Abstract
The amino acid-Target of Rapamycin (AA/TOR) and insulin pathways play a pivotal role in reproduction of female insects, serving as regulatory checkpoints that guarantee the sufficiency of nutrients for developing eggs. Being evolutionary older, the AA/TOR pathway functions as an initial nutritional sensor that not only activates nutritional responses in a tissue-specific manner, but is also involved in the control of insect insulin-like peptides (ILPs) secretion. Insulin and AA/TOR pathways also assert their nutritionally linked influence on reproductive events by contributing to the control of biosynthesis and secretion of juvenile hormone and ecdysone. This review covers the present status of our understanding of the contributions of AA/TOR and insulin pathways in insect reproduction.
Introduction
Development of chorionated eggs with a large quantity of nutrient reserves represents one of the evolutionary advances of insects that is responsible for their extraordinary success as terrestrial animals. Hence, reproductive events of female insects require a massive input of nutritionally-and energy-rich resources. The regulatory checkpoints exemplified by the amino acid-Target of Rapamycin (AA/TOR) and insulin pathways ensure the proper influx of nutrients for developing eggs. These pathways are obligatory for reproduction of all insects. Moreover, the AA/TOR as an evolutionary older pathway serves as a primary nutritional sensor activating secretion of insect insulin-like peptides (ILPs) and also triggering nutritional responses in the tissue-specific manner. The insulin/TOR pathway is involved in controlling biosynthesis of juvenile hormone (JH) and ecdysone (E), which in turn initiate yolk protein production (vitellogenesis) and egg maturation. The relative contribution of above mentioned signaling pathways differs depending on insects with various life strategies. The AA/TOR and insulin pathways play dual roles as mediators of nutritional status, one by directly affecting reproductive tissues, and another by controlling biosynthesis and secretion of juvenile hormone and ecdysteroids (Figure 1).
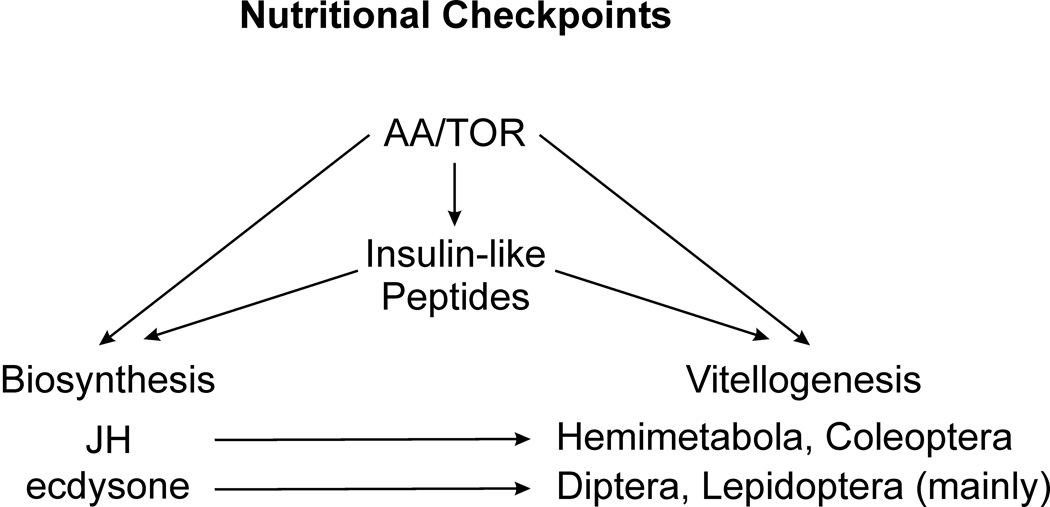
Figure 1.
Insect nutritional checkpoints. The serine/threonine kinase TOR pathway represents primary nutritional checkpoint via sensing amino acids (AAs). In Drosophila, AA/TOR regulates synthesis and secretion of Insulin-like Peptides (ILPs) and together with ILPs participates on biosynthesis of lipophilic hormones Juvenile hormone (JH) and ecdysone. AA/TOR, ILPs, JH and ecdysone regulate production of yolk proteins and their uptake into the oocytes, marked here as Vitellogenesis. Contributions of particular players differ substantially among insect orders. Vitellogenesis is controlled mainly by JH in Hemimetabola and Coleoptera and by ecdysone (20-Hydroxyecdysone) in Diptera and Lepidoptera orders.
The role of insulin and TOR in nutritional regulation of insect growth and development, particularly that of Drosophila melanogaster, has been studied in great detail [1, 2, 3]. These studies have laid a foundation for our understanding of the mechanisms underlying the nutritional control. In the last several years, progress has also been made in elucidating the mechanisms of the nutritional control of insect reproduction. These advances have provided important insights into the role of insulin and AA/TOR as nutritional sensors in multiple reproductive events.
The Amino Acid/TOR pathway as a nutritional sensor
The serine/threonine kinase TOR being the center of nutritional signaling is linked to nutritional sensing through AAs [4–7]. The presence of the AA/TOR pathway in unicellular Eukaryotes, such as yeast, indicates its early evolutionary origin [5–9]. Research suggests that in addition to glucose, AA/TOR controls synthesis and secretion of ILPs [10**, 11]. In adult Drosophila, ILP secretion is at least in part under the remote control of cytokine unpaired 2 produced by the fat body in response to nutritional signals [11, 12*]. Thus, it appears that AAs signaling through TOR represents a first order of nutritional signaling, particularly for insects requiring a protein meal for the initiation of egg production (Figure 2). TOR signaling is partitioned into two different pathways, TORC1 and TORC2, with only TORC1 being nutritionally sensitive [4–6]. AAs connect to TORC1 (hereafter TOR) through transmembrane AA transporters and the intracellular pathway that includes Ras-related small GTP-binding protein GTPases or Rags [7, 8]. Two types of Rags, Rag A/B and Rag C/D are involved in mediating AA signaling [8]. Ras-homolog enriched in brain GTPase (Rheb) is also an integral part of the pathway that activates TOR in response to AAs [7, 9]. In conjunction with phospholipase D1 and upon its loading with GTP, Rheb promotes TOR phosphorylation and stimulation [9].
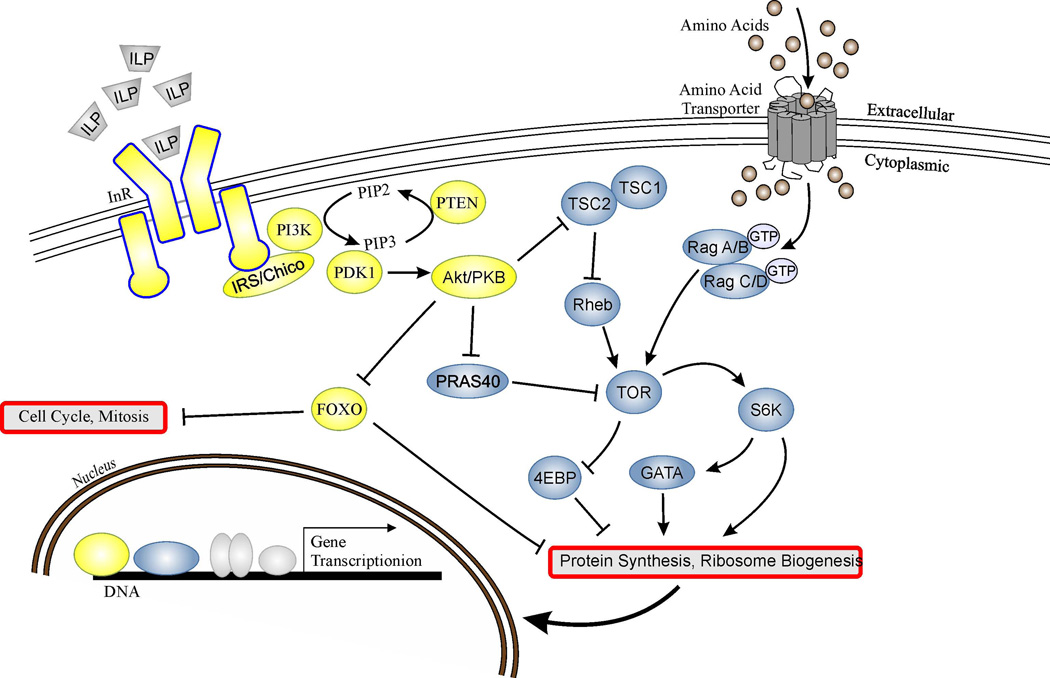
Figure 2.
Simplified outline of Insulin and AA/TOR signaling pathways. ILPs bind membrane Insulin receptor and activate Insulin pathway by series of protein phosphorylations to activate Akt/PKB. Akt phosphorylates FOXO, sequestrates it from the nucleus and allows cell cycle progression. Akt also activates TOR signaling pathway by inhibition of its repressors TSC 1/2 and PRAS40. Nutritional input in a form of AAs regulates TOR pathway through small Rag GTPases. Activated TOR kinase phosphorylates effector proteins such as 4EBP and S6K and thus stimulates protein synthesis, ribosome biogenesis and cell growth. Insulin factors are shown in yellow and TOR-pathway components in blue.
Despite of its importance, the relative contribution of the AA/TOR pathway compared to that of the insulin one in nutritional sensing has not been investigated in detail in insect reproduction. In many insects, intake of proteins serves as a key trigger for the initiation of egg development. It is particularly pronounced in blood-feeding species. In mosquitoes, in which only females feed on blood, egg development is arrested until a female takes a blood meal. Understanding of this phenomenon came from a realization that the AA signaling via TOR is responsible for de-repression of the egg developmental arrest [13**, 14]. AA transporters of the solute carrier 7 family are involved in the AA sensing mechanism, as shown by the resulting decrease in TOR signaling and fertility caused by RNA interference silencing of any family member in female mosquitoes [15*, 16–17]. Upon blood intake by Aedes aegypti female mosquitoes, the influx of signaling AAs, such as leucine, leads to activation of TOR, phosphorylating the translational activator S6K and the translational repressor 4E-BP [18]. Rheb silencing in A. aegypti females downregulates S6K phosphorylation and subsequently vitellogenin (Vg) gene expression [19*], while 4E-BP phosphorylation inhibits its translational repression function and allows protein synthesis and progression of vitellogenesis [18, 19*]. In the red flour beetle Tribolium castaneum, RNAi-mediated silencing of most members of the insulin and TOR signaling pathways either decreases expression of Vg2 or severely affects egg production. However, knockdown of Rheb lowers Vg2 mRNA levels by only 10–30%, suggesting that the insulin rather than the AA branch of the TOR pathway is essential for signaling in this insect [20].
Nutrient-sensitive TOR-mediated activation of S6K leads to translation, resulting in cell growth and differentiation and triggering various aspects of egg development in reproducing female insects. The TOR and S6K are involved in the regulation of the Vg gene expression by providing transcriptional and translational machineries required for this central reproductive event [14, 15*]. Park et al. [21**] have revealed that the TOR signaling pathway regulates the translation of a GATA transcription factor, which is an activator of the Vg gene, in a Rapamycin- and AA-dependent manner in A. aegypti. Upon blood ingestion by the female mosquito, massive translation of Aa-GATA occurs in the fat body and AaGATA binds to the Vg gene promoter, activating its transcription (Figure 3).
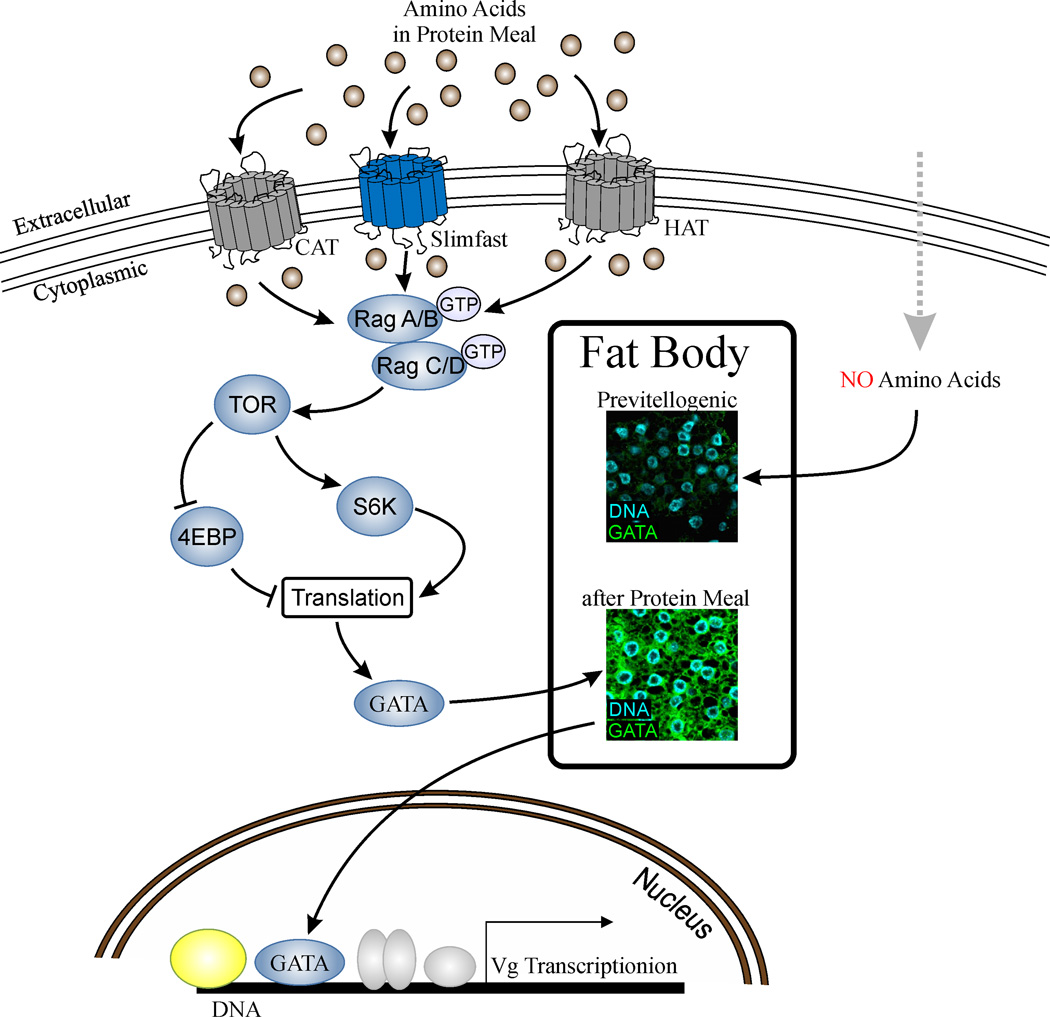
Figure 3.
Nutrient-sensitive activation of translation is a critical step for female reproduction. Amino acids released upon digestion of a protein-rich meal exert TOR-mediated activation of S6K [14, 15*] and lead to a massive translation of GATA transcription factor in the female FB [21**]. GATA protein binds to the Vg promoter and activates its transcription. The absence of signaling AAs in previtellogenic females or upon protein-poor meal is not sufficient to trigger GATA translation and consequentially Vg gene transcription. CAT = cationic amino acid transporter, HAT = heteromeric amino acid transporter. Inserts: the mosquito fat body before (upper panel) and after a blood meal (low panel). Immunocytochemistry with anti-GATA antibody and Dapi staining.
TOR plays an important role in regulating developmental or starvation-induced autophagy that are important for either programmed cell remodeling or tissue catabolism, respectively [22–24]. TOR interacts with the initiator of autophagy, ATG1, inhibiting its action in the presence of sufficient nutrients. However, the situation is reversed in the case of starvation, during which ATG1 suppresses TOR and initiates autophagy. Interestingly, at the end of the female A. aegypti reproductive cycle fat body undergoes programmed autophagy. TOR represses autophagy during the vitellogenic phase, preventing its premature triggering. Activation of programmed autophagy leading to the remodeling of the fat body is required for a normal switch to the second reproductive cycle (Figure 4) [25*]. Further studies should demonstrate whether a similar programmed autophagy occurs in other insects with cyclical reproduction.
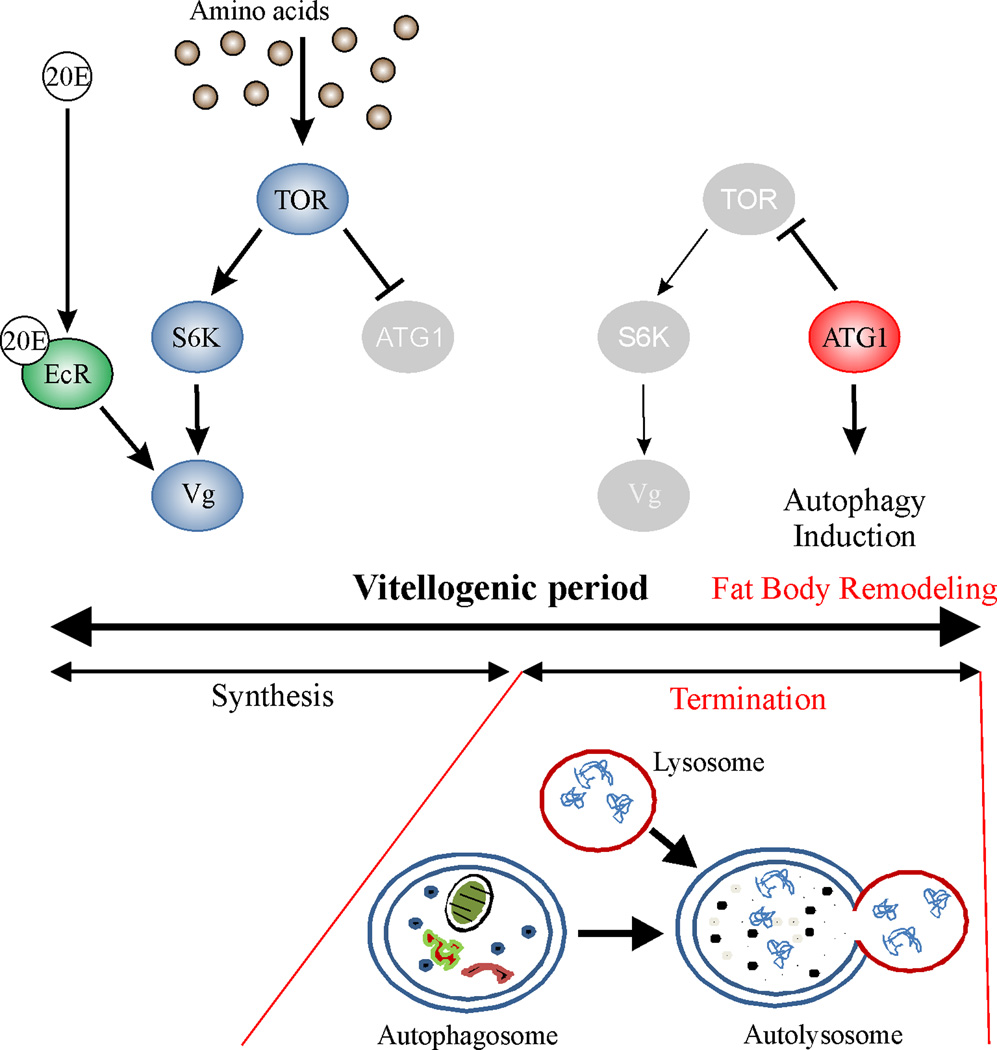
Figure 4.
The role of programmed autophagy in vitellogenesis. Vg synthesis is stimulated by AA/TOR and ecdysone pathways in the female mosquito A. aegypti fat body (FB). When active, TOR blocks FB autophagy by repressing the initiator of autophagy, Autophagy related 1 (ATG1) [25*]. Reduced TOR signaling is no more inhibiting ATG1 during the terminal phase of the vitellogenesis; instead, ATG1 is suppressing TOR and initiates autophagy. Fat body remodeling during terminal phase of vitellogenesis is a necessary step for the second gonadotropic cycle to occur. During the fat body remodeling, FB cell cytoplasm is being enclosed into autophagosomes, which fuse with lysosomes to become autolysosomes that digest their content into basic nutrients. Thus, autophagy allows lowering energetic costs until the next gonadotrophic cycle. 20E = 20-Hydroxyecdysone
A recent study has shown a possible crosstalk between wingless (Wnt) and TOR signaling pathways in A. aegypti vitellogenesis [26]. Depletion of Frizzled 2, the transmembrane Wnt receptor that is predominantly expressed in the mosquito fat body after a blood meal, causes a significant reduction in S6K phosphorylation and subsequently in Vg expression [27].
Insulin-like peptides (ILPs) as nutritional sensors
TOR is linked to nutritional sensing through not only AAs but also the insulin pathway, which is conserved in Protostome and Deutorostome animals [27, 28]. Insects possess multiple insulin-like peptides (ILPs), the number of which varies among different species [3, 27–33]. Although, some ILPs are functionally analogous to vertebrate insulin, the complete repertoire of their actions remains to be elucidated, particularly during reproduction. Drosophila ILPs regulate germline stem cell division, and germline cyst development rate and progression through vitellogenesis [34**]. In the mosquito A. aegypti, ILP3 is involved in stimulation of egg production following the intake of vertebrate blood [35*, 36]. Only one, in dipteran and lepidopteran, or two, in hymenopteran and hemipteran, tyrosine kinase transmembrane insulin receptors (InR) exist in insects [3, 27, 37]. The negative effect of InR RNAi-mediated silencing on reproductive events has been observed in several insects, suggesting involvement of the insulin pathway in the control of reproduction [20, 35*, 36, 38].
The insulin signaling that is conserved in the animal kingdom converges onto the main effector Akt/protein kinase B, which in turn suppresses the negative regulators of TOR, Tuberous Sclerosis Complex 1 and 2 (TSC1/2), thereby activating the TOR via its phosphorylation (Figure 2) [4, 5, 39]. Insulin/TOR regulates cell growth, protein synthesis and metabolism. In ovaries of D. melanogaster, insulin and TOR control the development of niche-stem units in a tissue-specific manner [40*, 41**, 42–43]. This ovary-specific action occurs via coupling of the insulin signaling with TOR by phosphorylating the 40-kDa proline-rich Akt substrate TOR inhibitor (PRAS40) [40*]. Moreover, this action of insulin signaling in Drosophila is required for ovarian production of yolk proteins, and the ovarian vitellogenesis is autonomous for the ovary independently from juvenile hormone and ecdysteroids [43].
The role of insulin and TOR in control of JH and ecdysone biosynthesis
Insulin/TOR pathway also asserts its nutritionally linked influence on reproductive events by contributing to the control of biosynthesis and secretion of JH and ecdysone.
In the German cockroach Blatella germanica, in which JH plays a role of major gonadotrophic hormone, TOR connects the nutritional status with JH biosynthesis and as a consequence vitellogenesis. TOR RNAi knockdown resulted in a severe inhibition of JH synthesis in adult female corpora allata (CA) mimicking starvation conditions. Under both TOR silencing and starvation, there was a significant reduction in mRNA levels of JH biosynthetic enzymes, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) synthase-1, HMG-CoA synthase-2, and HMG-CoA reductase [44*]. Moreover, a similar negative effect on JH biosynthesis and adult vitellogenesis has been obtained by means of InR RNAi in the penultimate and last instar nymphs of the same insect [38]. In female mosquitoes, JH controls posteclosion maturation and leads to reproductive competency and ability to feed on blood. The insulin/TOR pathway plays a role in the transduction of the nutritional information that controls JH synthesis in mosquitoes [45]. This pathway (partially) mediates transcription of the genes encoding JH biosynthetic enzymes regulating JH production [46*]. Incubation of Corpus Cardiacum-CA complexes from A. aegypti females in the presence of bovine insulin increases JH synthesis by 2- to 3-fold, but this is blocked by incubating with LY294002 or with rapamycin, insulin and TOR signaling inhibitors, respectively [46*]. In adult Drosophila, mutations in InR cause a decrease of JH titer [47].
The role of brain factors (neuropeptides) in activation of ecdysone production by ovaries in dipterans, in which ecdysteroids are the primary effectors of vitellogenesis and egg maturation, has been well established [48]. More recently, ILPs have been implicated in regulation of ovarian ecdysteroidogenesis [28, 35*, 49]. Female D. melanogaster mutants for InR exhibit reduced ecdysteroid synthesis [49]. Wen et al. [50**] have shown that two mosquito ILPs, ILP3 and ILP4, exhibit gonadotropic activity in blood-fed females, including the stimulation of ovaries to produce ecdysteroids and the uptake of yolk by primary oocytes. However, ILP3 and ILP4 do not cross compete in binding assays; ILP3 interacts with InR and ILP4 with an uncharacterized 54-kDa membrane protein. In addition, in female A. aegypti mosquitoes blood feeding triggers the release of ILPs and ovary ecdysteroidogenic hormone (OEH) from the brain. OEH binds to the recently identified putative receptor tyrosine kinase (RTK) orphan receptor AAEL001915 in A. aegypti ovarian follicle cells [51] and activates the insulin pathway protein kinase B/Akt in an InR-independent manner [52]. Moreover, AAs with either OEH or ILP3 stimulate synthesis and release of ecdysteroids from A. aegypti ovaries [52].
Conclusions and future directions
Recent progress has revealed critical roles of AA/TOR and insulin in the control of female insect reproduction. These pathways serve as sensors of the nutritional status of a reproducing insect. Research has shown that these pathways act at multiple levels affecting reproductive organs directly and indirectly through the control of gonadotrophic hormones, JH and ecdysone. However, more studies are required in non-Drosophilid and non-dipteran insects to evaluate cell- and tissue-autonomous and systemic functions of these pathways.
AAs act through TOR independently from insulin, connecting AA signaling to reproduction as well as triggering release of ILPs. Relative contributions of insulin and AA/TOR as nutritional sensors in different insects are unclear but most likely are tightly bound to various insect life strategies. It is conceivable that AA/TOR plays a more significant role in insects, in which protein intake is required for the initiation of egg production. More studies are required to clarify this question.
Our understanding of the nutritional regulation of insect reproduction is still incomplete. Despite the exceptional value of D. melanogaster as a model insect, it cannot serve as a universal research system because reproductive events in insects vary dramatically. There is an urgent need for the development of genetic tools and their applications for investigating nutritional regulation of reproduction in insects other than Drosophila. Limited genetic tools have been established for A aegypti, Tcastaneum, Bombyx mori and Apis mellifera [53–56]. However, thus far genetic tools have found only narrow use in the reproductive biology of non-Drosophilid insects [57–60]. Most investigations of these insects discussed above have been performed using systemic RNA interference for gene silencing. Although such studies have been valuable for initial understanding of insect reproductive biology, deeper insights are required utilizing novel genetic tools. Recent advancements in genetic engineering such as CRISPR/Cas9 [61–63] provide a new prospective in research of insect reproduction.
Highlights.
Amino acids/Target of Rapamycin pathway serves as a nutritional sensor that is essential for reproduction in female insects requiring protein-rich diet
Amino acids/Target of Rapamycin pathway regulates synthesis and secretion of ILPs as shown for Drosophila
A second nutritional checkpoint is represented by ILPs that are involved in regulation of vitellogenesis and metabolism of reproducing female insects
ILPs control the development of ovarian niche stem units in an autonomous manner, independent of JH and ecdysone as demonstrated in Drosophila
Insulin and TOR signaling pathways regulate JH and ecdysone biosynthesis providing a link to the nutritional status of a reproducing insect
Acknowledgments
This work was supported by NIH/NIAID Award 5R01 AI036959 to ASR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Vlastimil Smykal, Email: vlastimil.smykal@ucr.edu.
Alexander S. Raikhel, Email: alexander.raikhel@ucr.edu.
References
Papers of particular interest, published within the period review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Layalle S, Arquier N, Léopold P. The TOR couples nutrition and developmental timing in Drosophila. Dev Cell. 2008;15(4):568–577. doi: 10.1016/j.devcel.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Danielsen ET, Moeller ME, Revitz KE. Nutrient signaling and developmental timing of maturation. Curr Top Dev Biol. 2013;105:37–67. doi: 10.1016/B978-0-12-396968-2.00002-6. [DOI] [PubMed] [Google Scholar]
- 3.Koyama T, Mendes CC, Mirth CK. Mechanisms regulating nutrition-dependent developmental plasticity through organ-specific effects in insects. Front Physiol. 2013;4:263. doi: 10.3389/fphys.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics. 2011;189(4):1177–1201. doi: 10.1534/genetics.111.133363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J, Guan KL. Amino acid signaling in TOR activation. Annu Rev Biochem. 2011;80:1001–1032. doi: 10.1146/annurev-biochem-062209-094414. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Gong R, Xu Y. Control of cell growth: Rag GTPases in activation of TORC1. Cell Mol Life Sci. 2013;70(16):2873–2885. doi: 10.1007/s00018-012-1195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groenewoud MJ, Zwartkruis FJ. Rheb and Rags come together at the lysosome to activate mTORC1. Biochem Soc Trans. 2013;41(4):951–955. doi: 10.1042/BST20130037. [DOI] [PubMed] [Google Scholar]
- 9.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind Raptor and amino acid signaling to mTORC1. Science. 2008;320(5882):1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Léopold P. A nutritional sensor mechanism controls Drosophila growth. Cell. 2003;114(6):739–749. doi: 10.1016/s0092-8674(03)00713-x. A significant contribution to our understanding of AA/TOR nutritional signaling. The authors identified the amino acid transporter slimfast and demonstrated that the fat body functions as a nutrient sensor that acts systemically through a humoral mechanism.
- 11.Géminard C, Rulifson EJ, Léopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metabol. 2009;10(3):199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 12. Rajan A, Perrimon N. Drosophila cytokine unpaired 2 regulates physiological homeostasis by remotely controlling insulin secretion. Cell. 2012;151(1):123–137. doi: 10.1016/j.cell.2012.08.019. Characterization of the fat body-borne factor that is involved in the control of insulin secretion.
- 13. Hansen IA, Attardo GM, Park JH, Peng Q, Raikhel AS. Target of rapamycin-mediated amino acid signaling in mosquito anautogeny. Proc Natl Acad Sci U S A. 2004;101(29):10626–10631. doi: 10.1073/pnas.0403460101. In mosquitoes, the ecdysone controlled egg maturation is blocked until the intake of a protein (blood) meal. The study has recognized the significance of AA signaling through TOR in de-repression of this reproductive block.
- 14.Hansen IA, Attardo GM, Roy SG, Raikhel AS. Target of rapamycin-dependent activation of S6 kinase is a central step in the transduction of nutritional signals during egg development in a mosquito. J Biol Chem. 2005;280(21):20565–20572. doi: 10.1074/jbc.M500712200. [DOI] [PubMed] [Google Scholar]
- 15. Attardo GM, Hansen IA, Shiao SH, Raikhel AS. Identification of two cationic amino acid transporters required for nutritional signaling during mosquito reproduction. J Exp Biol. 2006;209(16):3071–3078. doi: 10.1242/jeb.02349. The study has identified amino acid transporters involved in the AA/TOR signaling. It also characterized a repertoire of signaling AAs involved in this pathway in a mosquito.
- 16.Hansen IA, Boudko DY, Shiao SH, Voronov DA, Meleshkevitch EA, Drake LL, Aguirre SE, Fox JM, Attardo GM, Raikhel AS. AaCAT1 of the yellow fever mosquito Aedes aegypti: a novel histidine-specific amino acid transporter from the SLC7 family. J Biol Chem. 2011;286(12):10803–10813. doi: 10.1074/jbc.M110.179739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carpenter VK, Drake LL, Aguirre SE, Price DP, Rodriguez SD, Hansen IA. SLC7 amino acid transporters of the yellow fever mosquito Aedes aegypti and their role in fat body TOR signaling and reproduction. J Insect Physiol. 2012;58(4):513–522. doi: 10.1016/j.jinsphys.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy SG, Raikhel AS. Nutritional and hormonal regulation of the TOR effector 4E-binding protein (4E-BP) in the mosquito Aedes aegypti. FASEB J. 2012;26(3):1334–1342. doi: 10.1096/fj.11-189969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roy SG, Raikhel AS. The small GTPase Rheb is a key component linking amino acid signaling and TOR in the nutritional pathway that controls mosquito egg development. Insect Biochem Mol Biol. 2011;41(1):62–69. doi: 10.1016/j.ibmb.2010.10.001. The paper characterized the role of Rheb in the AA/TOR pathway controlling mosquito vitellogenesis.
- 20.Parthasarathy R, Palli SR. Molecular analysis of nutritional and hormonal regulation of female reproduction in the red flour beetle Tribolium castaneum. Insect Biochem Mol Biol. 2011;41(5):294–305. doi: 10.1016/j.ibmb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park JH, Attardo GM, Hansen IA, Raikhel AS. GATA factor translation is the final downstream step in the amino acid/target-of-rapamycin-mediated vitellogenin gene expression in the anautogenous mosquito Aedes aegypti. J Biol Chem. 2006;281(16):11167–11176. doi: 10.1074/jbc.M601517200. This work has uncovered a link between the AA/TOR pathway and transcriptional machinery represented by GATA.
- 22.Di Bartolomeo S, Nazio F, Cecconi F. The role of autophagy during development in higher eukaryotes. Traffic. 2010;11(10):1280–1289. doi: 10.1111/j.1600-0854.2010.01103.x. [DOI] [PubMed] [Google Scholar]
- 23.Chang YY, Juhász G, Goraksha-Hicks P, Arsham AM, Mallin DR, Muller LK, Neufeld TP. Nutrient-dependent regulation of autophagy through the target of rapamycin pathway. Biochem Soc Trans. 2009;37(Pt 1):232–236. doi: 10.1042/BST0370232. [DOI] [PubMed] [Google Scholar]
- 24.Chang YY, Neufeld TP. Autophagy takes flight in Drosophila. FEBS Lett. 2010;584(7):1342–1349. doi: 10.1016/j.febslet.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bryant B, Raikhel AS. Programmed autophagy in the fat body of Aedes aegypti is required to maintain egg maturation cycles. PLoS One. 2011;6(11):e25502. doi: 10.1371/journal.pone.0025502. The study has uncovered the TOR role in regulation of the programmed autophagy, which is involved in the fat body remodeling during the termination of vitellogenesis.
- 26.Weng SC, Shiao SH. Frizzled 2 is a key component in the regulation of TOR signalingmediated egg production in the mosquito Aedes aegypti. Insect Biochem Mol Biol. 2015;61:17–24. doi: 10.1016/j.ibmb.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Badisco L, Van Wielendaele P, Vanden Broeck J. Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front Physiol. 2013;4:202. doi: 10.3389/fphys.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu Q, Brown MR. Signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- 29.Riehle MA, Fan Y, Cao C, Brown MR. Molecular characterization of insulin-like peptides in the yellow fever mosquito Aedes aegypti: expression, cellular localization, and phylogeny. Peptides. 2006;27(11):2547–2560. doi: 10.1016/j.peptides.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 30.Badisco L, Claeys I, Van Hiel M, Clynen E, Huybrechts J, Vandersmissen T, Van Soest S, Vanden Bosch L, Simonet G, Vanden Broeck J. Purification and characterization of an insulin-related peptide in the desert locust. J Mol Endocrinol. 2008;40(3):137–150. doi: 10.1677/JME-07-0161. [DOI] [PubMed] [Google Scholar]
- 31.Li B, Predel R, Neupert S, Hauser F, Tanaka Y, Cazzamali G, Williamson M, Arakane Y, Verleyen P, Schoofs L, et al. Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Res. 2008;18(1):113–122. doi: 10.1101/gr.6714008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aslam AF, Kiya T, Mita K, Iwami M. Identification of novel bombyxin genes from the genome of the silkworm Bombyx mori and analysis of their expression. Zoolog Sci. 2011;28(8):609–616. doi: 10.2108/zsj.28.609. [DOI] [PubMed] [Google Scholar]
- 33.Colombani J, Anderson DS, Léopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336(6081):582–585. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- 34. LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309(5737):1071–1073. doi: 10.1126/science.1111410. The study has identified the cell-autonomous role of insulin in the control of ovarian stem cells.
- 35. Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2008;105(15):5716–5721. doi: 10.1073/pnas.0800478105. The study characterized mosquito ILP3 that is involved in the control of egg development.
- 36.Gulia-Nuss M, Robertson AE, Brown MR, Strand MR. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLoS One. 2011;6(5):e20401. doi: 10.1371/journal.pone.0020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu HJ, Xue J, Lu B, Zhang XC, Zhuo JC, He SF, Ma XF, Jiang YQ, Fan HW, Xu JY, et al. Two insulin receptors determine alternative wing morphs in planthoppers. Nature. 2015;519(7544):464–467. doi: 10.1038/nature14286. [DOI] [PubMed] [Google Scholar]
- 38.Abrisqueta M, Süren-Castillo S, Maestro JL. Insulin receptor-mediated nutritional signalling regulates juvenile hormone biosynthesis and vitellogenin production in the German cockroach. Insect Biochem Mol Biol. 2014;49:14–23. doi: 10.1016/j.ibmb.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Hyun S. Body size regulation and insulin-like growth factor signaling. Cell Mol Life Sci. 2013;70(13):2351–2365. doi: 10.1007/s00018-013-1313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pallares-Cartes C, Cakan-Akdogan G, Teleman AA. Tissue-specific coupling between insulin/IGF and TORC1 signaling via PRAS40 in Drosophila. Dev Cell. 2012;22(1):172–182. doi: 10.1016/j.devcel.2011.10.029. The study characterized the mechanism of tissue-specific action of TOR.
- 41. Gancz D, Gilboa L. Insulin and Target of rapamycin signaling orchestrate the development of ovarian niche-stem cell units in Drosophila. Development. 2013;140(20):4145–4154. doi: 10.1242/dev.093773. A fundamental study demonstrating the critical role of ILP/TOR pathway in the development of stem cells in the Drosophila ovary.
- 42.Wei Y, Reveal B, Reich J, Laursen WJ, Senger S, Akbar T, Iida-Jones T, Cai W, Jarnik M, Lilly MA. TORC1 regulators Iml1/GATOR1 and GATOR2 control meiotic entry and oocyte development in Drosophila. Proc Natl Acad Sci U S A. 2014;111(52):5670–5677. doi: 10.1073/pnas.1419156112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Richard DS, Rybczynski R, Wilson TG, Wang Y, Wayne ML, Zhou Y, Partridge L, Harshman LG. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J Insect Physiol. 2005;51(4):455–464. doi: 10.1016/j.jinsphys.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 44. Maestro JL, Cobo J, Bellés X. Target of rapamycin (TOR) mediates the transduction of nutritional signals into juvenile hormone production. J Biol Chem. 2009;284(9):5506–5513. doi: 10.1074/jbc.M807042200. Characterization of the role of the TOR signaling in activation of JH biosynthesis in this basal insect, in which JH controls reproduction.
- 45.Clifton ME, Noriega FG. The fate of follicles after a blood meal is dependent on previtellogenic nutrition and juvenile hormone in Aedes aegypti. J Insect Physiol. 2012;58(7):1007–1019. doi: 10.1016/j.jinsphys.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pérez-Hedo M, Rivera-Perez C, Noriega FG. The insulin/TOR signal transduction pathway is involved in the nutritional regulation of juvenile hormone synthesis in Aedes aegypti. Insect Biochem Mol Biol. 2013;43(6):495–500. doi: 10.1016/j.ibmb.2013.03.008. Characterization of the role of the insulin/TOR signaling in activation of JH biosynthesis in a mosquito.
- 47.Tu M, Yin CM, Tatar M. Mutations in insulin signaling alter juvenile hormone synthesis in Drosophila melanogaster. Gen Comp Endocrinol. 2005;142:347–356. doi: 10.1016/j.ygcen.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 48.Brown MR, Sieglaff DH, Rees HH. Gonadal ecdysteroidogenesis in arthropoda: occurrence and regulation. Annu Rev Entomol. 2009;54:105–125. doi: 10.1146/annurev.ento.53.103106.093334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu MP, Yin CM, Tatar M. Impaired ovarian ecdysone synthesis of Drosophila melanogaster insulin receptor mutants. Aging Cell. 2002;1(2):158–160. doi: 10.1046/j.1474-9728.2002.00016.x. [DOI] [PubMed] [Google Scholar]
- 50. Wen Z, Gulia M, Clark KD, Dhara A, Crim JW, Strand MR, Brown MR. Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol Cell Endocrinol. 2010;328(1–2):47–55. doi: 10.1016/j.mce.2010.07.003. Demonstration that mosquito ILPs, ILP3 and ILP4, possess different binding properties with the former binding to InR and the latter to a 54-kDa membrane protein. However, both ILPs are involved in the control of ovarian ecdysteroidogenesis and accumulation of yolk proteins.
- 51.Vogel KJ, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone requires a receptor tyrosine kinase to activate egg formation in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2015;112(16):5057–5062. doi: 10.1073/pnas.1501814112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhara A, Eum JH, Robertson A, Gulia-Nuss M, Vogel KJ, Clark KD, Graf R, Brown MR, Strand MR. Ovary ecdysteroidogenic hormone functions independently of the insulin receptor in the yellow fever mosquito Aedes aegypti. Insect Biochem Mol Biol. 2013;43(12):1100–1108. doi: 10.1016/j.ibmb.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schinko JB, Weber M, Viktorinova I, Kiupakis A, Averof M, Klingler M, Wimmer EA, Bucher G. Functionality of the GAL4/UAS system in Tribolium requires the use of endogenous core promoters. BMC Dev Biol. 2010;10:53. doi: 10.1186/1471-213X-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kokoza VA, Raikhel AS. Targeted gene expression in the transgenic Aedes aegypti using the binary Gal4-UAS system. Insect Biochem Mol Biol. 2011;41(8):637–644. doi: 10.1016/j.ibmb.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Tan A, Xu J, Li Z, Zeng B, Ling L, You L, Chen Y, James AA, Huang Y. Site-specific, TALENs-mediated transformation of Bombyx mori. Insect Biochem Mol Biol. 2014;55C:26–30. doi: 10.1016/j.ibmb.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulte C, Theilenberg E, Müller-Borg M, Gempe T, Beye M. Highly efficient integration and expression of piggyBac-derived cassettes in the honeybee (Apis mellifera) Proc Natl Acad Sci U S A. 2014;111(24):9003–9008. doi: 10.1073/pnas.1402341111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kokoza VA, Martin D, Mienaltowski MJ, Ahmed A, Morton CM, Raikhel AS. Transcriptional regulation of the mosquito vitellogenin gene via a blood meal-triggered cascade. Gene. 2001;274(1–2):47–65. doi: 10.1016/s0378-1119(01)00602-3. [DOI] [PubMed] [Google Scholar]
- 58.Xu J, Wang YQ, Li ZQ, Ling L, Zeng BS, You L, Chen YZ, Aslam AF, Huang YP, Tan AJ. Functional characterization of the vitellogenin promoter in the silkworm, Bombyx mori. Insect Mol Biol. 2014;23(5):550–557. doi: 10.1111/imb.12102. [DOI] [PubMed] [Google Scholar]
- 59.Xu J, Bi H, Chen R, Aslam AF, Li Z, Ling L, Zeng B, Huang Y, Tan A. Transgenic characterization of two testis-specific promoters in the silkworm, Bombyx mori. Insect Mol Biol. 2015;24(2):183–190. doi: 10.1111/imb.12144. [DOI] [PubMed] [Google Scholar]
- 60.Lucas KJ, Roy S, Ha J, Gervaise AL, Kokoza VA, Raikhel AS. MicroRNA-8 targets the Wingless signaling pathway in the female mosquito fat body to regulate reproductive processes. Proc Natl Acad Sci U S A. 2015;112(5):1440–1445. doi: 10.1073/pnas.1424408112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma Y, Zhang L, Huang X. Genome modification by CRISPR/Cas9. FEBS J. 2014;281(23):5186–5193. doi: 10.1111/febs.13110. [DOI] [PubMed] [Google Scholar]
- 62.Dong S, Lin J, Held NL, Clem RJ, Passarelli AL, Franz AW. Heritable CRISPR/Cas9-mediated genome editing in the yellow fever mosquito Aedes aegypti. PLoS One. 2015;10(3):e0122353. doi: 10.1371/journal.pone.0122353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Basu S, Aryan A, Overcash JM, Samuel GH, Anderson MA, Dahlem TJ, Myles KM, Adelman ZN. Silencing of end-joining repair for efficient site-specific gene insertion after TALEN/CRISPR mutagenesis in Aedes aegypti. Proc Natl Acad Sci U S A. 2015;112(13):4038–4043. doi: 10.1073/pnas.1502370112. [DOI] [PMC free article] [PubMed] [Google Scholar]