Abstract
Background & objectives:
The Vitamin-D receptor (VDR) regulates vitamin D levels and calcium metabolism in the body and these are known to be associated with endocrine dysfunctions, insulin resistance and type-2 diabetes in polycystic ovarian syndrome (PCOS). Studies on VDR polymorphisms among PCOS women are sparse. We undertook this study to investigate the association pattern of VDR polymorphisms (Cdx2, Fok1, Apa1 and Taq1) with PCOS among Indian women.
Methods:
For the present study, 250 women with PCOS and 250 normal healthy control women were selected from Hyderabad city, Telangana, India. The four VDR polymorphisms were genotyped and analysed using ASM-PCR (allele specific multiple PCR) and PCR-RFLP (restriction fragment length polymorphism).
Results:
The genotype and allele frequency distributions of only Cdx2 showed significant difference between the PCOS cases and control women, indicating protective role of this SNP against PCOS phenotype. However, significant association was observed between VDR genotypes and some of the PCOS specific clinical/biochemical traits. For example, Fok1 showed a significant genotypic difference for the presence of infertility and Cdx2 genotpes showed association with testosterone levels. Further, the two haplotypes, ACCA and ACTA, were found to be significantly associated with PCOS indicating haplotype specific risk.
Interpretation & conclusions:
Although VDR polymorphisms have not shown significant association with PCOS, in view of functional significance of the SNPs considered, one cannot yet rule out the possibility of their association with PCOS. Further, specifically designed studies on large cohorts are required to conclusively establish the role of VDR polymorphisms in PCOS, particularly including data on vitamin D levels.
Keywords: Association, gene polymorphism, PCOS, SNP, vitamin-D, vitamin D receptor
Polycystic ovary syndrome (PCOS) is the most common endocrine-metabolic disorder affecting 5-10 per cent of women in reproductive age and is a common cause of anovulatory infertility1,2,3. It is a heterogeneous disease characterized by oligomenorrhoea due to increased ovarian and adrenal androgen secretion, hyperandrogenic symptoms such as hirsutism, acne and/or alopecia, menstrual irregularity, and polycystic ovaries. This disorder is also associated with an increased risk of hyperinsulinaemia, insulin resistance, type 2 diabetes mellitus, dyslipidaemia, and cardiovascular diseases4,5. Being a complex multigenic and heteroplasmy disease, various susceptibility genes of PCOS interact with each other and with the environmental factors and influence the development and manifestation of the syndrome.
Insulin resistance (IR), which is commonly present in women with PCOS, may play an important role in the long-term complications of PCOS6,7. Vitamin D levels play a critical role in metabolic modulations including calcium-phosphate (Ca-P) homeostasis, specifically in the regulation of insulin secretion by the β-cells8. Abnormalities in calcium balance may also be responsible, in part, for the arrested follicular development in women with PCOS and may even contribute to the pathogenesis of the PCOS syndrome. Recent studies suggest that vitamin D deficiency may be a causal factor in the pathogenesis of IR and the metabolic syndrome in PCOS but whether vitamin D is also related to endocrine parameters and fertility in PCOS is not established clearly9,10,11. Yildizhan et al12, however, correlated low serum 25-hydroxy vitamin D [25(OH) D] status with PCOS features such as testosterone and dehydroepiandrosterone (DHEAS) levels, luteinizing hormone/follicle stimulating hormone (LH/FSH) ratio, free androgen index, sex hormone-binding globulin (SHBG) and hirsutism score12. Hence, genes involved in insulin signaling pathway and vitamin D metabolism have been suggested as candidates for PCOS.
The vitamin D receptor (VDR) gene, also known as calcitriol receptor or NR1I1, is considered to be an important candidate gene for PCOS13. It is a ligand-activated transcription factor that mediates the genomic actions of vitamin D regulating several endocrine functions and cell functions including bone metabolism and calcium-phosphate homeostasis14,15. It is expressed in various tissues including skeletal, parathyroid as well as reproductive, and modulates the expression of several target genes to produce a variety of biological effects. The VDR gene is mapped to chromosomal locus 12q12-14 comprising pre-extensive promoter region and capable of generating multiple tissue-specific transcripts. Several VDR polymorphisms have been investigated for functional significance and potential effects on disease susceptibility to complex diseases such as osteoarthritis (OA), diabetes, cancer, high myopia, cardiovascular disease and tuberculosis16,17,18,19,20,21. A few studies have also been focussed on association of VDR gene in endocrine disorders including PCOS22,23,24.
The association of vitamin D and VDR variants such as Cdx2, Fok1, Apa1, Taq1 with endocrine, metabolic, and genetic aspects in PCOS has been reported indicating their strong functional role15,24,25. The present study was an attempt to examine the nature of association of the four functionally most relevant VDR polymorphisms- Cdx2 (rs11568820; exon 1e (G/A)), Fok1(rs2228570; exon 2 (C/T)), Apa1 (rs7975232; intron 8 (C/A)) and Taq1 (rs731236; exon 9 (T/C)) with PCOS among the Indian women from Hyderabad, Telangana, India.
Material & Methods
As part of a larger project undertaken by the Molecular Anthropology Group of the Biological Anthropology Unit, Indian Statistical Institute, Hyderabad, 250 women with PCOS and 250 normal healthy women as controls were enrolled for the study during 2008-2009. The PCOS patients were taken from the Gynaecology Clinic of the Osmania General Hospital, Hyderabad and Infertility Clinic, Anu's Test Tube Baby Centre, Hyderabad, as per the Rotterdam criteria, 200326 according to which any two of the following three conditions need to be fulfilled for the inclusion: (i) presence of clinical and/or biochemical signs of hyperandrogenism, (ii) infrequent periods with intermenstrual interval of more than 35 days, and (iii) polycystic ovaries, [an ovary with the ultrasound appearance of more than 10 subcapsular follicles (<10 mm in diameter) in the presence of prominent ovarian stroma was considered polycystic]. Patients with hyperprolactinaemia, thyroid and adrenal diseases, 21-hydroxylase deficiency, and androgen-secreting tumours were excluded. Hirsutism was defined as a Ferriman-Gallwey score of more than five27. Hormonal assays included serum levels of gonadotrophic hormones [luteinizing hormone (LH) and follicle stimulating hormone (FSH)], thyroid stimulating hormone (TSH) and testosterone (total). The ethnically matched normal women with no history of treatment for fertility, no evidence of clinical hyperandrogenism (hirsutism/acne/alopecia), and with normal menstrual cycles every 25-32 days were selected from the Family Planning Center of the Osmania hospital and healthy volunteers. The controls could not be screened for hormonal profile. BloodBlood samples (5 ml) were collected from the patients as well as controls after obtaining their informed written consent. The study protocol was approved by the Indian Statistical Institute Review Committee for Protection of Research Risks to Humans.
DNA isolation and genotyping: The genomic DNA was isolated from the blood samples by phenol-chloroform method28 and used for genotyping. A fresh working dilution was made for each DNA sample and was checked for the quality by electrophoresis in a 0.8 per cent agarose gel.
Genomic DNA samples were amplified and analysed for the four VDR gene single nucleotide polymorphisms (SNPs) using predesigned specific primers29,30,31,32. Analysis of Cdx2 polymorphism was done through ASM-PCR (allele specific multiple-PCR) using two sets of primers and PCR products were resolved on 2-3 per cent agarose gels. Other SNPs (Fok1, Apa1 and Taq1) were analysed through PCR-RFLP (restriction fragment length polymorphism) method wherein the amplified PCR products were digested under required conditions using respective restriction endonuclease enzymes (New England Biolabs, USA). Based on the presence or absence of restriction sites, variant RFLP products for each SNP were resolved on 3 per cent agarose gel to determine the genotypes. Details of the studied polymorphisms and PCR conditions are given in Table I, and RFLP conditions are presented in Table II.
Table I.
PCR condition and product size for vitamin D receptor single nucleotide polymorphisms 5’ and 3’ (VDR SNPs)
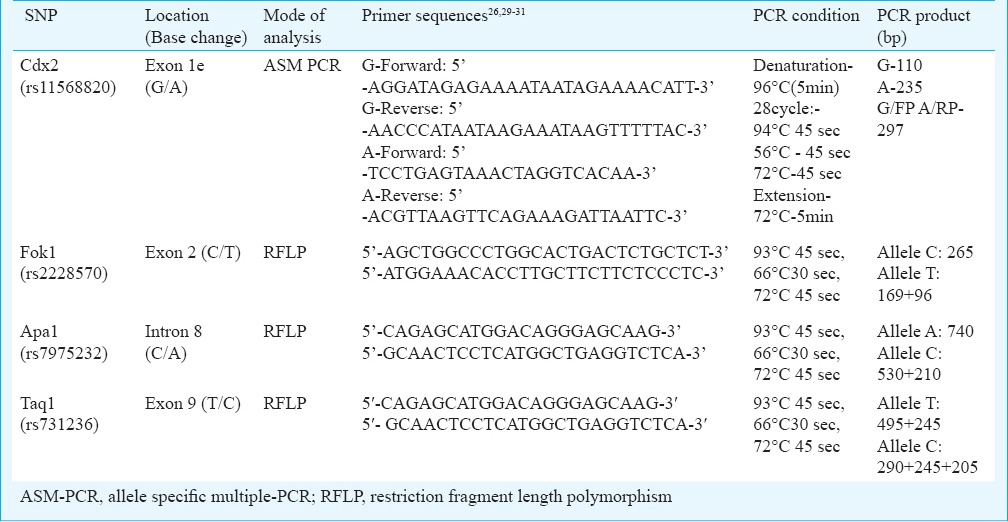
Table II.
Conditions for restriction digestion protocol for the three VDR polymorphisms
Statistical analysis: Allele and genotype frequencies were determined by the gene-counting method33. The statistical analyses were performed mainly with the help of SPSS statistical software, version 20.0, IBM SPSS, version 20 (SPSS, Inc., Chicago Il, USA). The departure from the Hardy-Weinberg equilibrium (HWE) was tested for each SNP by χ2 test (Pearson's goodness of fit test) as implemented in Pypop software- version-7.0 (Thomson Laboratories CA, USA). The cut-off P value for deviation from HWE was taken at <0.001 (0.0012 with a Bonferroni correction). The odds ratio with its significance and 95% confidence interval (95% CI) were estimated with age and body mass index (BMI) as covariates. The genotypic distribution for each polymorphism was also analyzed with respect to the clinical and biochemical traits among the women with PCOS using SPSS. Haploview-version-4.2 (Cambridge, USA, haploview@broad.mit.edu) and THESIAS softwares-version-3.1 (INSERM, Paris, France, david.tregouet@chups.jussieu.ft) were used to estimate linkage disequilibrium and generate haplotype frequencies. Power of the study was calculated using G*Power software-version 3.1. (University of Dusseldorf, Germany, http://www.psycho.uni-duesseldorf.de/abteilungen/aap/gpower3). Multivariate logistic regression analysis was carried out using R software -version-3.1.0. (R program version 2.15.2; R Foundation. http://www.r-project.org/).
Results
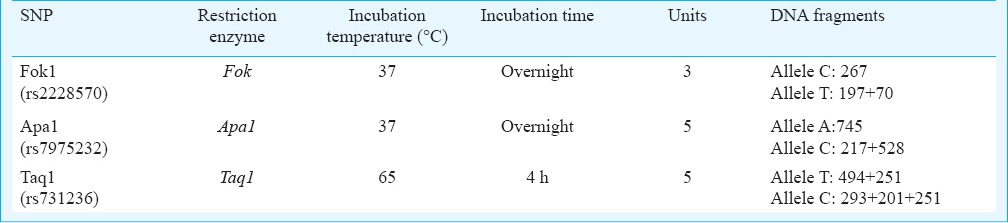
Genotype and allele frequency distributions: The genotype and allele frequency distributions for each of the four VDR polymorphisms are presented in Table III. Except for Cdx2 polymorphism, the frequency distribution of the genotypes as well as alleles was found to be similar between the cases and controls. In case of Cdx2, a significantly higher frequency of the heterozygous GA genotype as well as A allele was observed in cases compared with controls (P<0.001). While the normal homozygote was predominant as compared to the other two genotypes for Fok1 and Taq1, the heterozygote was more frequent than the homozygotes, normal or derived, in case of Apa1. There was a significant deviation in HWE values for Taq1 and Apa1 polymorphisms (P<0.001, and P=0.007, respectively) in the disease group suggesting genetic association. However, there was also significant deviation in HWE in control group for Cdx2 polymorphism (P<0.001). The deviation was still significant after testing for Bonferroni correction (adjusted P=0.0012).
Table III.
Allele and Genotype frequency (%) distributions for each of the four VDR polymorphisms among women with PCOS and normal controls
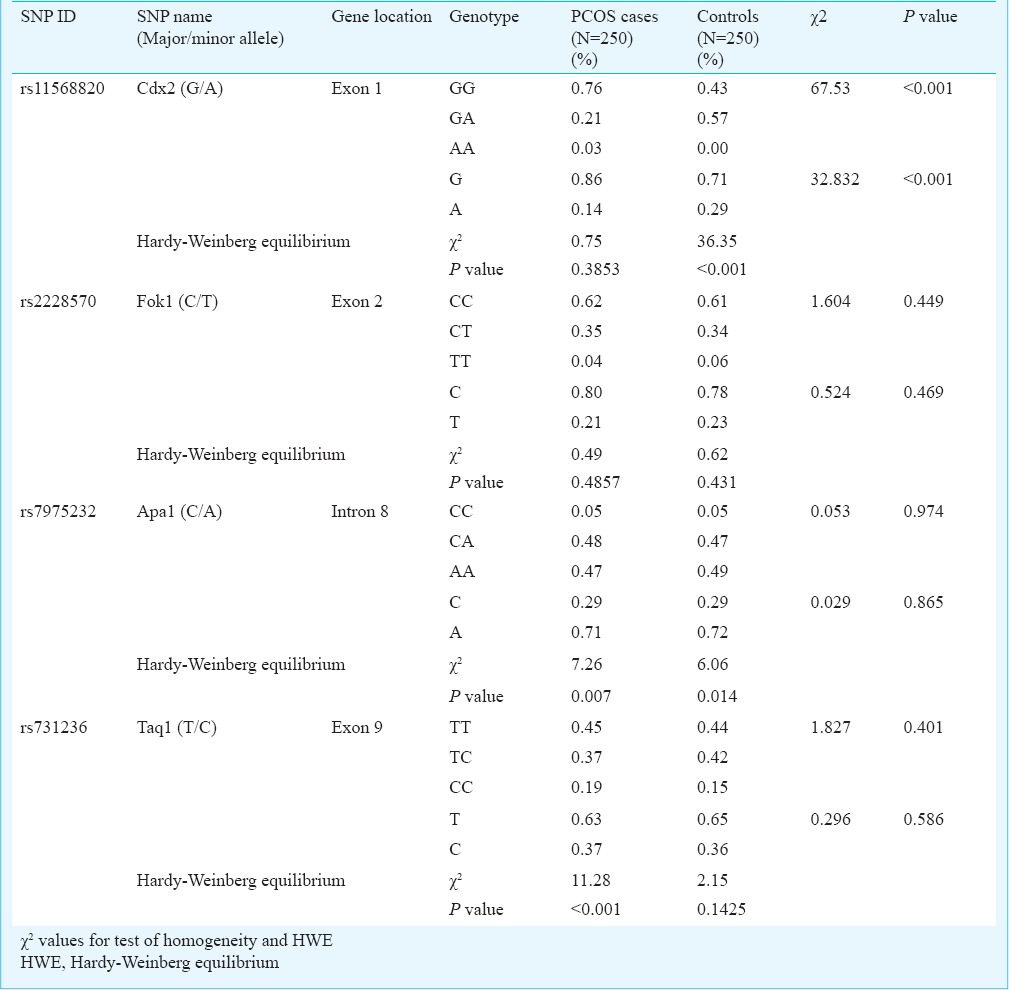
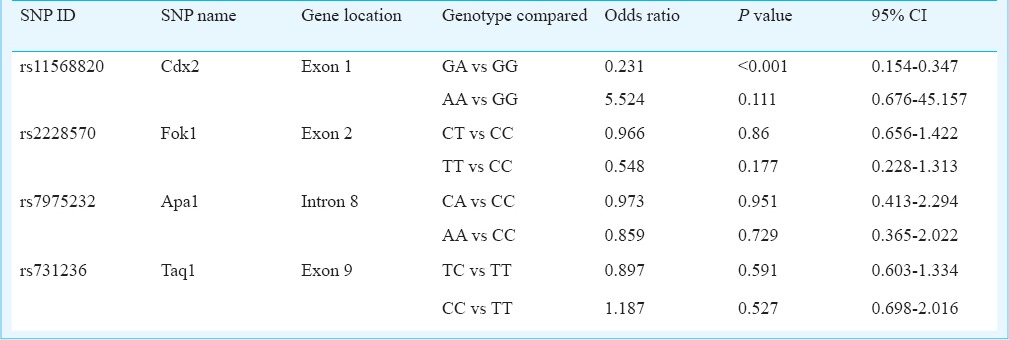
To study the pattern of association of PCOS with genetic variants, logistic regression analysis was carried out for each of the four polymorphisms individually taking the respective genotypes and alleles as categorical variable and the PCOS phenotype as the binary response variable with age and BMI as the covariates (Table IV). Significant results were obtained only in case of Cdx2 polymorphism, where the heterozygote genotype GA as well as the variant allele A seemed to confer significant protection (OR=0.231, 95% CI=0.154-0.307, P<0.001 and OR=0.434, 95%CI = 0.312-0.602, P<0.001, respectively) against developing PCOS. Given the odds ratios and sample size (250 cases and 250 controls), the post hoc power of the study (P=0.05) yielded statistical power (1-β error probability) of 100 per cent for our study.
Table IV.
Results of logistic regression analysis of PCOS on the genotypes of VDR gene polymorphisms with age and BMI as covariates
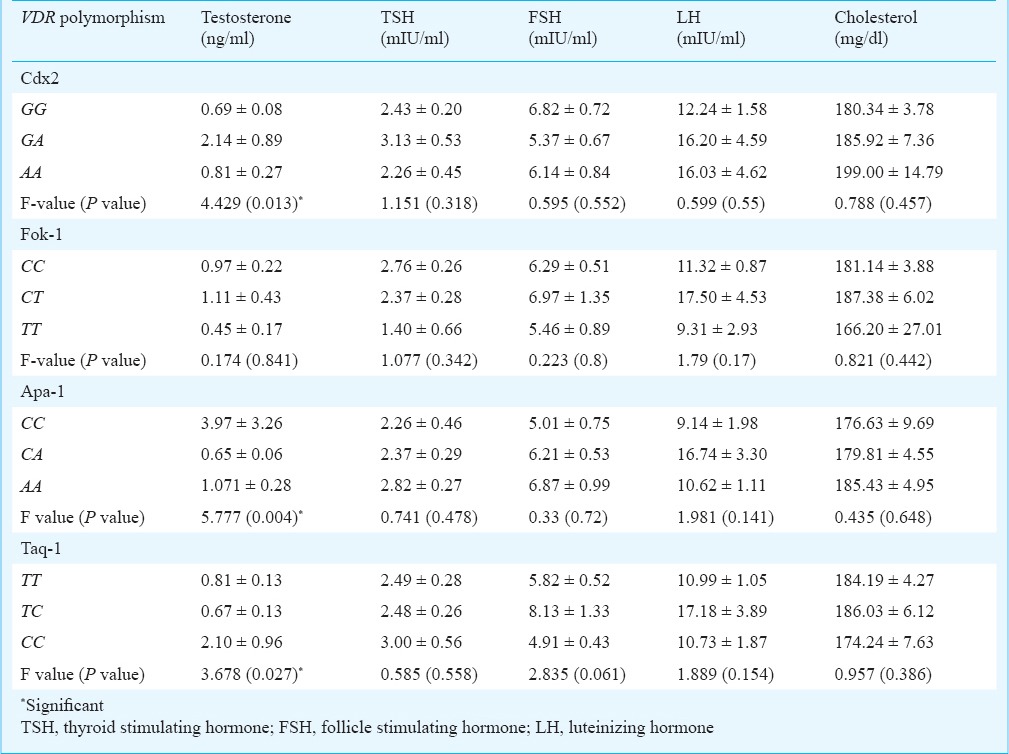
Genotype-wise analysis for clinical and biochemical traits in women with PCOS: The frequency distribution of the seven clinical traits, viz. hirsutism, infertility, alopecia, acne, acanthosis, cholesterol levels and testosterone levels were analyzed according to genotypes of each of the four VDR polymorphisms among the women with PCOS. While hirsutism, infertility, alopecia, acne and acanthosis were categorized by their presence or absence, cholesterol and testosterone levels were categorized based on the presence or absence of elevated levels of the respective hormones (cholesterol level of >200 mg/dl and testosterone level of >0.67 ng/ml considered elevated). Although variation in the occurrence of these clinical traits could be observed among the genotypes within each of the four SNPs, the differences were not significant.
In case of Cdx2 polymorphism, a relatively greater frequency of PCOS cases with homozygote genotype GG showed characteristic traits like hirsutism, acne, acanthosis and elevated cholesterol whereas in case of Fok1 polymorphism, PCOS cases with variant homozygote TT were in greater frequency for traits like hirsutism, infertility, alopecia and acne compared with other genotypes. In case of Apa1 polymorphism, a greater proportion of PCOS women with homozygote genotype CC were infertile compared with the other two genotypes. While a greater proportion of the heterozygotes showed alopecia and acanthosis traits, a higher proportion of women with PCOS with variant AA genotype showed elevated testosterone levels compared with other genotypes, though not significant. No significant results were obtained in the comparative analysis between the clinical traits and the Taq1 genotypes. However, women with PCOS with CC or CT genotype for this SNP were found to be relatively more hirsute than those with variant homozygote while infertility and alopecia were observed more among the variant homozygotes.
Table V shows biochemical traits according to genotypes of each of the four VDR polymorphisms. Of the five biochemical traits, only testosterone mean levels exhibited significant heterogeneity among the genotypes for Cdx2 (P=0.013), Apa1 (P=0.004) and Taq1 (P=0.27). Women with PCOS having heterozygote GA genotype for Cdx2 were found to have a relatively higher mean than those with homozygotes for testosterone as well as TSH levels. For Apa1, CC genotype exhibited the highest mean value of testosterone followed by genotypes AA and CA. The mean of TSH, FSH and cholesterol levels were found to be highest in PCOS cases with variant genotype AA. In case of Taq1, CC genotype exhibited the highest mean value of testosterone followed by genotypes TT and TC. The mean FSH, LH and cholesterol levels were found to be highest for TC genotype. None of the biochemical traits showed significant heterogeneity with respect to the Fok1 genotypes. However, PCOS cases with CT genotype were found to have a higher mean for testosterone, FSH, LH and cholesterol levels.
Table V.
Heterogeneity in the mean (± SE) values of different biochemical traits according to the genotypes of VDR polymorphisms in women with PCOS
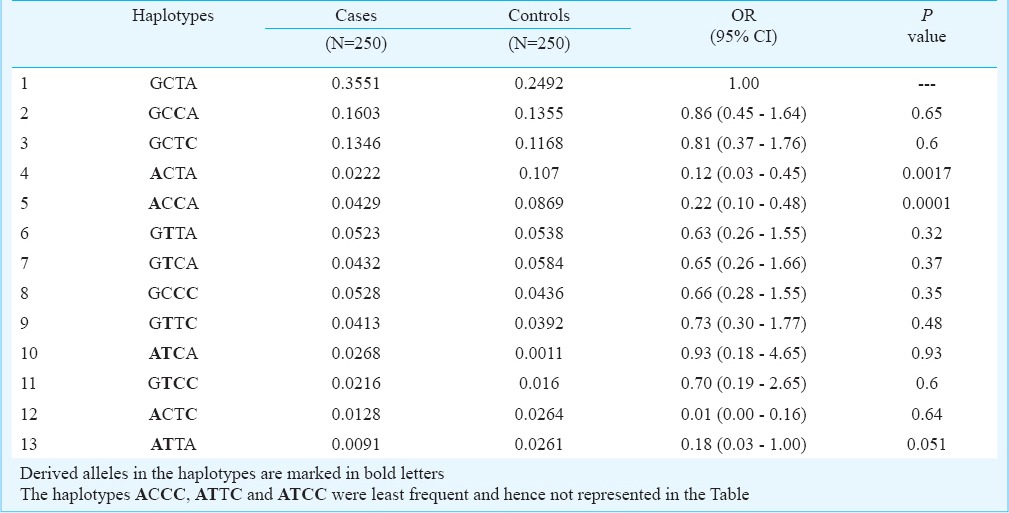
Pairwise LD estimates and association of haplotypes: The D’ values ranged between 0.02-0.487 among different pairs of VDR SNPs and none of these were significant suggesting that these SNPs are not in LD. Haplotypes based on the four VDR polymorphisms were constructed and analyzed for possible association with PCOS (Table VI). The order of SNPs in the haplotypes is given in the following order corresponding to Cdx2(G/A), Fok1(C/T), Taq1(T/C), Apa1(C/A). The frequency of GCCA and GCTC haplotypes was found to be slightly elevated in cases whereas ACCA and ACTA were more frequent among controls. However, only ACCA and ACTA haplotypes were found to be significantly associated in conferring protection against PCOS (Table VI).
Table VI.
Frequencies of haplotypes representing the four SNP loci: Cdx2, Fok1, Taq1, Apa1, in the respective order, among the cases and controls
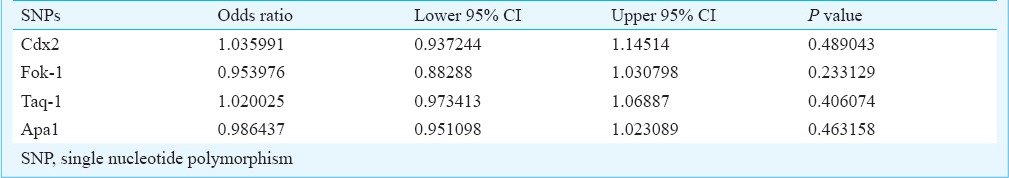
Multivariate logistic regression analysis: The results of multivariate logistic regression analysis including all the four SNPs are presented in Table VII. The odds ratios were calculated taking the wild-type homozygote as the reference. These results did not reveal any significant association of any of the four SNPs with PCOS.
Table VII.
Results of multivariate logistic regression analysis
Discussion
In this study we examined four VDR polymorphisms (Cdx2, Fok1, Apa1 and Taq1) among women with PCOS and in healthy controls and tested for their association with the PCOS phenotype. Cdx2 polymorphism is a G>A sequence variation in the 1a promoter region associated with transcriptional activity of the VDR gene18, which further regulates the transcription of about 3 per cent of the human genome including genes that are crucial for glucose metabolism. This SNP was found to be associated with testosterone levels in our study. Fok1 polymorphism defines the presence of T>C transition (ATG to ACG) in exon 2 of the VDR gene that results in altered protein length due to different translation initiation sites18. Taq1 polymorphism in exon 9 associated with rate of gene expression, is a T/C substitution (ATT to ATC) leading to a synonymous change at codon 352 (Isoleucine)18. In our study a significant difference was observed only in the Cdx2 genotypic and allelic frequency distribution between the PCOS cases and the controls which was in contrast to a study conducted in Austria where no significant difference was found24. No significant association was observed between the genotypes and the clinical traits in our study. However, women with GA and AA genotypes of Cdx2 polymorphism were found to be at a relatively higher risk for infertility. While Cdx2 GA genotype was found to confer high risk for alopecia, AA was more often found with elevated testosterone levels. It was suggested that Cdx2 A-allele carriers might have higher intestinal calcium absorption, because of the elevated expression of intestinal calcium channel proteins34. Calcium fluxes and regulation of intracellular calcium stores being essential in the regulation of insulin secretion by the β-cells, this polymorphism might be influencing β-cell function in PCOS.
For Fok1 polymorphism, similar to the earlier studies22,23,24, no significant association was found either at the genotype frequency level or with clinical/biochemical traits. However, it was evident from our results that the genotype TT was associated with the risk for infertility, alopecia, acne and elevated cholesterol levels suggesting that cases with this specific genotype might have higher risk for respective phenotypes of PCOS compared with others. For Apa1 polymorphism, genotype CC showed a risk for infertility, while variant genotype AA was associated with testosterone levels which was in contrast to an earlier study among Austrian women with PCOS where they demonstrated AA genotype to be associated with low testosterone levels24. The Taq1 genotype/allele distribution pattern in our study did not yield any significant results. However, similar to a previous study23, an increase in mean testosterone level was observed for CC genotype. Also, there was a significant variation in the testosterone levels and FSH levels with this genotype.
Apa1 and Taq1 polymorphisms are known to be linked with poly (A) microsatellite repeat in the gene, and can influence gene activity35. In earlier studies, it was found that two haplotypes AAT and GCC of Bsm1, Apa1 and Taq1 under the effect of Cdx2 and Fok1 produced different levels of VDR activity15,18, and GTGCC and ACAAT haplotypes produced more active VDR resulting in higher calcium absorption than GTAAT and ACGCC. Vitamin D is required for proper calcium absorption and calcium is needed for normal development of follicles each month, and, therefore, reduced calcium absorption might hamper follicular maturation thereby leading to cystic pattern of ovaries. It can also be deduced that haplotypes GTAAT and ACGCC are related to decreased calcium absorption thereby increasing the risk of PCOS. In the present study, two haplotypes- GCCA and GCTC showed elevated frequencies in the cases and these haplotypes might be related to the low VDR activity that could affect calcium absorption and follicular development in PCOS. The ACCA and ACTA haplotypes were observed to be relatively more frequent in controls implying that these might be associated with reduced risk for PCOS. The haplotype analysis is far more conclusive than the individual polymorphism analysis since specific haplotype patterns are expected to be associated with certain aspects like differential levels of calcium absorption which in turn reflects the variability in the VDR expression. Haplotypes reported to be responsible for increased calcium absorption indicating a higher VDR expression are expected to be more frequent among the control group.
In the multivariate logistic regression analysis of all the SNPs with age and BMI as covariates, none of the polymorphisms could reveal any significant association with PCOS. Further, the protective nature of Cdx2 shown in the individual gene analysis was nullified in the multivariate analysis, in the presence of other SNP variants of the VDR gene.
The VDR genetic variants were found to have an association with severity of clinical features of PCOS, but not the disease (except Cdx2) indicating that these variants were not directly related to its cause or risk but might influence the development of PCOS indirectly at the biochemical level through regulation of vitamin D or calcium levels.
To conclude, the findings of our study provide an understanding on the nature of association of VDR polymorphisms with PCOS susceptibility in the Indian women. However, the functional role of the VDR vis-a-vis vitamin D level needs to be established clearly. One of the lacunae of our study was lack of information on vitamin D levels of our patients and controls which would have given better and more precise insights into the role of VDR polymorphism in the pathophysiology of PCOS. Further studies specifically designed to understand the role of VDR polymorphisms vis-à-vis vitamin-D levels among the Indian women with PCOS are warranted in large cohorts of ethnically diverse populations of India so as to reach unequivocal conclusion on the role of VDR polymorphisms in PCOS. Functional studies based on the regulatory regions of this gene would also be required to help gain more meaningful insights on the precise aetiological role of VDR towards PCOS phenotype.
Acknowledgment
The authors thank Director, Indian Statistical Institute, Kolkata for logistic and financial support.
Footnotes
Conflicts of Interest: None.
References
- 1.Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 2.Diamanti KE, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, Spina GG, et al. A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab. 1999;84:4006–11. doi: 10.1210/jcem.84.11.6148. [DOI] [PubMed] [Google Scholar]
- 3.Asuncion M, Calvo RM, San Millan JL, Sancho J, Avila S, Escobar- Morreale HF. A prospective study of the prevalence of the polycystic ovary syndrome in unselected Caucasian women from Spain. J Clin Endocrinol Metab. 2000;85:2434–8. doi: 10.1210/jcem.85.7.6682. [DOI] [PubMed] [Google Scholar]
- 4.Maitra A, Pingle RR, Menon PS, Naik V, Gokral JS, Meherji PK. Dyslipidemia with particular regard to apolipoprotein profile in association with polycystic ovary syndrome: A study among Indian women. Int J Fertil Womens Med. 2001;46:271–7. [PubMed] [Google Scholar]
- 5.Legro RS, Gnatuk CL, Kunselman AR, Dunaif A. Changes in 3 glucose tolerance over time in women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2005;90:3236–42. doi: 10.1210/jc.2004-1843. [DOI] [PubMed] [Google Scholar]
- 6.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 7.Wehr E, Moller R, Horejsi R, Giuliani A, Kopera D, Schweighofer N, et al. Subcutaneous adipose tissue topography and metabolic disturbances in polycystic ovary syndrome. Wien Klin Wochenschr. 2009;121:262–9. doi: 10.1007/s00508-009-1162-2. [DOI] [PubMed] [Google Scholar]
- 8.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. Review: the role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wehr E, Pilz S, Schweighofer N, Giuliani A, Kopera D, Pieber TR, et al. Association of hypovitaminosis D with metabolic disturbances in polycystic ovary syndrome. Eur J Endocrinol. 2009;161:575–82. doi: 10.1530/EJE-09-0432. [DOI] [PubMed] [Google Scholar]
- 10.Ngo DT, Chan WP, Rajendran S, Heresztyn T, Amarasekera A, Sverdlov AL, et al. Determinants of insulin responsiveness in young women: Impact of polycystic ovarian syndrome, nitric oxide, and vitamin D. Nitric Oxide. 2011;25:326–30. doi: 10.1016/j.niox.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Lerchbaum E, Obermayer-Pietsch B. Vitamin D and fertility: a systematic review. Eur J Endocrinol. 2012;166:765–78. doi: 10.1530/EJE-11-0984. [DOI] [PubMed] [Google Scholar]
- 12.Yildizhan R, Kurdoglu M, Adali E, Kolusari A, Yildizhan B, Sahin HG, et al. Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet. 2009;280:559–63. doi: 10.1007/s00404-009-0958-7. [DOI] [PubMed] [Google Scholar]
- 13.Morteza B, Isa AR, Nima Hosseini J, Fariba N. Vitamin D receptor TaqI gene variant in exon 9 and polycystic ovary syndrome risk. Int J Fertil Steril. 2013;7:116–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Serge LF, Foldes AJ, Kaufman JM, Stepan JJ, Wahl DA, Adami S, et al. The vitamin D receptor gene and calcium metabolism. Trends Endocrinol Metab. 1998;9:259–65. doi: 10.1016/s1043-2760(98)00065-4. [DOI] [PubMed] [Google Scholar]
- 15.Fariba R, Aidin M, Shemirani AI, Mahmoudi T, Mohsen V, Nikzamir A, et al. Influence of gene variants related to calcium homeostasis on biochemical parameters of women with polycystic ovary syndrome. J Assist Reprod Genet. 2010;28:225–32. doi: 10.1007/s10815-010-9506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uitterlinden AG, Fang Y, Van Meurs JBJ, Leeuwen H, Pols HAP. Vitamin D receptor gene polymorphism in relation to vitamin D related disease states. J Ster Biochem Mol Biol. 2004;89:187–93. doi: 10.1016/j.jsbmb.2004.03.083. [DOI] [PubMed] [Google Scholar]
- 17.Solovieva S, Hirvonen A, Siivola P, Vehmas T, Luoma K, Hilkka R, et al. Vitamin D receptor gene polymorphisms and susceptibility of hand osteoarthritis in Finnish women. Arthritis Res Ther. 2006;8:20. doi: 10.1186/ar1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Köstner K, Denzer N, Müller CS, Klein R, Tilgen W. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29:3511–36. [PubMed] [Google Scholar]
- 19.Dilmec F, Uzer E, Akkafa F, Kose E, André BP. Detection of VDR gene ApaI and TaqI polymorphisms in patients with type 2 diabetes mellitus using PCR-RFLP method in a Turkish population. J Dia comp. 2009;24:186–91. doi: 10.1016/j.jdiacomp.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Sharma PR, Singh S, Jena M, Mishra G, Prakash R, Das PK, et al. Coding and non-coding polymorphisms in VDR gene and susceptibility to pulmonary tuberculosis in tribes, castes and Muslims of Central India. Infect Genet Evol. 2011;11:1456–61. doi: 10.1016/j.meegid.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Annamaneni S, Bindu CH, Reddy KP, Vishnupriya S. Association of vitamin D receptor gene start codon (Fok-1) polymorphism with high myopia. Oman J Ophthalmol. 2011;4:57–62. doi: 10.4103/0974-620X.83654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmoudi T. Genetic variation in the vitamin D receptor and polycystic ovary syndrome risk. Fertil Steril. 2009;92:1381–3. doi: 10.1016/j.fertnstert.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Fariba R, Aidin M, Atena IS, Touraj M, Mohsen V, Abdolrahim N, et al. Influence of gene variants related to calcium homeostasis on biochemical parameters of women with polycystic ovary syndrome. J Assist Reprod Genet. 2011;28:225–32. doi: 10.1007/s10815-010-9506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wehr E, Trummer O, Giuliani A, Gruber HJ, Pieber TR, Pietsch BO. Vitamin D-associated polymorphisms are related to insulin resistance and vitamin D deficiency in polycystic ovary syndrome. Eur J Endocrinol. 2011;164:741–9. doi: 10.1530/EJE-11-0134. [DOI] [PubMed] [Google Scholar]
- 25.Zadeh-Vakili A, Ramezani Tehrani F, Daneshpour MS, Zarkesh M, Saadat N, Azizi F. Genetic polymorphism of vitamin D receptor gene affects the phenotype of PCOS. Gene. 2013;515:193–6. doi: 10.1016/j.gene.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 26.The Rotterdam ESHRE/ASRM-sponsored PCOS Concensus Workshop Group. Revised 2003 Concensus on diagnostic criteria and long term health risk related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 27.Ferriman D, Purdie AW. The aetiology of oligomenorrhoea and/or hirsuties: a study of 467 patients. Postgrad Med J. 1983;59:17–20. doi: 10.1136/pgmj.59.687.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritschi EF, Maniatis T. New York: Cold Spring Horbor Laboratory Press; 1989. Molecular cloning: A laboratory manual. [Google Scholar]
- 29.Park BS, Park JS, Lee DY, Youn JI, Kim IN. Vitamin D receptor polymorphism is associated with psoriasis. J Invest Dermatol. 1999;112:113–6. doi: 10.1046/j.1523-1747.1999.00482.x. [DOI] [PubMed] [Google Scholar]
- 30.Selvaraj P, Chandra G, Sunil MK, Reetha AM, Narayanan PR. Association of vitamin D receptor gene variants of BsmI, ApaI and FokI polymorphisms with susceptibility or resistance to pulmonary tuberculosis. Curr Sci. 2003;84:1564–8. [Google Scholar]
- 31.Hemant Kumar B, Rituraj K, Aggarwal CG, Sunaina G, Madhukar S, Lakshma Nayak V, et al. Vitamin D receptor (Fok1, Bsm1 and Taq1) gene polymorphisms and type 2 diabetes mellitus: a north indian study. Indian J Med Sci. 2009;63:187–94. [PubMed] [Google Scholar]
- 32.Marozik P, Mosse I, Alekna V, Rudenko E, Tamulaitienë M, Ramanau H, et al. Association between polymorphisms of VDR, Col1a1, and lCt genes and bone mineral density in Belarusian women with severe postmenopausal osteoporosis. Medicina (Kaunas) 2013;49:177–83. [PubMed] [Google Scholar]
- 33.Ceppellini R, Siniscalo M, Smith CAB. The estimation of gene frequencies in a random-mating population. Ann Hum Genet. 1955;20:97–115. doi: 10.1111/j.1469-1809.1955.tb01360.x. [DOI] [PubMed] [Google Scholar]
- 34.Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, et al. Duodenal calcium absorption in vitamin D receptor-knockout mice: functional and molecular aspects. Proc Natl Acad Sci USA. 2001;98:13324–9. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCullough ML, Stevens VL, Diver WL, Feigelson HS, Rodriguez C, Bostick RM, et al. Vitamin D pathway gene polymorphisms, diet, and risk of postmenopausal breast cancer: a nested case-control study. Breast Cancer Res. 2007;9:9. doi: 10.1186/bcr1642. [DOI] [PMC free article] [PubMed] [Google Scholar]


