Abstract
Background & objectives:
Hepatitis B virus (HBV), human immunodeficiency virus (HIV), hepatitis C virus (HCV) and syphilis infections pose a great threat to blood safety. This study was undertaken to investigate the seroprevalence of serologic markers for transfusion transmitted infections (TTIs) among blood donors at a hospital based blood centre in north India over a period of nine years.
Methods:
The results of serologic markers for TTIs (HBsAg, anti-HCV, anti-HIV and syphilis) of all blood donations (both voluntary and replacement) at our hospital from January 2005 to December 2013 were screened. Additional analysis was conducted to examine the prevalence trends associated with each of the positive marker.
Results:
The data of 180,477 donors [173,019 (95.86%) males and 7,458 (4.13%) females] were analyzed. Replacement donations [174,939 (96.93%)] represented the majority whereas, only 5,538 (3.06%) donations were from the voluntary donors. The risk of blood being reactive was three times higher in male donors when compared with the female donors. The risk of blood being reactive for one or more infectious markers was 2.1 times higher in replacement donors when compared with the voluntary donors. Seropositivity of HIV, HBsAg, HBcAb, syphilis showed a significant decreasing trend (P<0.05) while there was an increasing trend in HCV infection which was insignificant.
Interpretation & conclusions:
This study reflects that the risk of TTIs has been decreased over time with respect to HIV, HBV and syphilis, but the trends for HCV remains almost the same in blood donors. Blood transfusion remains a risk factor for the spread of blood-borne infections. Therefore, improvements are needed to strengthen both safety and availability of blood.
Keywords: HBV, HCV, HIV, prevalence, syphilis, transfusion transmitted infections
The transfusion of blood and its components is one of the most essential procedures in the health care delivery in the present scenario. Transfusion transmitted infections (TTIs) can be caused by various microorganisms which may be present in the blood being transfused. The major globally prevalent TTIs are caused by human immunodeficiency virus (HIV), hepatitis B virus (HBV), hepatitis C virus (HCV), Treponema pallidum and malaria parasite.
In India, it is mandatory to screen blood donors for HIV, hepatitis B, hepatitis C, syphilis and malaria1. The donor screening strategies include taking the elaborate medical history, performing preliminary clinical examination and screening for infectious markers. The infectious markers include anti HIV (1 and 2) antibodies, hepatitis B surface antigen (HBsAg), anti-hepatitis C virus antibodies, and malaria antigens, such as histidine rich protein (HRP) and pan-aldolase. VDRL (venereal disease research laboratory)/RPR (rapid plasma reagin) test is done for anticardiolipin antibodies. The testing for anti-hepatitis B core antibody (HBcAb) is optional. The screening for these infectious markers is performed using rapid diagnostic tests and ELISA. Nucleic acid testing (NAT) is done at only a few centers in the country2. Though these strategies have been effective, but transmission of diseases still occurs, primarily because of the inability of the test to detect the disease in the ‘window’ period of infection, immunologically variant viruses, immune-silent carriers and inadvertent laboratory testing errors3. TTIs remain a major concern to patients, physicians and policy makers. Earlier we reported the seroprevalence of HIV as 0.24 per cent among blood donors of north India during an 11 year period4. HCV seroprevalence among the blood donors in the same hospital was found to be 0.39 per cent during 2001-20115.
The present study was carried out with the aim to find out the seroprevalence of infectious markers and their trends among the blood donors a hospital based blood transfusion service set up in north India over a period of nine years.
Material & Methods
The present study was carried out at the department of Transfusion Medicine, Indraprastha Apollo Hospitals, New Delhi, India, retrospectively from January 1, 2005 to December 31, 2013 over a period of nine years. Ethical clearance for the study was obtained by the institutional review board. All blood donors (voluntary and replacement) who donated blood at this hospital during the study period were included in this study. The donors who donated repeatedly were counted only once. Information regarding age, sex, number of previous donations, type of donation (replacement/voluntary) and infectious markers status of each donor was obtained from the records. Aphaeresis donations were not included in the study.
Blood analysis: At our centre the donated blood is screened for HBV, HCV, HIV, malaria and syphilis markers. ELISA is performed on a fully automated platform EVOLIS Walk away system (Biorad, USA) using fourth generation kits for anti-HIV 1 and 2 antibodies and HIV 1 antigen (Genscreen HIV1/2, Bio-Rad), third generation ELISA kits for anti-HCV antibodies (Monolisa, Biorad, USA), hepatitis B surface antigen (HBsAg) (Monolisa™ HBsAg ULTRA, BIO-RAD) and anti-HBc antibodies- IgG+IgM (Monolisa™ Anti-HBc PLUS, BIO-RAD). All samples testing positive by ELISA are repeat tested in duplicate using the same ELISA kit and repeat reactive samples are considered as true reactive. RPR card test (CARBOGEN, Tulip Diagnostics Inc., India) was used for detection of syphilis.
Individual donor nucleic acid testing (ID-NAT) was performed for all donors using Procleix® Ultrio® assay (Gen-Probe, CA, USA) and further discriminatory assays were performed for the all initial ID-NAT reactive samples to differentiate between HIV RNA, HBV DNA and HCV RNA.
Statistical analysis: The data were analyzed using SPSS version 20.0 (SPSS. Inc., USA) Source, Country. Seroprevalence of TTIs between males and females, replacement and voluntary donors was compared using chi-square test. For analysis of trend of the TTIs partial linear regression was used.
Results
The data of 180,477 donors who donated blood during the study period were analyzed. Among them, 173,019 (95.86%) were male donors and 7,458 (4.14%) were female donors. Replacement donations 174,939 (96.93%)) represented the majority whereas, only 5,538 (3.06%) donations were from the voluntary donors (VD).
The overall seroprevalences of HIV, HBsAg, HBcAb, HCV and syphilis were 440 (0.24%); 2,138 (1.18%); 17,815 (9.87%); 790 (0.43%); and 421(0.23%), respectively (Table I). There were 21,604 (11.9%) infectious markers positive donors during the study period. When replacement and voluntary donors were compared with respect to the seroprevalence of the infectious markers it was observed that HCV, HBV and syphilis were significantly higher in replacement donors (P<0.05), whereas no such significant difference was noted with HIV. The risk of blood being reactive for one or more infectious markers was 2.1 times higher in replacement donors when compared with the voluntary donors (Table II). Similarly, it was seen that HCV, HBV and syphilis were significantly higher in male donors (P<0.05). The risk of blood being reactive was three times higher in male donors when compared with the female donors (Table II).
Table I.
Seroprevalences of various infectious markers in blood donors (2005-2013)
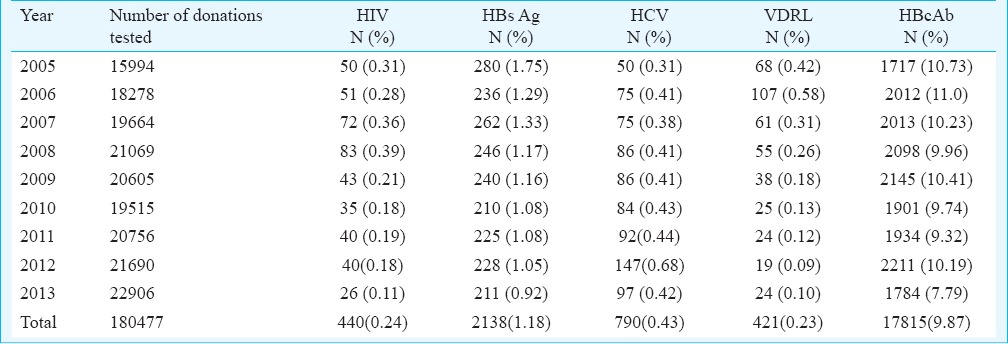
Table II.
Comparison between replacement and voluntary donors, and male and female donors
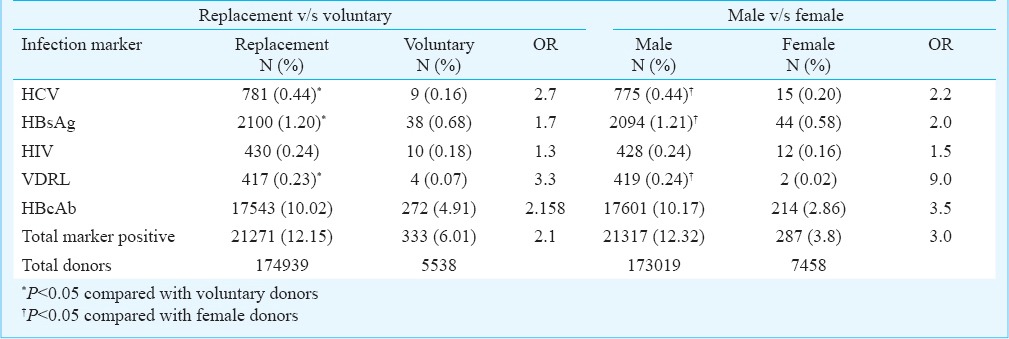
The trends in the seroprevalences of HIV, HBsAg, HBcAb, HCV and syphilis during the study period are shown in the Figure. Seropositivity of HIV, HBsAg, HBcAb, syphilis showed a significant decreasing trend (P<0.01) whereas there was an increasing but insignificant trend in the HCV infection.
Figure.
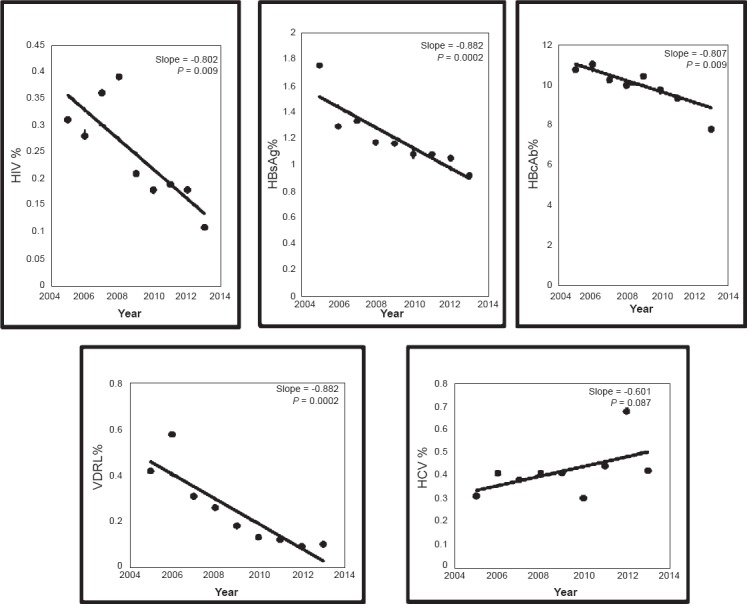
The trends in the seroprevalences of HIV, HBsAg, HBcAb, HCV and syphilis.
The seroprevalences of co-infectious markers were as follows: HIV and syphilis was seen in 13 (0.007%) donors, HIV and HCV in two (0.001%) donors. HBcAb was detected in 85 of the HIV positive donors (85/440= 19.3%), 79 of syphilis positive donors (79/421=18.7%) and 170 of the HCV positive donors (170/790=21.5%). HBcAb was positive in 1812 of 2138 HBsAg positive individuals. Co-infection of HBV, HCV and HIV was found in none.
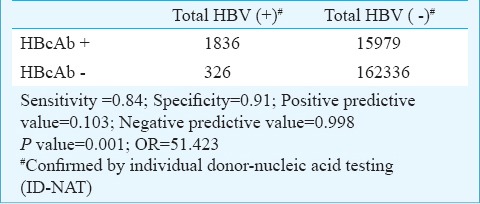
HBcAb as a marker of infection of HBV infection had a low predictive sensitivity of 84 per cent and a moderate specificity of 91 per cent. It had a high negative predictive value of 99.8 per cent and a very low positive predictive value of 10.3 per cent. An individual positive for HBcAb had 51 times more risk of having HBV infection compared with a HBcAb negative individual (Table III).
Table III.
Prelevance of HBcAb as a marker of HBV infection
Discussion
In the western world the transmission rates of HIV, HBV, HCV and syphilis through blood transfusion have been reported to be around 1 in 2-5 million, 1 in 0.5-1million, 1 in 2-4 million, 6 in a million, respectively6,7. The seroprevalences of various infectious markers from different parts of the India are given in Table IV8,9,10,11,12,13. The findings of the present study were also similar to that of other studies.
Table IV.
Comparison of seroprevalences (%) of infectious markers from various studies
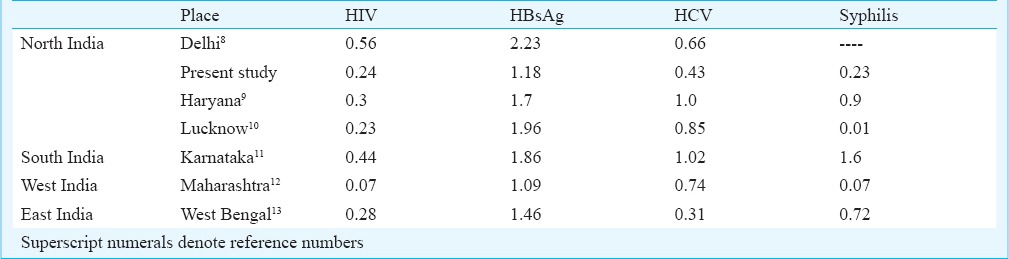
The National AIDS Control Organization (NACO) has reported an overall prevalence of HIV of 0.36 per cent (2006 estimate) in India14. In the present study, almost similar values have been found. World Health Organization has placed India in the intermediate zone (2-7% prevalence rates) of prevalence of hepatitis B15. Seroprevalence of HBsAg in our blood donors was 1.18 per cent. It is important to emphasize that this seroprevalence may be an underestimate of the infection risk due to the fact that the cases of occult hepatitis are not taken into consideration. Discarding core (HBcAb) reactive blood provides a safety blanket to an extent in such a situation but it is a double edged sword16 as it causes the wastage of around 10 per cent of the blood units. The prevalence of core (IgM+ IgG) reactive individuals was 9.87 per cent in our study. It is neither prudent nor cost-effective to use a test like HBcAb (total=IgM + IgG) as an infectious marker because it has a low sensitivity (84%), moderate specificity (91%) and a poor positive predictive value (10%). Issue of the core window (where the only marker of the infection is core antibody) can be better taken care of by an additional only-IgM core antibody test17.
Other than testing for syphilis, VDRL/RPR test acts as an additional safety as it prevents the blood of individuals with high risk behaviour (n=329 in this study) from being transfused. Detecting anti-cardiolipin antibodies is neither a specific nor a sensitive test for syphilis.
Studies7,18,19 have shown high seropositivity rates of TITs in replacement donors compared to voluntary donors, a similar finding was noted in our study. The risk of having TTIs in the replacement donors was 1.5 to 2.5 times more when compared with the voluntary donors. This emphasizes the importance of repeat, non-remunerated, regular voluntary donations. In India, the voluntary donations are to the tune of 60-70 per cent, but all are not repeat, non-remunerated, regular voluntary donations. At hospital based blood banks the majority (>90%) of the donors are replacement donors. In such a scenario it becomes difficult to guarantee the safety of blood.
A recent study from Kolkata, India, has shown a high seropositivity rate in male donors compared to female donors20. A similar finding was noted in our study. The risk of having TTIs in the male donors was three times more when compared with the female donors.
According to UNAIDS, between 1996 and 2010 the rate of new HIV infections fell by 56 per cent21. In concordance to this there was a significant fall in the seroprevalence of HIV over the study period in our study. HBV and syphilis infections also showed a significant decrease over the years, whereas there was a slight increase in the seroprevalence of HCV infection in the blood donors. At present there is no vaccination available against this disease, there is no effective treatment, it has a very high acute to chronic conversion rate and it spreads through various routes22. All these factors make HCV a more dreaded disease.
In conclusion, our analysis showed that the risk of TTIs decreased with respect to HIV, HBV and syphilis, but the seroprevalence of HCV was on a rise in our blood donors. There is a need for a comprehensive nationwide programme to tackle HCV in blood donors. The study reinforces that voluntary donors are safer than the replacement donors, hence recruitment of more donors into the repeat-regular voluntary donors pool will be a major step towards safe blood.
References
- 1.Blood bank. Central Drugs Standard Control Organization. Guidelines for blood banks (updated 2012 Jul 26) [accessed on June 10, 2014]. Available from: www.cdsco.nic.in/forms/list .
- 2.Hans R, Marwaha N. Nucleic acid testing-benefits and constraints. Asian J Transfus Sci. 2014;8:2–3. doi: 10.4103/0973-6247.126679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes H, D’souza PF, D’souza PM. Prevalence of transfusion transmitted infections in voluntary and replacement donors. Indian J Hematol Blood Transfus. 2010;26:89–91. doi: 10.1007/s12288-010-0044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Makroo RN, Chowdhry M, Bhatia A, Arora B, Rosamma N L. Prevalence of HIV among blood donors in a tertiary care centre of north India. Indian J Med Res. 2011;134:950–3. doi: 10.4103/0971-5916.92640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makroo RN, Walia RS, Chowdhry M, Bhatia A, Hegde V, Rosamma NL. Seroprevalence of anti-HCV antibodies among blood donors of north India. Indian J Med Res. 2013;138:125–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Bihl F, Castelli D, Marincola F, Dodd RY, Brander C. Transfusion-transmitted infections. J Transl Med. 2007;5:25. doi: 10.1186/1479-5876-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiavetta JA, Maki E, Gula CA, Newman A. Estimated risk of transfusion transmitted infection in the Canadian blood supply (1987-1996) Vox Sang. 2000;78(Suppl 1):360. [Google Scholar]
- 8.Pahuja S, Sharma M, Baitha B, Jain M. Prevalence and trends of markers of hepatitis C virus, hepatitis B virus and human immunodeficiency virus in Delhi blood donors.a hospital based study. Jpn J Infect Dis. 2007;60:389–91. [PubMed] [Google Scholar]
- 9.Arora D, Arora B, Khetarpal A. Seroprevalence of HIV, HBV, HCV and syphilis in blood donors in Southern Haryana. Indian J Pathol Microbiol. 2010;53:308–9. doi: 10.4103/0377-4929.64295. [DOI] [PubMed] [Google Scholar]
- 10.Chandra T, Kumar A, Gupta A. Prevalence of transfusion transmitted infections in blood donors: an Indian experience. Trop Doct. 2009;39:152–4. doi: 10.1258/td.2008.080330. [DOI] [PubMed] [Google Scholar]
- 11.Srikrishna A, Sitalakshmi S, Damodar P. How safe are our safe donors. Indian J Pathol Microbiol. 1999;42:411–6. [PubMed] [Google Scholar]
- 12.Giri PA, Deshpande JD, Phalke DB, Karle LB. Seroprevalence of transfusion transmissible infections among voluntary blood donors at a tertiary care teaching hospital in rural area of India. J Family Med Prim Care. 2012;1:48–51. doi: 10.4103/2249-4863.94452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharya P, Chandra PK, Datta S, Banerjee A, Chakraborty S, Rajendran K, et al. Significant increase in HBV, HCV, HIV and syphilis infections among blood donors in West Bengal, Eastern India 2004-2005: exploratory screening reveals high frequency of occult HBV infection. World J Gastroenterol. 2007;13:3730–3. doi: 10.3748/wjg.v13.i27.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National AIDS Control Organisation. [accessed on May 15, 2014]. Available from: www.naco.gov.in/NACO/NACO_Action/Media_Press_Release/
- 15.World health organization South-East Asia Regional Office. Prevention of hepatitis B in India-an overview. [accessed on June 10, 2014]. Available from: whqlibdoc.who.int/searo/2002/SEA_Hepat.-5.pdf .
- 16.Makroo RN, Chowdhry M, Bhatia A, Arora B, Rosamma NL. Hepatitis B core antibody testing in Indian blood donors: A double-edged sword! Asian J Transfus Sci. 2012;6:10–3. doi: 10.4103/0973-6247.95043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar H, Gupta PK, Jaiprakash M. The role of anti-HBc IgM in screening of blood donors. Med J Armed Forces India. 2007;63:350–2. doi: 10.1016/S0377-1237(07)80013-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh K, Bhat S, Shastry S. Trend in seroprevalence of hepatitis B virus infection among blood donors of coastal Karnataka, India. J Infect Dev Ctries. 2009;3:376–9. doi: 10.3855/jidc.246. [DOI] [PubMed] [Google Scholar]
- 19.Singh B, Verma M, Kotru M, Verma K, Batra M. Prevalence of HIV and VDRL seropositivity in blood donors of Delhi. Indian J Med Res. 2005;122:234–6. [PubMed] [Google Scholar]
- 20.Karmakar PR, Shrivastava P, Ray TG. Seroprevalence of transfusion transmissible infections among blood donors at the blood bank of a Medical College of Kolkata. Indian J Public Health. 2014;58:61–4. doi: 10.4103/0019-557X.128172. [DOI] [PubMed] [Google Scholar]
- 21.UNAIDS World AIDS Day report 2011. [accessed on June 10, 2014]. Available from: www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/unaidspublication/2011/jc2216_Worldaidsday_report_2011_en.pdf .
- 22.World health organization. Hepatitis C Fact sheet No. 164. [accessed on July 10, 2015]. Available from: www.who.int/mediacentre/factsheets/fs164/en/


