Abstract
The role of low grade systemic inflammation as evidenced by elevated high sensitivity C-reactive protein (hsCRP) levels in the pathogenesis of atherosclerotic vascular disease has been intensely investigated through observational studies and clinical trials in the past two decades. On the basis of evidence that has accrued, hsCRP measurement has been integrated into the Reynolds risk scoring system to predict cardiovascular risk. The JUPITER trial proved the benefit of statins in cardiovascular risk reduction in patients with low grades of systemic inflammation and ‘normal’ cholesterol levels. However, substantial evidence has been generated from western studies. We, therefore, conducted a scoping review for studies done in India with a view to identify gaps in evidence and make further recommendations. Most Indian studies had small sample sizes and short term follow ups. There were no large population based prospective studies where patients were followed up for long periods of time for major cardiovascular end points. An analysis of the hsCRP level from the control arms of case-control studies derived a mean hsCRP value of 1.88 mg/l, which is higher than the western population where values < 1 mg/l are classified as low cardiovascular risk. Further large prospective cohort studies with longer term follow ups are essential before we can make further recommendations to integrate hsCRP into risk prediction models for cardiovascular disease prevention.
Keywords: Atherosclerosis, cardiovascular disease, hsCRP, India, inflammation, myocardial infarction
Introduction
The role of inflammation in the pathogenesis of atherosclerosis has been firmly established in the past two decades. Numerous studies, both observational (nested case control and prospective cohort) and randomized controlled trials (RCTs) have shown an association of pro-inflammatory biomarkers with incident hypertension, metabolic syndrome, coronary artery disease (CAD), acute coronary syndrome (ACS), peripheral artery disease, stroke and recurrent coronary and cerebrovascular events1,2,3,4. Approximately 25 large observational studies published since the 1990s have established high sensitivity C-reactive protein (hsCRP), a biomarker of inflammation, as an independent predictor for CAD. A meta-analysis of these observational studies showed that people in the top quartile for hsCRP levels had an odds ratio (OR) of 1.5 compared with those in the lowest quartile for major cardiovascular events, after adjusting for established risk factors5. Apart from observational studies, several RCTs evaluating statins such as Pravastatin or Atorvastatin Evaluation and Infection Therapy- Thrombolysis in Myocardial Infarction 22 (PROVE-IT TIMI-22)6, Cholesterol and Recurrent Events (CARE)7, The Pravastatin Inflammation/CRP Evaluation (PRINCE)8, Aggrastat- to- Zocor (A to Z)9 and Justification for the Use of Statins in Primary Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)10 indicate that cardiovascular benefits are more apparent when systemic inflammation (as evidenced by hsCRP reduction) is reduced in addition to intensive low-density lipoprotein cholesterol (LDL-C) lowering. The A to Z trial9 demonstrated that the best clinical outcomes occurred when the hsCRP levels were lowered below 2 mg/l in addition to LDL-C lowering to < 70 mg/dl. An imbalance between pro- and anti-inflammatory factors contributes to the atherosclerotic process. Inflammatory processes have an effect on the integrity of the fibrous cap in atherosclerotic plaque. Pro-inflammatory processes involving innate and adaptive immune mechanisms weaken the fibrous cap, causing a predisposition towards its rupture2. Interferon-γ (IFN-γ) elaborated by activated T cells suppresses collagen production by smooth muscles cells of the arterial wall. This is coupled with enhanced collagen degradation in the fibrous cap mediated by the matrix metalloproteinase enzymes (MMP-1, MMP-8, MMP-13) synthesized by activated macrophages. These processes enhance the friability of the fibrous cap11.
Currently it is being tested whether interventions to suppress the inflammatory process at “key points” in the inflammatory cascade can modify the atherosclerotic process and reduce clinical events.
What are CRP and hsCRP?
C-reactive protein (CRP) is a member of the pentraxin family of proteins. It is an acute phase reactant synthesized mainly by the liver. Serum CRP levels are elevated in response to acute infections, inflammatory conditions and trauma. In these clinical situations, the serum CRP levels rise rapidly generally beyond 10 mg/l with a concomitant elevation of erythrocyte sedimentation rates (ESR)12. CRP has a relatively long half-life of 18 to 20 h, owing to its stable pentraxin structure. In addition, CRP levels are stable as these do not exhibit diurnal variations or variations in relation to food intake. In the past decade, high-sensitivity assays with rapid turnaround times for measurement have become available. High-sensitivity assay techniques such as immunonephelometry, immunoturbidimetry, high-sensitivity enzyme-linked immunosorbent assay (ELISA) and resonant acoustic profiling (RAP) can detect CRP with a sensitivity range of 0.01 to 10 mg/ l13. These high-sensitivity assays help quantify low grades of systemic inflammation, in the absence of overt systemic inflammatory or immunologic disorders. The hsCRP assays have been standardized across several commercial platforms and can be accurately measured from fresh or frozen plasma14. The hsCRP is the most widely evaluated biomarker in the quest for an ideal biomarker for global cardiovascular disease (CVD) risk prediction. It has been incorporated into the Reynolds Risk Scoring system for global CVD risk prediction in women and along with a parental history of premature myocardial infarction can reclassify 50 per cent of all women in the ATP III intermediate risk category (annual CVD risk of 5 to 10%) and 10 to 20 per cent into higher or lower 10-year risk categories, with improved accuracy15. On the basis of data obtained from population based studies, the AHA/CDC (American Heart Association/Centres for Disease Control) Working Group on markers of inflammation in CVD has classified serum hsCRP levels <1, 1–3 and >3 mg/l as low-, intermediate-, and high-risk groups for global CVD, respectively13. The Working Group recommends conducting two hsCRP assays two weeks apart in a fasting or a non-fasting state in a metabolically stable patient with no obvious signs of infection or inflammation that could confound results.
Although hsCRP has largely been the central focus, other inflammatory markers such as tumour necrosis factor (TNF)-α, interleukin (IL)-6, IL-7 and the matrix metalloproteinases have also been associated with the atherosclerotic process2. Several factors, however, make hsCRP an attractive biomarker for cardiovascular risk prediction.
Is hsCRP just a marker of inflammation or does it play a causative role?
A debated controversy in this area has been whether hsCRP contributes to the atherosclerotic process or is merely a marker of inflammation. The hsCRP has been noted to have opsonizing properties, increasing the recruitment of monocytes into atheromatous plaque and also inducing endothelial dysfunction by suppressing basal and induced nitric oxide release. The hsCRP per se has also been found to increase the expression of vascular endothelial plasminogen activator inhibitor-1 (PAI-1) and other adhesion molecules and alter LDL uptake by macrophages11. However, interventions that directly inhibit hsCRP would have to be evaluated before conclusively establishing hsCRP as a direct contributor to the atherosclerotic process. Mendelian randomization studies have hinted at a causal relationship between hsCRP genotypes and atherosclerotic CVD, though stronger evidence of causality is required16.
What were the lessons from JUPITER?
JUPITER – a primary prevention trial10 sought to evaluate the utility of a statin in reducing major adverse cardiovascular events in patients with normal to low cholesterol levels (LDL-C <130 mg/dl) but with high hsCRP levels (>2 mg/l). A total of 17,802 apparently healthy men and women were randomized to receive either rosuvastatin 20 mg or placebo. The trial was stopped prematurely within a median follow up duration of 1.9 yr, as rosuvastatin produced a significant reduction in the pre-specified primary composite end point of myocardial infarction, stroke, cardiovascular death, arterial revascularization and unstable angina. Rosuvastatin was shown to reduce LDL-C levels by 50 per cent and hsCRP levels by 37 per cent. The overarching question that this trial posed was whether the beneficial effects on cardiovascular end points were due to lipid lowering alone, suppression of inflammation alone (as demonstrated by hsCRP reduction), or a combination of both mechanisms. JUPITER did not address the question of whether selective suppression of the inflammatory process could also achieve beneficial effects. The ongoing Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) trial17 attempts to provide better clarity on the questions raised by the JUPITER trial.
What can CANTOS tell us?
The CANTOS (ClinicalTrials.gov Identifier NCT01327846) trial addresses this controversy; it evaluates if selective inhibition of IL-1β with canakinumab can reduce cardiovascular death, non-fatal myocardial infarction and stroke in stable post-myocardial infarction patients at high risk for recurrent events as evidenced by serum hsCRP >2 mg/l. Phase 2 trials with canakimumab have shown that upstream inhibition of IL-1β resulted in dose dependent 50 per cent reductions in downstream biomarkers, CRP and IL-6 levels, without lowering lipid levels or blood pressures18. The results of the trial are expected in 2018.
The large numbers of Western studies that have evaluated the link between hsCRP and cardiovascular disease, prompted a scoping review of the studies conducted in India linking the inflammatory hypothesis in general and hsCRP in particular, with metabolic syndrome and CVD. The objectives of this article are to review the studies linking the inflammatory hypothesis with diabetes mellitus, metabolic syndrome and atherosclerosis in the South Asian/Indian population, to identify gaps in evidence and to make recommendations for further work in this important area in the Indian context.
Literature search methods
The search was done using PubMed and Google Scholar. We used the following search strings: hsCRP in India AND type 2 diabetes mellitus AND cardiovascular disease, hsCRP in Indian patients, hsCRP in Indian population, hsCRP in India AND cardiovascular disease. No limits were set in order to retrieve a maximum number of articles.
A total of 71 articles were retrieved from PubMed and 1400 articles were retrieved from Google Scholar. The abstracts were reviewed and a total of 27 articles (14 from PubMed and 13 from Google Scholar) that had hsCRP measurements in patients with cardiovascular disease and/or type 2 diabetes mellitus, were selected. Studies conducted in Indian patients that explored the relationship between hsCRP as a marker of inflammation and outcomes such as type 2 diabetes mellitus, impaired glucose tolerance and primary or secondary prevention of coronary artery disease were included. Studies that were not conducted in India, evaluated inflammatory markers other than hsCRP or evaluated the relationship of hsCRP with outcomes other than the ones specified were excluded.
The studies included
Of the 24 studies (Table), 12 (50%) were case-control studies, six (25%) were cohort studies and six (25%) were cross-sectional studies. Of the six cohort studies, four (66.6%) were comparative studies performed in a nested cohort of patients, one (16.6%) was a retrospective and one (16.6%) was a prospective cohort study. Eight (33.3%) studies evaluated the utility of hsCRP as a predictor for diabetes mellitus, nine (37.5%) evaluated hsCRP levels in patients with metabolic syndrome including diabetes, six (25%) studies correlated hsCRP levels with abdominal adiposity and body mass index. Ten (41.6%) studies reported the utility of hsCRP as a predictor of CAD and two (8.3%) studies as cerebrovascular disease. Only five (20.8%) of the 24 studies have large sample sizes (n > 1000) of which only one was a prospective study and three were case-control studies. None of the studies evaluated parameters such as sensitivity, specificity, positive and negative predictive values of hsCRP in cardiovascular risk prediction.
Table.
Summary of Indian studies evaluating hsCRP as a risk predictor
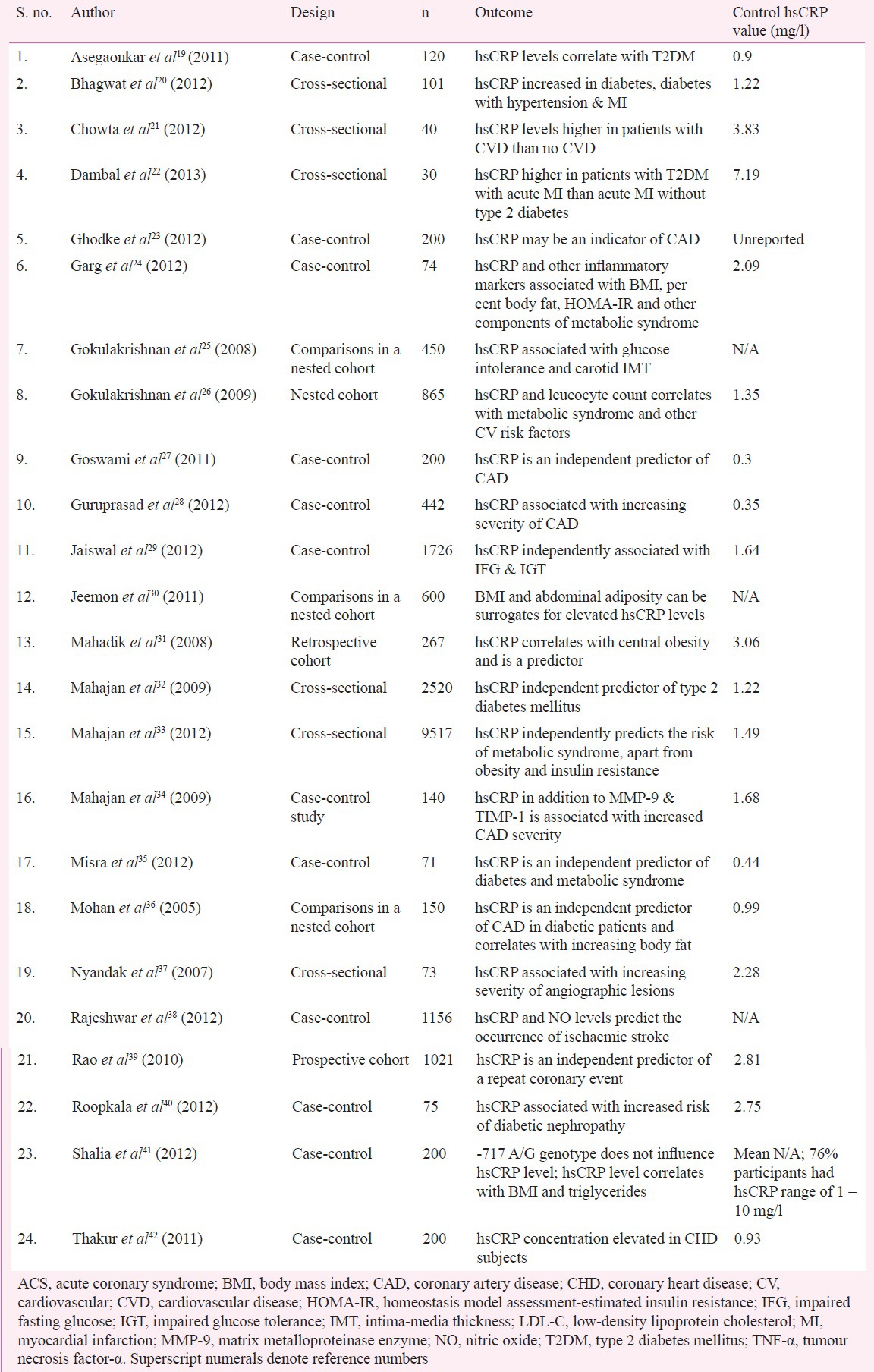
Of the 24 studies, control group patients in at least 13 (54.2%) studies had hsCRP levels in the intermediate to high risk group level (>1 mg/l), indicating that the basal concentration of hsCRP is high in Indians. An analysis of the control arm of the various studies derives a mean hsCRP value of 1.88 mg/l. In studies with subjects having established CVD, the hsCRP values varied from 2.46 to 9.3 mg/l. Similar results have been found among Asian Indians living in the United Kingdom, where Indians were found to have 17 per cent higher CRP values compared with Europeans43, among Indians living in the United States as compared with Caucasians44, and among Indians in Singapore as compared with the Chinese and the Malays45. The included studies employed a wide range of analytical techniques for hsCRP estimation; eight (33%) studies employed ELISA, six (25%) employed nephelometry, seven (29%) used turbidimetry, two (8.3%) used chemiluminescence and in one study latex agglutination test was used for quantification. This could have contributed to the varying values seen across these studies.
Implications of the Indian studies so far
The hsCRP was found to be an independent predictor of diverse end points ranging from obesity, type 2 diabetes mellitus, metabolic syndrome, increased carotid intima-media thickness, stable CAD, first acute coronary event, and recurrent CVD events. The larger studies have mainly evaluated the association of hsCRP and risk factors for CVD, diabetes mellitus and glucose intolerance. Mahajan et al32, in a study of 2,520 subjects, reported hsCRP to be an independent predictor of type 2 diabetes mellitus (OR, 1.66; 95% CI, 1.21 – 2.28, P=0.002). In another study, a cross-sectional survey of 9,517 subjects32, the authors again found an association between hsCRP levels and metabolic syndrome, obesity and insulin resistance (OR, 1.65; 95% CI, 1.41 – 1.92). Jaiswal et al29 in a case-control study of 1,726 subjects, reported hsCRP to be independently associated with impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) (OR, 2.60; 95% CI, 1.56 – 5.34). Studies with clinical CVD events included a case-control study by Rajeshwar et al38 (1,156 subjects; hsCRP levels predict ischaemic stroke), Goswami et al27 (200 subjects; hsCRP is an independent predictor of CAD) and Guruprasad et al28 (442 subjects; hsCRP levels are associated with an increasing severity of CAD). A prospective cohort study by Rao et al39 with 1,021 subjects, of whom 772 had established CAD and the rest were controls, found that hsCRP was an independent predictor of repeat coronary events.
The published studies from India have thus reported an association between hsCRP and metabolic syndrome, IGT, diabetes mellitus, CAD, and stroke. These studies used different designs and methods of estimating hsCRP and at times used arbitrary cut-off levels. A majority of these studies had small sample sizes and were case-control, cross-sectional or retrospective cohort studies. It is, therefore, not possible to define normal values and cut-off levels as identifiers of risk specifically for the Indian population from these studies. This is particularly important as current evidence points to elevated basal levels of hsCRP even in the normal control group patients. If one has to define a specific value and range as normal for Indian subjects and cut-off values for estimation of risk for CVD, data from large high-quality studies are needed to permit the construction of a receiver operating characteristic (ROC) curve. To achieve this, it is important to initiate large prospective cohort studies with standardization of diagnostic tests across sites and adequate follow up of participants for cardiovascular outcomes to derive risk cut-off values in the Indian population. Such studies are also needed to estimate the role of hsCRP versus other risk factors such as lipids to justify the recommendation and/or of routine measurement of hsCRP in estimating the risk for CVD in Indian patients.
Conclusion
Multiple small Indian studies employing varying designs have found an association between hsCRP and coronary artery disease, diabetes mellitus and the metabolic syndrome. The normal or basal values of hsCRP are likely higher in the Indian population. Larger prospective cohort studies employing standardized hsCRP measurement assays with adequate follow up duration are required to derive risk cut-off values for CVD in the Indian population.
Footnotes
Conflicts of Interest: Dr Prem Pais is the National Leader for the CANTOS study in India. The other authors declare no conflicts of interest.
References
- 1.Hak AE, Stehouwer CD, Bots ML, Polderman KH, Schalkwijk CG, Westendorp IC, et al. Associations of C-reactive protein with measures of obesity, insulin resistance, and subclinical atherosclerosis in healthy, middle-aged women. Arterioscler Thromb Vasc Biol. 1999;19:1986–91. doi: 10.1161/01.atv.19.8.1986. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–95. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, et al. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–44. doi: 10.1161/01.cir.98.9.839. [DOI] [PubMed] [Google Scholar]
- 4.Elkind MS, Tai W, Coates K, Paik MC, Sacco RL. High-sensitivity C-reactive protein, lipoprotein-associated phospholipase A2, and outcome after ischemic stroke. Arch Intern Med. 2006;166:2073–80. doi: 10.1001/archinte.166.19.2073. [DOI] [PubMed] [Google Scholar]
- 5.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Morrow DA, Rose LM, Rifai N, Cannon CP, Braunwald E. Relative efficacy of atorvastatin 80 mg and pravastatin 40 mg in achieving the dual goals of low-density lipoprotein cholesterol <70 mg/dl and C-reactive protein <2 mg/l: an analysis of the PROVE-IT TIMI-22 trial. J Am Coll Cardiol. 2005;45:1644–8. doi: 10.1016/j.jacc.2005.02.080. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–5. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- 8.Albert MA, Danielson E, Rifai N, Ridker PM. PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001;286:64–70. doi: 10.1001/jama.286.1.64. [DOI] [PubMed] [Google Scholar]
- 9.Wiviott SD, de Lemos JA, Cannon CP, Blazing M, Murphy SA, McCabe CH, et al. A tale of two trials: a comparison of the post-acute coronary syndrome lipid-lowering trials A to Z and PROVE IT-TIMI 22. Circulation. 2006;113:1406–14. doi: 10.1161/CIRCULATIONAHA.105.586347. [DOI] [PubMed] [Google Scholar]
- 10.JUPITER Study Group. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 11.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–13. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 12.Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem. 2004;279:48487–90. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 13.Roberts WL. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: laboratory tests available to assess inflammation--performance and standardization: a background paper. Circulation. 2004;110:e572–6. doi: 10.1161/01.CIR.0000148986.52696.07. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297:611–9. doi: 10.1001/jama.297.6.611. [DOI] [PubMed] [Google Scholar]
- 16.Shen J, Ordovas JM. Impact of genetic and environmental factors on hsCRP concentrations and response to therapeutic agents. Clin Chem. 2009;55:256–64. doi: 10.1373/clinchem.2008.117754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM, Thuren T, Zalewski A, Libby P. Interleukin-1b inhibition and the prevention of recurrent cardiovascular events: rationale and design of the Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) Am Heart J. 2011;162:597–605. doi: 10.1016/j.ahj.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 18.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. CANTOS Pilot Investigative Group. Effects of interleukin-1beta inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo controlled trial. Circulation. 2012;126:2739–48. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 19.Asegaonkar SB, Marathe A, Tekade ML, Cherekar L, Bavikar J, Bardapurkar J, et al. High-sensitivity C-reactive protein: a novel cardiovascular risk predictor in type 2 diabetics with normal lipid profile. J Diabetes Complications. 2011;25:368–70. doi: 10.1016/j.jdiacomp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Bhagwat R, Gupte A, Yadav KS. Diagnostic utility of hs-CRP in coronary heart disease. Int J Mol Biol. 2012;3:36–9. [Google Scholar]
- 21.Chowta MN, Adhikari PM, Sinha R, Acharya SD, Gopalakrishna HN, Ramapuram JT. Highly sensitive C reactive protein in patients with metabolic syndrome and cardiovascular disease. Ann Trop Med Public Health. 2012;5:98–102. [Google Scholar]
- 22.Dambal A, Padaki S, Herur A, Kashinakunti S, Manjula R. High sensitivity C-reactive protein in patients of acute myocardial infarction with type-2 diabetes mellitus-A cross-sectional study. [accessed on September 15, 2015]. Available from: www.omicsonline.org/scientific-reports/srep570.php .
- 23.Ghodke SS, Padalkar RK, Bhagat SS, Ghone RA, Patil SM. hs- CRP: A “Golden Marker” of inflammation and coronary artery disease. Int J Health Sci Res. 2012;2:42–6. [Google Scholar]
- 24.Garg MK, Dutta MK, Brar KS. Inflammatory markers in metabolic syndrome. Int J Diabetes Dev Ctries. 2012;32:131–7. [Google Scholar]
- 25.Gokulakrishnan K, Deepa R, Mohan V. Association of high sensitivity C-reactive protein (hsCRP) and tumour necrosis factor-alpha (TNF-alpha) with carotid intimal medial thickness in subjects with different grades of glucose intolerance--the Chennai Urban Rural Epidemiology Study (CURES-31) Clin Biochem. 2008;41:480–5. doi: 10.1016/j.clinbiochem.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Gokulakrishnan K, Deepa R, Sampathkumar R, Balasubramanyam M, Mohan V. Association of leukocyte count and hsCRP with metabolic abnormalities in subjects with normal glucose tolerance (CURES - 64) J Assoc Physicians India. 2009;57:27–32. [PubMed] [Google Scholar]
- 27.Goswami B, Tayal D, Tyagi S, Mallika V. Assessment of insulin resistance, dyslipidemia and inflammatory response in North Indian male patients with angiographically proven coronary artery disease. Minerva Cardioangiol. 2011;59:139–47. [PubMed] [Google Scholar]
- 28.Guruprasad S, Rajasekhar D, Subramanyam G, Srinivasa Rao PV, Vanajakshamma V, Latheef K. High sensitivity C-reactive protein levels across spectrum and severity of coronary artery disease. J Clin Sci Res. 2012;3:126–30. [Google Scholar]
- 29.Jaiswal A, Tabassum R, Podder A, Ghosh S, Tandon N, Bharadwaj D. Elevated level of C-reactive protein is associated with risk of prediabetes in Indians. Atherosclerosis. 2012;222:495–501. doi: 10.1016/j.atherosclerosis.2012.02.034. [DOI] [PubMed] [Google Scholar]
- 30.Jeemon P, Prabhakaran D, Ramakrishnan L, Gupta R, Ahmed F, Thankappan K, et al. Sentinel Surveillance in Industrial Populations Study Group. Association of high sensitive C-reactive protein (hsCRP) with established cardiovascular risk factors in the Indian population. Nutr Metab (Lond) 2011;8:1–8. doi: 10.1186/1743-7075-8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahadik SR, Deo SS, Mehtalia SD. Relation of C-reactive protein with the components of metabolic syndrome in Asian Indian subjects. Diabetes Metab Syndr. 2008;2:29–35. [Google Scholar]
- 32.Mahajan A, Tabassum R, Chavali S, Dwivedi OP, Bharadwaj M, Tandon N, et al. High-sensitivity C-reactive protein levels and type 2 diabetes in urban North Indians. J Clin Endocrinol Metab. 2009;94:2123–7. doi: 10.1210/jc.2008-2754. [DOI] [PubMed] [Google Scholar]
- 33.Mahajan A, Jaiswal A, Tabassum R, Podder A, Ghosh S, Madhu SV, et al. Elevated levels of C-reactive protein as a risk factor for metabolic syndrome in Indians. Atherosclerosis. 2012;220:275–81. doi: 10.1016/j.atherosclerosis.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 34.Mahajan N, Malik N, Bahl A, Sharma Y, Dhawan V. Correlation among soluble markers and severity of disease in non-diabetic subjects with pre-mature coronary artery disease. Mol Cell Biochem. 2009;330:201–9. doi: 10.1007/s11010-009-0134-1. [DOI] [PubMed] [Google Scholar]
- 35.Misra DP, Das S, Sahu PK. Prevalence of inflammatory markers (high-sensitivity C-reactive protein, nuclear factor- êB, and adiponectin) in Indian patients with type 2 diabetes mellitus with and without macrovascular complications. Metab Syndr Relat Disord. 2012;10:209–13. doi: 10.1089/met.2011.0044. [DOI] [PubMed] [Google Scholar]
- 36.Mohan V, Deepa R, Velmurugan K, Premalatha G. Association of C-reactive protein with body fat, diabetes and coronary artery disease in Asian Indians: the Chennai Urban Rural Epidemiology Study (CURES-6) Diabet Med. 2005;22:863–70. doi: 10.1111/j.1464-5491.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- 37.Nyandak T, Gogna A, Bansal S, Deb M. High sensitive C-reactive protein (hs-CRP) and its correlation with angiographic severity of coronary artery disease (CAD) J Indian Acad Clin Med. 2007;8:217–21. [Google Scholar]
- 38.Rajeshwar K, Kaul S, Al-Hazzani A, Babu MS, Balakrishna N, Sharma V, et al. C-reactive protein and nitric oxide levels in ischemic stroke and its subtypes: correlation with clinical outcome. Inflammation. 2012;35:978–84. doi: 10.1007/s10753-011-9401-x. [DOI] [PubMed] [Google Scholar]
- 39.Rao VS, Kadarinarasimhiah NB, John S, Hebbagodi S, Shanker J, Kakkar VV. Usefulness of C-reactive protein as a marker for prediction of future coronary events in the asian Indian population: Indian atherosclerosis research study. Int J Vasc Med. 2010;2010:1–80. doi: 10.1155/2010/389235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roopakala MS, Pawan HR, Krishnamurthy U, Wilma Delphine Silvia CR, Eshwarappa M, Prasanna Kumar KM. Evaluation of high sensitivity C-reactive protein and glycated hemoglobin levels in diabetic nephropathy. Saudi J Kidney Dis Transpl. 2012;23:286–9. [PubMed] [Google Scholar]
- 41.Shalia K, Savant S, Haldankar VA, Nandu T, Pawar P, Divekar S, et al. Study of C-reactive protein and myocardial infarction in the Indian population. Indian J Clin Biochem. 2012;27:74–82. doi: 10.1007/s12291-011-0164-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thakur S, Gupta S, Parchwani H, Shah V, Yadav V. Hs-CRP - a potential marker for coronary heart disease. Indian J Fundam Appl Life Sci. 2011;1:1–4. [Google Scholar]
- 43.Chambers JC, Eda S, Bassett P, Karim Y, Thompson SG, Gallimore JR, et al. C-reactive protein, insulin resistance, central obesity, and coronary heart disease risk in Indian Asians from the United Kingdom compared with European whites. Circulation. 2001;104:145–50. doi: 10.1161/01.cir.104.2.145. [DOI] [PubMed] [Google Scholar]
- 44.Chandalia M, Cabo-Chan AV, Jr, Devaraj S, Jialal I, Grundy SM, Abate N. Elevated plasma high-sensitivity C-reactive protein concentrations in Asian Indians living in the United States. J Clin Endocrinol Metab. 2003;88:3773–6. doi: 10.1210/jc.2003-030301. [DOI] [PubMed] [Google Scholar]
- 45.Dalan R, Jong M, Chan SP, Hawkins R, Choo R, Lim B, et al. High-sensitivity C-reactive protein concentrations among patients with and without diabetes in a multiethnic population of Singapore: CREDENCE Study. Diabetes Metab Syndr Obes. 2010;3:187–95. [PMC free article] [PubMed] [Google Scholar]


