Abstract
The Bvg-regulated promoters for the fimbrial subunit genes fim2 and fim3 of B. pertussis behave differently from each other both in vivo and in vitro. In vivo Pfim2 is significantly stronger than Pfim3, even though predictions based on the DNA sequences of BvgA binding motifs and core promoter elements would indicate the opposite. In vitro Pfim3 demonstrated robust BvgA~P-dependent transcriptional activation, while none was seen with Pfim2. This apparent contradiction was investigated further. By swapping sequence elements we created a number of hybrid promoters and assayed their strength in vivo. We found that, while Pfim3 promoter elements upstream of the +1 transcriptional start site do indeed direct Bvg-activated transcription more efficiently than those of Pfim2, the overall promoter strength of Pfim3 in vivo is reduced due to sequences downstream of +1 that inhibit transcription more than 250-fold. This element, the DRE (downstream repressive element), was mapped to the 15 bp immediately downstream of the Pfim3 +1. Placing the DRE in different promoter contexts indicated that its activity was not specific to fim promoters, or even to Bvg-regulated promoters. However it does appear to be specific to Bordetella species in that it did not function in E. coli.
Keywords: Bordetella pertussis, global virulence regulation, virulence gene promoters, promoter architecture, transcriptional activation
Introduction
Whooping cough caused by Bordetella pertussis is a highly contagious respiratory disease that is persistent in developing countries and reemerging in developed countries, despite well accepted vaccination programs. As with many Gram-negative bacterial pathogens, adherence of B. pertussis to the host respiratory tract is thought to play an important role in establishing and maintaining infection (Mooi et al., 1992; Cotter et al., 1998). Filamentous hemagglutinin (FHA) and fimbriae are two of the most well-recognized adhesins produced in B. pertussis (Relman et al., 1989; Weiss & Goodwin, 1989) and have been included as antigen components in current acellular pertussis vaccines (Poolman & Hallander, 2007).
In B. pertussis the serologically distinct fimbriae 2 and fimbriae 3 are composed primarily of the major subunit proteins Fim2 and Fim3. Interestingly, while the chaperone and usher proteins FimB and FimC, and the minor subunit and tip adhesin FimD, are encoded in the same operon as filamentous hemagglutinin (Locht et al., 1992; Willems et al., 1992), the genes encoding the pilin major structural subunits are at widely separated chromosomal locations (Stibitz and Garletts, 1992). Each of these fimbrial subunits undergoes phase-variation at a rate much higher than random mutation. The mutational change leading to altered expression of fim2 or fim3 is the addition or deletion of one basepair within a monotonic stretch of Cs in the promoters Pfim2 and Pfim3, presumably due to slipped-strand mispairing during replication (Willems et al., 1990). We have previously determined an optimum length of this C-stretch to permit maximal transcription of fim2 and fim3 (12 and 15 Cs, respectively), as well as a normally silent fimX. In the latter case, inactivation was due to a much shorter C-stretch in its promoter that presumably could not switch on spontaneously by the insertion of one or small number of basepairs (Chen et al., 2010).
Expression of virulence genes in B. pertussis, and in the related species B. bronchiseptica and B. parapertussis, is regulated globally by a two-component system comprising the membrane-spanning sensor histidine-kinase BvgS and the phospho-accepting response regulator BvgA (Weiss et al., 1983; Aricó et al., 1989; Stibitz and Yang, 1991). In standard culture media at 37°C BvgA, phosphorylated by BvgS, activates the transcription of multiple virulence genes (fha, cya, ptx, etc.) while it represses a separate group of genes known as vrgs (Knapp & Mekalanos, 1988). A third class, exemplified by the gene bipA, is maximally expressed under conditions intermediate between those resulting in maximal activation and repression. This is due to activation when levels of BvgA~P are low or moderate, and repression when they are high (Williams et al., 2005). Unlike many other two-component sensor kinases, the ground state of BvgS appears to be “on”. Specific activators of BvgS have never been identified, and recent crystallographic evidence suggests an active conformation in the absence of ligands (Herrou et al., 2010). However, the Bvg− state, in which the BvgS kinase is inactive, can be induced by the addition of compounds including MgSO4 or nicotinic acid or by culture at reduced temperatures (Lacey, 1960; Boulanger et al., 2013). This phenomenon is known as modulation.
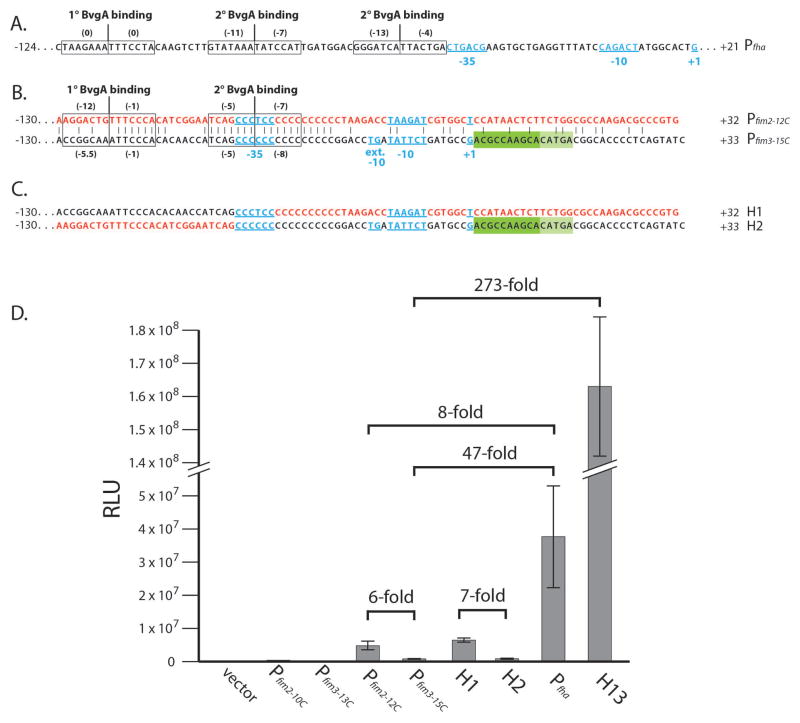
Even among Bvg-activated genes, the kinetics of gene activation after a shift from Bvg− to Bvg+ mode are different, and depend on the affinity and/or number of BvgA~P binding sites. These features of promoter architecture dictate a given promoter’s response to the different levels of BvgA~P encountered following a shift (Scarlato et al., 1991). For example, as shown in Fig. 1A, the early gene promoter Pfha, which has one strong primary and two weak secondary binding sites for BvgA~P (Boucher et al., 2001a; Boucher et al., 2003), is activated at the initial stages of induction when BvgA~P level is low; whereas the late gene promoter Pptx that is activated by BvgA~P binding to six sites of moderate strength is activated hours later when high levels of BvgA~P have been reached (Scarlato et al., 1991, Boulanger et al., 2013). It has long been known that fimbrial production is ultimately controlled by BvgAS at two points. One is through the direct binding of BvgA~P to Pfha. This in turn activates the transcription of Pfha, which co-transcribes, as part of the fha operon, the fimBCD genes required for pilus secretion, assembly, and function. The other control point, expression of the unlinked pilin structural genes fim2 and fim3, was also shown over two decades ago to be ultimately BvgAS-regulated (Willems et al., 1990). However, we have confirmed only recently that this regulation involves direct binding of BvgA~P to the fim promoters (Chen et al., 2010). As summarized in Fig. 1B, each of these promoters has one primary, stronger, upstream binding site and one secondary, weaker, downstream binding site in a BvgA binding geometry generally similar to that of Pfha. However, unlike Pfha, in which the proximal secondary BvgA~P binding site is juxtaposed to the −35 region, the secondary binding site of the fim promoters is centered on the −35 region, which is contained within the C-stretch. Further study (Decker et al., 2011) on the spatial interrelationships of BvgA~P, RNAP, and Pfim3-15C promoter DNA, revealed that Region 4 of the sigma factor of RNAP retains its normal location relative to the −35 region of the promoter despite the highly non-consensus sequence (CCCCCC vs. the consensus TTGACA). We also previously demonstrated that the −15TG−14, present in Pfim3 but not Pfim2, contributes significantly to Pfim3 activity. The presence of this functional extended −10 motif in a promoter similar to Pfim2 is inconsistent with the weaker transcriptional activity we observed from Pfim3 in vivo (Chen et al., 2010). Here we report that Pfim3-15C promoter sequences down to and including the transcription start at +1 direct stronger transcription than their counterparts in Pfim2-12C. However, a repressive element (DRE), present immediately downstream of the transcription start, represses this strong Pfim3-15C activity. Furthermore, inhibition of transcription by the DRE is not specific to Bvg-regulated promoters, but is specific to Bordetella species, suggesting that it may involve an intrinsic property of Bordetella RNAP or the involvement of a species-specific co-factor.
Fig. 1. Pfim3-15C transcriptional activity in B. pertussis.
(A). Sequence of Pfha. The −35 region, −10 element, and transcription start site +1 are in blue underlined. The numbers at each end indicate the extent of cloned fragments. The BvgA binding sites are boxed, with a vertical line to indicate the center, and with numbers in parentheses that denote scores of the individual half-sites using an algorithm to assess BvgA~P binding strength (Chen et al., 2010, Merkel et al., 2003).
(B). Sequence comparison of Pfim2-12C (in red) and Pfim3-15C (in black). Identical residues are denoted by vertical dashes. The −35 region, extended −10 −15TG−14 motif, −10 element, and transcription start site +1 are in blue underlined. Positions +1 to +15 constituting the DRE are indicated by a dark and light green rectangles to indicate its repressive effect observed in the deletion or swapping analysis. The numbers at each end and the BvgA binding sites are indicated as in (A)
(C). Cloned hybrid sequence for H1 and H2 with colors as in (B) to indicate sequence source.
(D). B. pertussis strain BP536 harboring chromosomally integrated pSS3967 containing no insert (control), or different promoter constructs were grown on BG agar and analyzed for light production by luciferase as described in the Experimental Procedures. Values are given in arbitrary units (RLU for relative light units). Data averaged from at least four assays were used in the calculation of standard deviations as indicated by error bars.
Results
Pfim3 exhibits strong transcriptional activity in vitro but not in vivo
Previously, using promoter-luciferase fusions integrated as single copies at an arbitrary but constant location in the chromosome of B. pertussis strain BP536, we showed that the C-stretch-length-optimized fim promoters Pfim2-12C and Pfim3-15C were activated directly by BvgA (Chen et al., 2010). These promoter sequences demonstrated identical spacing of their main features, from the upstream primary BvgA binding sites to the core features of their inferred −35 regions (concentric with secondary BvgA binding sites), −10 regions, and transcription starts. Only Pfim3 contains the extended −10 sequence −15TG−14, which has been shown to be required for robust Pfim3-15C transcription (Chen et al., 2010; Decker et al., 2011; Fig. 1B for Pfim2-12C and Pfim3-15C). In this study, in order to understand the relationships between promoter structure and promoter activity, we compared Pfim2-12C, Pfim3-15C, and the known Bvg-regulated strong promoter Pfha. These promoters and all hybrids or variants thereof were examined in single copies of an “ectopic” promoter-luciferase fusion in B. pertussis BP536 as previously reported (Chen et al., 2010). Relative promoter strengths are presented in Fig. 1D. As expected, luciferase activity was undetectable with the pSS3967 vector containing no promoter (control), or with the non-permissive shortened C-stretch promoters Pfim2-10C and Pfim3-13C. With optimized C-stretch promoters, Pfim2-12C was ~6-fold more active than Pfim3-15C, indicating that it is a stronger promoter in vivo, consistent with our previous observation (Chen et al., 2010). Relative to the strong promoter Pfha, transcription from both Pfim2-12C and Pfim3-15C was much weaker, with ~8-fold and ~47-fold decreased activity, respectively (Fig. 1D), suggesting that fim promoters, especially Pfim3-15C, are weaker than Pfha, one of the strongest B. pertussis promoters described to date.
In contrast, the levels of transcription observed from these promoters in vitro using E. coli RNAP and BvgA~P were quite different. While transcription from the optimized fim3 promoter Pfim3-15C was comparable to that from Pfha, (Chen et al., 2010; Decker et al., 2011), we were unable to detect transcription from Pfim2-12C (data not shown), consistent with our observation that in vivo Pfim2-12C is a weak promoter relative to Pfha (Fig. 1D). Taking together our observations from in vivo and in vitro studies, it was evident that Pfim3-15C is highly competent at promoting transcription in vitro, but not in vivo in B. pertussis.
Pfim3 possesses a downstream repressing element (DRE) that inhibits its transcription in vivo in B. pertussis
Given the disparity between the in vivo and in vitro transcriptional activities of Pfim3-15C, we speculated that it possesses sequence feature(s), not found in Pfim2-12C, that are either stimulatory in vitro or repressive in vivo, or that Pfim2 possesses features with opposing activities. As shown in Fig. 1B, a sequence comparison of Pfim3 vs Pfim2 suggests two significant differences that could affect promoter activity. First, Pfim3 appears to possess a primary BvgA-binding site of higher affinity than that of Pfim2. In both promoters, the upstream, primary, high-affinity BvgA-binding site is composed of two heptads, each directing the binding of one monomer of a BvgA-dimer. In both, the rightward, downstream heptad is nearly identical and very close to the consensus high-affinity sequence. However, the leftward, upstream heptad of Pfim3 is predicted to be of higher affinity than that of Pfim2 as determined by the application of an algorithm based on mutational studies (Boucher et al., 2001b, Merkel et al, 2003). This heptad in Pfim3 has a moderately good score of −5.5, while that of Pfim2 has a poor score of −12. The secondary binding sites of both promoters are nearly identical and coincide at 10 of 14 bp with the C-stretch. The second notable difference in the fim2 vs fim3 promoter sequences is within core promoter itself where Pfim3 possesses an extended −10 −15TG−14 region. This element is known to contribute to promoter activity in a number of systems, including Pfim3 (Decker et al., 2011). Thus, two sequence features of Pfim3 are predictive of higher promoter activity relative to Pfim2, and are consistent with the higher level of Pfim3-15C activity observed in vitro. The fact that these features fail to result in a stronger overall promoter in vivo is consistent with the hypothesized existence of an in vivo inhibitory sequence element unique to Pfim3 in B. pertussis.
To map the positions of functional sequence differences, we constructed hybrid promoter fragments by swapping segments of Pfim2-12C and Pfim3-15C. We first constructed two hybrid promoters by swapping BvgA-binding regions, to create H1 containing the upstream BvgA binding regions of Pfim3-15C together with the downstream core region of Pfim2-12C, and H2 with the complementary hybrid sequence (depicted in Fig. 1C). Readouts of transcriptional activity by light production in vivo are shown in Fig. 1D. Pairwise comparisons of these results indicate that core promoter and/or downstream sequences are most important. For example, substitution of that region in Pfim3-15C with the corresponding sequences from Pfim2-12C results in a substantial increase (H1) whereas the complementary substitution to Pfim2 results in a decrease (H2). This is in contrast to the lack of effects of BvgA binding site substitutions in that Pfim3-15C and H2 have a similar level of activity, as do Pfim2-12C and H1. Taken together these results indicate that key differences between the two promoters are downstream of the BvgA binding sites. It also indicates that the fim2 promoter contains the more optimal combination of core promoter, transcriptional start, and downstream sequences, an observation that is inconsistent with the presence of the extended −10 element in Pfim3, but not Pfim2.
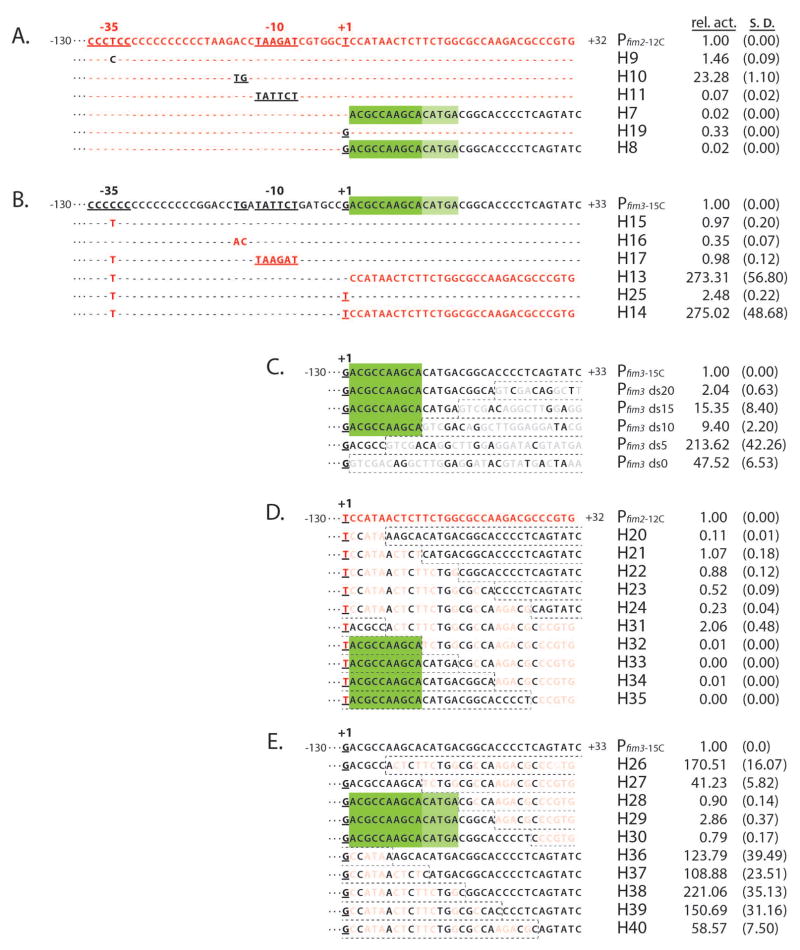
To functionally map relevant sequence features we constructed additional swaps of more specific sequence features. As shown in Figs. 2A and 2B, these were: 1) the T (in Pfim2) in the −35 region/secondary BvgA-binding site/C-stretch (H9 and H15), 2) the extended −10 element (H10 and H16), 3) the −10 element (H11 and H17), 4) downstream sequences (H7 and H13), 5) just the +1 initiating nucleotide (H19 and H25), and 6) +1 plus downstream sequences (H8 and H14). Although there were some changes in activity when upstream promoter sequences were swapped (as detailed below), the most dramatic differences arose when the sequences just downstream of +1 were exchanged. Addition of the Pfim2 downstream sequences to Pfim3 (H13 and H14) increased transcription of Pfim3 over 250-fold (also seen in Fig. 1D for H13 activity relative to Pfim3-15c) while the reciprocal substitutions (H7 and H8) reduced transcription 50-fold (relative activity 0.02). The effect of these substitutions was not influenced significantly by the identity of the initiating nucleotide and substitution of the +1 nucleotide itself (H19 and H25) had only a three-fold effect in either direction. These results indicate that the downstream sequences of Pfim3 are repressive or that those of Pfim2 have a stimulatory effect. To address this question directly, we deleted the downstream sequences of Pfim3 in an incremental manner. The adjacent pSS3967 vector sequence that replaces the deleted sequences in each construct is shown in faded gray with the exception of nucleotides identical to those in Pfim3, at a given position. As shown in Fig 2C, leaving either 10 or 15 bp of the native downstream sequence intact maintained a moderate repressive effect, while reduction to 0 or 5 bp had a profound stimulatory effect. We highlighted the first 10 bp (ACGCCAAGCA) in dark green to indicate its repressive effect observed in the deletion study. These results indicate that Pfim3 harbors a repressive element, not that Pfim2 harbors a stimulatory one. We have termed this element the DRE for downstream repressive element.
Fig. 2. DRE represses Pfim3-15C transcription in B. pertussis.
Transcriptional activities of fim hybrid promoters were assessed in vivo. Promoter fragments comprising sequences from −130 to +32 (Pfim2) or +33 (Pfim3) were cloned into the luxCDABE transcriptional vector pSS3967, the resulting constructs were inserted into the chromosome of BP536 at a constant, ectopic, location, and light output was measured as described in Experimental Procedures. Promoter activities normalized to the wild-type in each set are presented to the right, with values for standard deviation in parentheses. Based on the data in each panel, the inferred extent of the DRE is indicated by green rectangles
(A & B). Relevant portions of the nucleotide sequences of the fim2 promoter (A) and the fim3 promoter (B) are presented with sequence elements exchanged to create hybrid promoters indicated below and in contrasting color. Unchanged nucleotides are depicted by dashes.
(C). Deletion analysis of the downstream region of Pfim3. Downstream sequences were deleted to varying extents. Vector sequences substituting for promoter sequences are boxed by dashed lines. Nucleotides identical at a given position to those in the wildtype fim3 promoter are shown in black, and those that are different are depicted in light gray.
(D & E). Hybrid promoters were derived by substituting, to varying extents, sequences downstream of +1 with those of the alternate promoter. Substituting sequences are boxed by dashed lines with those differing at a given position shaded out.
To accurately localize DRE, we swapped 5-bp incrementally, from both directions, of the +1 downstream regions of Pfim2-12C and Pfim3-15C, as depicted in Fig. 2D and 2E. Among the DRE swapped-in variants in Pfim2-12C, only H32-H35, containing at least 10 bp, in dark green, of the immediate downstream sequence of Pfim3-15C, nearly abolished Pfim2-12C activity (Fig. 2D). As shown in Fig. 2E, of the reciprocal substitutions of Pfim3, only H28-H30 (Fig. 2E), retaining at least 15 bp, 5 of them marked in light green (CATGA), of the immediate downstream sequence of Pfim3-15C, maintained the repressive effect. These data localize the DRE to the 15 bp (in both dark and light green) immediately downstream of the initiation nucleotide of Pfim3.
The profound changes observed with the DRE were not seen with other swaps. The “T” swaps in the C-stretch, H9 and H15, did not change promoter activity significantly, consistent with the previous finding of little or no influence of the sequence content of the C-stretch on promoter function (Chen et al., 2010). Results with the −10 and extended −10 were qualitatively as expected although the quantitative levels were unexpected. For example, while hybrid H10, in which Pfim2 acquired the extended −10 sequence, showed a 23-fold increase in activity, in keeping with the importance of this element, the reciprocal exchange (H16) had only a three-fold negative effect on Pfim3. Similarly, the substitution of the −10 element of Pfim2 with that of Pfim3 (H11) resulted in a 14-fold reduction, suggesting that the latter was weaker, while the reciprocal exchange (H17) had no effect on Pfim3. These results suggest that function of the −10 region may not be entirely independent of the effects of the DRE (see below), and that the Pfim3-15C −10 element (TATTCT) is somewhat weaker than the Pfim2-12C −10 element (TAAGAT), despite their possession of four matches to the consensus of canonical −10 (TATAAT), including the most crucial nucleotides TA---T (Hook-Barnard & Hinton, 2007).
DRE represses promoter activity in a manner that is independent of BvgA-promoter binding and BvgAS regulation
Pfim2-12C and Pfim3-15C, whose activity can be repressed by DRE, constitute a distinct subset of BvgA-activated promoters. These promoters contain a unique architectural feature in that the most downstream (secondary) BvgA-binding site is concentric with the position expected of a bona fide −35 element (see Fig. 1B). Binding of BvgA~P to this secondary binding site is essential for promoter activation, and our previous analyses suggest that productive contacts with RNAP are made by this BvgA dimer (Decker et al., 2011). Specifically, we have demonstrated that, in an active ternary transcriptional complex, the alpha subunit C-terminal domain, Region 4 of sigma, and BvgA~P are all localized spatially to the same linear segment of promoter DNA, but to different faces of the helix. This architecture is in contrast to that of the BvgA-activated promoters of other genes including fha, ptx, cya, prn and bvgR, in which the most downstream secondary BvgA-binding site is juxtaposed to, but is not overlapping, the putative −35 element. Also, as shown recently, Pfim3-15C plays host to BvgA and RNAP in a conformational complex different from that at PfhaB (Decker et al., 2011). Given the discovery of the DRE in context of the unique architecture of the fim promoters, we sought to determine if its effects could be seen on other BvgA-regulated or non-BvgA-regulated promoters.
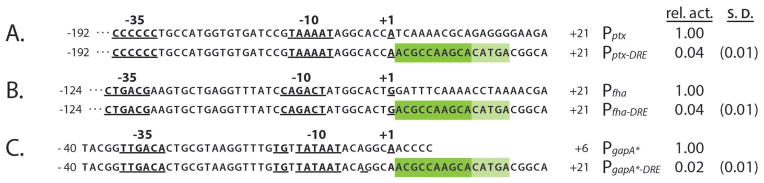
To this end, we tested the DRE repression effect on the BvgA-activated promoters Pfha and Pptx. We constructed promoters in which 20 bp of the DRE-containing region (+1 to +21 of Pfim3), was placed immediately downstream of the transcription starts of Pfha and Pptx. Compared to the wild type Pptx and PfhaB the resulting Pptx-DRE and PfhaB-DRE both showed dramatically reduced activity (Fig. 3). Effective DRE repression of other BvgA-activated promoters thus strongly suggests that the unusual fim-like promoter architecture apparently dictating atypical specific interactions between BvgA and RNAP is not required for DRE to function as a repressive element. In addition, since the distance from the DRE to the positions of BvgA binding sites varied in these constructs, we speculated that BvgA-promoter binding is not required for DRE function, but rather that RNAP, without the transcriptional activator, interacts with DRE to block transcription. To test this hypothesis we used a BvgA independent promoter that is a mutational variant of the gapA promoter described by (Thouvenot et al., 2004). This promoter variant, here termed PgapA*, has consensus −35 and −10 elements as well as the extended −10 −15TG−14 dinucleotide. As shown in Fig. 3, PgapA* was active in B. pertussis, but in constructs where DRE sequences were placed in the same context as previously, i.e. directly downstream of the initiating nucleotide, activity was greatly reduced. This observation of similar DRE repression effects observed on both BvgA-dependent and BvgA-independent promoters demonstrates that the DRE does not require interaction with BvgA for it to manifest its repressive effects.
Fig. 3. Universal promoter repression by DRE in B. pertussis.
Wild-type ptx (A), fha (B), and gapA* (C) promoters were compared to those in which sequences downstream of +1 were substituted with those of Pfim3, which contain DRE. All promoters were assessed, and data are presented, as described for Fig. 2.
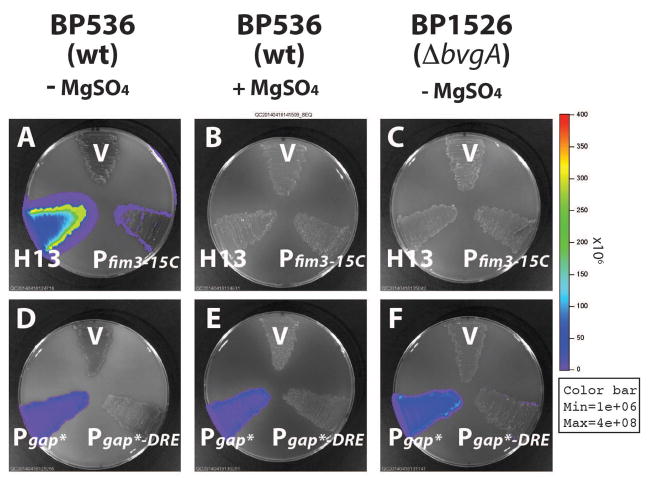
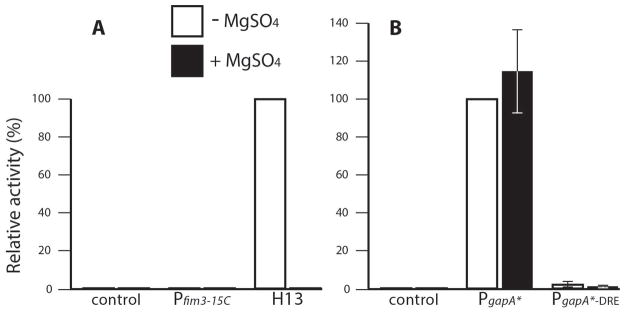
Although it was clear that specific BvgA-binding was not directly involved in DRE-mediated repression it was still possible that DRE repression was one of the many aspects globally regulated by BvgAS in B. pertussis. To address this we included the modulator MgSO4 in growth media. In B. pertussis grown in modulating media, BvgA is present in its unphosphorylated form (Boulanger et al., 2013). It therefore cannot activate BvgA-dependent promoters. We first tested the effect of modulation on the DRE effect on a BvgA-dependent promoter. As shown in Fig. 4A and Fig. 4B, the weak transcription of Pfim3-15C was totally abolished in the presence of MgSO4 in wild type B. pertussis strain BP536, thus demonstrating its BvgA-dependent behavior. Removal of DRE from Pfim3-15C, in hybrid promoter H13, did not affect the level of modulation, which remained complete, thus demonstrating that the DRE does not interfere with MgSO4 modulation, nor is modulation required. However, in this case, we could not discern if the repressive effects of DRE were subject to BvgAS regulation. To address this question we tested the effect of modulation on DRE’s ability to repress a BvgA-independent promoter. As shown Fig. 4D and Fig. 4E, PgapA* activity in wild type B. pertussis BP536, as expected, is constitutive and independent of BvgA regulation, whereas PgapA*-DRE remained repressed in the presence of MgSO4. In addition, we used a bvgA deletion B. pertussis strain BP1526 and observed the same patterns of activities of all the tested promoters as that in the modulated condition (Fig. 4C vs. Fig. 4B; Fig. 4F vs. Fig. 4E). The observed constitutive repression effect of DRE on PgapA* suggests that it is unlikely that DRE-activity is regulated as part of the BvgAS global virulence regulon.
Fig. 4. BvgAS-independent repression by DRE in B. pertussis.
The effects of DRE under different growth conditions and genetic backgrounds were determined by comparing Pfim3-15C with its DRE-free variant H13 (A, B and C), and PgapA* with its DRE-containing variant PgapA*-DRE (D, E and F). B. pertussis strains BP536 (wild type) were grown on BG agar supplemented with 50 mM MgSO4 (B and E) or with no MgSO4 added (A, and D). B. pertussis strains BP1526 (ΔbvgA) were grown on BG agar supplemented without 50 mM MgSO4 (C and F). Light output was measured as described in Experimental Procedures. Error bars indicate standard deviation as calculated from the results of four independent assays.
DRE function is conserved and specific to Bordetella species
It has been known that B. pertussis is different from its ancestor species B. bronchiseptica in many aspects, such as pertussis toxin production, utilizable growth conditions, and host range, due to the result of genomic changes (Parkhill et al., 2003; Mattoo & Cherry, 2005 and ref. therein). Consequently, we compared sequences downstream of the transcriptional start of Pfim3 among Bordetella species and found that the DREs are identical in sequence (data not shown). To check if the DRE is also functionally conserved, we tested the effect of DRE in B. bronchiseptica strain RB50 by using DRE-containing promoters integrated as promoter-lux fusions in the same way as in B. pertussis strain BP536. We found that both Pfim3-15C and PgapA*-DRE are similarly repressed in both B. pertussis and B. bronchiseptica (Fig. 5). Therefore, DRE is conserved not only in its sequence but also in its function within Bordetella species.
Fig. 5. Repression by DRE is observed in Bordetella species, but not in E. coli in vivo.
Plasmids containing Pfim3-15C, Pfim3-15C lacking DRE (H13), PgapA*, or PgapA* containing DRE (PgapA-DRE) were introduced in single copy in the chromosome of B. pertussis strain BP536 and B. bronchiseptica strain RB50, or as plasmid multi-copies in E. coli strain SM10. The resulting strains were grown at 37°C on BG plates for Bordetella strains, or on LB plates for the E. coli strain, and analyzed for the light output as described in Experiment Procedures. The activity of the DRE-containing promoter (solid box, Pfim3-15C, PgapA-DRE) is expressed as a percentage of that of the DRE-free promoter (open box, H13, PgapA) in each species. Error bars indicate standard deviation as calculated from the results of four independent assays.
However, DRE was not functional when tested in E. coli. When we introduced PgapA* and PgapA*-DRE into E. coli strain SM10 as plasmid-encoded luciferase-fusions, no repressive effect of the DRE was observed, with the difference being only ~1.9-fold. We did not use Pfim3-15C for this test since it is not transcribed in E. coli. Failure to repress PgapA* promoter activity in E. coli suggests that functioning of DRE involves a difference between the Bordetella and E. coli RNAPs, an unknown factor present only in Bordetella but not in E. coli, or both.
Discussion
The genetic organization of genes involved in fimbrial biosynthesis in B. pertussis is notable in two respects. First, while the genes coding for the chaperone, usher, and adhesin proteins are encoded within the same operon as the filamentous hemagglutinin (Willems et al., 1992; Locht et al., 1992), genes for the major fimbrial structural protein are at unlinked locations. Second, these genes, fim2, fim3, and fimX, are capable of undergoing independent phase-variation by changes in the length of a run of C residues in their promoters (Willems et al., 1990). While it has been known for sometime that fimbrial expression is ultimately regulated by the bvgAS locus, the demonstration that BvgA interacts directly with fim promoters is relatively recent (Chen et al., 2010; Decker et al., 2011). While the fimX promoter appears to be essentially silenced due to a too-short C-stretch, a comparison of the fim2 and fim3 promoters, each optimized in the length of their C-stretch for maximal expression, has revealed a large difference in promoter strength, with the fim2 promoter exhibiting approximately 6-fold higher activity in vivo.
This discrepancy is puzzling for two reasons. One is that the fim3 promoter is predicted, on the basis of its core promoter elements, to be the stronger promoter than fim2 promoter. Although each promoter has a nearly identical, very poor, −35 region (CCCCCC or CCCTCC, due to its location within the C-stretch), the fim3 promoter has the extended −10 motif consisting of a TG dinucleotide one basepair upstream of the −10 element. This element, combined with a strong −10, has been shown to compensate for the absence of a −35 element and to contribute to promoter strength when a −35 is present (reviewed in Hook-Barnard & Hinton, 2007). The −10 elements of the two promoters would both be expected to be moderately strong and roughly equal, since each contains the most highly conserved nucleotides in the pattern TA - - - T. Therefore, the fim3 promoter, with its extended −10 −15TG−14 dinucleotide, would be predicted to be a stronger promoter in vivo. However, the opposite was observed. A second puzzling discrepancy was revealed in in vitro transcription experiments. In vitro, the fim3 promoter shows strong BvgA~P-dependent transcriptional activity, although none has been detected from the fim2 promoter (Chen et al., 2010; Decker et al., 2011; Decker, unpublished).
To understand these discrepancies, we created a number of hybrid promoters, with key sequence segments exchanged between Pfim2-12C and Pfim3-15C, and measured their activities in vivo. Our results demonstrated that the 15 bp sequence just downstream of the transcription start site of Pfim3 (called here DRE), contains a repressive cis-element that significantly attenuates transcription in vivo. Furthermore, DRE activity is not BvgA-dependent, but it is dependent on some aspect specific to Bordetella since DRE inhibition was observed in both B. pertussis and B. bronchiseptica, but not in E. coli, in vivo. The apparent discrepancies cited in the previous paragraph can now be understood. Based only on core promoter elements and predicted BvgA binding strength, Pfim2 should be weaker than Pfim3, accounting for the lack of detectable activity in vitro. In vivo, where the DRE inhibits transcription only of Pfim3, the opposite is true.
In other in vitro work using purified E. coli RNAP or B. pertussis RNAP, we have shown that the presence of the DRE results in a novel inhibitory transcription complex at Pfim3 when non-phosphorylated BvgA is present, but not in the presence of BvgA~P (Boulanger et al., unpublished). These results might appear puzzling in light of the in vivo work here. However, the results of both studies are consistent with the idea that the DRE is an inhibitory sequence whose function is affected by as yet unknown conditions or factors in vivo. Furthermore, even though it is not yet clear how DRE inhibits transcription in vivo or forms an inhibitory complex in vitro, it is clear that the DRE has a dramatic affect on transcription, presumably at the step of initiation. Previously, work in E. coli has identified sequences just downstream of the +1 start that affect the promoter strength in vitro and in vivo (Kammerer et al., 1986), but the mechanism of this action has not been determined. In addition, extensive work has characterized sequences immediately downstream of the transcription start site of some lambdoid phage promoters that are needed for σ-dependent pausing and the loading of an antitermination factor, such as λ Q protein (reviewed in Perdue & Roberts, 2011). However, the DRE sequence does not appear to share significant similarities with the sequences described in either of these systems. Thus, an investigation of DRE function in vivo and in vitro is expected to elucidate both the complex regulation of fimbrial gene expression and a novel mechanism of regulation during promoter clearance.
Experimental procedures
Bacterial strains and culture conditions
Bacterial strains and plasmids used in this study are listed in Table 1. E. coli strains were grown in LB broth or on LB agar. B. pertussis and B. bronchiseptica strains were grown on BG agar (Chen et al., 2010). The antibiotics used in LB for E. coli strains were ampicillin, 100 μg ml−1; gentamicin, 5 μg ml−1. The antibiotics used in BG agar for B. pertussis and B. bronchiseptica strains were streptomycin, 50 μg ml−1; gentamicin, 10 μg ml−1.
Table 1.
Bacterial strains and plasmid used in this study
| Strain or plasmid | Relevant features | Source or reference |
|---|---|---|
| E. coli | ||
| DH5 | High-efficiency transformation | Bethesda Research Laboratories |
| SM10 | Mobilization of RK2 oriT plasmids | Simon et al. (1983) |
| B. pertussis | ||
| Tohama I | Patient isolate | Kasuga, et al. (1954) |
| BP536 | Tohama I, StrR, NalR | Stibitz and Yang (1991) |
| BP1526 | BP536, fha-lacZ, ptx-phoA, ΔbvgA-1215 | Chen, et al. (2010) |
| Plasmids | ||
| pSS3967 | luxCDABE promoter assay vector | Chen, et al. (2010) |
| pQC1023 | pSS3967:: Pfim2-10C | Chen, et al. (2010) |
| pQC1025 | pSS3967:: Pfim2-12C | Chen, et al. (2010) |
| pSS4159 | pSS3967:: Pfim3-13C | Chen, et al. (2010) |
| pQC1157 | pSS3967:: Pfim3-15C | Chen, et al. (2010) |
| pHn | pSS3967:: hybrids (Pfim2-12C/Pfim3-15C) | This study |
| pQC1440 | pSS3967:: Pfim3-15C-ds0 | This study |
| pQC1441 | pSS3967:: Pfim3-15C-ds5 | This study |
| pQC1442 | pSS3967:: Pfim3-15C-ds10 | This study |
| pQC1443 | pSS3967:: Pfim3-15C-ds15 | This study |
| pQC1444 | pSS3967:: Pfim3-15C-ds20 | This study |
| pQC1840 | pSS3967:: Pptx a | This study |
| pQC1841 | pSS3967:: Pptx-DRE | This study |
| pQC1843 | pSS3967:: Pfha b | This study |
| pQC1844 | pSS3967:: Pfha-DRE | This study |
| pQC1631 | pSS3967:: PgapA* | This study |
| pQC1761 | pSS3967:: PgapA*-DRE | This study |
Cloned ptx fragment contains nucleotides 3988042 to 3988253 of the B. pertussis Tohama I genome and extends, relative to the start of transcription, from −192 to +21.
Cloned fhaB fragment contains nucleotides 1968586 to 1968731 of the B. pertussis Tohama I genome and extends, relative to the start of transcription, from −124 to +21.
Plasmid and strain constructions
The promoters H1 - H40, which are hybrids of Pfim2-12C and Pfim3-15C were constructed using a variation of the method of Stemmer and Morris with the hybrid promoters cloned into the promoter assay vector pSS3967, between EcoRI and SalI sites upstream of luxCDABE (Stemmer & Morris, 1992; Chen et al., 2010). The generated hybrid sequences are depicted in Fig. 1B and Fig. 2ABDE. Plasmids pQC1440 to pQC1444 (Fig. 2C) are identical to pQC1157 (pSS3967::Pfim3-15C), except that the regions downstream were shortened by PCR amplification with different downstream primers. Plasmids pQC1840 (pSS3967::Pptx) and pQC1843 (pSS3967::PfhaB) contain PCR-generated EcoRI and SalI promoter fragments of ptx and fhaB, respectively, in pSS3967. Plasmid pQC1631 contains PgapA*, with the sequence tacggTTGACActgcgtaaggtttgTGtTATAATacaggcAacccc (−35 element, extended −10 motif, −10 element and +1 in uppercase), inserted as two complementary oligonucleotides in pSS3967. Subsequently, the +2 to +21 downstream regions of Pptx and PfhaB, and +2 to +6 downstream region of PgapA* were replaced by the +2 to +21 downstream sequence of Pfim3-15C (ACGCCAAGCACATGACGGCA), to generate plasmids pQC1841 (pSS3967::Pptx-DRE), pQC1844 (pSS3967::PfhaB-DRE), and pQC1761 (pSS3967::PgapA*-DRE). E. coli DH5α was used as a host for cloning.
To introduce the promoter-lux fusions into Bordetella, the pSS3967-based plasmids were integrated, respectively, in single copy at an ectopic site in the chromosome of B. pertussis strain BP536 or BP1526, and B. bronchiseptica strain RB50 using E. coli strain SM10 as a donor in conjugation, as previously described (Chen et al., 2010).
In vivo luciferase activity assay
Bacterial strains harboring promoter-lux fusions in pSS3967 were streaked in sectors and grown at 37°C for 48 h for B. pertussis strain BP536, for 24 h for B. bronchiseptica strain RB50 on BG agar (streptomycin 50 μg ml−1; gentamicin, 10 μg ml−1), or for 24 h for E. coli strain SM10 on LB plate (ampicillin 100 μg ml−1; gentamicin, 5 μg ml−1). To modulate BvgAS-mediated regulation in B. pertussis, 50 mM MgSO4 was included in the BG agar. The light output was revealed and analyzed as described previously (Chen et al., 2010). The data, averaged from at least 4 assays, were presented as a relative to the wild type promoter control strain or to the most luminescent strain on a given plate.
References
- Aricó B, Miller JF, Roy C, Stibitz S, Monack D, Falkow S, Gross R, Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989;86 doi: 10.1073/pnas.86.17.6671. 6671-6675-6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher PE, Maris AE, Yang MS, Stibitz S. The response regulator BvgA and RNA polymerase alpha subunit C-terminal domain bind simultaneously to different faces of the same segment of promoter DNA. Mol Cell. 2003;11:163–173. doi: 10.1016/s1097-2765(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Boucher PE, Yang MS, Schmidt DM, Stibitz S. Genetic and biochemical analyses of BvgA interaction with the secondary binding region of the fha promoter of Bordetella pertussis. J Bacteriol. 2001a;183:536–544. doi: 10.1128/JB.183.2.536-544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher PE, Yang MS, Stibitz S. Mutational analysis of the high-affinity BvgA binding site in the fha promoter of Bordetella pertussis. Mol Microbiol. 2001b;40:991–999. doi: 10.1046/j.1365-2958.2001.02442.x. [DOI] [PubMed] [Google Scholar]
- Boulanger A, Chen Q, Hinton DH, Stibitz S. In vivo phosphorylation dynamics of the Bordetella pertussis virulence-controlling response regulator BvgA. Mol Microbiol. 2013;88:156–72. doi: 10.1111/mmi.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Decker KB, Boucher PE, Hinton D, Stibitz S. Novel architectural features of Bordetella pertussis fimbrial subunit promoters and their activation by the global virulence regulator BvgA. Mol Microbiol. 2010;77:1326–1340. doi: 10.1111/j.1365-2958.2010.07293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PA, Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PA, Yuk MH, Mattoo S, Akerley BJ, Boschwitz J, Relman DA, Miller JF. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect Immun. 1998;66:5921–5929. doi: 10.1128/iai.66.12.5921-5929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker KB, Chen Q, Hsieh ML, Boucher P, Stibitz S, Hinton DM. Different requirements for sigma Region 4 in BvgA activation of the Bordetella pertussis promoters P(fim3) and P(fhaB) J Mol Biol. 2011;409:692–709. doi: 10.1016/j.jmb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrou J, Bompard C, Wintjens R, Dupre E, Willery E, Villeret V, Locht C, Antoine R, Jacob-Dubuisson F. Periplasmic domain of the sensor-kinase BvgS reveals a new paradigm for the Venus flytrap mechanism. Proc Natl Acad Sci U S A. 2010;107:17351–17355. doi: 10.1073/pnas.1006267107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook-Barnard IG, Hinton DM. Transcription Initiation by Mix and Match Elements: Flexibility for Polymerase Binding to Bacterial Promoters. Gene Regul Syst Bio. 2007;1:275–293. [PMC free article] [PubMed] [Google Scholar]
- Kammerer W, Deuschle U, Gentz R, Bujard H. Functional dissection of Escherichia coli promoters: information in the transcribed region is involved in late steps of the overall process. EMBO J. 1986;5:2995–3000. doi: 10.1002/j.1460-2075.1986.tb04597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga T, Nakase Y, Ukishima K, Takatsu K. Studies on Haemophilus pertussis. V. Relation between the phase of bacilli and the progress of the whooping-cough. Kitasato Arch Exp Med. 1954;27:57–62. [PubMed] [Google Scholar]
- Knapp S, Mekalanos JJ. Two trans-acting regulatory genes (vir and mod) control antigenic modulation in Bordetella pertussis. J Bacteriol. 1988;170:5059–5066. doi: 10.1128/jb.170.11.5059-5066.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey BW. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C, Geoffroy MC, Renauld G. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 1992;11:3175–3183. doi: 10.1002/j.1460-2075.1992.tb05394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel TJ, Boucher PE, Stibitz S, Grippe VK. Analysis of bvgR expression in Bordetella pertussis. J Bacteriol. 2003;185(6):902–6912. doi: 10.1128/JB.185.23.6902-6912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooi FR, Jansen WH, Brunings H, Gielen H, van der Heide HG, Walvoort HC, Guinee PA. Construction and analysis of Bordetella pertussis mutants defective in the production of fimbriae. Microb Pathog. 1992;12:127–135. doi: 10.1016/0882-4010(92)90115-5. [DOI] [PubMed] [Google Scholar]
- Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, Holden MT, Churcher CM, Bentley SD, Mungall KL, Cerdeno-Tarraga AM, Temple L, James K, Harris B, Quail MA, Achtman M, Atkin R, Baker S, Basham D, Bason N, Cherevach I, Chillingworth T, Collins M, Cronin A, Davis P, Doggett J, Feltwell T, Goble A, Hamlin N, Hauser H, Holroyd S, Jagels K, Leather S, Moule S, Norberczak H, O’Neil S, Ormond D, Price C, Rabbinowitsch E, Rutter S, Sanders M, Saunders D, Seeger K, Sharp S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Unwin L, Whitehead S, Barrell BG, Maskell DJ. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- Perdue SA, Roberts JW. Sigma(70)-dependent transcription pausing in Escherichia coli. J Mol Biol. 2011;412:782–792. doi: 10.1016/j.jmb.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Poolman JT, Hallander HO. Acellular pertussis vaccines and the role of pertactin and fimbriae. Expert Rev Vaccines. 2007;6:47–56. doi: 10.1586/14760584.6.1.47. [DOI] [PubMed] [Google Scholar]
- Relman DA, Domenighini M, Tuomanen E, Rappuoli R, Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989;86:2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlato V, Arico B, Prugnola A, Rappuoli R. Sequential activation and environmental regulation of virulence genes in Bordetella pertussis. EMBO J. 1991;10:3971–3975. doi: 10.1002/j.1460-2075.1991.tb04967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram-negative bacteria. Biotechnology. 1983;1:784–789. [Google Scholar]
- Stemmer WP, Morris SK. Enzymatic inverse PCR: a restriction site independent, single-fragment method for high-efficiency, site-directed mutagenesis. Biotechniques. 1992;13:214–220. [PubMed] [Google Scholar]
- Stibitz S, Garletts T. Derivation of a physical map of the chromosome of Bordetella pertussis Tohama I. J Bacteriol. 1992;174(7):770–7777. doi: 10.1128/jb.174.23.7770-7777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibitz S, Yang MS. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173:4288–4296. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouvenot B, Charpentier B, Branlant C. The strong efficiency of the Escherichia coli gapA P1 promoter depends on a complex combination of functional determinants. Biochem J. 2004;383:371–382. doi: 10.1042/BJ20040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AA, Hewlett EL, Myers GA, Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983;42(3):3–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss AA, Goodwin MS. Lethal infection by Bordetella pertussis mutants in the infant mouse model. Infect Immun. 1989;57:3757–3764. doi: 10.1128/iai.57.12.3757-3764.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems R, Paul A, van der Heide HG, ter Avest AR, Mooi FR. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J. 1990;9:2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems RJ, van der Heide HG, Mooi FR. Characterization of a Bordetella pertussis fimbrial gene cluster which is located directly downstream of the filamentous haemagglutinin gene. Mol Microbiol. 1992;6:2661–2671. doi: 10.1111/j.1365-2958.1992.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Williams CL, Boucher PE, Stibitz S, Cotter PA. BvgA functions as both an activator and a repressor to control Bvg phase expression of bipA in Bordetella pertussis. Mol Microbiol. 2005;56:175–188. doi: 10.1111/j.1365-2958.2004.04526.x. [DOI] [PubMed] [Google Scholar]