Abstract
Background & objectives:
The two major genotypic markers that distinguish community acquired (CA) from hospital acquired (HA) methicillin resistant Staphylococcus aureus (MRSA) isolates are the architecture of mobile genetic element (SCCmec type) and presence of panton valentine leukocidin (PVL) toxin. This study was conducted to determine the molecular characteristics of CA- and HA- MRSA and methicillin sensitive S. aureus (MSSA) isolates in Sikkim.
Methods:
A total of 150 clinical isolates of S. aureus isolated from various clinical specimens were subjected to duplex (mec-A and pvl gene) and multiplex (SCCmec typing) PCR.
Results:
Of the 150 isolates, 53 (35.33%) and 66 (44%) were positive for mec-A (MRSA) and pvl genes, respectively. Thirty eight (25.33%) met the definition of CA-MRSA and 15 (10%) of HA-MRSA and the remaining 63 (42%) and 34 (22.66%) as CA- and HA-MSSA, respectively. No significant difference was seen in the distribution of PVL toxin in MRSA and MSSA isolates, but it was significantly (P<0.001) high in overall MRSA isolates than in MSSA. The majority of the MRSA isolates showed a double amplification band of SCCmec type III plus V (54.71%), and only a fewer isolates were amplified by single DNA fragments of type I (1.88%), III (3.77%), IVa (1.88%) and V (11.32%). SCCmec types I, III, IVa, were found only in HA-MRSA isolates, whereas type V in both the CA- and HA-MRSA. AST pattern showed that 18.42 per cent (7/38) and 46.66 per cent (7/15) were multidrug resistant (MDR)-CA-MRSA and MDR-HA-MRSA, respectively.
Interpretation & conclusions:
The present results show that SCCmec type V MRSA has been on the rise, and genotypic markers such as pvl gene detection used for the differentiation of these clinically distinct isolates of MRSA may not be reliable.
Keywords: CA-MRSA, CA-MSSA, HA-MRSA, HA-MSSA, pvl gene, SCCmec element
The architecture of staphylococcal cassette chromosome mec (SCCmec) type and presence of panton valentine leukocidin (PVL) toxin are the two important genotypic markers that differentiate community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) strains from hospital-acquired (HA)-MRSA1. Based on SCCmec types, HA-MRSA usually carried large SCCmec element; typess I, II and III (34-67kb)2 but CA-MRSA harboured newly described smaller SCCmec element type IV (24kb)3 or less frequently V or a variant VT4. The PVL toxin is widely associated with the presence of SCCmec types IV and sporadically with SCCmec type V or VT but not with SCCmec type, I, II or III1. High association of PVL toxin in CA-MRSA strains is thought to have evolved via methicillin-sensitive S. aureus (MSSA) strains acquiring the lukS-PV and lukF-PV genes for PVL production and the resulting PVL positive MSSA gaining methicillin resistance through integration of the smaller, more mobile SCCmec types IV or V1.
The present study was undertaken to compare the molecular characteristics of community- and hospital-acquired MRSA and MSSA isolates in Sikkim by detecting two major genotypic markers; presence of pvl gene and SCCmec type, and to determine the diversity of SCCmec type of MRSA strains circulating in this region.
Material & Methods
Settings and bacterial isolates: A total of 150 S. aureus isolates obtained from the various clinical specimens of blood (10), sputum (1), throat swab (1) and pus (138) submitted to the department of Microbiology of Sikkim Manipal Institute of Medical Sciences (SMIMS) and Sir Thutob Namgyal Memorial (STNM) hospital at Gangtok, Sikkim, during the period from September 2009 to March 2011, were studied. The study was approved by the ethics committee of SMIMS.
Confirmation and storage of S. aureus isolates: All these isolates were confirmed as S. aureus by using standard techniques5. The isolates were inoculated into the semi-solid nutrient agar and stored at -20°C until further study.
Case definition: HA-MRSA was defined as one cultured from a clinical specimen obtained ≥72 h after patient's hospital admission or whose sources of isolation were associated with risk factors for HA-MRSA infection (e.g. recent hospitalization, recent surgery, residence in a long-term care facility, drug use)6, within one year of MRSA isolation date. CA- MRSA isolate was defined as one cultured < 72 h of a patient's hospital admission, or whose sources of isolation were not associated with risk factors for HA-MRSA infection. In a similar manner HA- and CA-MSSA were defined8.
Exclusion criteria: Duplicate isolates from the same patients, even if the site of infection was different during the sample collection time frame were excluded from the study.
DNA isolation: The DNA was extracted by using the HiPurA™ Bacterial and Yeast Genomic DNA Miniprep Purification Spin kit (Hi-Media, Mumbai) as per the manufacturer's instructions.
Duplex PCR for detection of mec-A and pvl genes: The primer pairs (Desalted) for mec-A and pvl genes were taken from the published sequence by Oliveria et al7 and McClure et al8, respectively. Primers were blasted and commercially obtained from Sigma-Aldrich Pvt. Ltd., Bangalore. PCR was performed by using Multiplex PCR kit (Qiagen, Hilden, Germany) with slight modification of final reaction volume of 25 μl (12.5 µl mastermix, 2.5 µl primer mix, 3µl of DNA template and 7µl of RNase-free water). Thermocycling conditions and visualization of products were done as per the manufacturer's instructions. Reference strains ATCC 43300 and 25923 were used as positive and negative controls for mec-A gene, respectively and ATCC 43300 was used as negative control for pvl gene.
Multiplex PCR: Primers - Primers were selected from the published sequence of the SCCmec types I-III (Oliveria et al)7, IVa -IVb (Okuma et al)9, IVc-IVd (Hisata et al)10, V (Zang et al)11 and were commercially obtained from Sigma-Aldrich Pvt., Ltd., Bengaluru.
Preparation of primer mix - The preparation of primer mix was done as per manufacturer's instructions of multiplex PCR kit (Qiagen). For multiplex PCR, the primers were divided into two sets: set A was designed to amplify SCCmec type I, II, III, V and mec-A, whereas set B was designed to amplify SCCmec IVa, IVb, IVc, IVd and mec-A. The mec-A gene was included in the protocol as an internal positive control.
SCCmec typing - To ensure the individual primer pairs were adequate for the amplification of all loci (gene fragments), the single-target PCR protocol11 with each individual primer pair was conducted prior to the multiplex PCR optimization. Then PCR was performed by using Qiagen Multiplex PCR kit with slight modification. Reaction was run in two sets with two different sets of primers mix. Multiplex PCR with set A primers consisting of 12.5 µl mastermix, 2.5 µl primer mix (set A), 3µl of DNA template and 7µl of RNase-free water with total reaction volume of 25µl. Multiplex primer set B included the same constituents as in set A except for the primers mix (set B). Thermocycling conditions and visualization of products were done as per the manufacturer's instructions.
Antibiotic susceptibility testing of MRSA isolates- Kirby-Bauer disc diffusion method12 was performed with following antibiotics discs (Hi-Media, Mumbai); penicillin-G (10 units), co-trimoxazole (25 µg), erythromycin (15 µg), ofloxacin (5 µg), gentamicin (10 µg), linezolid (30 µg/ml), rifampicin (5 µg), chloramphenicol (30 µg), fusidic acid (30 µg). Five discs in one and four in another agar plate were tested. The testing conditions and interpretation of the test was done as per Clinical and Laboratory Standards Institute (CLSI) criteria13. MRSA isolates resistant to ≥ three non-beta lactam antibiotics were classified as multidrug-resistant MRSA (MDR-MRSA)14.
Statistical analysis: Categorical variables were analyzed using Chi-square and Fisher's exact tests using IBM SPSS Statistics 20 (IBM Corporation, USA).
The ethical clearance was taken from the Institutional ethics committee, SMIMS.
Results
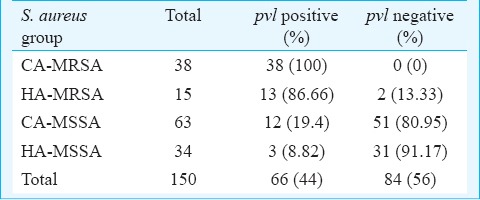
Duplex PCR: Out of 150 S. aureus isolates, 53 (35.33%) and 66 (44%) showed amplifications with mec-A and pvl genes, respectively (Fig. 1). Among 150 S. aureus isolates, 38 (25.33%) met the definition of CA-MRSA and 15 (10%) of HA-MRSA and rest of CA-MSSA and HA-MSSA (Table I). All MRSA isolates were positive for pvl barring two HA-MRSA isolates. Among MSSA, 12.37 per cent (12/97) and 3.09 per cent (3/97) were pvl positive CA-MSSA and pvl positive HA-MSSA, respectively. The presence of PVL toxin was significantly higher (P<0.001) in overall MRSA population (77.27% or 51/66) than that in MSSA (22.72% or 15/66).
Fig. 1.
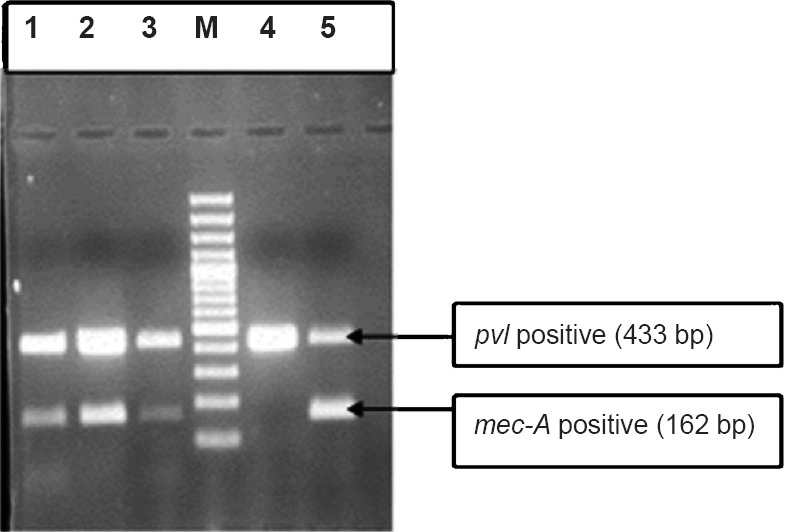
Duplex PCR (mec-A and pvl gene). Lanes 1,2,3,5 = Positive mec-A (162bp) and pvl (433bp), M= Marker (100bp DNA ladder), Lane 4 =Negative mec-A (162bp) positive pvl (433 bp).
Table I.
Distribution of pvl gene in CA- and HA-MRSA and MSSA isolates
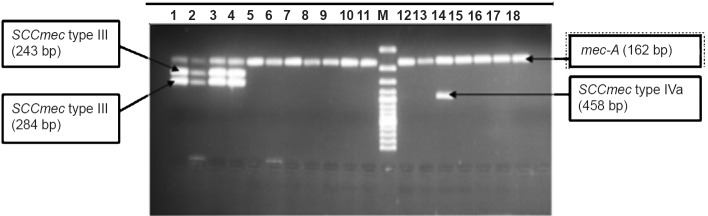
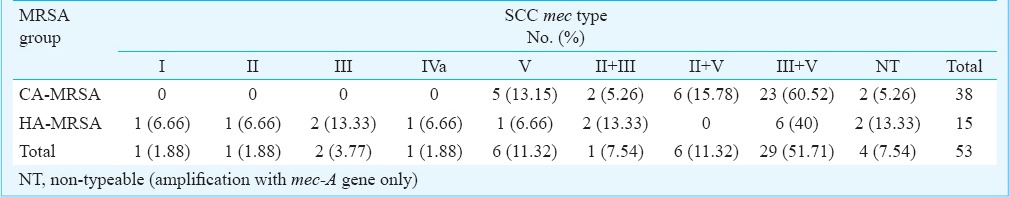
SCCmec typing: SCCmec typing patterns of MRSA isolates (Figs 2 and 3), and its distribution in two groups of MRSA (CA- and HA-MRSA) is shown in Table II. All MRSA isolates tested were found to be positive for internal control (mec-A) included in every batch of testing. The SCCmec types distributions in HA-MRSA patients were as follows: (i) patients developed infections ≥ 72 h of hospital admission (n=3), one each type I, II and II+III (ii) patients with previous history of hospital admission (n=9), one each type III, IVa, V, II+III, non-typeable and four isolates harboured combination band of III+V (iii) patients with previous history of antibiotic intake (n=3), III+V (two) and non-typeable (one).
Fig. 2.

SCCmec typing of MRSA isolates. Lanes 1,2,3,5 = mec-A (162 bp), SCCmec type III (243 bp) and type V (325 bp) positive. Lane 4= mec-A (162bp) and SCCmec type V (325 bp) positive.
Fig. 3.
SCCmec typing of MRSA isolates. Lanes 1,2,3,4 (reaction mixture of Set A primer mix run): Positive for mec-A (162 bp), SCCmec type II (284 bp) and type III (243 bp). Lane 5-18 (reaction mixture of Set B primer mix run)= Positive for mec-A gene. Lane 14= Positive for mec-A (162bp) and SCCmec type IVa (458 bp).
Table II.
SCCmec type of CA- and HA-MRSA isolates
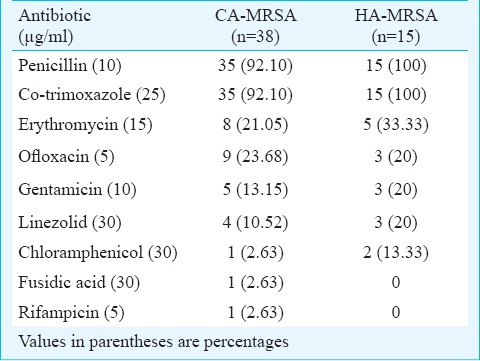
Antimicrobial susceptibility pattern of MRSA isolates: The results of in vitro susceptibility testing of CA- and HA-MRSA isolates are given in Table III. High percentage of CA-MRSA (92.10%) and all isolates of HA-MRSA were resistant to penicillin and co-trimoxazole. Among CA- and HA-MRSA, 18.42 per cent (7/38) and 46.66 per cent (7/15) were found to be multi-drug resistant (MDR), respectively. SCCmec types of MDR-HA-MRSA isolates were as follows: type I (one), III (two), V (one), II+III (one) and III +V (two). Similarly, SCCmec types of MDR-CA-MRSA were type V (two), II+III (one), II+V (two) and III+V (two).
Table III.
Antibiotic resistance pattern of CA- and HA-MRSA isolates
Discussion
In the present study, we evaluated the presence of PVL toxin as a marker of CA-MRSA isolates. Several studies have reported the pvl as a reliable marker for CA-MRSA strains15,16 and a few studies17,18 have reported the presence of the PVL toxin more in MSSA than MRSA. Our finding is in agreement with the reports from Ireland19 and Finland20.
In this study, SCCmec typing revelead that MRSA isolates with double DNA fragment bands (73.58%) were much higher than single fragment (18.86%), majority (54.71%) had the combination of SCCmec type III plus V. Similarly, Zang et al11 reported the presence of double bands in 1.1 per cent of clinical isolates of MRSA of these combinations; SCCmec type III +IVc (one), type I+II (two) and type II+IVc (two isolates). The percentage of non-typeable MRSA isolates in our study was 7.54 per cent, which was higher than the earlier reports from Korea (1.35%)21, Canada (1.77%)11, India (4%)22. In contrast, a study from Taiwan reported non-typeable MRSA as high as 80.95 per cent23.
SCCmec type III has been reported to be the predominant MRSA in Asian continent except Korea and Japan22,25. The present study showed low occurrence of type III MRSA isolates in Sikkim (3.8%) compared to that reported from other parts of India22,25,26,27. D'souza et al16 from Mumbai, India, reported that SCCmec type V (41.01%) was higher than type III (24.55%). Our study suggests that SCCmec type V seems to be an emerging MRSA in this part of India.
In our study only one HA-MRSA isolate was found to be positive for type IVa. On the contrary, a study from Mumbai, India, reported presence of SCCmec type IV in 34 per cent of MRSA isolates collected during a three year period. In earlier studies24,27 conducted on the MRSA isolates collected from Indian hospitals have not reported type IV MRSA isolates.
The association of specific SCCmec type in CA- and HA-MRSA isolates has been well characterized, and formed the basis of molecular definition1. In the present study, the SCCmec types IV and V which are typically associated with CA-MRSA, were also found in HA-MRSA isolates. Yu et al28 reported type IV in HA-MRSA isolates (12.5%). Gonzalez et al29 also reported CA-MRSA isolates (SCCmec type IV) as an important cause of healthcare-associated infections. This indicates that HA-MRSA with typical molecular characteristics of CA-MRSA (SCCmec type IVa, V and PVL positive) have emerged as an important cause of healthcare-associated infections. Therefore, differentiation of MRSA based on these genotypic markers would not be reliable in near future. However, these findings need to be validated on a large number of isolates.
Apart from genotypic markers, MRSA is also categorized based on the susceptibility pattern to various antibiotics30. Based on this definition, CA-MRSA has wider spectrum of susceptibility to antibiotics compare to HA-MRSA31 and multidrug resistance is a phenotypic marker for nosocomial strain14. In support of this a study has reported 33 per cent of MRSA isolates as MDR-MRSA, where CA-MRSA isolates were less likely to be resistance to antibiotics than HA-MRSA isolates14. Fey et al31 reported 87.5 per cent of HA-MRSA as MDR, whereas no MDR was found among the CA-MRSA isolates. In our study also, occurrence of MDR-MRSA was more in HA-MRSA than CA-MRSA.
In conclusion, pvl may no longer be a reliable marker for CA-MRSA isolates, rather all MRSA may be important reservoir of PVL toxin, and SCCmec type V MRSA is emerging as predominant isolates over most prevalent type III/IIIA. Multidrug resistance seen among CA-MRSA indicates the changing epidemiological and microbiological characteristics of MRSA in the community and hospitals in this part of India.
Acknowledgment
Authors thank the Dean, Sikkim Manipal Institute of Medical Sciences, Dr Samar Hussain Naqvi (Genetix-Biotech) and faculty and technical staffs of the department of Microbiology of SMIMS and STNM hospitals, and acknowledge Dr Amit Chakrabarti, Professor, Department of Pharmacology, SMIMS for his help in statistical analysis.
References
- 1.Boyle-Vavra S, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus: the role of Panton-Valentine Leukocidin. Lab Invest. 2007;87:3–9. doi: 10.1038/labinvest.3700501. [DOI] [PubMed] [Google Scholar]
- 2.Ito T, Katayama Y, Asada K, Mori N, Tsutsumimoto K, Tiensasitorn C, et al. Structural comparison of three types of staphylococcal cassette chromosome mec integrated in the chromosome in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:1323–36. doi: 10.1128/AAC.45.5.1323-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma XX, Ito T, Tiensasitorn C, Jamklang M, Chongtrakool P, Boyle-Vavra S, et al. Novel type of staphylococcal cassette chromosome mec identified in community-acquired methicillin-resistant Staphylococcus aureus strains. Antimicrob Agents Chemother. 2002;46:1147–52. doi: 10.1128/AAC.46.4.1147-1152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle-Vavra S, Ereshefsky B, Wang CC, Daum RS. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel Staphylococcal chromosome cassette mec (SCCmec) type V T or SCCmec Type IV. J Clin Microbiol. 2005;43:4719–30. doi: 10.1128/JCM.43.9.4719-4730.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baird D. Staphylococcus: cluster-forming gram-positive cocci. In: Collee JG, Fraser AG, Marmion BP, Simmons A, editors. Mackie & McCartney practical medical microbiology. 14th ed. Churchill Livingstone: New York; 1996. pp. 245–61. [Google Scholar]
- 6.Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–8. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid-identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:2155–61. doi: 10.1128/AAC.46.7.2155-2161.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McClure J, Conly JM, Lau V, Elsayed S, Louie T, Hutchins W, et al. Novel multiplex PCR assay for detection of the staphylococcal virulence marker panton-valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from -resistant staphylococci. J Clin Microbiol. 2006;44:1141–4. doi: 10.1128/JCM.44.3.1141-1144.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuma K, Iwakawa K, Turnidge JD, Grubb WB, Bell JM, O’Brien FG, et al. Dissemination of new methicillin-resistant staphylooccus aureus clones in the community. J Clin Microbiol. 2002;40:4289–94. doi: 10.1128/JCM.40.11.4289-4294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hisata K, Kuwahara-Arai K, Yamanoto M, Ito T, Nakatomi Y, Cui L, et al. Dissemination of methicillin-resistant Staphylococci among healthy Japanese children. J Clin Microbiol. 2005;43:3364–72. doi: 10.1128/JCM.43.7.3364-3372.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang K, McClure JA, Elsayed S, Louie T, Conly JM. Novel multiplex PCR assay for characterization and concomitant subtyping of staphylococcal cassette chromosome mec types I to V in methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43:5026–33. doi: 10.1128/JCM.43.10.5026-5033.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–6. [PubMed] [Google Scholar]
- 13.28. No1. Wayne, PA: CLSI; 2008. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing; Performance standards for antimicrobial susceptibility testing; 18 th informational supplement. Approved standard M100-S18. [Google Scholar]
- 14.Charlebois ED, Perdreau-Remington F, Kreiswirth B, Bangsberg DR, Ciccarone D, Diep BA, et al. Origins of community strains of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2004;39:47–54. doi: 10.1086/421090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald RR, Antonishyn NA, Hansen T, Snook LA, Nagle E, Mulvey MR, et al. Development of a triplex real-time PCR assay for detection of Panton-Valentine leukocidin toxin genes in clinical isolates of methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 2005;43:6147–9. doi: 10.1128/JCM.43.12.6147-6149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’souza N, Rodriques C, Mehta A. Molecular characterization of methicillin-resistant Staphylococcus aureus with emergence of epidemic clones of sequence type (ST)22 and ST 772 in Mumbai, India. J Clin Microbiol. 2010;48:1806–11. doi: 10.1128/JCM.01867-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goering RV, Shawar RM, Scangarella NE, O’Hara FP, Amrine-Madsen H, West JM, et al. Molecular epidemiology of methicillin-resistant and methicillin-susceptible Staphylococcus aureus isolates from global clinical trials. J Clin Microbiol. 2008;46:2842–7. doi: 10.1128/JCM.00521-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campbell SJ, Deshmukh HS, Nelson CL, Bae IG, Stryjewski ME, Federspiel JJ, et al. Genotypic characteristics of Staphylococcus aureus isolates from a multinational trial of complicated skin and skin structure infections. J Clin Microbiol. 2008;46:678–84. doi: 10.1128/JCM.01822-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roosney AS, Shore AC, Morgan PM, Fitzgibbon MM, O’Connell B, Coleman DC. The emergence and importation of diverse genotypes of methicillin-resistant Staphylococcus aureus (MRSA) harboring the Panton-Valentine Leukocidin gene (pvl) reveal that pvl is a poor marker for community-acquired MRSA strains in Ireland. J Clin Microbiol. 2007;45:2554–63. doi: 10.1128/JCM.00245-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kardén-Lilja M, Ibrahem S, Vuopio-Varkila J, Salmenlinna S, Lyytikäinen O, Siira L, et al. Panton-Valentine leukocidin genes and staphylococcal chromosomal cassette mec types amongst Finnish community-acquired methicillin-resistant Staphylococcus aureus strains, 1997–1999. Eur J Clin Microbiol Infect Dis. 2007;26:729–33. doi: 10.1007/s10096-007-0334-0. [DOI] [PubMed] [Google Scholar]
- 21.Ko KS, Lee JY, Suh JY, Oh WS, Peck KR, Lee NY, et al. Distribution of major genotypes among methicillin-resistant Staphylococcus aureus clones in Asian countries. J Clin Microbiol. 2005;43:421–6. doi: 10.1128/JCM.43.1.421-426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arakere G, Nadig S, Ito T, Ma XX, Hiramatsu K. A novel type-III staphylococcal cassette chromosome mec (SCCmec) variant among Indian isolates of methicillin-resistant Staphylococcus aureus. FEMS Microbiol lett. 2009;292:141–8. doi: 10.1111/j.1574-6968.2008.01482.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang CC, Lo WT, Chu ML, Siu LK. Epidemiological typing of community-acquired methicillin-resistant Staphylococcus aureus isolates from children in Taiwan. Clin Infect Dis. 2004;39:481–7. doi: 10.1086/422642. [DOI] [PubMed] [Google Scholar]
- 24.Chongtrakool P, Ito T, Ma XX, Kondo Y, Trakulsomboon S, Tiensasitorn C, et al. Staphylococcal cassette chromosome mec (SCCmec) typing of methicillin-resistant Staphylococcus aureus strains isolated in 11 Asian countries: a proposal of new nomenclature for SCCmec elements. Antimicrob Agents Chemother. 2006;50:1001–12. doi: 10.1128/AAC.50.3.1001-1012.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shabir S, Hardy KJ, Abbasi WS, McMurray CL, Malik SA, Wattal C, et al. Epidemiological typing of methicillin resistant Staphylococcus aureus isolates from Pakistan and India. J Med Microbiol. 2010;59:330–7. doi: 10.1099/jmm.0.014910-0. [DOI] [PubMed] [Google Scholar]
- 26.Jeshina J, Surekha K. Molecular characterization of methicillin resistant Staphylococcus aureus strains isolated in Kerala, South India. Curr Res Bacteriol. 2009;2:1–6. [Google Scholar]
- 27.Nadig S, Namburi P, Raghunath D, Arakere G. Genotyping of methicillin-resistant Staphylococcus aureus isolates from Indian hospitals. Curr Sci. 2006;91:1364–9. [Google Scholar]
- 28.Yu F, Chen Z, Liu C, Zhang X, Lin X, Chi S, et al. Prevalence of Staphylococcus aureus carrying Panton-Valentine leukocidin genes among isolates from hospitalised patients in China. Clin Microbiol Infect. 2008;14:381–4. doi: 10.1111/j.1469-0691.2007.01927.x. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez BE, Rueda AM, Shelburne SA, 3rd, Musher DM, Hamill RJ, Hulten KG. Community-associated strains of methicillin-resistant Staphylococcus aureus as the cause of healthcare-associated infection. Infect Control Hosp Epidemiol. 2006;27:1051–6. doi: 10.1086/507923. [DOI] [PubMed] [Google Scholar]
- 30.Benoit SR, Estivariz C, Mogdasy C, Pedreira W, Galiana A, Galiana A, et al. Community strains of methicillin-resistant Staphylococcus aureus as potential cause of healthcare-associated infections, Uruguay, 2002-2004. Emerg Infect Dis. 2008;14:1216–23. doi: 10.3201/eid1408.071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fey PD, Said-Salim B, Rupp ME, Hinrichs SH, Boxrud DJ, Davis CC, et al. Comparative molecular analysis of community- or hospital-acquired methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2003;47:196–203. doi: 10.1128/AAC.47.1.196-203.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]