Abstract
Background
Intracranial volume (ICV) has been proposed as a measure of maximum lifetime brain size. Accurate ICV measures require neuroimaging which is not always feasible for epidemiologic investigations. We examined head circumference as a useful surrogate for intracranial volume in older adults.
Methods
99 older adults underwent Magnetic Resonance Imaging (MRI). ICV was measured by Statistical Parametric Mapping 8 (SPM8) software or Functional MRI of the Brain Software Library (FSL) extraction with manual editing, typically considered the gold standard. Head circumferences were determined using standardized tape measurement. We examined estimated correlation coefficients between head circumference and the two MRI-based ICV measurements.
Results
Head circumference and ICV by SPM8 were moderately correlated (overall r=0.73, men r=0.67, women r=0.63). Head circumference and ICV by FSL were also moderately correlated (overall r=0.69, men r=0.63, women r=0.49).
Conclusions
Head circumference measurement was strongly correlated with MRI-derived ICV. Our study presents a simple method to approximate ICV among older patients, which may prove useful as a surrogate for cognitive reserve in large scale epidemiologic studies of cognitive outcomes. This study also suggests the stability of head circumference correlation with ICV throughout the lifespan.
Keywords: Head circumference, intracranial volume, magnetic resonance imaging, biomarkers, older adults, elderly
Introduction
Brain size may passively protect against long-term effects of aging and disease, helping compensate for and preserve cognitive function (Mori et al., 1997; Reynolds et al., 1999). The exact mechanism for how this occurs remains unclear, but may be due to the complexity of neurons, synaptic connections, parenchymal density and co-morbidities (Henneberg, 1998). Intracranial volume (ICV) represents the peak, total volume of white and grey matter plus cerebrospinal fluid (CSF) (Pfefferbaum et al., 1994). Because of its association with maximum lifetime brain size, it has been proposed as a predictive measure of cognitive reserve (Katzman et al., 1988; Schofield et al., 1997). A number of studies have found that patients with larger brains or ICV by autopsy or imaging had preserved cognitive function (MacLullich et al., 2002; Schofield et al., 1995). Accurate ICV measures, however, currently require complex, time-consuming neuroimaging technology and analysis (Filippi et al., 2000). Thus, efficient correlates like head circumference have been proposed for ICV, as appropriate surrogate measures particularly for epidemiologic clinical studies in resource-scarce regions of the world.
There are limited previous studies on the relationship between head circumference and ICV (Jorgensen et al., 1961). Wolf et al. demonstrated that parenchymal volume, easily calculated from MRI-derived head circumference, is closely correlated with ICV even among patients with cognitive impairment (Wolf et al., 2004; Wolf et al., 2003). There are, however, many physiological factors besides brain size that can affect head circumference, including skull thickness, temporal wasting and estrogen deficiency (Gurwitz et al., 2002; Wolf et al., 2004). These factors gain increasing prominence as people age. While sophisticated neuroimaging means of determining ICV exist, head circumference remains a quick, efficient strategy that is more readily available. In two population-based cross sectional studies, head circumference was inversely related to the prevalence of dementia in low and middle-income regions internationally although this may have been due to epidemiologic or statistical limitations such as reverse causality and selective mortality (Jotheeswaran et al., 2011; Prince et al., 2011). Our study is the first to assess the correlation of simple head circumference measurement with estimated ICV by MRI among older adults. We examine whether this surrogate measure may be useful as a research tool for large-scale epidemiologic studies among the geriatric population.
Methods
Study Design
Our study population is a subsample of the Successful Aging after Elective Surgery (SAGES) study, a five-year prospective, observational study of postoperative delirium at Beth Israel Deaconess Medical Center (BIDMC), Brigham and Women’s Hospital (BWH), and Hebrew SeniorLife in Boston, Massachusetts. Study design and eligibility criteria have been reported previously (Schmitt et al., 2012). Inclusion criteria included: age ≥70 years old, English-speaking, undergoing major elective surgery at BIDMC or BWH. Exclusion criteria included diagnosis of dementia by medical record screening or patient report; cognitive impairment defined by score ≤69 or education-adjusted equivalent on the Modified Mini-Mental State (3MS) examination (Teng and Chui, 1987); terminal disease; total blindness; severe deafness; MRI contraindication; present neurological illness, alcohol or illicit drug abuse. All study procedures were approved by the institutional review boards at BIDMC, BWH and Hebrew SeniorLife. All subjects gave written informed consent.
Head Circumference
All SAGES participants underwent head circumference measurements using standardized approaches by trained research staff, blinded to MRI results. The research staff asked participants to remove headwear and placed the inflexible paper tape measure snugly around the participant’s head. The measurement extended from just above the eyebrows outwards to the most posterior occipital protuberance. Two measurements were taken, and the largest reading recorded in centimeters.
MRI Acquisition
Approximately one-third of the enrolled SAGES participants elected to undergo MRI imaging prior to surgery. 99 pre-surgical MRI scans were analyzed for the present study as a convenience sample. All subjects were imaged at the BIDMC Radiology Department on a 3-Tesla HDxt MRI scanner (General Electric Medical Systems, Milwaukee, WI) using a standard 8-channel head coil. MRI imaging was acquired 1–4 months before head circumference measurement.
The MRI protocol included the following image acquisitions used for ICV evaluation:
High Resolution three-dimensional (3D) anatomic imaging – Magnetization prepared fast gradient echo (MPRAGE) 3D T1-weighted sequence with TR of 7.9ms, TE of 3.2ms, 15 degree flip angle and 32kHz-bandwidth, a coronal acquisition plane with 24x19 field of view, 0.94mm in-plane resolution, 1.4mm slices, preparation time of 1100ms with repeated saturation at the beginning of the SAT period, and an adiabatic inversion pulse 500ms before imaging.
High Resolution 3D T2-weighted imaging – A 3D fast spin echo sequence (Cube™) was acquired in the sagittal plane with TR 3s, effective TE 63.6ms, echo train length 100, bandwidth 62.5, 1mm in-plane resolution and 128 1.6mm slices.
Fluid Attenuated Inversion Recovery (FLAIR) – An axial acquisition with TR/TE of 9000/156 and T1 of 2250 was employed. Seventy 2mm slices were acquired with no interslice gap and interleaved acquisition. Nominal in-plane resolution was 1mm.
MRI Analysis
MRI-derived ICV was determined using two different methods. The first method involved Statistical Parametric Mapping (SPM8) software (Wellcome Institute). The New Segmentation Method provided by this software, an extension of unified segmentation method, allows for automated whole brain segmentation (Ashburner and Friston, 2005) of the T1-weighted images; then grey and white matter and CSF components were totaled to yield brain volume measurement.
ICV was also determined using Functional Magnetic Resonance Imaging of the Brain Software Library (FSL) software. First, bias-field correction to account for field inhomogeneities was performed using 3DSlicer software (www.slicer.org) (Fedorov et al., 2012). ICV masks were then segmented from T2-weighted images using Brain Extraction Tool (BET) of FSL (Smith, 2002; Smith et al., 2004; Smith et al., 2002) and subsequently reviewed and manually corrected by a neuroimaging expert (TH) using axial T2-weighted images as reference. This method for determining ICV is generally considered the gold standard by experts in neuroimaging (Smith et al., 2004; Smith et al., 2002). A second neuroimaging expert reviewed and manually corrected ten ICV masks independently to assess inter-rater reliability.
The neuroimaging experts who performed the processing and analysis of the images were blinded to the head circumference measurement and clinical data.
Statistical Analysis
Data analysis for this study was performed on the first 99 patients enrolled in the SAGES study and participating in the MRI substudy. We measured associations between head circumference and MRI indices and between the MRI indices, using Pearson correlation coefficients. Discrepant cases were determined by fitting a linear regression to head circumference and ICV, allowing for a main effect and interaction with gender. The threshold for significance was p <0.05 in all analyses.
Results
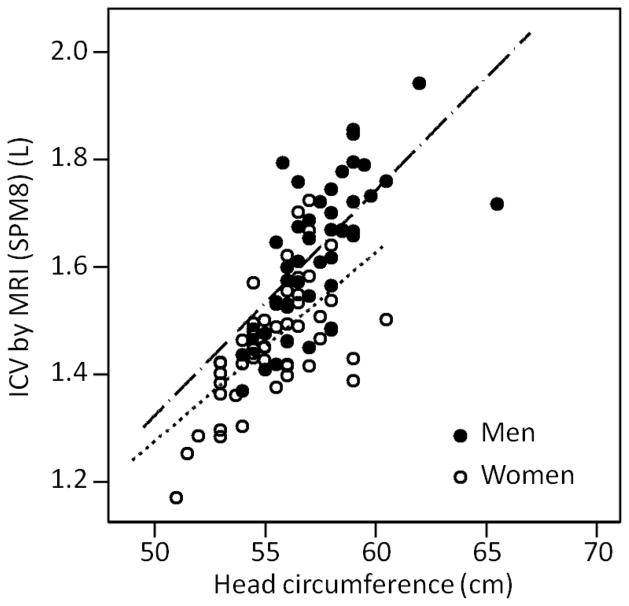
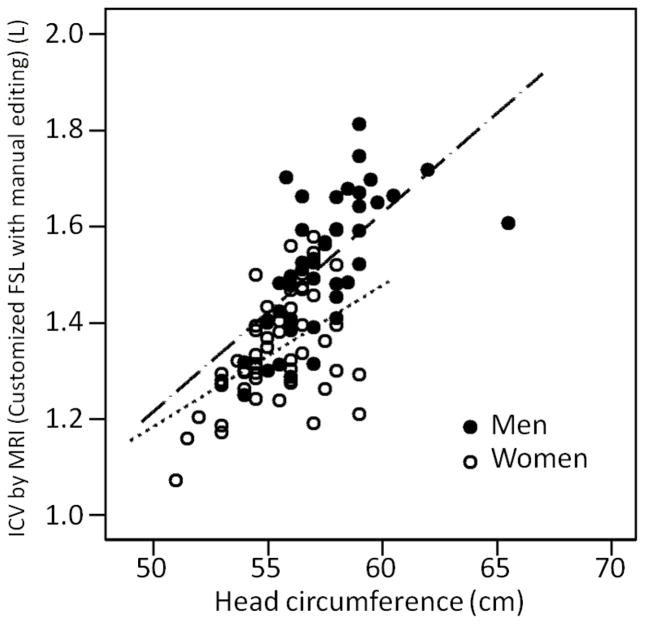
Baseline characteristics of the 99 subjects are summarized in Table 1. The mean time elapsed between MRI acquisition and head circumference measurement was 50 days +/− 17 (range 29–127 days). The correlation between head circumference and MRI ICV, and between the two MRI ICV measures, is summarized in Table 2. There was high positive correlation between head circumference and SPM8 software-calculated ICV overall (r=0.73, p<0.01) (Figure 1). There was also moderately high correlation between head circumference and SPM8-calculated ICV for women (r=0.67, p<0.01) and men (r=0.63, p<0.01) (Figure 1). There was high positive correlation between head circumference and ICV derived from FSL software plus manual editing (r=0.69, p<0.01) (Figure 2). The correlation was higher among men (r=0.63, p<0.01) but moderate among women (r=0.49, p<0.01) (Figure 2). The two measures of MRI ICV had very high correlation overall (r=0.96, p<0.01), among women (r=0.91, p <0.01) and men (r=0.96, p<0.01) (Table 2). Mean residual (by sex) was evaluated for all 99 study participants; there was an overall overestimation of ICV for women and underestimation of ICV for men by head circumference.
Table 1.
Baseline characteristics of study participants *
| Characteristic | Women (n = 55) | Men (n = 44) |
|---|---|---|
| Age: At baseline assessment, mean years (SD) | 75.9 (4.2) | 76.0 (4.4) |
| Non-white or Hispanic race, n (%) | 15 (27) | 2 (3.6) |
| African-American or Black, n (%) | 9 (16) | 0 (0.0) |
| Education, mean years (SD) | 14.5 (2.5) | 15.3 (2.7) |
| SF-12 physical composite, T-score, mean (SD) | 31.6 (10.1) | 39.6 (9.8) |
| Height, cm, mean (SD) | 160.1 (7.5) | 175.5 (7.8) |
| Head circumference, cm, mean (SD) | 55.4 (2.0) | 57.4 (2.2) |
SD = standard deviation; SF-12 = Short Function-12; cm = centimeters
Table 2.
The relationship between head circumference and ICV by MRI *
| Analysis Method | Women (n=55) | Men (n=44) | Overall (n=99) |
|---|---|---|---|
| Pearson Correlation Coefficient (r) | |||
|
| |||
| SPM8 software | 0.67 | 0.63 | 0.73 |
| FSL software plus manual editing | 0.49 | 0.63 | 0.69 |
| ICV from FSL vs. ICV from SPM8 | 0.91 | 0.96 | 0.96 |
p < 0.01 in all correlations
ICV = intracranial volume; MRI = Magnetic Resonance Imaging; SPM8 = Statistical Parametric Mapping 8; FSL = Functional Magnetic Resonance Imaging of the Brain Software Library
Figure 1. The relationship between head circumference and ICV by MRI (SPM8).
The correlation between head circumference and MRI derived ICV (SPM8 analysis) for men was r = 0.67 (n = 44), for women r = 0.63 (n = 55) and overall r = 0.73 (n = 99) with p < 0.01 for all relationships. SPM8 = Statistical Parametric Mapping 8; ICV = intracranial volume; MRI = magnetic resonance imaging; L = liters; cm = centimeters. Linear functions are: ICV(SPM8) = −0.85 + 0.57×female + 0.0413×HC + −0.012×female×HC.
Figure 2. The relationship between head circumference and ICV by MRI (customized FSL with manual editing).
The correlation between head circumference and MRI derived ICV (FSL with manual editing) for men was r = 0.63 (n = 44), for women r = 0.49 (n = 55) and overall r = 0.69 (n = 99) with p < 0.01 for all relationships. FSL = Functional Magnetic Resonance Imaging of the Brain Software Library; ICV = intracranial volume; MRI = magnetic resonance imaging; L = liters; cm = centimeters. Linear functions are: ICV(FSL) = −0.78 + 0.37×female + 0.0420×HC + −0.0082×female×HC.
A very relevant concern is the potential for bias in estimating associations between exposures or outcomes and head circumference measurements as variously defined. To evaluate this possibility, we regressed standardized intracranial volume assessed with SPM8, FSL and approximated with head circumference on height in 101 bootstrap samples and examined the variability in the regression coefficients. The mean standardized coefficient for SMP8 ICV and height was (standard deviation, SD = 0.085); FSL was similar ( ) and head circumference consistently lower (in 91% of 101 replications ). Using the mean for SPM8 as the standard, the root mean squared error for SPM8 was 0.085, FSL was 0.089, and was 0.133 for head circumference. Therefore, there may be some attenuation of effects and increased variability that would accompany the use of head circumference relative to MRI measured intracranial volume. There are sample size and statistical power concerns as well. For example, the minimum sample size needed to detect a correlation of 0.55 is n = 22, compared to n = 33 to detect a correlation of 0.46.
Discussion
This study of community-dwelling older adults scheduled for elective surgery demonstrates a moderately strong correlation between head circumference and ICV measured by MRI in older adults. The correlation was present regardless of which type of MRI analysis. SPM8 for ICV segmentation uses T1-weighted scans in which both CSF and cortical bone appear darkened, making it less accurate and more prone to inadvertently including extra dural tissue in the ICV calculation. Thus, the SPM8-calculated ICV likely correlates better with head circumference but is less accurate because it is more influenced by overall head size. FSL with manual editing is considered the gold standard for ICV segmentation because of the expert review and high T2-signal contrast between CSF and meninges, allowing for more accurate segmentation. This does, however, make FSL with manual editing a more time consuming and less feasible approach for some studies.
The relatively weaker correlation between women’s head circumference and ICV by both MRI analysis methods could be expected, given the frequent presence of more hair in older women compared to men, women’s hairstyles and male-pattern hair loss. Previous literature found correlations between head circumference and ICV to be r=0.5–0.8 (Brandt, 1985; Graves et al., 1996), similar to our results. Most prior studies involved younger individuals without many co-morbidities. There was also a statistically significant overestimation of female ICV by head circumference and underestimation of male ICV in our study population.
To our knowledge, this is the first study to examine the correlation between head circumference and ICV among older patients. We demonstrated that even in this older population, head circumference is a quick and accurate approximation of ICV. This study suggests the stability of head circumference correlation with ICV throughout the lifespan. Several important strengths of this study include the large sample size (one of the largest to date addressing this topic), clinically rich data collection and two methods of ICV neuroimaging analysis lending robustness to our findings.
Limitations of our study include the fact that only the larger measurement of head circumference measurement was recorded, which may have decreased precision and precluded calculating a coefficient of variation. One previous study used multi-dimension measurements – height, width and length – of the human skull instead of head circumference to approximate intracranial volume (Sahin et al., 2007). They also found good correlations with actual intracranial volumes possibly because their dimensions are less susceptible to hair thickness and may characterize the shape of the entire skull better. There may also be potential limitations to generalizability. Our study is a subsample selected from surgical schedules of two large academic hospitals; study participants were generally highly functional, well-educated and not racially diverse. Thus, our findings will need future validation in larger and more heterogeneous populations.
This study helps bolster the research utility of head circumference as a simple, quick prognostic surrogate for ICV even among older persons. We do show some evidence that MRI measures of ICV are more accurate than measures derived from simple head circumference, and the use of the simple measures comes at a cost of increased variability and lower statistical power. Our study lays the groundwork for more sophisticated predictive models correlating head circumference and ICV. Head circumference can serve an important role for community-based epidemiologic studies, where MRI studies are not feasible, as an estimate of ICV and brain volume. This measurement may have particular usefulness in large-scale studies of dementia and cognitive function (Mortimer, 2012) internationally. This study addresses some of the selective mortality and reverse causality concerns raised by other researchers regarding head circumference correlations with dementia (Jotheeswaran et al., 2011; Prince et al., 2011). Finally, the correlation between head circumference and cognitive reserve represents a rich area for exploration in future studies (Mortimer et al., 2003).
Acknowledgments
The authors thank the patients, family members, nurses, physicians, and dedicated staff who participated in the SAGES study.
This work was supported by the following sources of funding - Grants No. P01AG031720 (SKI), K07AG041835 (SKI) and K24AG035075 (ERM) from the National Institute on Aging. Dr. Hshieh is supported by an NIH funded T32 Training Grant (AG000158). Dr. Inouye holds the Milton and Shirley F. Levy Family Chair. The funding sources had no role in the study design, collection/analysis/ interpretation of data, writing of the report, or decision to submit for publication.
Footnotes
Conflict of Interest: None
All co-authors had no potential financial or personal conflicts of interest.
Author Contributions: We affirm that all the co-authors listed contributed significantly to the preparation of this manuscript and approve this final version to be published.
Dr. Hshieh substantially contributed to the analysis and interpretation of data, drafting the article and revising it critically for important intellectual content. Ms. Fox and Mr. Kosar contributed to the acquisition of data, analysis and interpretation of data and revising the article critically. Drs. Cavallari and Guttmann contributed to the acquisition of data, analysis and interpretation of data and revising the article critically. Dr. Alsop contributed to the conception and design, analysis and interpretation of data and revising the article critically. Dr. Schmitt contributed to the acquisition of data, analysis and interpretation of data and revising the article critically. Dr. Jones contributed to the acquisition of data, analysis and interpretation of data and revising the article critically. Dr. Marcantonio contributed to the conception and design, acquisition of data, analysis and interpretation of data and revising the article critically. Dr. Inouye contributed to the conception and design, acquisition of data, analysis and interpretation of data, drafting the article and revising it critically as well as obtaining funding and administrative support.
References
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Brandt I. Growth dynamics of low-birth-weight infants. Acta Paediatr Scand Suppl. 1985;319:38–47. doi: 10.1111/j.1651-2227.1985.tb10107.x. [DOI] [PubMed] [Google Scholar]
- Fedorov A, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–1341. doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippi M, Rovaris M, Iannucci G, Mennea S, Sormani MP, Comi G. Whole brain volume changes in patients with progressive MS treated with cladribine. Neurology. 2000;55:1714–1718. doi: 10.1212/wnl.55.11.1714. [DOI] [PubMed] [Google Scholar]
- Graves AB, Mortimer JA, Larson EB, Wenzlow A, Bowen JD, McCormick WC. Head circumference as a measure of cognitive reserve. Association with severity of impairment in Alzheimer’s disease. Br J Psychiatry. 1996;169:86–92. doi: 10.1192/bjp.169.1.86. [DOI] [PubMed] [Google Scholar]
- Gurwitz D, Chapman J, Weizman A. Head circumference and incident Alzheimer’s disease: modification by apolipoprotein E. Neurology. 2002;58:1440. doi: 10.1212/wnl.58.9.1440. [DOI] [PubMed] [Google Scholar]
- Henneberg M. Evolution of the human brain: is bigger better? Clin Exp Pharmacol Physiol. 1998;25:745–749. doi: 10.1111/j.1440-1681.1998.tb02289.x. [DOI] [PubMed] [Google Scholar]
- Jorgensen JB, Paridon E, Quaade F. The correlation between external cranial volume and brain volume. Am J Phys Anthropol. 1961;19:317–320. doi: 10.1002/ajpa.1330190402. [DOI] [PubMed] [Google Scholar]
- Jotheeswaran AT, Williams JD, Stewart R, Prince MJ. Could reverse causality or selective mortality explain associations between leg length, skull circumference and dementia? A South Indian cohort study. Int Psychogeriatr. 2011;23:328–330. doi: 10.1017/S1041610210001171. [DOI] [PubMed] [Google Scholar]
- Katzman R, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- MacLullich AM, Ferguson KJ, Deary IJ, Seckl JR, Starr JM, Wardlaw JM. Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology. 2002;59:169–174. doi: 10.1212/wnl.59.2.169. [DOI] [PubMed] [Google Scholar]
- Mori E, et al. Premorbid brain size as a determinant of reserve capacity against intellectual decline in Alzheimer’s disease. Am J Psychiatry. 1997;154:18–24. doi: 10.1176/ajp.154.1.18. [DOI] [PubMed] [Google Scholar]
- Mortimer JA. The Nun Study: risk factors for pathology and clinical-pathologic correlations. Curr Alzheimer Res. 2012;9:621–627. doi: 10.2174/156720512801322546. [DOI] [PubMed] [Google Scholar]
- Mortimer JA, Snowdon DA, Markesbery WR. Head circumference, education and risk of dementia: findings from the Nun Study. J Clin Exp Neuropsychol. 2003;25:671–679. doi: 10.1076/jcen.25.5.671.14584. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Prince M, et al. Leg length, skull circumference, and the prevalence of dementia in low and middle income countries: a 10/66 population-based cross sectional survey. Int Psychogeriatr. 2011;23:202–213. doi: 10.1017/S1041610210001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds MD, Johnston JM, Dodge HH, DeKosky ST, Ganguli M. Small head size is related to low Mini-Mental State Examination scores in a community sample of nondemented older adults. Neurology. 1999;53:228–229. doi: 10.1212/wnl.53.1.228. [DOI] [PubMed] [Google Scholar]
- Sahin B, et al. Comparison of four methods for the estimation of intracranial volume: a gold standard study. Clin Anat. 2007;20:766–773. doi: 10.1002/ca.20520. [DOI] [PubMed] [Google Scholar]
- Schmitt EM, et al. Novel risk markers and long-term outcomes of delirium: the successful aging after elective surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13:818 e811–810. doi: 10.1016/j.jamda.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PW, Logroscino G, Andrews HF, Albert S, Stern Y. An association between head circumference and Alzheimer’s disease in a population-based study of aging and dementia. Neurology. 1997;49:30–37. doi: 10.1212/wnl.49.1.30. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Mosesson RE, Stern Y, Mayeux R. The age at onset of Alzheimer’s disease and an intracranial area measurement. A relationship. Arch Neurol. 1995;52:95–98. doi: 10.1001/archneur.1995.00540250103019. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith SM, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–489. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- Wolf H, et al. Structural correlates of mild cognitive impairment. Neurobiol Aging. 2004;25:913–924. doi: 10.1016/j.neurobiolaging.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Wolf H, Kruggel F, Hensel A, Wahlund LO, Arendt T, Gertz HJ. The relationship between head size and intracranial volume in elderly subjects. Brain Res. 2003;973:74–80. doi: 10.1016/s0006-8993(03)02552-6. [DOI] [PubMed] [Google Scholar]