able to recognize cancer cell by forming a Ca2+-dependent cytotoxic IS with the cancer cell and perform a killing mechanism either through the release of lytic granules and granzymes, or by the activation of Fas-FasLigand receptors (known as death receptors).2 Efficient CRAC channels and the resulting increase in the cytosolic Ca2+ concentration are necessary for adherence to the target cell as well as its recognition.56 The adhesion molecule, particularly lymphocyte function-associated antigen 1 (LFA-1) integrin is essential for this process and interacts with Ca2+ in diverse ways.3 This includes inside-out (transmission of the regulatory signals originating within the cytoplasm to the external ligand-binding domain of the receptor) signaling-based LFA-1 activation or outside-in (transmission of chemical signals into the cell) signaling via LFA-1.5 Interaction between CTL and epithelial tumor cell is integrin-dependent and promotes maturation of the cytotoxic IS and modulates anti-tumor CTL response.56 Additionally, LFA-1 activation is implicated in mitochondria positioning at the IS in order to control Ca2+-influx through CRAC/ORAI Ca2+ channels.57, 58 It has recently been shown that store-operated Ca2+ release driven by ORAI1 is crucial for lytic granule exocytosis in NK cells and CTLs as well as production of cytokines (TNF-α and IFN-γ) by NK cells.59 Furthermore, delineation of the accurate STIM-ORAI1 ratio could be a feature of the killing efficiency of CTL and NK cells.3 Ca2+ does not directly play a role in the formation of the IS, but it has enormous effect in controlling the duration and kinetics of the cytotoxic IS between killer immune and cancer cell.2 Along with the depolarizing nature of cancer cells, Ca2+ concentration can also be a marker of the action of a killer T cell. Small fluctuations from the external Ca2+ (~1.2 mM) range of a cancerous tissue can indicate the influence of cancer cell killing by CTL or NK cells.60, 61
Ion Channels in Cancer
Ion channels comprise an important factor influencing the formation and development of tumors. Such malignant transformation leads to enhanced proliferation, abnormal differentiation, impaired apoptosis, and finally uncontrolled migration and invasion (Table 1). This is often associated with altered levels of ion channel expression as well as their activity in the mutated cancer cells.62 The role of ion channels in pathogenesis of various diseases including cancer and its treatment has been extensively studied. The major types of ion channels implicated in carcinogenesis are presented below.
Table 1. The role of distinct ion channels in cancer development and progression.
| Ion channels | Expression profile | Cancer type | References |
|---|---|---|---|
| Proliferation of cancer cells | |||
| Shaker-like K+ channels (Kv1.1, Kv1.3, Kv1.5) | Gene and protein upregulation | Glioma, breast cancer, lung cancer, pancreas cancer, prostate cancer, lymphoma | 64, 123 |
| EAG K+ channels (EAG1, EAG2) | Gene and protein upregulation | Medulloblastoma, breast cancer, head and neck cancer, melanoma, gastrointestinal tract cancer | 65, 66, 67 |
| EAG-related K+ channels (HERG/Kv11.1) | Gene and protein upregulation | Melanoma, neuroblastoma, breast cancer | 68 |
| Ca2+-activated K+ channels (KCa3.1) | Gene and protein upregulation | Glioma, breast cancer, ovarian cancer, prostate cancer, melanoma | 124, 125, 126, 127 |
| TRP (TRPC6, TRPV6, TRPM7, TRPM8) | Gene and protein upregulation | Breast cancer, prostate cancer, head and neck cancer, human glioblastoma cell line | 89, 95, 96, 97, 128, 129 |
| P2Y (P2Y2), P2X (P2X7), P2U | Gene and protein upregulation | Melanoma, colorectal cancer cells, lung cancer cells | 101, 130, 131 |
| SOCs (ORAI1/STIM1) | Gene and protein downregulation | Lung cancer cells, cervical cancer | 113, 132 |
| SOCs (ORAI1/STIM1) | Gene and protein upregulation | Cervical cancer, glioblastoma cells | 113, 133 |
| Cell migration and metastasis | |||
| EAG K+ channels (EAG1/ Kv10.1) | Gene and protein upregulation | Migration of breast cancer cells | 134 |
| Ca2+-activated K+ channels (KCNMA1, SK3/ORAI1, KCa1.1, KCa3.1) | Gene and protein upregulation | Breast cancer→metastasis to brain Breast cancer→bone metastasis Migration of glioma cells, transformed renal epithelial cells and breast cancer cells | 75, 76, 77, 78, 135 |
| Kir channels (Kir3.1/GIRK1) | Gene and protein upregulation | Primary breast cancer→axillary lymph node metastasis | 81 |
| TRP (TRPM7, TRPM8, TRPV1, TRPV6) | Gene and protein upregulation | Lung cancer cells, primary breast cancer, prostate cancer cells, squamos carcinoma, hepatoblastoma | 90, 91, 97, 136, 137, 138 |
| P2X (P2X7) | Gene and protein upregulation | Breast cancer cell line | 139 |
| SOCs (ORAI1/STIM1) | Gene and protein upregulation | Breast cancer, cervical cancer, hepatocarcinoma, glioblastoma | 111, 112, 113, 140 |
| Tumor angiogenesis | |||
| EAG K+ channels (EAG1) | Gene and protein upregulation | Breast cancer and other solid tumors | 65, 66 |
| TRP (TRPC6 ) | Gene and protein upregulation | Human glioblastoma cell line | 88, 94, 141 |
| SOCs (ORAI1/STIM1) | siRNA- or dominant-negative mutant-mediated knockdown | VEGF-induced angiogenesis observed in tumors | 141, 142 |
| Apoptosis resistance | |||
| Shaker-like K+ channels (Kv1.3) | Gene and protein upregulation | Large B-cell lymphoma, glioma | 64 |
| TRP (TRPA1) | Gene and protein upregulation | Lung cancer cell line | 143 |
| P2X (P2X7) | Gene and protein downregulation | Breast cancer, melanoma | 104 |
| SOCs (ORAI1) | siRNA-mediated knockdown | Prostate cancer cell line | 109, 144 |
Voltage-gated K+ channels
Shaker-like
Shaker-type of voltage-gated K+ channels regulate cell cycle progression by four mechanisms such as controlling membrane potential oscillations, controlling the cell volume dynamics, controlling calcium signaling and promoting malignant growth through the migratory pathway. Influence of voltage-dependent K+ channels in the early stages of cancer development confirms the evidence for the overexpression of these channel proteins in cells exposed to chemical carcinogens.61 It has been shown that voltage-gated K+ channels affect tumor cell proliferation through the regulation of the membrane potential. As an example, overexpression of Kv1.1 and Kv1.3 are found in glioma, lymphoma, breast, lung, pancreas and prostate cancer.49, 63 Furthermore, Kv1.3 channel overexpression is also linked with resistance to apoptosis as shown by the upregulation of Kv1.3 expression in diffuse large B-cell lymphoma and glioma.64
EAG channels
The EAG subfamily of voltage-gated K+ channels is divided into three distinct groups including EAG (EAG1/ Kv10.1; EAG2/ Kv10.2), EAG-like K+ (ELK) and EAG-related (HERG/ Kv11.1). EAG1 overexpression has showed tumorigenic potential and poor overall patient survival in multiple cancer types.65 Additionally, EAG1 plays a significant role in cell proliferation and tumor angiogenesis.66 Another member of the EAG subfamily of voltage-gated K+ channels, particularly EAG2, regulates cell volume dynamics important for cell cycle progression and cell proliferation in medulloblastoma.67 Similar to EAG1, HERG overexpression is found in brain, breast, gastrointestinal tract, head and neck, kidney, lung, melanoma, ovary, and thyroid cancers.63 Moreover, HERG expression correlates with TNF-mediated tumor cell proliferation.68
K2P channels
K2P channels are typically constitutively open as 'leak channels' in order to stabilize the negative membrane potential. A member of this family, K2P5.1 (TASK-2 or KCNK5) plays a major role in the regulation of cell volume, which requires the interplay with Ca2+ and Cl- channels. This kind of swelling-activated channel is implicated highly in cancer cell physiology.69 Overexpression of K2P9.1 (TASK-3 or KCNK9) and K2P3.1 (TASK-1 or KCNK3) is found in breast, gastrointestinal tract, lung, adrenal cancers and melanoma.70 Additionally, overexpression of K2P9.1 in breast cancer cell lines promotes tumorigenesis and confers resistance to hypoxia and serum withdrawal.71 In general, rapidly proliferating cancer cells are more depolarized in nature with a membrane potential varying from −20 to 40 mV.72 Therefore, membrane depolarization plays a functional role in tumor progression inducing DNA synthesis and promoting mitotic activities, which in turn leads to tumor invasion.73 As potassium conductance is the major regulatory factor in maintaining relatively depolarized state of the cell, the roles of potassium channel inhibitors in controlling polarization phenomenon of tumor cells remains to be revealed.
Ca2+-activated K+ channels
Ca2+-activated K+ channels are regulated by Ca2+ concentration inside the cells. This kind of channels has a major role in cancer metastasis process, which cause >90% of cancer deaths.74 Tumor metastasis is a dynamic process involving mobilization of primary tumor cells by migration into other non-tumoral regions. Thus, ion channels are involved in migration, which plays a major role in the initiation of metastasis process.75 As an example, BKCa and SKCa channels are implicated in metastasis as they have been shown to promote breast cancer cell migration.76 Furthermore, SKCa channels form a complex with the ORAI1 channel for localized calcium entry within lipid rafts in order to enhance cancer cell migration and metastasis.77 In general, overexpression of Kca1.1 and Kca3.1 has been shown in bone, brain, breast, ovary, pancreas cancers and brain, gastrointestinal tract, melanoma and prostate cancers. Interestingly, application of Kca1.1 and Kca3.1 channel inhibitors decreases the migration of human glioma and experimental transformed renal epithelial cells respectively.78, 79
Kir channels
As mentioned above, Kir channels allow for easy movement of K+ into the cell. They are activated by PIP2, but they can also be modulated by other regulatory factors such as ATP (ATP-sensitive K+ channels) and G-proteins (G protein-gated Kir channels) or by some non-specific regulators including polyamines, kinases, pH and Na+ ions.80
The mRNA upregulation of the G-protein regulated inward-rectifier K+ (GIRK) channel called Kir3.1 (GIRK1) has been shown in invasive breast cancer and non-small-cell lung cancer. Additionally, overexpression of GIRK1 in both types of tumors was correlated with poor prognosis for the patients.81, 82
TRP channels
TRP cation channels have been implicated in various pathological states including cancer due to their role as intracellular Ca2+ release channels. Recent studies have shown the association of TRP channels with various cancer types such as melanoma83 (TRPM1), prostate cancer84, 85, 86 (TRPV2, TRPV6, TRPM8), hepatoblastoma87 (TRPV1) and glioblastoma88, 89 (TRPC6). Besides the roles of volume control and motility, TRPM8 channel serves as a potential marker for metastatic prostate cancer.84 Another TRP channel that has been implicated in enhanced motility and metastasis of cancer cells is TRPM7 channel.90, 91 Furthermore, TRP channels are also involved in angiogenesis,92, 93, 94 thus their inhibitors might be considered a good pharmaceutical target for cancer therapy. TRPV6, TRPM7 and TRPM8 are also associated with proliferation of breast and prostate cancer cells.95, 96, 97 Interestingly, sustained Ca2+ flux through TRP channels can itself be a diagnostic marker for a cancer cell and can be inhibited with a TRP channel inhibitor.98, 99
Purinergic Receptors
The ATP-dependent activity of P2X7 channel is associated with various physiological functions including cell proliferation, cell death and cytokine secretion. Recent studies have implicated the role of P2X and P2Y receptors in B cell leukemia,100 melanoma and colorectal cancer.101, 102, 103 Targeting the P2X7 receptor by selective P2X7 agonists as well as P2X7 antagonists in cancer has shown anti-tumor effect.101, 104 Furthermore, the effect of ATP infusion in patients with advanced lung cancer has proven the potential of ATP, which might become an anti-cancer agent in the future.105, 106, 107, 108 However, larger studies are required in order to verify these findings.
Store-operated calcium channels (SOCs)
SOC-mediated sustained increase in the cytosolic Ca2+ has shown to trigger apoptosis in tumor cells.109 STIM1-ORAI1 driven store-operated calcium entry seems to be indispensable for migration and metastasis of breast cancer, cervical cancer and hepatocarcinoma, which was potently blocked by the store-operated calcium entry inhibitor.110, 111, 112, 113 Moreover, CRAC channels are implicated in VEGF-activated Ca2+ influx promoting angiogenesis, which might be crucial for cancer progression.111
Ion Channel Modulators
Ion channels are often overexpressed in numerous types of tumors and their altered activity plays a significant role in apoptosis resistance, proliferation and metastasis of cancer cells. Thus, blocking the activity of ion channels seems to be an obvious strategy to impair cancer growth. However, such treatment is not as straightforward as it may look. When targeting ion channels, we aim at efficient killing of cancer cells without causing toxic effects in other tissues expressing the same or related channels. A vast amount of known ion channels blockers are used to treat cardiac arrhythmias or epilepsy (anticonvulsants);114 thus, incorporating them into oncology is accompanied by the risk of heart or nervous system disorders.
Unspecificity of ion channel blockers is still a big challenge that needs to be overwhelmed to avoid serious side effects during oncological treatment. Specific inhibition can be obtained by developing monoclonal blocking antibodies, antisense oligonucleotides, small interfering RNAs, peptide toxins and novel small organic compounds.115 As discussed by Arcangeli and Becchetti, to improve the efficiency of ion channels targeting cancer, one should also focus on finding inhibitors recognizing conformational changes in ion channels (e.g., open channel versus close channel). So far, such an approach was found to be possible in a case of lamotrigine and lidocaine that preferentially target open and inactivated voltage-gated Na+ channels, without distinguishing other conformational states.116 Similar property exhibits in R-roscovitine recognizing open HERG channel.117
Interesting alternative for conventional ways of targeting ion channels in cancer treatment are some dietary compounds.118 Curcumin, resveratrol (grape polyphenol), docosahexaenoic acid (omega-3) and epigallocatechin gallate (catechin from green tea) extract were shown to modulate ion channels activity and suppress migration and growth of breast and ovarian cancer cells.119, 120, 121, 122 Other examples of targeting ion channels in cancer and immune cells are presented in Table 2.
Table 2. Ion channel blockers in immune and cancer cells.
| Ion channel blocker | Ion channel | Cell type | Comments | References |
|---|---|---|---|---|
| Margatoxin (MgTX) Charybdotoxin (CTX) | Kv1.3 | T lymphoctyes, Jurkat cells | Antiproliferative effect in T-lymphoytes, regulation of immunoresponsiveness | 145, 146 |
| TRAM-34, NS6180, ShK-186 | Kv1.3, KCa3.1 | NK cells, leukemia cells | Inhibition of KCa3.1 increased the degranulation of adherent NK cells and their ability to kill K562 leukemia cells | 147 |
| R-roscovitine | Kv1.3, Kv2.1, Kv4.2, HERG (Kv11.1) | Leukemia | Roscovitine is well known cyclin-dependent kinase inhibitor | 148, 149 |
| mAb56 | EAG1 (Kv10.1) | Pancreas carcinoma, breast cancer | Inhibition of tumor cell growth both in vitro and in vivo. | 150 |
| Way 123,398 | HERG (Kv11.1) | Colorectal cancer | Reduced cell migration of H630, HCT and HCT8 cells; unaffected growth of HEK 293 cells | 151 |
| Way 123,398; CsCl; E4031 | HERG (Kv11.1) | Acute myeloid leukemia | Impaired cell proliferation. | 152, 153 |
| Cisapride | HERG (Kv11.1) | Gastric cancer | Inhibition of cells entering S phase from G1 phase of the cell cycle. | 154 |
| Verapamil | ERG (Kv11.1) | Lung cancer, melanoma, colon cancer | Increased survival rate for patients treated with verapamil+chemotherapy | 155, 156 |
| UNBS0 (Cardenolide) | Na+/K+ ATPase | Glioblastoma | Decrease in intracellular ATP concentration leads to autophagy in glioma cells UNBS0 shows anti-proliferative activity in vitro in 58 human cancer cell lines | 18, 157 |
| Tetrodotoxin (TTX) | Nav1.5, Nav1.6 Voltage-gated Na+ channels | Human melanoma, macrophages, breast cancer | TTX and shRNA knockdown of Nav1.6 has inhibitory effects on both cellular invasion of macrophages and melanoma cells | 158, 159 |
| Charybdotoxin (CTX) | Kir (IK1) | Human melanoma | Reduced migration of melanoma cells treated with CTX | 160 |
| Zinc, methanandamide | K2P9.1 (TASK-3) | Ovarian cancer | Reduction in cell proliferation and increase in apoptosis | 161 |
Conclusions and Future Perspectives
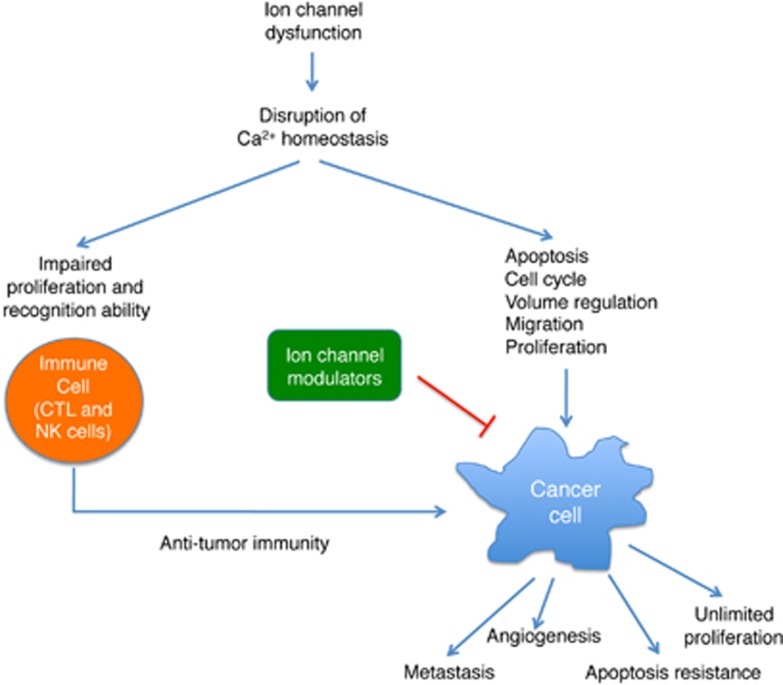
The main task of the immune system is to defend against attacks by foreign invaders including bacteria, viruses, fungi, parasites and other microorganisms. It has been shown by the researchers from both immunology and oncology fields that cancer cells are also recognized by the immune system, and their proliferation can be controlled immunologically. Alterations in ion channel-based Ca2+ signaling are linked to the behavior of cancer cells. Recent studies indicate the significance of ion channels and Ca2+ signaling in activation of cancer killing immune cells as well as cancer progression. Generation of an appropriate Ca2+ response, which is induced by recognition of a tumor antigen is driven by above-described ion channels (Figure 2). Regulation of certain features of cancer cells by decreasing the activity of ion channel proteins is still under investigation. The market success of Ambien (GABAA receptor inhibitor for the treatment of insomnia) and Norvasc (Ca2+ channel blocker used to lower blood pressure and to treat angina pectoris) have energized the drug market to explore more the ion channel field searching for new therapeutics including cancer therapy. Nevertheless, the ion channel-based treatment comprises still far unused anti-cancer strategy. Thus, future research will focus on ion channels as therapeutic target in order to inhibit proliferation of cancer cells and promote their apoptosis together with modulation of cancer-specific cytotoxicity of immune cells. Furthermore, studies involving mutating ion channels in cancer using animal models should uncover novel insights into the ion channel function in tumorigenesis.
Figure 2.
The influence of ion channels on the interaction between the immune system and cancer as well as their role in neoplastic transformation
Acknowledgments
E.W. and A. C-P. kindly acknowledge support from the Integrative Regenerative Medicine Center (IGEN).
Glossary
- ATP
adenosine 5'-triphosphate
- BKCa
big-conductance calcium-activated potassium channel
- CRAC
calcium release-activated channels
- CTL
cytotoxic T-lymphocyte
- IFN-γ
interferon gamma
- IKCa
intermediate-conductance calcium-activated potassium channel
- IL-2
interleukin-2
- IL-16
interleukin-16
- IS
immune synapse
- IP3
inositol trisphosphate
- KCa
calcium-activated potassium channel
- Kir
inwardly rectifying potassium channels
- Kv
voltage-gated potassium channel
- LFA-1
lymphocyte function-associated antigen 1
- NFAT
nuclear factor of activated T cells
- NK
natural killer cell
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PLCγ1
phospholipase Cγ1
- P2X
purinergic receptor 2X
- SKCa
small-conductance calcium-activated potassium channel
- SOCE
store-operated calcium entry
- STIM1/2
stromal interaction molecule 1/2
- TASK1/3
TWIK-related acid-sensitive K+ channels 1/3
- TCR
T-cell receptor
- TNF-α
tumor necrosis factor alpha
- TRP
transient receptor potential
The authors declare no conflict of interest.
Footnotes
Edited by G Dewson
References
- 1Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 2000; 1: 11–21. [DOI] [PubMed] [Google Scholar]
- 2Dustin ML, Long EO. Cytotoxic immunological synapses. Immunol Rev 2010; 235: 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3Prevarskaya N, Skryma R, Shuba Y. Ion channels and the hallamrks of cancer. Trends Mol Med 2010; 16: 107–121. [DOI] [PubMed] [Google Scholar]
- 4Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 5Schwarz EC, Qu B, Hoth M. Calcium, cancer and killing: the role of calcium in killing cancer cells by cytotoxic T lymphocytes and natural killer cells. Biochim Biophys Acta 2013; 1833: 1603–1611. [DOI] [PubMed] [Google Scholar]
- 6Weidinger C, Shaw PJ, Feske S. STIM1 and STIM2-mediated Ca(2+) influx regulates antitumor immunity by CD8(+) T cells. EMBO Mol Med 2013; 5: 1311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7Meier T, Polzer P, Diederichs K, Welte W, Dimroth P. Structure of the rotor ring of F-Type Na+-ATPase from Ilyobacter tartaricus. Science 2005; 308: 659–662. [DOI] [PubMed] [Google Scholar]
- 8Murata T, Yamato I, Kakinuma Y, Leslie AG, Walker JE. Structure of the rotor of the V-Type Na+-ATPase from Enterococcus hirae. Science 2005; 308: 654–659. [DOI] [PubMed] [Google Scholar]
- 9Hannaert-Merah Z, Combettes L, Coquil JF, Swillens S, Mauger JP, Claret M, Champeil P. Characterization of the co-agonist effects of strontium and calcium on myo-inositol trisphosphate-dependent ion fluxes in cerebellar microsomes. Cell Calcium 1995; 18: 390–399. [DOI] [PubMed] [Google Scholar]
- 10Feske S, Skolnik EY, Prakriya M. Ion channels and transporters in lymphocyte function and immunity. Nat Rev Immunol 2012; 12: 532–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11Takuwa N, Iwamoto A, Kumada M, Yamashita K, Takuwa Y. Role of Ca2+ influx in bombesin-induced mitogenesis in Swiss 3T3 fibroblasts. J Biol Chem 1991; 266: 1403–1409. [PubMed] [Google Scholar]
- 12Nordstrom T, Nevanlinna HA, Andersson LC. Mitosis-arresting effect of the calcium channel inhibitor SK&F 96365 on human leukemia cells. Exp Cell Res 1992; 202: 487–494. [DOI] [PubMed] [Google Scholar]
- 13Takuwa N, Zhou W, Kumada M, Takuwa Y. Ca2+/calmodulin is involved in growth factor-induced retinoblastoma gene product phosphorylation in human vascular endothelial cells. FEBS Lett 1992; 306: 173–175. [DOI] [PubMed] [Google Scholar]
- 14MacKenzie AB, Surprenant A, North RA. Functional and molecular diversity of purinergic ion channel receptors. Ann NY Acad Sci 1999; 868: 716–729. [DOI] [PubMed] [Google Scholar]
- 15Putney Jr JW, Broad LM, Braun FJ, Lievremont JP, Bird GS. Mechanisms of capacitative calcium entry. J Cell Sci 2001; 114: 9. [DOI] [PubMed] [Google Scholar]
- 16Putney JW Jr. A model for receptor-regulated calcium entry. Cell Calcium 1986; 7: 1–12. [DOI] [PubMed] [Google Scholar]
- 17Shuttleworth TJ. Arachidonic acid activates the noncapacitative entry of Ca2+ during [Ca2+]i oscillations. J Biol Chem 1996; 271: 21720–21725. [DOI] [PubMed] [Google Scholar]
- 18Lefranc F, Kiss R. The sodium pump alpha1 subunit as a potential target to combat apoptosis-resistant glioblastomas. Neoplasia 2008; 10: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19Mijatovic T, Roland I, Van Quaquebeke E, Nilsson B, Mathieu A, Van Vynckt F et al. The alpha1 subunit of the sodium pump could represent a novel target to combat non-small cell lung cancers. J Pathol 2007; 212: 170–179. [DOI] [PubMed] [Google Scholar]
- 20Gao YD, Hanley PJ, Rinne S, Zuzarte M, Daut J. Calcium-activated K (+) channel (K(Ca)3.1) activity during Ca(2+) store depletion and store-operated Ca(2+) entry in human macrophages. Cell Calcium 2010; 48: 19–27. [DOI] [PubMed] [Google Scholar]
- 21Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci USA 1993; 90: 6295–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22Lis A, Peinelt C, Beck A, Parvez S, Monteilh-Zoller M, Fleig A et al. CRACM1 CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr Biol 2007; 17: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23DeHaven WI, Smyth JT, Boyles RR, Putney JW Jr. Calcium inhibition and calcium potentiation of Orai1, Orai2, and Orai3 calcium release-activated calcium channels. J Biol Chem 2007; 282: 17548–17556. [DOI] [PubMed] [Google Scholar]
- 24Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell Jr JE et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 2005; 15: 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S et al. STIM1 an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 2005; 169: 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 2008; 454: 538–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27Meuth SG, Bittner S, Meuth P, Simon OJ, Budde T, Wiendl H. TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 critically influence T lymphocyte effector functions. J Biol Chem 2008; 283: 14559–14570. [DOI] [PubMed] [Google Scholar]
- 28Wulff H, Castle NA, Pardo LA. Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov 2009; 8: 982–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29Harmar AJ, Hills RA, Rosser EM, Jones M, Buneman OP, Dunbar DR et al. IUPHAR-DB: the IUPHAR database of G protein-coupled receptors and ion channels. Nucleic Acids Res 2009; 37: D680–D685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30Yellen G. The voltage-gated potassium channels and their relatives. Nature 2002; 419: 35–42. [DOI] [PubMed] [Google Scholar]
- 31Judge SI, Lee JM, Bever Jr CT, Hoffman PM. Voltage-gated potassium channels in multiple sclerosis: overview and new implications for treatment of central nervous system inflammation and degenration. J Rehabil Res Dev 2006; 43: 111. [DOI] [PubMed] [Google Scholar]
- 32Leonard RJ, Garcia ML, Slaughter RS, Reuben JP. Selective blockers of voltage-gated K+ channels depolarize human T lymphocytes: mechanism of the antiproliferative effect of charybdotoxin. Proc Natl Acad Sci USA 1992; 89: 10094–10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA et al. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem 2000; 275: 37137–37149. [DOI] [PubMed] [Google Scholar]
- 34Di L, Srivastava S, Zhdanova O, Ding Y, Li Z, Wulff H et al. Inhibition of the K+ channel KCa3.1 ameliorates T cell-mediated colitis. Proc Natl Acad Sci U S A 2010; 107: 1541–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35Hu L, Wang T, Gocke AR, Nath A, Zhang H, Margolick JB et al. Blockade of Kv1.3 potassium channels inhibits differentiation and granzyme B secretion of human CD8+ T effector memory lymphocytes. PloS One 2013; 8: e54267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36Panyi G, Vamosi G, Bacso Z, Bagdany M, Bodnar A, Varga Z et al. Kv1.3 potassium channels are localized in the immunological synapse formed between cytotoxic and target cells. Proc Natl Acad Sci U S A 2004; 101: 1285–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37Koshy S, Wu D, Hu X, Tajhya RB, Huq R, Khan FS et al. Blocking KCa3.1 channels increases tumor cell killing by a subpopulation of human natural killer lymphocytes. PloS One 2013; 8: e76740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38Perier F, Radeke CM, Vandenberg CA. Primary structure and characterization of a small-conductance inwardly rectifying potassium channel from human hippocampus. Proc Natl Acad Sci USA 1994; 91: 6240–6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39Judge SI, Lee JM, Bever Jr CT, Hoffman PM. Voltage-gated potassium channels in multiple sclerosis: Overview and new implications for treatment of central nervous system inflammation and degeneration. J Rehabil Res Dev 2006; 43: 111. [DOI] [PubMed] [Google Scholar]
- 40Kurschner C, Yuzaki M. Neuronal interleukin-16 (NIL-16): a dual function PDZ domain protein. J Neurosci 1999; 19: 7770–7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41Tsai KL, Chang HF, Wu SN. The inhibition of inwardly rectifying K+ channels by memantine in macrophages and microglial cells. Cell Physiol Biochem 2013; 31: 938–951. [DOI] [PubMed] [Google Scholar]
- 42Srivastava R, Aslam M, Kalluri SR, Schirmer L, Buck D, Tackenberg B et al. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med 2012; 367: 115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43Goldstein SA, Bockenhauer D, O'Kelly I, Zilberberg N. Potassium leak channels and the KCNK family of two-P-domain subunits. Nat Rev Neurosci 2001; 2: 175–184. [DOI] [PubMed] [Google Scholar]
- 44Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem 2007; 76: 387–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45Wenning AS, Neblung K, Strauss B, Wolfs MJ, Sappok A, Hoth M et al. TRP expression pattern and the functional importance of TRPC3 in primary human T-cells. Biochim Biophys Act 2011; 1813: 412–423. [DOI] [PubMed] [Google Scholar]
- 46Vennekens R, Nilius B. Insights into TRPM4 function, regulation and physiological role. Handb Exp Pharmacol 2007: 269–285. [DOI] [PubMed]
- 47Launay P, Cheng H, Srivatsan S, Penner R, Fleig A, Kinet JP. TRPM4 regulates calcium oscillations after T cell activation. Science 2004; 306: 1374–1377. [DOI] [PubMed] [Google Scholar]
- 48Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J 2009; 23: 1685–1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y et al. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 2010;: 3475–3484. [DOI] [PMC free article] [PubMed]
- 50Padeh S, Cohen A, Roifman CM. ATP-induced activation of human B lymphocytes via P2-purinoceptors. J Immunol 1991; 146: 1626–1632. [PubMed] [Google Scholar]
- 51Baricordi OR, Ferrari D, Melchiorri L, Chiozzi P, Hanau S, Chiari E et al. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood 1996; 87: 682–690. [PubMed] [Google Scholar]
- 52Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR et al. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell 2005; 16: 3260–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat Immunol 2008; 9: 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54Matsumoto M, Fujii Y, Baba A, Hikida M, Kurosaki T, Baba Y. The calcium sensors STIM1 and STIM2 control B cell regulatory function through interleukin-10 production. Immunity 2011; 34: 703–714. [DOI] [PubMed] [Google Scholar]
- 55Feske S. STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol Rev 2009; 231: 189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56Franciszkiewicz K, Le Floc'h A, Boutet M, Vergnon I, Schmitt A, Mami-Chouaib F. CD103 or LFA-1 engagement at the immune synapse between cytotoxic T cells and tumor cells promotes maturation and regulates T-cell effector functions. Cancer Res 2013; 73: 617–628. [DOI] [PubMed] [Google Scholar]
- 57Quintana A, Hoth M. Mitochondrial dynamics and their impact on T cell function. Cell Calcium 2012; 52: 57–63. [DOI] [PubMed] [Google Scholar]
- 58Quintana A, Pasche M, Junker C, Al-Ansary D, Rieger H, Kummerow C et al. Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J 2011; 30: 3895–3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59Maul-Pavicic A, Chiang SC, Rensing-Ehl A, Jessen B, Fauriat C, Wood SM et al. ORAI1-mediated calcium influx is required for human cytotoxic lymphocyte degranulation and target cell lysis. Proc Natl Acad Sci USA 2011; 108: 3324–3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60Fierro L, Parekh AB. Substantial depletion of the intracellular Ca2+ stores is required for macroscopic activation of the Ca2+ release-activated Ca2+ current in rat basophilic leukaemia cells. J Physiol 2000; 522(Pt 2): 247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J Physiol 1993; 465: 359–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62Lang F, Stournaras C. Ion channels in cancer: future perspectives and clinical potential. Philos Trans R Soc Lond B Biol Sci 2014; 369: 20130108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63Ousingsawat J, Spitzner M, Puntheeranurak S, Terracciano L, Tornillo L, Bubendorf L et al. Expression of voltage-gated potassium channels in human and mouse colonic carcinoma. Clin Cancer Res 2007; 13: 824–831. [DOI] [PubMed] [Google Scholar]
- 64Preussat K, Beetz C, Schrey M, Kraft R, Wolfl S, Kalff R et al. Expression of voltage-gated potassium channels Kv1.3 and Kv1.5 in human gliomas. Neurosci Lett 2003; 346: 33–36. [DOI] [PubMed] [Google Scholar]
- 65Pardo LA, Stuhmer W. Eag1: an emerging oncological target. Cancer Res 2008; 68: 1611–1613. [DOI] [PubMed] [Google Scholar]
- 66Downie BR, Sanchez A, Knotgen H, Contreras-Jurado C, Gymnopoulos M, Weber C et al. Eag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumors. J Biol Chem 2008; 283: 36234–36240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67Huang X, Dubuc AM, Hashizume R, Berg J, He Y, Wang J et al. Voltage-gated potassium channel EAG2 controls mitotic entry and tumor growth in medulloblastoma via regulating cell volume dynamics. Genes Dev 2012; 26: 1780–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B et al. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res 2002; 62: 4843–4848. [PubMed] [Google Scholar]
- 69Barriere H, Belfodil R, Rubera I, Tauc M, Lesage F, Poujeol C et al. Role of TASK2 potassium channels regarding volume regulation in primary cultures of mouse proximal tubules. J Gen Physiol 2003: 177–190. [DOI] [PMC free article] [PubMed]
- 70Williams S, Bateman A, O'Kelly I. Altered expression of two-pore domain potassium (K2P) channels in cancer. PloS One 2013; 8: e74589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71Mu D, Chen L, Zhang X, See LH, Koch CM, Yen C et al. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell 2003; 3: 297–302. [DOI] [PubMed] [Google Scholar]
- 72Yang M, Brackenbury WJ. Membrane potential and cancer progression. Front Physiol 2013; 4: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73Morokuma J, Blackiston D, Adams DS, Seebohm G, Trimmer B, Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Natl Acad Sci USA 2008; 105: 16608–16613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011: 646–674. [DOI] [PubMed]
- 75Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev 2012; 92: 1865–1913. [DOI] [PubMed] [Google Scholar]
- 76Khaitan D, Sankpal UT, Weksler B, Meister EA, Romero IA, Couraud PO et al. Role of KCNMA1 gene in breast cancer invasion and metastasis to brain. BMC Cancer 2009; 9: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77Chantome A, Potier-Cartereau M, Clarysse L, Fromont G, Marionneau-Lambot S, Gueguinou M et al. Pivotal role of the lipid Raft SK3-Orai1 complex in human cancer cell migration and bone metastases. Cancer Res 2013; 73: 4852–4861. [DOI] [PubMed] [Google Scholar]
- 78Schwab A, Schuricht B, Seeger P, Reinhardt J, Dartsch PC. Migration of transformed renal epithelial cells is regulated by K+ channel modulation of actin cytoskeleton and cell volume. Pflugers Arch 1999; 438: 330–337. [DOI] [PubMed] [Google Scholar]
- 79Kraft R, Krause P, Jung S, Basrai D, Liebmann L, Bolz J et al. BK channel openers inhibit migration of human glioma cells. Pflugers Arch 2003; 446: 248–255. [DOI] [PubMed] [Google Scholar]
- 80Xie LH, John SA, Ribalet B, Weiss JN. Activation of inwardly rectifying potassium (Kir) channels by phosphatidylinosital-4,5-bisphosphate (PIP2): interaction with other regulatory ligands. Prog Biophys Mol Biol 2007; 94: 320–335. [DOI] [PubMed] [Google Scholar]
- 81Stringer BK, Cooper AG, Shepard SB. Overexpression of the G-protein inwardly rectifying potassium channel 1 (GIRK1) in primary breast carcinomas correlates with axillary lymph node metastasis. Cancer Res 2001; 61: 582–588. [PubMed] [Google Scholar]
- 82Takanami I, Inoue Y, Gika M. G-protein inwardly rectifying potassium channel 1 (GIRK 1) gene expression correlates with tumor progression in non-small cell lung cancer. BMC Cancer 2004; 4: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83Duncan LM, Deeds J, Hunter J, Shao J, Holmgren LM, Woolf EA et al. Down-regulation of the novel gene melastatin correlates with potential for melanoma metastasis. Cancer Res 1998; 58: 5–20. [PubMed] [Google Scholar]
- 84Prevarskaya N, Zhang L, Barritt G. TRP channels in cancer. Biochim Biophys Acta 2007; 1772: 937–946. [DOI] [PubMed] [Google Scholar]
- 85Monet M, Lehen'kyi V, Gackiere F, Firlej V, Vandenberghe M, Roudbaraki M et al. Role of cationic channel TRPV2 in promoting prostate cancer migration and progression to androgen resistance. Cancer Res 2010; 70: 1225–1235. [DOI] [PubMed] [Google Scholar]
- 86Zhang L, Barritt GJ. TRPM8 in prostate cancer cells: a potential diagnostic and prognostic marker with a secretory function? Endocr Relat Cancer 2006; 13: 27–38. [DOI] [PubMed] [Google Scholar]
- 87Vriens J, Janssens A, Prenen J, Nilius B, Wondergem R. TRPV channels and modulation by hepatocyte growth factor/scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium 2004; 36: 19–28. [DOI] [PubMed] [Google Scholar]
- 88Chigurupati S, Venkataraman R, Barrera D, Naganathan A, Madan M, Paul L et al. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res 2010; 70: 418–427. [DOI] [PubMed] [Google Scholar]
- 89Ding X, He Z, Zhou K, Cheng J, Yao H, Lu D et al. Essential role of TRPC6 channels in G2/M phase transition and development of human glioma. J Natl Cancer Inst 2010; 102: 1052–1068. [DOI] [PubMed] [Google Scholar]
- 90Gao H, Chen X, Du X, Guan B, Liu Y, Zhang H. EGF enhances the migration of cancer cells by up-regulation of TRPM7. Cell Calcium 2011; 50: 559–568. [DOI] [PubMed] [Google Scholar]
- 91Middelbeek J, Kuipers AJ, Henneman L, Visser D, Eidhof I, van Horssen R et al. TRPM7 is required for breast tumor cell metastasis. Cancer Res 2012; 72: 4250–4261. [DOI] [PubMed] [Google Scholar]
- 92Bates DO, Hillman NJ, Williams B, Neal CR, Pocock TM. Regulation of microvascular permeability by vascular endothelial growth factors. J Anat 2002; 200: 581–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93Hamdollah Zadeh MA, Glass CA, Magnussen A, Hancox JC, Bates DO. VEGF-mediated elevated intracellular calcium and angiogenesis in human microvascular endothelial cells in vitro are inhibited by dominant negative TRPC6. Microcirculation 2008; 15: 605–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94Ge R, Tai Y, Sun Y, Zhou K, Yang S, Cheng T et al. Critical role of TRPC6 channels in VEGF-mediated angiogenesis. Cancer Lett 2009; 283: 43–51. [DOI] [PubMed] [Google Scholar]
- 95Lehen'kyi V, Flourakis M, Skryma R, Prevarskaya N. TRPV6 channel controls prostate cancer cell proliferation via Ca(2+)/NFAT-dependent pathways. Oncogene 2007; 26: 7380–7385. [DOI] [PubMed] [Google Scholar]
- 96Guilbert A, Gautier M, Dhennin-Duthille I, Haren N, Sevestre H, Ouadid-Ahidouch H. Evidence that TRPM7 is required for breast cancer cell proliferation. American journal of physiology. Am J Physiol Cell Physiol 2009; 297: C493–C502. [DOI] [PubMed] [Google Scholar]
- 97Yang ZH, Wang XH, Wang HP, Hu LQ. Effects of TRPM8 on the proliferation and motility of prostate cancer PC-3 cells. Asian J Androl 2009; 11: 157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98Wertz IE, Dixit VM. Characterization of calcium release-activated apoptosis of LNCaP prostate cancer cells. J Biol Chem 2000; 275: 11470–11477. [DOI] [PubMed] [Google Scholar]
- 99Tapia-Vieyra JV, Mas-Oliva J. Apoptosis and cell death channels in prostate cancer. Arch Med Res 2001; 32: 175–185. [DOI] [PubMed] [Google Scholar]
- 100Adinolfi E, Melchiorri L, Falzoni S, Chiozzi P, Morelli A, Tieghi A et al. P2X7 receptor expression in evolutive and indolent forms of chronic B lymphocytic leukemia. Blood 2002; 99: 706–708. [DOI] [PubMed] [Google Scholar]
- 101White N, Butler PE, Burnstock G. Human melanomas express functional P2 X(7) receptors. Cell Tissue Res 2005; 321: 411–418. [DOI] [PubMed] [Google Scholar]
- 102Cheng PN, Leung YC, Lo WH, Tsui SM, Lam KC. Remission of hepatocellular carcinoma with arginine depletion induced by systemic release of endogenous hepatic arginase due to transhepatic arterial embolisation, augmented by high-dose insulin: arginase as a potential drug candidate for hepatocellular carcinoma. Cancer Lett 2005; 224: 67–80. [DOI] [PubMed] [Google Scholar]
- 103Coutinho-Silva R, Stahl L, Cheung KK, de Campos NE, de Oliveira Souza C, Ojcius DM et al. P2X and P2Y purinergic receptors on human intestinal epithelial carcinoma cells: effects of extracellular nucleotides on apoptosis and cell proliferation. Am J Physiol Gastrointest Liver Physiol 2005; 288: G1024–G1035. [DOI] [PubMed] [Google Scholar]
- 104Roger S, Pelegrin P. P2X7 receptor antagonism in the treatment of cancers. Expert Opin Investig Drugs 2011; 20: 875–880. [DOI] [PubMed] [Google Scholar]
- 105Agteresch HJ, Burgers SA, van der Gaast A, Wilson JH, Dagnelie PC. Randomized clinical trial of adenosine 5'-triphosphate on tumor growth and survival in advanced lung cancer patients. Anticancer Drugs 2003; 14: 639–644. [DOI] [PubMed] [Google Scholar]
- 106Haskell CM, Wong M, Williams A, Lee LY. Phase I trial of extracellular adenosine 5'-triphosphate in patients with advanced cancer. Med Pediatr Oncol 1996; 27: 165–173. [DOI] [PubMed] [Google Scholar]
- 107Haskell CM, Mendoza E, Pisters KM, Fossella FV, Figlin RA. Phase II study of intravenous adenosine 5'-triphosphate in patients with previously untreated stage IIIB and stage IV non-small cell lung cancer. Invest New Drugs 1998; 16: 81–85. [DOI] [PubMed] [Google Scholar]
- 108Beijer S, Hupperets PS, van den Borne BE, Eussen SR, van Henten AM, van den Beuken-van Everdingen M et al. Effect of adenosine 5'-triphosphate infusions on the nutritional status and survival of preterminal cancer patients. Anti-cancer Drugs 2009; 20: 625–633. [DOI] [PubMed] [Google Scholar]
- 109Flourakis M, Lehen'kyi V, Beck B, Raphael M, Vandenberghe M, Abeele FV et al. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Disease 2010; 1: e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110Huang X, Jan LY. Targeting potassium channels in cancer. J Cell Biol 2014; 206: 151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111Yang S, Zhang JJ, Huang XY. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell 2009; 15: 124–134. [DOI] [PubMed] [Google Scholar]
- 112Yang N, Tang Y, Wang F, Zhang H, Xu D, Shen Y et al. Blockade of store-operated Ca(2+) entry inhibits hepatocarcinoma cell migration and invasion by regulating focal adhesion turnover. Cancer Lett 2013; 330: 163–169. [DOI] [PubMed] [Google Scholar]
- 113Chen YF, Chiu WT, Chen YT, Lin PY, Huang HJ, Chou CY et al. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc Natl Acad Sci U S A 2011; 108: 15225–15230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114Wood JN, Boorman J. Voltage-gated sodium channel blockers; target validation and therapeutic potential. Curr Top Med Chem 2005; 5: 529–537. [DOI] [PubMed] [Google Scholar]
- 115Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem 2009; 16: 66–93. [DOI] [PubMed] [Google Scholar]
- 116Kaczorowski GJ, McManus OB, Priest BT, Garcia ML. Ion channels as drug targets: the next GPCRs. J Gen Physiol 2008; 131: 399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117Ganapathi SB, Kester M, Elmslie KS. State-dependent block of HERG potassium channels by R-roscovitine: implications for cancer therapy. Am J Physiol Cell Physiol 2009; 296: C701–C710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118Frede J, Fraser SP, Oskay-Ozcelik G, Hong Y, Ioana Braicu E, Sehouli J et al. Ovarian cancer: Ion channel and aquaporin expression as novel targets of clinical potential. Eur J Cancer 2013; 49: 2331–2344. [DOI] [PubMed] [Google Scholar]
- 119Ji C, Cao C, Lu S, Kivlin R, Amaral A, Kouttab N et al. Curcumin attenuates EGF-induced AQP3 up-regulation and cell migration in human ovarian cancer cells. Cancer Chemother Pharmacol 2008; 62: 857–865. [DOI] [PubMed] [Google Scholar]
- 120Lee MH, Choi BY, Kundu JK, Shin YK, Na HK, Surh YJ. Resveratrol suppresses growth of human ovarian cancer cells in culture and in a murine xenograft model: eukaryotic elongation factor 1A2 as a potential target. Cancer Res 2009; 69: 7449–7458. [DOI] [PubMed] [Google Scholar]
- 121Isbilen B, Fraser SP, Djamgoz MB. Docosahexaenoic acid (omega-3) blocks voltage-gated sodium channel activity and migration of MDA-MB-231 human breast cancer cells. Int J Biochem Cell Biol 2006; 38: 2173–2182. [DOI] [PubMed] [Google Scholar]
- 122Yan C, Yang J, Shen L, Chen X. Inhibitory effect of Epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch Gynecol Obstet 2012; 285: 459–467. [DOI] [PubMed] [Google Scholar]
- 123Comes N, Bielanska J, Vallejo-Gracia A, Serrano-Albarras A, Marruecos L, Gomez D et al. The voltage-dependent K(+) channels Kv1.3 and Kv1.5 in human cancer. Front Physiol 2013; 4: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci 2004; 25: 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125Pardo LA, Stuhmer W. The roles of K(+) channels in cancer. Nat Rev Cancer 2014; 14: 39–48. [DOI] [PubMed] [Google Scholar]
- 126Liu X, Chang Y, Reinhart PH, Sontheimer H, Chang Y. Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J Neurosci 2002;: 1840–1849. [DOI] [PMC free article] [PubMed]
- 127Grossinger EM, Weiss L, Zierler S, Rebhandl S, Krenn PW, Hinterseer E et al. Targeting proliferation of chronic lymphocytic leukemia (CLL) cells through KCa3.1 blockade. Leukemia 2014; 28: 954–958. [DOI] [PubMed] [Google Scholar]
- 128Jiang J, Li MH, Inoue K, Chu XP, Seeds J, Xiong ZG. Transient receptor potential melastatin 7-like current in human head and neck carcinoma cells: role in cell proliferation. Cancer Res 2007; 67: 10929–10938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129Tsavaler L, Shapero MH, Morkowski S, Laus R. Trp-p8 a novel prostate-specific gene, is up-regulated in prostate cancer and other malignancies and shares high homology with transient receptor potential calcium channel proteins. Cancer Res 2001; 61: 3760–3769. [PubMed] [Google Scholar]
- 130Hopfner M, Lemmer K, Jansen A, Hanski C, Riecken EO, Gavish M et al. Expression of functional P2-purinergic receptors in primary cultures of human colorectal carcinoma cells. Biochem Biophys Res Commun 1998; 251: 811–817. [DOI] [PubMed] [Google Scholar]
- 131Schafer R, Sedehizade F, Welte T, Reiser G. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am J Physiol Lung Cell Mol Physiol 2003; 285: L376–L385. [DOI] [PubMed] [Google Scholar]
- 132Hou MF, Kuo HC, Li JH, Wang YS, Chang CC, Chen KC et al. Orai1/CRACM1 overexpression suppresses cell proliferation via attenuation of the store-operated calcium influx-mediated signalling pathway in A549 lung cancer cells. Biochim Biophys Act 2011; 1810: 8–84. [DOI] [PubMed] [Google Scholar]
- 133Liu H, Hughes JD, Rollins S, Chen B, Perkins E. Calcium entry via ORAI1 regulates glioblastoma cell proliferation and apoptosis. Exp Mol Pathol 2011; 91: 753–760. [DOI] [PubMed] [Google Scholar]
- 134Hammadi M, Chopin V, Matifat F, Dhennin-Duthille I, Chasseraud M, Sevestre H et al. Human ether a-gogo K(+) channel 1 (hEag1) regulates MDA-MB-231 breast cancer cell migration through Orai1-dependent calcium entry. J Cell Physiol 2012; 7: 3837–3846. [DOI] [PubMed] [Google Scholar]
- 135Ruggieri P, Mangino G, Fioretti B, Catacuzzeno L, Puca R, Ponti D et al. The inhibition of KCa3.1 channels activity reduces cell motility in glioblastoma derived cancer stem cells. PloS One 2012; 7: e47825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136Okamoto Y, Ohkubo T, Ikebe T, Yamazaki J. Blockade of TRPM8 activity reduces the invasion potential of oral squamous carcinoma cell lines. Int J Oncol 2012; 40: 1–40. [DOI] [PubMed] [Google Scholar]
- 137Waning J, Vriens J, Owsianik G, Stuwe L, Mally S, Fabian A. A novel function of capsaicin-sensitive TRPV1 channels: involvement in cell migration. Cell Calcium 2007; 42: 17–25. [DOI] [PubMed] [Google Scholar]
- 138Dhennin-Duthille I, Gautier M, Faouzi M, Guilbert A, Brevet M, Vaudry D et al. High expression of transient receptor potential channels in human breast cancer epithelial cells and tissues: correlation with pathological parameters. Cell Physiol Biochem 2011; 28: 813. [DOI] [PubMed] [Google Scholar]
- 139Jelassi B, Chantome A, Alcaraz-Perez F, Baroja-Mazo A, Cayuela ML, Pelegrin P et al. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene 2011; 30: 2108. [DOI] [PubMed] [Google Scholar]
- 140Motiani RK, Hyzinski-Garcia MC, Zhang X, Henkel MM, Abdullaev IF, Kuo YH et al. STIM1 and Orai1 mediate CRAC channel activity and are essential for human glioblastoma invasion. Pflugers Arch 2013; 465: 9–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141Munaron L, Genova T, Avanzato D, Antoniotti S, Fiorio Pla A. Targeting calcium channels to block tumor vascularization. Recent Pat Anticancer Drug Discov 2013; 8: 27–37. [DOI] [PubMed] [Google Scholar]
- 142Li J, Cubbon RM, Wilson LA, Amer MS, McKeown L, Hou B et al. Orai1 and CRAC channel dependence of VEGF-activated Ca2+ entry and endothelial tube formation. Circ Res 2011; 108: 0–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143Schaefer EA, Stohr S, Meister M, Aigner A, Gudermann T, Buech TR. Stimulation of the chemosensory TRPA1 cation channel by volatile toxic substances promotes cell survival of small cell lung cancer cells. Biochem Pharmacol 2013; 85: 426–438. [DOI] [PubMed] [Google Scholar]
- 144Skryma R, Mariot P, Bourhis XL, Coppenolle FV, Shuba Y, Vanden Abeele F et al. Store depletion and store-operated Ca2+ current in human prostate cancer LNCaP cells: involvement in apoptosis. J Physiol 2000; 527: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145Garcia-Calvo M, Leonard RJ, Novick J, Stevens SP, Schmalhofer W, Kaczorowski GJ et al. Purification, characterization, and biosynthesis of margatoxin, a component of Centruroides margaritatus venom that selectively inhibits voltage-dependent potassium channels. J Biol Chem 1993; 268: 18866–18874. [PubMed] [Google Scholar]
- 146Helms LM, Felix JP, Bugianesi RM, Garcia ML, Stevens S, Leonard RJ et al. Margatoxin binds to a homomultimer of K(V)1.3 channels in Jurkat cells. Comparison with K(V)1.3 expressed in CHO cells. Biochemistry 1997; 36: 3737–3744. [DOI] [PubMed] [Google Scholar]
- 147Koshy S, Wu D, Hu X, Tajhya RB, Huq R, Khan FS et al. Blocking KCa3.1 channels increases tumor cell killing by a subpopulation of human natural killer lymphocytes. PloS One 2013; 8: e76740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148Buraei Z, Schofield G, Elmslie KS. Roscovitine differentially affects CaV2 and Kv channels by binding to the open state. Neuropharmacology 2007; 52: 883–894. [DOI] [PubMed] [Google Scholar]
- 149Meijer L, Borgne A, Mulner O, Chong JP, Blow JJ, Inagaki N et al. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur J Biochem 1997; 243: 527–536. [DOI] [PubMed] [Google Scholar]
- 150Gomez-Varela D, Zwick-Wallasch E, Knotgen H, Sanchez A, Hettmann T, Ossipov D et al. Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res 2007; 67: 7343–7349. [DOI] [PubMed] [Google Scholar]
- 151Lastraioli E, Guasti L, Crociani O, Polvani S, Hofmann G, Witchel H et al. herg1 gene and HERG1 protein are overexpressed in colorectal cancers and regulate cell invasion of tumor cells. Cancer Res 2004; 64: 606–611. [DOI] [PubMed] [Google Scholar]
- 152Pillozzi S, Brizzi MF, Balzi M, Crociani O, Cherubini A, Guasti L et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia 2002; 16: 1791–1798. [DOI] [PubMed] [Google Scholar]
- 153Pillozzi S, Brizzi MF, Bernabei PA, Bartolozzi B, Caporale R, Basile V et al. VEGFR-1 (FLT-1), beta1 integrin, and hERG K+ channel for a macromolecular signaling complex in acute myeloid leukemia: role in cell migration and clinical outcome. Blood 2007; 110: 8–0. [DOI] [PubMed] [Google Scholar]
- 154Shao XD, Wu KC, Hao ZM, Hong L, Zhang J, Fan DM. The potent inhibitory effects of cisapride, a specific blocker for human ether-a-go-go-related gene (HERG) channel, on gastric cancer cells. Cancer Biol Ther 2005; 4: 295–301. [DOI] [PubMed] [Google Scholar]
- 155Millward MJ, Cantwell BM, Munro NC, Robinson A, Corris PA, Harris AL. Oral verapamil with chemotherapy for advanced non-small cell lung cancer: a randomised study. Br J Cancer 1993; 67: 1031–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156Yohem KH, Clothier JL, Montague SL, Geary RJ, Winters AL 3rd, Hendrix MJ et al. Inhibition of tumor cell invasion by verapamil. Pigment Cell Res 1991; 4: 225–233. [DOI] [PubMed] [Google Scholar]
- 157Van Quaquebeke E, Simon G, Andre A, Dewelle J, El Yazidi M, Bruyneel F et al. Identification of a novel cardenolide (2''-oxovoruscharin) from Calotropis procera and the hemisynthesis of novel derivatives displaying potent in vitro antitumor activities and high in vivo tolerance: structure-activity relationship analyses. J Med Chem 2005; 48: 849–856. [DOI] [PubMed] [Google Scholar]
- 158Carrithers MD, Chatterjee G, Carrithers LM, Offoha R, Iheagwara U, Rahner C et al. Regulation of podosome formation in macrophages by a splice variant of the sodium channel SCN8A. J Biol Chem 2009; 284: 8114–8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res 2005; 11: 5381–5389. [DOI] [PubMed] [Google Scholar]
- 160Schwab A, Reinhardt J, Schneider SW, Gassner B, Schuricht B. K (+) channel-dependent migration of fibroblasts and human melanoma cells. Cell Physiol Biochem 1999; 9: 126–132. [DOI] [PubMed] [Google Scholar]
- 161Innamaa A, Jackson L, Asher V, Van Shalkwyk G, Warren A, Hay D et al. Expression and prognostic significance of the oncogenic K2P potassium channel KCNK9 (TASK-3) in ovarian carcinoma. Anticancer Res 2013; 33: 1–8. [PubMed] [Google Scholar]