Abstract
Background & objectives:
Injecting drug use is a major route of hepatitis C virus (HCV) infection in India, but there may be other risk factors also. This study was carried out to determine the seroprevalence of anti-HCV antibody in injecting drug users (IDUs) vs. non-IDUs (NIDUs), and to study the risk estimates for HCV seropositivity in the total sample of substance users with regard to various demographic, clinical, behavioural and personality factors.
Methods:
The IDUs (n = 201) and NIDUs (n = 219) were assessed for demographic, clinical and behavioural information, and were rated on instruments for severity of dependence, risk behaviour and personality profiles. Anti-HCV antibody was tested by ELISA and confirmed by recombinant immunoblot assay (RIBA) test.
Results:
Almost one-third of the IDUs (64 of 201; 31.8%) were positive for anti-HCV antibody, as opposed to only seven (3.2%) of the NIDUs. The four risk factors strongly associated with HCV positivity in multivariate analysis were sharing syringe [Exp(B) 75.04; 95%CI 18.28-307.96; P<0.001], reuse of injection accessories (16.39; 3.51-76.92; P<0.001), blood transfusion (5.88; 1.63-21.23; P=0.007) and IDU status (3.60; 1.26-10.31; P=0.017). Other variables less strongly but significantly associated with HCV positivity were multiple sex partners, opioid dependence, risk behaviour scores, impulsivity, and lower age of onset of drug use.
Interpretation & conclusions:
Our study showed a high seroprevalence of anti-HCV antibody in IDUs. In the substance users, HCV positivity was significantly and independently associated with several clinical, behavioural, and personality risk factors.
Keywords: Hepatitis C virus (HCV), injecting drug use, risk behaviour, personality
Hepatitis C virus (HCV) infection is a blood borne infection which can be the harbinger of chronic hepatic illnesses such as chronic active hepatitis, hepatic cirrhosis and hepatocellular carcinoma1. In India, a few population based studies have been reported from different regions with prevalence of HCV infection ranging from 0.09 to 7.89 per cent2. The common modalities of spread of hepatitis C infection are blood transfusion, injection drug use, unsafe therapeutic injections and health care related procedures2. Injection drug use is the main mode of transmission of HCV in developed countries, transmitted through blood-to-blood contact, either via direct or indirect sharing of injecting equipment3. In India, blood transfusions and unsafe therapeutic injections were the predominant modalities of transmission of HCV2. However, after HCV screening of blood products was made mandatory in India, injection drug use is gradually becoming the major route of HCV infection. A systematic review of 1125 articles reported around 10 million injecting drug users (IDUs) being HCV positive with Eastern Europe, East Asia, and Southeast Asia having the largest infected populations4. Indian studies have reported HCV seropositivity in IDUs to be in the range of 20 to 90 per cent; there are pockets of very high HCV seroprevalence in India, otherwise the range is moderate (30-50%) compared to western studies (40-90%)5.
There may be several reasons for high prevalence of these infections in IDUs. High risk behaviours are common in IDUs and include needle sharing, unsafe disposal and inappropriate cleaning of needles as well as unsafe sex with limited condom use6. It is important to study specific risk factors in Indian scenario as different drug-using populations differ in their epidemiologic risk factors and reflect the dominant culture of their group7. Further, studying personality measures in this context is important because risk behaviours often arise within the matrix of personality profiles and psychological characteristics like high sensation seeking and impulsivity8. It becomes imperative to study these personality and attitudinal measures in relation to HCV infection in injecting as well as non-injecting drug users. Such studies are lacking in India.
A previous study from our centre reported the seroprevalence of HCV infection in a small sample of IDUs (n = 103) and compared risk behaviours in the HCV positive vs. negative subjects, but only among the IDUs, not the entire sample of substance users9. No risk behaviour inventory or risk scores were used, and no personality profiles relevant to risk behaviour were studied9. Hence we aimed to find the seroprevalence of anti-HCV antibody in a large and non-overlapping sample of IDUs and non-IDUs (NIDUs) and to study various risk behaviours and relevant personality profiles of all substance users for contracting HCV infection. The specific objectives of this study were to find out the seroprevalence of anti-HCV antibody in IDUs versus NIDUs attending a de-addiction centre in north India, and to study the risk estimates for HCV seropositivity in the total sample of substance users with regard to various demographic, clinical, behavioural and personality factors.
Material & Methods
The study was carried out at the Postgraduate Institute of Medical Education and Research (PGIMER), a multispecialty tertiary care teaching hospital at Chandigarh, India. The study protocol was approved by the Institute Ethics Committee. Informed written consent was obtained from the patients prior to study. The study population comprised all patients who were registered in the Drug De-addiction and Treatment Centre (DDTC), department of Psychiatry, PGIMER, Chandigarh, during the study period (May 2010 to February 2012). The sample was drawn from this population.
The index group in the study sample comprised 201 patients (25.4±5.8 yr), all males with ICD-10-diagnosed opioid dependence10 using injectable substances (IDUs) attending the DDTC. The control group included 219 male NIDUs (30.8 ± 7.6 yr) attending DDTC. Patients with mental retardation or organic brain syndrome (both determined by a clinical interview and examination by a qualified psychiatrist), or those who refused to give informed consent were excluded. None of the patients reported in this study were part of the previously published study from our centre9. The main substances used by IDUs were buprenorphine and heroin, while those used by the NIDUs were non-injectable street heroin (smack), codeine, dextropropoxyphene, opium and poppy husk (bhukki).
Baseline instruments were administered as follows:
-
(i)
Socio-demographic data sheet - covered age, sex, marital status, educational status, family income, occupational status, religion, family type, locality, distance from the DDTC and the source of referral.
-
(ii)
Clinical data sheet - covered the types of substance use, age of onset, durations of use and dependence, history of injecting drug use, type of injection drug, duration, mode, and route of injecting drug use.
-
(iii)
Drug related harm - measured on a 4-point scale from 0 (none) to 3 (severe) for seven domains: health (physical or psychiatric), occupation (absenteeism, suspension, unemployment, etc.), finance (debts, etc.), legal (arrests), family (impaired inter-personal relationships), marital (separation, divorce) and social (restriction of social circle, ostracism).
-
(iv)
Severity of Dependence Scale (SDS) - a five-item scale reported to be a reliable and valid screening instrument for dependence and a measure of dependence severity in adults across several substance classes was used11.
-
(v)
Modified Sensation Seeking Scale - Indian adaptation of Sensation seeking scale standardized on an Indian population having 40 items and good test-retest reliability and internal consistency was used. The scale has 4 sub-scales: thrill and adventure seeking, experience seeking, boredom susceptibility and disinhibition12.
-
(vi)
Barratt Impulsiveness Scale, Version 11 (BIS-11) - measures impulsivity in terms of three domains: motor impulsiveness, non-planning impulsiveness and cognitive impulsiveness. The BIS-11 has 30 items scored on a 4-point scale and possible scores range from 30 to 120. The BIS-11 was translated into local language and back translated to English to ensure the translated version matched the original scale. The translated version of BIS-11 in local language was administered to the subjects13.
-
(vii)
Risk Behaviour Inventory (RBI) - it is a simple, 12-item, validated instrument for self-report on various risk behaviours, both sharing of injection and sexual risk related. Answers are scored as ‘1’ depending on the response. Total score is calculated by adding all scores of 1; higher score indicates greater risk14.
-
(viii)
HIV Risk Taking Behaviour Scale (HRTBS)- it consists of 11 items, each item being chosen to address a specific HIV risk taking behaviour. All items are scored on a 0-5 scale, with a higher score indicating a higher degree of risk-taking. The scale includes two sub-scales to measure injecting and sexual behaviour15.
The participants were required to give a venous blood sample of 5 ml, which was obtained by a trained laboratory technician using standard aseptic procedure. The blood samples were tested for hepatitis C antibody using ELISA method (SP-NANBASE c-96 3.0, General Biologicals Corporation, Taiwan). HCV reactive samples by ELISA were confirmed by supplemental HCV RIBA (recombinant immunoblot assay) test as false positive results or optical density (OD) value in HCV ELISA representing in grey zones had to be ruled out by serology. Only the confirmed positives were included in this study. The confirmed HCV seropositive subjects were referred to Hepatology services for further investigations and management. The samples were also tested for HBsAg (hepatitis B surface antigen) and anti-HIV antibody as additional measures (HIVASE 1+2, General Biologicals Corporation, Taiwan; ERBA LISA hepatitis B, Transasia Bio Medicals).
Statistical analysis: Chi square test with continuity correction or Fisher exact test for discrete variables and independent sample t test for continuous variables were used for group comparisons. Logistic regression analysis was used to determine the risk factors of HCV infection, with their risk estimates for the entire sample. Initially, HCV positive and HCV negative individuals were compared (irrespective of their IDU/NIDU status), on all the variables. The two groups differed significantly on a large number of variables. From this list, variables were chosen according to their potential biological or psychosocial relevance as risk factors. These were entered into a logistic regression model as independent variables by the “Forward Stepwise (Conditional)” method (SPSS, Inc., USA), with HCV status (negative vs. positive) as the dependent variable, in order to statistically adjust for the intercorrelations among the many so-called independent variables. The odds ratios [Exp(B)] with 95 % confidence intervals (95%CI) were calculated for all those variables that were independently and significantly associated with HCV positivity, to arrive at risk estimates. Analysis was done by SPSS version 14 for Windows (SPSS, Inc., USA).
Results
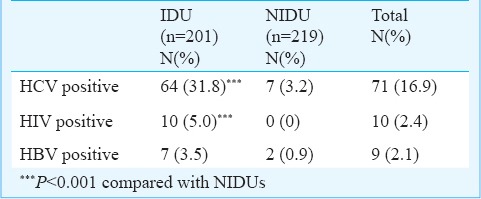
Seroprevalence of anti-HCV antibody: Almost one-third of the individuals in the IDU group (64 of 201; 31.8%) were positive for anti-HCV antibody, as opposed to only seven of 219 (3.2%) of the NIDUs. Only 10 participants in the IDU group (5.0%) and none in the NIDU group were positive for HIV antibody. Similarly, only seven (3.5%) of IDUs and two (0.9%) of NIDUs tested positive for HBsAg. The group differences (IDU vs. NIDU) on HCV and HIV seropositivity were significant (P<0.001; Table I).
Table I.
HCV, HBV and HIV testing results in injecting vs. non-injecting drug users (IDU vs. NIDU)
Risk factors, with risk estimates, of HCV infection in substance users: When the entire sample (IDU plus NIDU) was analysed using bivariate analysis, HCV positive subjects (n = 71) differed significantly from HCV negative subjects (n = 349) on a large number of variables: (i) demographic (HCV positive subjects were younger, with less education and family income, less often employed, and more often from rural locality), (ii) clinical (HCV positive subjects had more frequent IDU status, dependence on opioids, alcohol, cannabis and tobacco, lower age of onset of substance use, lower duration but higher severity of dependence, and more drug-related problems in health, finance, social, legal and total scores), (iii) risk-related behaviour (parenteral route of drug use, sharing syringe, needle, other injection accessories (mixer/vial/cotton), reuse of accessories, blood transfusion, multiple sex partners, sex with commercial sex workers, sex with strangers, higher RBI and HRTBS scores), and (iv) personality variables (higher sensation seeking in terms of disinhibition, boredom susceptibility and total scores on SSS, and motor, non-planned and total scores on BIS).
As many of these variables were inter-correlated, and to find out the risk factor profile and strength of these variables after taking into account their inter-correlations, a subset of these variables was chosen according to their potential biological or psychosocial relevance as risk factors and entered into a forward stepwise logistic regression model. The model with the best goodness-of-fit (Hosmer-Lemeshow test chi-square value 12.966; P = 0.113) predicted 57.6 per cent of the variance (Nagelkerke R2 = 0.576).
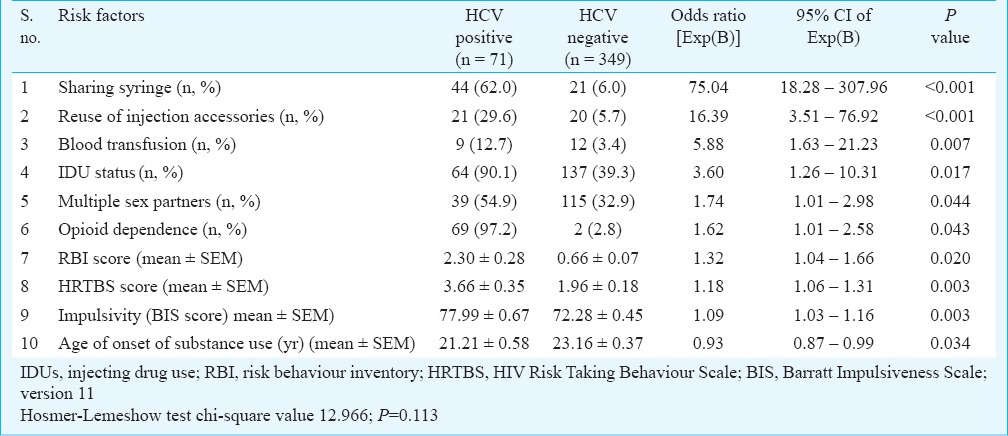
Table II shows the risk factors associated with HCV positivity in the final model, with their values, odds ratios and 95 % confidential interval (CI). Ten variables were retained in the final model. The four risk factors strongly associated with HCV positivity in the multivariate analysis were sharing syringe [Exp(B) 75.04; 95% CI 18.28-307.96; P<0.001], reuse of injection accessories (16.39; 3.51-76.92; P<0.001), blood transfusion (5.88; 1.63-21.23; P=0.007) and IDU status (3.60; 1.26-10.31; P=0.017). Other variables less strongly but still significantly associated with HCV positivity were multiple sex partners, opioid dependence; risk behaviour scores, impulsivity, and lower age of onset of drug use. Variables not included in the final model (likely because of their inter-correlations with the retained variables, which had stronger associations with the dependent variable) included age, duration of drug use, dependence on drugs other than opioids, route of use, sharing needles and other injection accessories, sex with commercial sex workers or strangers, and sensation seeking.
Table II.
Risk estimation of demographic, clinical, behavioural and personality factors for association with HCV seropositivity in the total sample: multivariate analysis using logistic regression
Discussion
The seroprevalence of anti-HCV antibody among IDUS in the current study (31.8%) was lower than that of 45.6 per cent found in an earlier study from our centre9. However, it was well within the range of 20-90 per cent reported from India5. Only 3.2 per cent of non-IDUs were found seropositive for HCV. This was similar to that reported earlier from our centre9 but low compared to another study from India (27%)16. However, this low seropositivity is also a cause for concern in view of the community seroprevalence of anti-HCV antibody of around one per cent in India5.
The seroprevalence of HBsAg in IDUs was negligible in comparison to seroprevalence of anti-HCV antibody (3.5% vs. 31.8%). Prior studies have also reported higher seroprevalence of HCV than that of HBV in IDUs17. Only five per cent of the IDUs were found positive for HIV antibody. This was similar to the overall HIV seroprevalence (7.14%) in IDU population in the country18. A study conducted in Punjab State reported higher HIV infection prevalence (29%) and HCV positivity (49%) in IDUs, especially those from the border districts19.
The four risk factors found strongly associated with HCV positivity in the multivariate analysis were sharing syringe, reuse of injection accessories, blood transfusion and IDU status. Impulsivity emerged as a significant and independent risk association with HCV positivity. It was of concern that lower age of onset of drug use was independently associated with HCV risk. Sexual risk behaviours (multiple sex partners in our study) are known to be associated with HCV positivity in India, as recently reported in commercial sex workers from Nagaland20.
Our study had some limitations. It focused only on treatment seeking population; hence the results may not be a true representation of prevalence and profile in the community. Further, genotype and RNA load of HCV were not tested because of resource constraints, and liver function tests and other hepatic pathology were also not reported here.
In conclusion, high seroprevalence of anti-HCV antibody was observed in the IDUs. In the substance users, HCV positivity was significantly and independently associated with several clinical, behavioural, and personality risk factors. The burden of HCV positivity in substance users, especially IDUs has obvious implications for patients, clinicians and policy makers. Further research is needed to characterize risk behaviour, risk perception and risk networks with particular focus on HCV transmission in substance users in India.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research, New Delhi, for financial support.
Footnotes
Conflicts of Interest: None.
References
- 1.Kew MC. Hepatitis B and C viruses and hepatocellular carcinoma. Clin Lab Med. 1996;16:395–406. [PubMed] [Google Scholar]
- 2.Mukhopadhya A. Hepatitis C in India. J Biosci. 2008;33:465–73. doi: 10.1007/s12038-008-0065-0. [DOI] [PubMed] [Google Scholar]
- 3.Thorpe LE, Ouellet LJ, Hershow R, Bailey SL, Williams IT, Williamson J, et al. Risk of hepatitis C virus infection among young adult injection drug users who share injection equipment. Am J Epidemiol. 2002;155:645–53. doi: 10.1093/aje/155.7.645. [DOI] [PubMed] [Google Scholar]
- 4.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–83. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basu D. Overview of substance abuse and hepatitis C virus infection and coinfections in India. J Neuroimmune Pharmacol. 2010;5:496–506. doi: 10.1007/s11481-010-9227-6. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SS, Desai M, Srikrishnan AK, Thamburaj E, Vasudevan CK, Kumar MS, et al. The profile of injection drug users in Chennai, India: Identification of risk behaviours and implications for Interventions. Subst Use Misuse. 2010;45:354–67. doi: 10.3109/10826080903452447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celentano DD, Latimore AD, Mehta SH. Variations in sexual risks in drug users: emerging themes in a behavioral context. Curr HIV/AIDS Rep. 2008;5:212–8. doi: 10.1007/s11904-008-0030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Essock SM, Dowden S, Constantine NT, Katz L, Swartz MS, Meador KG, et al. Five-Site Health and Risk Study Research Committee. Risk factors for HIV, hepatitis B, and hepatitis C among persons with severe mental illness. Psychiatr Serv. 2003;54:836–41. doi: 10.1176/appi.ps.54.6.836. [DOI] [PubMed] [Google Scholar]
- 9.Basu D, Kumar V, Sharma AK, Barnwal PK, Mattoo SK. Seroprevalence of anti-hepatitis C virus (anti-HCV) antibody and HCV-related risk in injecting drug users in northern India: Comparison with non-injecting drug users. Asian J Psychiatry. 2013;6:52–5. doi: 10.1016/j.ajp.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 10.10th ed. Geneva: WHO; 1992. World Health Organization (WHO). The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. [Google Scholar]
- 11.Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W, et al. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine, and amphetamine users. Addiction. 1995;90:607–14. doi: 10.1046/j.1360-0443.1995.9056072.x. [DOI] [PubMed] [Google Scholar]
- 12.Basu D, Varma VK, Malhotra S, Malhotra A. Sensation seeking scale: Indian adaptation. Indian J Psychiatry. 1993;35:155–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 14.Ottomanelli G, Kramer TH, Bihari B, Fine J, Heller S, Mosely JA. AIDS-related risk behaviors among substance abusers. Int J Addict. 1990;25:291–9. doi: 10.3109/10826089009056212. [DOI] [PubMed] [Google Scholar]
- 15.Darke S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. AIDS. 1991;5:181–5. doi: 10.1097/00002030-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari R, Aggarwal A, Devi P. Seroprevalence of hepatitis B, hepatitis C and human immunodeficiency viruses amongst drug users in Amritsar. Indian J Med Microbiol. 2006;24:151–2. doi: 10.4103/0255-0857.25223. [DOI] [PubMed] [Google Scholar]
- 17.Mahanta J, Borkakoty B, Das HK, Chelleng PK. The risk of HIV and HCV infections among injection drug users in northeast India. AIDS Care. 2009;21:1420–4. doi: 10.1080/09540120902862584. [DOI] [PubMed] [Google Scholar]
- 18.National Aids Control Organisation. Ministry of Health and Family Welfare Government of India. HIV sentinel surveillance 2012-13: a technical brief. [accessed on February 20, 2014]. Available from: http://www.naco.gov.in/upload/Surveillance/Reports%20&%20Publication/HSS%202012-13_%20A%20Technical%20Brief.pdf .
- 19.Panda S, Roy T, Pahari S, Mehra J, Sharma N, Singh G, et al. Alarming epidemics of human immunodeficiency virus and hepatitis C virus among injection drug users in the northwestern bordering state of Punjab, India: prevalence and correlates. Int J STD AIDS. 2013;25(Suppl):596–606. doi: 10.1177/0956462413515659. [DOI] [PubMed] [Google Scholar]
- 20.Barua P, Mahanta J, Medhi GK, Dale J, Paranjape RS, Thongamba G. Sexual activity as risk factor for hepatitis C virus (HCV) transmission among the female sex workers in Nagaland. Indian J Med Res. 2012;136(Suppl):30–5. [Google Scholar]


