Abstract
Some memories of the events of our lives have a long shelf-life—they remain accessible to recollection even after long delays. Yet many other of our experiences are forgotten, sometimes very soon after they take place. In spite of the prevalence of forgetting, theories of the development of episodic and autobiographical memory largely ignore it as a potential source of variance in explanation of age-related variability in long-term recall. They focus instead on what may be viewed as positive developmental changes, that is, changes that result in improvements in the quality of memory representations that are formed. The purpose of this review is to highlight the role of forgetting as an important variable in understanding the development of episodic and autobiographical memory. Forgetting processes are implicated as a source of variability in long-term recall due to the protracted course of development of the neural substrate responsible for transformation of fleeting experiences into memory traces that can be integrated into long-term stores and retrieved at later points in time. It is logical to assume that while the substrate is developing, neural processing is relatively inefficient and ineffective, resulting in loss of information from memory (i.e., forgetting). For this reason, focus on developmental increases in the quality of representations of past events and experiences will tell only a part of the story of how memory develops. A more complete account is afforded when we also consider changes in forgetting.
Keywords: autobiographical memory, forgetting, long-term recall
Forgetting is fundamental. Most of the time, we take it for granted that much of what we once remembered eventually (if not sooner) will be forgotten. We even plan for forgetting—creating notes and electronic reminders of things that we do not want to forget, but know that we otherwise would. Yet in spite of the prevalence of forgetting, theories of the development of memory largely ignore it as a potential source of variance in explanation of the course of change in long-term memory for past events—so-called episodic or autobiographical memory. Instead, focus is on what may be viewed as positive developmental changes, that is, changes that result in improvements in the quality of memory representations that are formed. The purpose of this review is to remove forgetting from the shadows and bring it into the spotlight of attention. The major argument of the review is that focus on developmental increases in the quality of representations of past events and experiences will tell only a part of the story of how memory develops. A more complete account is afforded when we also consider changes in forgetting (see Bauer, 2015, for a review).
Complementary Processes in Development of Memory
There is no doubt that over the course of childhood, memory for past events and experiences gets better. The early history of the developmental study of memory is replete with examples of age-related improvements in task performance (Bauer & Fivush, 2014). The trend is apparent whether one considers incidental memories, such as those formed over the course of everyday life, or deliberate and strategic remembering (see Bauer, 2013, for a review). The changes typically are viewed in terms of positive developments in the quality of the representations of past events that are formed, in terms of improvements in component abilities, or both. For example, we attribute better retention over time to more veridical encoding (e.g., Ornstein, Baker-Ward, & Naus, 1988), to more nuanced differentiation of the details of one event or experience relative to another (e.g., Bauer & Lukowski, 2010; Riggins, 2014), to greater precision locating events in time (Friedman, 2014) and place (Lourenco & Frick, 2014), to more robust and autonomous retrieval processes (e.g., Roebers, 2014), and to increases in autonoetic awareness (Tulving, 2005; Wheeler, 2000), to name a few. All of these changes contribute to the formation of memory representations that are of higher quality, through addition of more, better elaborated, and more tightly integrated features (Bauer, 2015). The result is a higher quality memory trace and more robust remembering.
Critically, increases in the quality of the representations of past events and experiences are only one side of the mnemonic coin. The other side of the coin—the complement to positive change—is negative change in the representation, change that results in forgetting. Forgetting has been recognized as a force in memory since the beginning of scientific study of the faculty. Famously, Ebbinghaus (1885) carefully tested and documented the course of forgetting of lists of nonsense syllables over time, revealing highly replicable retention (and forgetting) functions. Yet forgetting has been largely ignored in developmental theories. With notable exceptions (e.g., Brainerd, Reyna, Howe, & Kingma, 1990), most developmental scientists do not feature forgetting processes in theories of “what develops” in memory for specific events or experiences. That is, they do not attend to developmental changes in the rate at which memory traces are forgotten. The argument developed in this review (see also Bauer, 2015) is that focus on positive change alone fails to account for why the development of memory takes the forms that it does. The argument applies to episodic memory in general, and to autobiographical memory—memory for personally relevant past events—and explanation of the phenomenon of childhood amnesia, in particular. Briefly, to explain the shape of the distribution of episodic and autobiographical memories in childhood and beyond, we must consider that over the course of development, there are increases in the quality of memory traces and there are decreases in the vulnerability of mnemonic traces to forgetting.
It is noteworthy that consideration of complementary processes in shaping the course of development is not unique to memory. An excellent example of consideration of complementary processes is in brain development. Throughout prenatal and early postnatal development, there are increases in neurons, dendrites and axons, synapses, myelin, glial, and other “positive” events that form the brain (e.g., Gilmore, Shi, Woolson, Knickmeyer, Short, Lin, Zhu, et al., 2011; Seress & Ábrahám, 2008). Equally importantly, there are “regressive” events as well. The best-known example is the number of synapses: the adult complement of synapses is reached only after an initial period of over-production, followed by pruning (Huttenlocher, 1979; Huttenlocher & Dabholkar, 1997). In part as a result, cortical thickness first increases and then decreases, reaching adult levels only in adolescence (Gogtay, Giedd, Lusk, Hayashi, Greenstein, Vaituzis, Thompson, et al., 2004). The fact that regressive events take place at different times in different structures and regions of the brain is recognized as an important part of the story of why cognitive processes develop as they do (e.g., Østby, Tamnes, Fjell, & Walhovd, 2011; Gogtay et al., 2004). It is further recognized that focus on one or the other of these trends—either positive events or regressive events—and ignoring the other, results in an incomplete understanding of the factors and forces that shape brain development.
Why accounts for the relative lack of attention to the “regressive” event of forgotting as a source of developmental change in episodic and autobiographical memory? The blunt answer is that, historically, there was no role for forgetting because very little was deemed to be remembered and thus available to be forgotten. By analogy to brain development, there was no overproduction and thus no need to consider pruning. This argument applied to the capacity to remember specific events and experiences—so-called episodic memory—in infancy and to the capacity to remember personally relevant or significant events (i.e., autobiographical events) and experiences in the preschool and early school years. In infancy, based largely on Piagetian theory (e.g., 1962), mental life was considered to be confined to the “here and now” world of present objects and ongoing events. It was not until the middle to end of the second year of life that infants were thought to have developed the symbolic capacity to recall objects, events, or other entities in their absence. Without the capacity for recall, no memories of episodes, events, or experiences were formed or retained and thus there was no need to postulate a role for forgetting.
Beyond infancy, the capacity to remember specific past events and experiences was recognized but the ability to form autobiographical or personal memories was thought to be a later developmental achievement. Autobiographical memory differs from “run of the mill” episodic memory (memory for what, where, and when: Tulving, 1985) in the extent to which the memories are personally relevant or significant, and to which individuals take a personal, subjective, or evaluative perspective on the events (e.g., Fivush, 2012; Fivush & Zaman, 2014). Traditional developmental perspectives suggest that for the first 5 to 7 years of life, the capacity to create this particular type of episodic memory is either lacking or substantially underdeveloped. In some cases, the missing ingredient is posited to be a general cognitive deficit; in other cases, it is something more specific (discussed in detail below). Regardless of the nature of the deficit, the explanation is the same: to the extent that memories of specific events are formed early in life, they lack features of later-developing autobiographical memories. This perspective provides a ready account for infantile or childhood amnesia (Freud, 1905/1953)—the relative paucity of autobiographical memories for the first 3 to 4 years of life, with a gradually increasing number of such memories over the first decade (see Bauer, 2008, 2014). The explanation is that adults recall few (to no) autobiographical memories from this period because during the period, no such memories were formed. As was the case for episodic memory in infancy, there is no role for forgetting because there are no personal or autobiographical memories to be forgotten.
The major premise of this review is that episodic memories are evident even in infancy, and that autobiographical memories are evident even in the period eventually obscured by childhood amnesia. To explain developmental changes in the memory function and why the distribution of autobiographical memories looks the way that it does, we must focus on factors that improve the quality of memory representations, to be sure, yet we also must focus on developmental changes that affect the vulnerability of memory traces to forgetting. In the balance of this review, I focus primarily on forgetting. I do so because, as already noted, forgetting has been relatively neglected in the developmental literature, yet as discussed in sections to follow, there are strong reasons to believe that it is a major source of age-related variance in episodic and autobiographical memory function. Before turning to forgetting, I provide a brief review of evidence of remembering.
Memory in Infancy and Early Childhood
As implied above, consideration of forgetting is necessary only if there is something to be forgotten. If no memories are formed, there is no need to explain why they are not apparent. Evidence that even infants form and retain episodic memories, and that preschool and early school age children form and retain autobiographical memories, has been reviewed in detail elsewhere (e.g., Bauer, 2007, 2013, 2014, 2015; Lukowski & Bauer, 2014). For this reason, I provide a brief review here.
Memory for Specific Episodes in Infancy
The notion that infants were unable to recall specific past events and experiences came under empirical scrutiny with development of elicited and deferred imitation techniques. Deferred imitation was introduced into the literature by Piaget (1962). He reported on his daughter, Jacqueline’s, deferred imitation of a cousin’s temper tantrum. Piaget took Jacqueline’s re-enactment of the temporal tantrum after a delay as evidence of her ability to re-present the experience symbolically and thus of the capacity to recall the past. In the 1980s, the task was brought under experimental control (e.g., Bauer & Mandler, 1989; Bauer & Shore, 1987; Meltzoff, 1985), and since has been used to examine a number of attributes of infants’ memories, including whether the memories are of unique events, and whether the basic attributes of episodic memories—what, where, and when (Tulving, 1985)—are evident.
In imitation-based tasks, props are used to demonstrate individual actions or sequences of actions that infants then are permitted to imitate either immediately, after a delay, or both. Based on their behavior in these tasks, it is clear that infants remember what, where, and when, elements regarded as defining of episodic memories. Memory for the “what” of events is apparent in the first year of life (e.g., Carver & Bauer, 1999, 2001), through infants’ reproduction of the actions they see modeled. By the second year of life, infants reliably recall the individual target actions of multi-step sequences even over delays (e.g., Bauer & Leventon, 2013; Bauer, Wenner, Dropik, & Wewerka, 2000). Infants also remember the specific features of the objects on which the actions were performed. That is, they reliably identify the objects they saw used to model sequences, even when the objects are presented among distracters of perceptually different but functionally similar props (Bauer & Dow, 1994; Lechuga, Marcos-Ruiz, & Bauer, 2001). Memory for the specific features of the props used to produce events is associated with higher levels of recall over a delay (Bauer & Lukowski, 2010). Infants also seemingly bind objects to specific events. Infants who witness a prop used in one sequence and then see the same prop used differently in another sequence exhibit impaired performance, relative to infants who see the prop used in only one sequence (Wiebe & Bauer, 2005). Infants also remember the “where” and “when” of events. They correctly identify the locations in which specific events took place, even over delays of 1 and 3 months (Lukowski, Lechuga, & Bauer, 2011). They also remember the “when” of events, as evidenced by reliable ordered recall of multi-step sequences (e.g., Bauer & Leventon, 2013; Bauer et al., 2000). Thus although infants cannot verify their episodic memories verbally, they reveal them in their behavior.
Memory for Autobiographical Experiences in Childhood
As noted above, autobiographical memory is distinguished from episodic memory by the personal, subjective, or evaluative perspective placed on the event and the memory thereof. Traditional developmental perspectives suggest that for the first 5 to 7 years of life, the capacity to create this particular type of episodic memory is either lacking or substantially underdeveloped.
Traditional accounts: Missing ingredients
One hypothesized reason for the late emergence of autobiographical memory is that it awaits development of a self-concept around which memories can be organized (e.g., see Howe, 2014, for a review). Other accounts suggest that it is not a lack of a physical self-concept that precludes early memories that are autobiographical, but rather, a subjective self who takes personal perspective on life events and evaluates them for their significance to the self (e.g., Fivush, 2012; Fivush & Zaman, 2014). Without these developments, there can be no auto in episodic memories and thus no autobiographical memory. Other conceptualizations suggest that for the first 5 to 7 years of life, children lack autonoetic consciousness. The absence of this form of consciousness makes it impossible for them to recognize that the source of their mental experience is a representation of a past event (e.g., Perner & Ruffman, 1995). The lack of autonoetic consciousness also means that children cannot mentally travel in time to re-live past events and experiences, a feature thought to accompany both episodic and autobiographical retrieval (e.g., Suddendorf, Nielson, & van Gehlen, 2011; Tulving, 2005; Wheeler, 2000). Although each of these explanations implicates a different specific component ability, they have in common the perspective that young children’s memories are lacking in qualities that typify the autobiographical memories formed by older children and adults. The general argument is that children begin to form, retain, and later retrieve memories that are autobiographical only once the conceptual ingredients that are missing from early memories become available.
An especially influential and well-articulated example of the perspective that the capacity for autobiographical memory is late to develop was provided by Nelson and Fivush (2004; see Figure 1 of the original publication, for a depiction). Nelson and Fivush (2004) suggested that it is not until 5 years of age that the many general cognitive and specific conceptual dimensions required for encoding, retention, and later retrieval of memories have reached a sufficient level of development to support autobiographical memory. Among the elements necessity for autobiographical memory, they note developments in self-concept, language and narrative, theory of mind, understanding of time and place, subjective sense of self, mental time travel, and autonoetic awareness, and others. In each case, the capacities undergo development from infancy through early childhood, eventually culminating in the capacity to form autobiographical memories. Until that time, children may have semantic and perhaps even episodic memories, but their memories are not autobiographical. This provides a ready account for childhood amnesia: memories formed in the first 5 to 7 years are not autobiographical, thus explaining the relative paucity among adults of autobiographical memories from this life period.
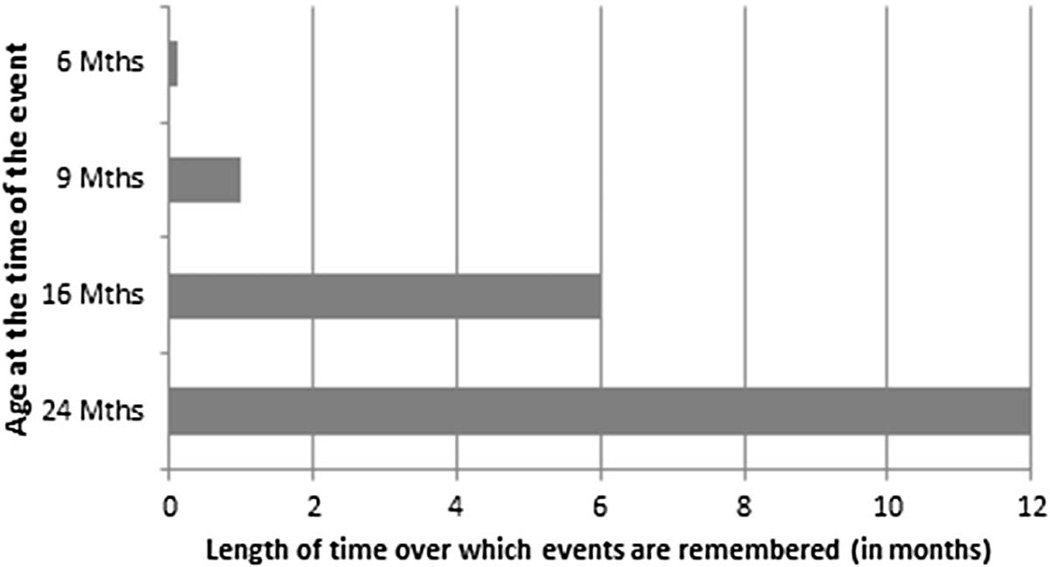
Figure 1.
Schematic depiction of the length of time over which infants retain memories of specific past events from ages 6 to 36 months. The retention interval that infants tolerate increases dramatically over the first two years of life.
Consistent with the suggestion that early memories are not autobiographical is the observation that for much of early childhood, children frequently omit one or more of the elements that we associate with autobiographical memory, seemingly licensing the conclusion that they also are missing from the underlying memory representation, and thus, in turn, rendering them non-autobiographical. Specifically, a full autobiographical report features a number of elements, including not only what, where, and when, but also who was involved in the event, why the event unfolded as it did, and how the participants reacted to it in terms of their emotions, thoughts, or evaluations of the experience. The latter element is especially important to establishing the self-referential nature of autobiographical memories.
Evidence of early autobiographical competence
Although children’s narratives may be less than convincing of the autobiographical nature of the memories that give rise to them, a different perspective is gained by looking beyond children’s verbal reports. Using nonverbal imitation-based tasks described earlier, researchers have found evidence of memory for unique events even in the first year of life. Not only do infants recall what, where, and when, they also evidence behavior that indicates that they have some understanding of why events unfold as they do (e.g., Bauer, 1992). When they reproduce events, they exclude actions that are irrelevant to the outcome or goal of the event. And as they approach and enter the third year of life and gain the fluency to provide verbal descriptions of events experienced in imitation-based tasks, children spontaneously verbalize about who took part in the events (who) and they provide evaluative comments on the activities in which they engaged (subjective perspective; Bauer & Wewerka, 1997). These behaviors make clear that sometimes well before they provide linguistic evidence, children encode, retain, and later retrieve memory representations that feature each of the individual elements associated with autobiographical memory (see Bauer, 2007, 2015; Bauer & Leventon, 2013, for discussions).
Beyond infancy, memory processes improve to the point that children remember unique experiences, even over substantial delays. Children also provide more frequent and consistent evidence that they remember the events from their own personal, self-referential perspective. For example, Hamond and Fivush (1991) found that children who experienced a trip to Disneyworld when they were 36 or 48 months of age remembered the event even 18 months later. In Bauer and Larkina (2014b), children 3 years of age at the time of events remembered in excess of 60% of them over delays of as many as 3 years. In this same age period, children become increasingly accurate and reliable in determining which of two events occurred earlier and in justifying their choices (Pathman, Larkina, Burch, & Bauer, 2013). They also gain command of the use of conventional indices of time, such as calendars (e.g., Friedman, Reese, & Dai, 2010) and seasons (Bauer, Burch, Scholin & Güler, 2007; Bauer & Larkina, 2014a), to locate when personally relevant event occurred. Children also become increasingly proficient at remembering the location in which they experienced specific past events (Bauer, Doydum, Pathman, Larkina, Güler, & Burch, 2012; Bauer, Stewart, White, & Larkina, in press). These changes mean that more events are stored with more, better elaborated, and more tightly integrated elements of autobiographical memories: unique events, with distinctive features, accurately located in time and place.
Children’s early memories also are self-referential. Between 18 and 24 months of age, children first begin to make reference to themselves in past events (Howe & Courage, 1993, for discussion). Throughout the preschool years, children develop a more self-oriented or subjective perspective on experience, as evidenced by increasingly frequent references to their own (and others’) emotional and cognitive states (see Fivush & Zaman, 2014). References to the emotional and cognitive states of the experiencer indicate the sense of personal ownership and unique perspective that is characteristic of autobiographical memories.
Summary
Children’s early verbal reports of past events frequently omit one or more of the elements that mark a memory as autobiographical. Yet this does not mean that the elements are missing from the underlying representation: absence of evidence does not constitute evidence of absence. Indeed, review of the corpus of studies with infant participants reveals evidence of memory for all of the features or elements characteristic of autobiographical memory: what, where, when, as well as why and how (i.e., evaluation; subjective perspective). Moreover, from virtually as early as they can talk, infants make reference to themselves in past events. Although no one instance of memory may feature all of the elements, they are apparent across memory “reports.” As infants become children, more and more representations feature all of the elements associated with autobiographical memory. As discussed elsewhere (Bauer, 2007, 2012, 2014, 2015), the net effect is that more and more memories are recognized as autobiographical.
The Role of Forgetting in the Development of Memory
The literature just reviewed makes clear that infants remember specific episodes and that children form autobiographical or personal memories. Throughout infancy and childhood, there are age-related changes in memory, such that both episodic and autobiographical memory become more robust, reliable, and more fully elaborated (see Bauer, 2007, 2013, 2015, for reviews). It is tempting to explain the changes in terms of positive developmental events associated with the strengthening of memory traces such as those implicated in traditional accounts of the development of autobiographical memory (e.g., self concept). Yet just as in brain development, positive events alone cannot explain the course of development. This is especially apparent in the case of autobiographical memory—if self-referential memories of past events are present from early in life and just get stronger and stronger with development, what explains childhood amensia? Clearly, “regressive” events also must come into play. In the case of memory, the regressive event is forgetting. In this section, I outline the conceptual argument for including forgetting among the ingredients in the explanation of the development of memory. I then provide empirical evidence of the role of forgetting in shaping the development of memory.
Why Forgetting is a Candidate Source of Age-related Variance
The motivation to focus on forgetting as a candidate source of age-related variance in episodic and autobiographical memory is two fold. First, memories are non-trivial to form and retain. They begin their lives as distributed representations created by fleeting experiences. Preservation of them over the long term requires substantial processing involving an integrated network of structures. Second, the neural structures and network that are responsible for the transformation of experience into enduring memory representations undergo a protracted course of development. Early in life, when the structures and networks are less well developed, the processes that they subserve may be expected to operate less efficiently and thus less effectively. As a consequence, information that otherwise would be retained is lost from the memory trace, leaving the traces more vulnerable to forgetting. The net effect is that youth is a risk factor for memory. Moreover, the younger the child, the greater the risk that memory traces will be lost to forgetting. Before describing the course of development of the neural substrate responsible for memory, I provide a brief review of the processes involved in memory trace construction, storage, and retrieval. Consideration of the processes and the number of “moving parts” involved—any of which is subject to fail—helps to make clear why focus on the developmental status of the neural substrate is important to explanation of the course of develoment of memory across childhood.
Memory processes
As described in Bauer (2015), a common metaphor for memory is a file cabinet stuffed full of file folders. Each folder contains the record of a past event. To recall any given event or experience, one finds the right folder, takes it out of the cabinet, opens it, and reads off what happened in the course of the event. Yet memory is nothing like a file cabinet and there are no file folders with records of experience. Rather, memory representations are made up of individual elements of experience that are encoded in synaptic connections between individual neurons in various regions of the cortex. Though we experience events as integrated wholes, at the neural level, they are widely distributed patterns of activity representing the different aspects of the experience, such as the sights, sounds, and movement. To live on as “memories,” the patterns of activity must be stabilized and integrated, processes carried out by a multi-component neural network that includes structures in the neocortex and the medial-temporal lobe. Later retrieval of a representation involves re-creation of the pattern of neural activity that gave rise to the experience in the first place (for reviews, see Eichenbaum & Cohen, 2001; Kandel & Squire, 2000; Manns & Eichenbaum, 2006; Rubin, 2005, 2006; Winocur & Moscovitch, 2011; Zola & Squire, 2000). Critically, each of these steps is a source of vulnerability, even in adulthood.
They carry even greater risk in childhood. The first step in creation of a memory is encoding. The process of encoding begins with registration of different aspects of experience in different cortical areas. For example, cell fields in primary visual cortex register form, color, and motion; cell fields in primary auditory cortex register attributes of sounds; and cell fields in primary somatosensory cortex register tactile information. The inputs from these primary sensory cortices are further processed in unimodal sensory association areas where they are integrated into whole percepts of what the object or event looks like, sounds like, and feels like, respectively. Unimodal association areas in turn send (project) the information to polymodal or multimodal posterior-parietal, anterior-prefrontal, and limbic-temporal association areas. The coordinated activity of these cortical areas gives rise to experience of a coherent event.
For fleeting experience to endure as a lasting memory, the distributed trace must undergo a process of stabilization and the trace must be integrated into long-term storage. This process of consolidation entails neurochemical and neuroanatomical changes that create a physical record of the experience (McGaugh, 2000). It depends on the coordinated actions of medial-temporal lobe structures and cortical association areas. Specifically, inputs from cortical association areas are projected to perirhinal and parahippocampal structures in the medial-temporal lobes. These cortices are thought to serve as an “intermediate-term memory” on which the hippocampus proper operates. It is in the hippocampus proper that all of the different components of the event are bound into a single representation (Manns & Eichenbaum, 2006). The “binding” takes place as a result of iterative processing of the conjunctions and relations among the stimuli that gave rise to the event. That is, the pattern is regularly “refreshed” by additional neural signaling among the hippocampus, the surrounding medial-temporal cortices, and the association areas. The iterative processing also maintains and strengthens the linkages between the distributed cortical representations that make up the entire event. At the same time, it supports integration of the new information with that previously stored (e.g., McKenzie & Eichenbaum, 2011), based on overlapping or shared elements. The result is an entire pattern of interconnection of new information with old. Critically, the process of consolidation takes time—on the order of days to weeks to months (e.g., Wixted, 2004). Until traces are well consolidated, they are vulnerable to disruption and interference and thus forgetting.
Finally, when a memory is retrieved, the pattern of neural activity that represents the event is reactivated. Thus retrieval of information from long-term stores is accomplished by the same circuits as were involved in initial registration of the experience, including the hippocampus and surrounding cortices, amygdala (for emotional events), visual and other cortices, and lateral and medial prefrontal cortex (see Gilboa, 2004; Rubin, 2005, 2006, for reviews; see Maguire, 2001; Svoboda, McKinnon, & Levine, 2006, for meta-analyses). Neuroimaging studies reveal that during the initial phases of memory search and access the more anterior, frontal-temporal components of this network are especially active, whereas in the later phases of retrieval—as the trace is elaborated—the more posterior, occipital and parietal regions are especially active (e.g., Daselaar, Rice, Greenberg, Cabeza, LaBar, & Rubin, 2008; McCormick, St-Laurent, Ty, Valiante, & McAndrews, 2013; St. Jacques, Kragel, & Rubin, 2011).
Neural structures and networks undergo protracted development
Efficient and effective stabilization and integration of widely distributed representations of experience into long-term memories requires a “well oiled” machine, involving numerous neural structures that are integrated into functional networks. Yet the “machine” undergoes a protracted course of development, and it is logical to assume that while it is developing, neural processing is relatively inefficient and ineffective, resulting in loss of information from memory (i.e., forgetting; Bauer, 2015). There are a number of reviews of the protracted course of development of the neural structures and network involved in memory trace formation, consolidation and storage, and retrieval (e.g., Bauer, 2009, 2013; Ghetti & Lee, 2014; Nelson, de Haan, & Thomas, 2006). For this reason, I review it only briefly here.
As noted above, registration of experience, consolidation and storage of memory traces, and their subsequent retrieval, all depend on cortical regions. Over the course of development, there are substantive changes in cortical structures. As noted earlier, cortical thickness first increases and then decreases to adult levels. The inverted-U shaped function extends well into adolescence (Gogtay et al., 2004). Moreover, the process is apparent across all of the major regions of the cortex (frontal, temporal, parietal, and occipital), though the specific timing differs by region.
The medial-temporal structures that are involved in consolidation and storage of memory traces as well as their retrieval also undergo a protracted course of development. Within the dentate gyrus of the hippocampus, which is the “route in” to the cell fields of the hippocampus, the number of synapses increases to peak levels in the second year of life and reaches adult levels at 4 to 5 years of age (Seress & Ábrahám, 2008). The hippocampus itself does not exhibit the same dramatic and extended increase and decrease as the cortical regions. However, there is evidence that the distribution of volume changes over development, such that anterior regions lose thickness and posterior regions of the hippocampus gain thickness. The processes continue well into adolescence (Gogtay, Nugent, Herman, Ordonez, Greenstein, & Hayashi, 2006).
There also are dramatic changes in connectivity within and between neural structures over development. There are pronounced changes in myelination both within the medial-temporal lobes, and in the hippocampus in particular (Ábrahám, Vincze, Jewgenow, Veszprémi, Kravják, Gömöri, et al., 2010; Benes, Turtle, Khan, & Farol, 1994), as well as between prefrontal and medial-temporal structures (see Ghetti & Lee, 2014, for a review). Unlike cortical thickness, which exhibits inverted-U shaped function, network connectivity increases linearly with age (Østby, Tamnes, Fjell, Westlye, Due-Tønnessen, Walhovd, 2009; Schneider, Il’yasov, Hennig, & Martin, 2004). The changes continue well into the second decaded of life. These wide-spread and protracted changes can be expected to have important consequences for memory function.
Forgetting in Memory for Specific Past Events: Infancy and Early Childhood
In this section, I focus on three logical consequences of the nature of memory representations and of the developmental status of the neural substrate responsible for them. First, as just noted, because the neural structures and networks responsible for memory undergo a protracted course of development that extends into the second decade of life, we would expect greater vulnerability of memory in younger relative to older children, resulting in age-related differences in how long memories are retained. Second, because memory traces must be stabilized and integrated into long-term storage—and the facts that consolidation processes take time and that traces are vulnerable throughout the period of consolidation—we would expect to see age-related differences in retention even with age-related variability in encoding controlled. That is, information should be differentially lost from memory, even as it is being stabilized and integrated with long-term stores. Third, the amount of information lost in the course of stabilization and integration should explain unique variance in long-term recall. In essence, the more information that succumbs to decay and interference, the less that will remain available for storage and thus retrieval. I evaluate these expectations using examples from the literature on episodic memory in infancy. In the section to follow, I explore the implications of forgetting for autobiographical memory and its development, and the phenomenon of childhood amnesia.
The Vulnerability of Memory as a Function of Age
Perhaps the most dramatic age-related changes in memory function in infancy is in the length of time over which memories are retained. The changes indicate decreases in the vulnerability of memory traces with age. Based on performance on imitation-based tasks, described earlier, the length of time over which infants retain memories of specific past events increases over the first two years of life. As suggested by inspection of Figure 1, in the first year, retention is limited to hours and days, whereas by the end of the second year of life, infants remember novel experiences over delays of 12 months or more (Bauer et al., 2000; see Bauer, 2009, 2013, and Lukowski & Bauer, 2014, for summaries). These data lend themselves to interpretation in terms of differential forgetting: there is greater vulnerability of memory in younger relative to older infants, resulting in age-related differences in how long memories are retained.
Age-related Differences even with Encoding Controlled
The data in Figure 1 are consistent with interpretation in terms of differential vulnerability of memory traces as a function of age. They also are consistent with an alternative explanation in terms of encoding. Specifically, it may be that older infants appear to remember over longer delays because they created higher quality memory representations in the first place. Though certainly a logical alternative, explanation in terms of differential encoding cannot account for age-related differences in retention when encoding-related vulnerability is controlled. To test this possibility, in a series of studies in my laboratory, we have controlled encoding statistically (Bauer, Van Abbema, & de Haan, 1999), and by bringing infants to a criterion level of learning (Bauer, Güler, Starr, & Pathman, 2011; see also Howe & Courage, 1997). We also have controlled encoding-related variability through matching of initial performance. Even with these controls, older infants exhibit less forgetting over time, relative to younger infants.
To illustrate the phenomenon, I use data from Bauer et al. (2000), in which elicited imitation was used to test recall of infants 13, 16, and 20 months of age both immediately and over delays of 1 to 12 months (with different infants tested at delays of 1, 3, 6, 9, and 12 months). Prior to imposition of the delay, the infants were exposed to novel multi-step event sequences. As a measure of encoding, we tested immediate imitation of some of the sequences. From the larger sample of 16- and 20-month-olds who had been assigned to delay intervals of 1, 3, and 6 months, Bauer (2005) created matched subsamples, such that 16- and 20-month-olds in the subsamples exhibited identical levels of performance prior to the delay, thus controlling encoding-related variability. When tested after 1 month, the age groups exhibited comparable levels of forgetting. However, after 3 months and 6 months, the younger infants exhibited more forgetting, relative to the older infants. The patterns cannot be explained by differential retrieval success, as older infants also exhibited greater relearning of the sequences, relative to the younger infants. Thus even with encoding controlled, younger infants forget more rapidly than older infants (see Bauer, 2005, for additional data on 13- and 16-month-olds).
Forgetting Explains Unique Variance in Long-term Recall
Differential retention is necessary for an explanation of developmental differences in long-term recall in terms of forgetting processes, but it is not sufficient. For forgetting to play a major role in explanation of patterns of long-term recall, it must explain unique variance in the outcome. We have found that it does, not only in infancy, but into the preschool years as well.
In Pathman and Bauer (2013), we exposed infants to a number of multi-step event sequences in an imitation-based task. We then used subsets of the events to “probe” the integrity of the memory traces of the events at four different delay intervals: 0 delay, as a test of encoding, and then after 15 minutes, 48 hours, and 2 weeks. We then tested long-term recall after 6 weeks. As expected, we observed forgetting over the course of the delay. We also observed that each of the “probes” accounted for variance in long-term recall. Importantly, only the 15-minute and 48-hour probes explained unique variance; encoding-related variability did not explain unique variance. Thus measures of the amount of forgetting during the period of consolidation and storage (after 15 minutes and 48 hours) explained unique variance in retrieval after a long delay (6 weeks; see also Bauer et al., 1999; and Bauer et al., 2011). A similar pattern of findings was apparent in Bauer, Larkina, and Doydum (2012) among 3- and 4-year-old children. In the preschool study, a measure of performance 1 week after encoding explained unique variance in recall after 5 weeks, whereas encoding-related variability did not. Work by Brainerd, Howe, and colleagues indicates that forgetting accounts for significant variance in recall throughout childhood (e.g., Brainerd et al., 1990; Howe & O’Sullivan, 1997).
Summary
The literature just reviewed makes clear that in the domain of episodic memory, forgetting is critical for understanding remembering. In the first two years of life, there are pronounced age-related differences in the rate at which information is lost from memory. The rate of forgetting is accelerated in younger relative to older infants. The differences cannot be explained by differential encoding. Moreover, the amount of information lost from memory within minutes to days after experience of events accounts for significant variance in long-term recall. Similar findings are obtained in the preschool years, and beyond. In the next section, I take the study of forgetting into the domain of autobiographical or personal memory. As will become apparent, forgetting helps to explain the shape of the distribution of autobiographical memories across the lifespan, including the phenomenon of childhood amnesia.
Forgetting in Autobiographical Memory: Childhood Amnesia
As argued in Bauer (2015), forgetting processes, and age-related changes in the vulnerability of memory traces to forgetting, have implications for one of the most robust phenomena in the memory literature, namely, childhood amnesia. After describing the phenomenon in adults and children, I provide data on the role of forgetting in explaining the phenomenon.
Childhood Amnesia: Adults
Late in the 19th century, Miles (1893) conducted a survey of adults’ childhood experiences. Among other things, the survey inquired as to the earliest event the respondents could remember, and how old they were at the time. This and subsequent similar surveys (e.g., Dudycha & Dudycha, 1933a, 1933b; Henri & Henri, 1895, 1896, 1898; Kihlstrom & Harackiewicz, 1982) have produced one of the most consistent and robust findings in the psychological literature. As illustrated in Table 1, Panel a, the average age of earliest memory for a specific past event among adults in Western cultures is age 3 to 4 years (see, for example, Wang, 2006, 2014, for discussions of cross-cultural differences in average age of earliest memory). Moreover, the average age of earliest memory is consistent whether the source of data is a survey, free recall (e.g., Bauer, Tasdemir-Ozdes, & Larkina, 2014; Waldfogel, 1948; Weigle & Bauer, 2000; West & Bauer, 1999), or response to a cue word prompt (e.g., Bauer & Larkina, 2014a; Rubin & Schulkind, 1997; though see Wang, Conway, & Hou, 2004, for evidence that repeated probes can produce earlier estimates).
Table 1.
Average Age of Earliest Memory among Samples of Adults (Panel a) and Children (Panel b)
| Study | Age of earliest memory (years) | |
|---|---|---|
| Panel a: Adults | ||
| Miles (1893) | 3.04 | |
| Dudycha & Dudycha (1933) | 3.58 | |
| Waldfogel (1948) | 3.50 | |
| Kihlstrom & Harackiewicz (1982) | 3.24 | |
| West & Bauer (1999) | 3.33 | |
| Bauer & Larkina (2013) | 3.59 | |
| Bauer, Tasdemir-Ozdes, & Larkina (2014) | 3.24 | |
| Panel b: Children | Age (years) | Age of earliest memory (years) |
| Peterson et al. (2005) | 6–9 | 3.00 |
| Bauer et al. (2007) | 7–10 | 3.67 |
| Bauer & Larkina (2013) | 7–11 | 3.67 |
| Peterson et al. (2005) | 10–13 | 3.67 |
| Reese et al. (2010) | 14 | 3.50 |
| Peterson et al. (2005) | 14–16 | 3.75 |
| Peterson et al. (2005) | 17–19 | 3.50 |
Note: Child data are from Bauer, Burch, Scholin, and Güler (2007); Bauer and Larkina (2014b); Peterson, Grant, & Boland (2005); and Reese, Jack, and White (2010).
The average age of earliest memory also is robust to age-cohort effects. That is, the same general pattern is obtained from individuals 60 to 70 years of age at the time the memories are elicited and 20 years of age at the time the memories are prompted (Rubin & Schulkind, 1997; see, Rubin, 2000, for review). The similarity is observed even though for younger adults, many fewer years have passed since childhood. The same average age of earliest memory is found even when respondents are asked to remember a specific event the date of which is clearly known, such as the birth of a younger sibling, or a hospitalization, for example (e.g., Sheingold & Tenney, 1982 Usher & Neisser, 1993). Thus seemingly regardless of age at inquiry or method of inquiry, prior to age 3 to 4 years, most adults have few if any memories of specific past events from their own lives.
Another characteristic feature of the “amnesia” that adults experience for early life events is that between the ages of 5 and 7 years, adults have a smaller number of autobiographical memories than would be expected based on forgetting alone. It is only from later in the first decade of life that most adults are able to recall a significant number of past events that are spatially and temporally localized, and which have some degree of personal relevance or significance. Although this “peculiar amnesia of childhood” (Freud, 1920/1935) is considered an adult phenomenon, as discussed below, there is a small but increasing body of evidence that by the end of the first decade of life, children also begin to experience it.
Though there are numerous studies of adults’ earliest memories, there are relatively fewer that are relevant to the shape of the distribution of memories across the lifespan. Indeed, the primary data on which is based the claim that before age 7 years, adults have a smaller number of autobiographical memories than would be expected based on forgetting alone, are derived from Rubin (1982); the shape of the distribution was empirically tested by Wetzler and Sweeney (1986). Rubin (1982) asked young adults to think of past events related to each of over 100 cue words (e.g., dog, table, book). He also asked them to estimate their age at the time of the event. To the data, Wetzler and Sweeney fitted a power function that in many investigations (e.g., Crovitz & Schiffman, 1974; Rubin & Wenzel, 1996; Rubin, Wetzler, & Nebes, 1986) has been shown to capture the distribution of memories across the lifespan. Wetzler and Sweeney found that the power function was a poor fit to data from birth to age 6 years, implying accelerated forgetting of memories from ages 6 and below. Memories from age 7 years were excluded from the analysis because age 7 years was considered the “inflection point” for childhood amnesia: after age 7 years, the rate of forgetting is assumed to be adult-like. Consistent with this suggestion, Wetzler and Sweeney found that the power function was a good fit to data from age 8 to adulthood (see Bauer, 2007, 2008, 2012, for additional discussions).
Childhood Amnesia: Children
As just described, childhood amnesia was first identified in adults. Moreover, for over 100 years, the data documenting the amnesia came exclusively from adults. That is, there were no data on the age of earliest memory or the distribution of early autobiographical memories in children. Beginning in the 21st century, data from children began to appear. As reflected in Table 1, Panel b, the data indicate that childhood amnesia is apparent in children as young as 6 to 9 years of age (Peterson, Grant, & Boland, 2005). Indeed, the data on the age of earliest memory among children are surprisingly similar to those among adults.
Accelerated Loss from Memory
The data on childhood amnesia in children present a paradox: why is it that children—who begin forming memories of specific past events even in infancy—themselves suffer from childhood amnesia? The explanation developed here (see also Bauer, 2015), is that early in life, the rate of forgetting is accelerated relative to later in life. As a result, though memories are formed, they are disproportionally forgotten, resulting in the phenomenon of childhood amnesia. Test of this hypothesis requires documenting memories created in the period eventually obscured by childhood amnesia and then prospectively tracking them across the boundary of the amnesia, to determine whether they are still remembered. Few studies fit this bill because most are retrospective in nature. That is, they are probes of what is retained from childhood, not what is lost. The few studies that focus on what is lost are discussed next; they reveal the emergence of childhood amnesia in childhood.
Emergence of childhood amnesia
One of the first prospective investigations of children’s memories from early childhood was conducted by Fivush and Schwarzmueller (1998). When children were 8 years of age, they were asked to recall events they had experienced at each of the ages of 3½ and 4 years and 5 and 6 years. The number of events that the 8-year-olds remembered decreased from 92% from the ages of 5 and 6 years to 77% from the ages of 3½ and 4 years. A similar trend was reported by Cleveland and Reese (2008), though the levels of memory were lower than those observed by Fivush and Schwarzmueller. Specifically, when children were 66 months of age (5½ years), they were asked to recall events that they had experienced at each of the ages of 19, 25, 32, 40, and 65 months. The number of events that the 5½-year-olds remembered decreased steadily as the retention interval increased from 1, 26, 34, 41, to 47 months in the past. The children recalled roughly 80% of events they had experienced 1 month in the past but fewer than 40% of events experienced 47 months in the past (at the age of 19 months). Both of these studies provide evidence that as time goes by, more and more events are lost from memory. Yet in both studies, events with the longest delays also had the earliest ages of encoding (i.e., 19 months in Cleveland & Reese, 2008). Thus it is not possible to determine whether forgetting was a result of the age of the children at the time of experience of the events or the length of the delay. Also, only Fivush and Schwarzmueller (1998) documented the fates of memories over the boundary of childhood amnesia (i.e., beyond age 7 years).
In Van Abbema and Bauer (2005) and Bauer and Larkina (2014b), we varied the retention interval, but held the age at encoding constant, thereby allowing for examination of fates of early memories over time. Specifically, when children were 3 years of age, we recorded conversations between children and their mothers as they discussed a number of events from the recent past. Based on their contributions to the conversations, the children clearly remembered the events. Thus we had documentation of memories from the age period corresponding to that from which adults report their earliest memories. We then tested different subgroups of the children again roughly 2, 3, 4, 5, or 6 years later, at the ages of 5, 6, 7, 8, or 9 years of age—ages at which, based on adult data, we would expect to see evidence of childhood amnesia. The later interviews were conducted by experimenters (rather than the children’s mothers). The data indicate a role for forgetting in explanation of the onset of the amnesia. Whereas the children 5 to 7 years of age remembered more than 60% of the events from age 3 years, the 8- and 9-year-olds remembered fewer than 40% of the events. Moreover, whereas a maximum of 6% of children ages 5, 6, and 7 years recalled none of the events from age 3 years, 37% of 8-year-olds and 25% of 9-year-olds recalled none of the early-life events.
There also is evidence that younger children forget more rapidly than older children. In Morris, Baker-Ward, and Bauer (2010), we examined recall of recent events by children ages 4, 6, and 8 years. One year later, when the children were 5, 7, and 9 years of age, we tested their recall of the events from 1 year in the past. The children who had been the oldest at the time of the events remembered 90% of them 1 year later (at 8 and 9 years of age, respectively). In contrast, the children who had been the youngest at the time of the events remembered only approximately 70% of them 1 year later (at 4 and 5 years of age, respectively). This pattern is strong evidence that within the period eventually obscured by childhood amnesia, the rate of forgetting is more accelerated among younger relative to older children.
Research by Peterson and colleagues (Peterson, Warren, & Short, 2011) provides additional evidence of the emergence of childhood amnesia in childhood. They interviewed children 4 to 13 year of age about their “earliest memory”; they interviewed the same children again 2 years later. Over the 2 years between interviews, the average age of earliest memory in the sample increased from 32.0 months to 39.6 months. Moreover, at the second interview, many of the children nominated a different “earliest” memory. Indeed, among the children 4 to 5 years at the time of the initial interview, fewer than 10% nominated the same event at both interviews. Children 12 to 13 years of age at the first interview were more consistent, yet still reported the same event only roughly 40% of the time. These patterns stand in sharp contrast to those we have observed in research with adults. Specifically, over the same delay, the age of earliest memory reported by adults does not change, and more than 80% of them nominate the same event as their “earliest” memory (Bauer et al., 2014). The pattern among children is suggestive of a “moving target” of earliest memory. In contrast, the corpus of adults is seemingly more stable.
The power function
The likely explanation for the greater stability of the corpus of early memories among adults (Bauer et al., 2014), relative to children (Peterson et al., 2011), is that among adults, the rate at which memories are forgotten slows over time. As described earlier, the results of retrospective studies in which adults are asked to provide memories in response to cue words (dog, table, book) indicate that the distribution of memories from ages 8 onward is well characterized by the power function (Crovitz & Schiffman, 1974; Rubin, 1982; Rubin et al., 1986). As discussed by Rubin and Wenzel (1996), the power function (e.g., Wickelgren, 1974, 1975) implies that equal ratios of time (t1/t2 = t3/t4) will result in equal ratios of recall (recall1/recall2 = recall3/recall4). The result is that as time goes by, the rate of forgetting slows down, presumably as a result of memory trace consolidation (see, for example, Wixted, 2004, for discussion).
The exponential function
The power function provides a good fit to adults’ data from ages 8 onward, but it does not provide a good fit to the distribution of autobiographical memories generated by children ages 7 to 11. Instead, as reported in Bauer et al. (2007) and Bauer and Larkina (2014a), the exponential—rather than the power—function provides a better fit to data from children. Rather than a reduction in the rate of forgetting over time, the exponential function implies an accelerated rate of forgetting that remains constant over time. In the first study to suggest accelerated forgetting (Bauer et al., 2007), we used the cue word technique to examine the distribution of autobiographical memories in children 7 to 10 years of age. The children successfully generated memories in response to the cue words and based on parental report, accurately dated them. Relative to the power function, the exponential function provided a better fit to the distribution of memories produced by the children (see Table 2).
Table 2.
Fit Indices for Exponential and Power Functions of the Distribution of Autobiographical Memories Elicited by Cue Words for Children 7 to 11 Years of Age and Adults
| Age Group | Fit by Function | |||
|---|---|---|---|---|
| Study | Overall | Individual age groups |
Exponential | Power |
| Bauer et al. (2007) | Children 7 to 10 years | .98 | .95 | |
| Bauer & Larkina (2013) | Children 7 to 11 years | .94 | .82 | |
| 7-year-olds | .97 | .94 | ||
| 8-year-olds | .92 | .87 | ||
| 9-year-olds | .89 | .84 | ||
| 10-year-olds | .88 | .82 | ||
| 11-year-olds | .86 | .72 | ||
| Adults | .65 | .91 | ||
| College students | .61 | .84 | ||
| Middle-age Adults | .70 | .93 | ||
Note: For each age group, the best fit function is highlighted by a box. Data are from Bauer, Burch, Scholin, and Güler (2007); and Bauer and Larkina (2013).
The same pattern was obtained in an independent study by Bauer and Larkina (2014a), in which we included not only children but adults as well, thus affording a within-study comparison of the distributions of memories. We tested 20 children at each of the ages of 7, 8, 9, 10, and 11 years (100 children total), as well as two groups of adults: college students and middle-aged adults. As reflected in Table 2, the data from the children provided a replication of the results of Bauer et al. (2007). For the entire sample of children and for each group of children (7-, 8-, 9-, 10-, and 11-year-olds) separately, the best fitting function to the distribution was the exponential. In contrast, for both the college students and middle-aged adults, the power function provided a superior fit to the distributions of memories. These data clearly suggest that as old as age 11 years, the distribution of children’s autobiographical memories is not adult-like. In contrast to adults, children experience exponential forgetting.
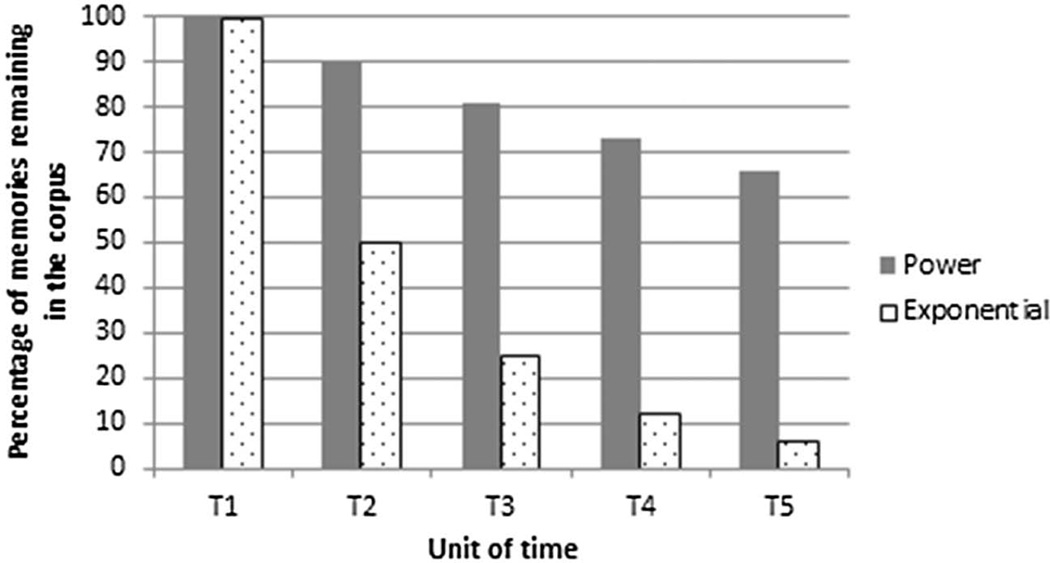
The exponential function has important implications for the expected distribution of memories over the lifespan. Specifically, it implies a constant half-life such that over each unit of time (e.g., a month) the number of memories in the corpus decreases by one-half. For example, if Time 1 recall was of 100 memories, then recall at Times 2, 3, and 4 would be of 50, 25, and 12.5 memories, respectively (Figure 2, dappled bars). Figure 2 makes clear the contrast between a distribution of memories characterized by the exponential function and a distribution characterized by the power function. The distributions differ both in terms of the initial rate of forgetting, and in terms of the number of memories lost from the corpus with each unit of time. For both adults and children, many events are lost from memory virtually immediately after experience of them. Importantly, for adults, the rate of forgetting slows over time, with individual memories becoming less vulnerable to disruption and interference, resulting in a relatively stable corpus (e.g., Wixted, 2004, for discussion). Children experience a sharp initial decline in the number of memories in the corpus (T2). Unlike adults, the rate at which children forget does not slow down over time—it is exponential. As a consequence, the pool of memories available for recollection is ever-shrinking, suggesting that memories do not consolidate (see Bauer, 2012, 2015; and Bauer et al., 2007; and Bauer & Larkina, 2014a, for discussions).
Figure 2.
Schematic depiction of the number of memories in the corpus that survive over each hypothetical unit of time (T1–T5) in distributions characterized by the power function (dark bars) and the exponential function (dappled bars).
Another important implication of an exponential rate of forgetting is that memories that survive the initial ravages of time may nevertheless eventually succumb to forgetting. This helps to explain the observations of Bauer and Larkina (2014b), in which children has robust memory for past events as many as 3 years after experience of them, yet by the time 4 to 5 years had passed, they were no longer as accessible. The phenomenon also helps to explain the findings of Peterson et al. (2011), in which it was observed that both the age of earliest memory and the specific event nominated as the “earliest” memory were unstable. The suggestion is that over the 2 years that intervened between interviews, early memories continued to be forgotten. As a result, the age of earliest memory had no place to go but up (to get older: from 32.0 to 39.6 months). With some early memories no longer accessible, the likelihood of sampling the same memory repeatedly over time decreases.
New Perspective on Characteristic Distribution
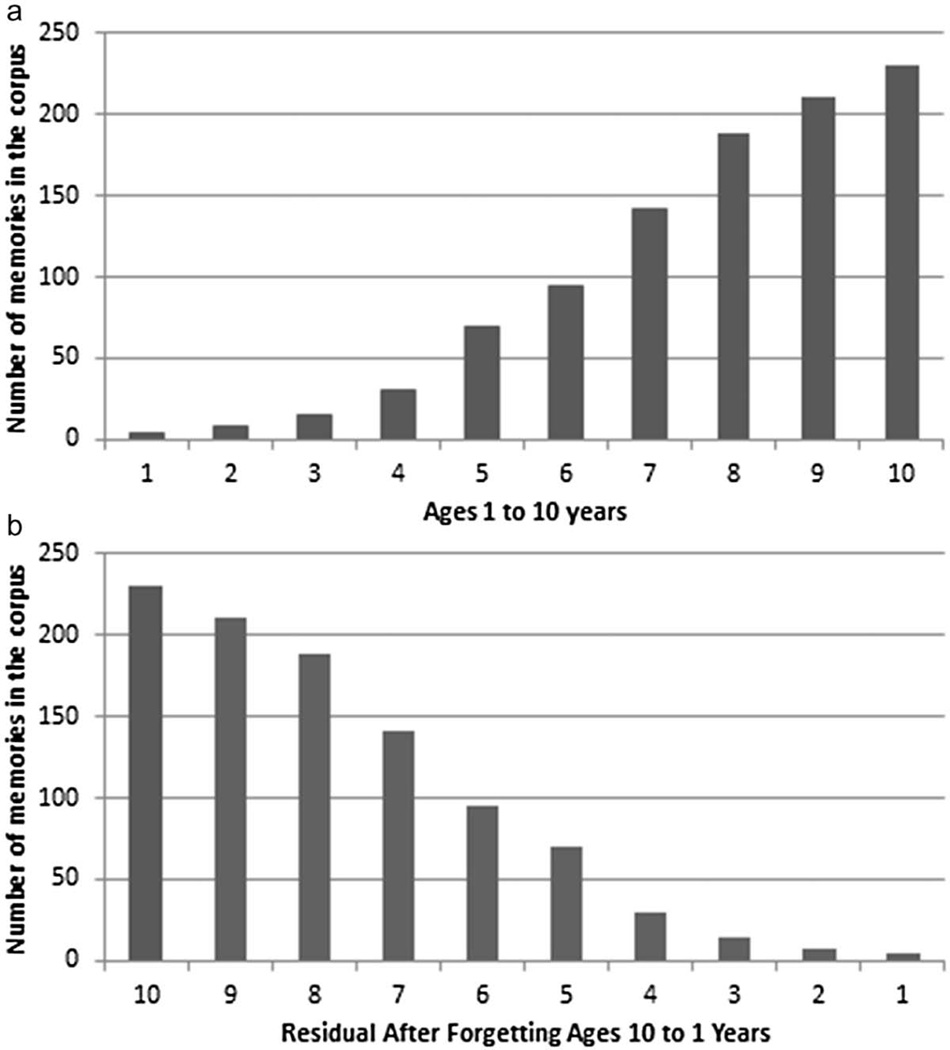
Consideration of the central role of forgetting in shaping the corpus of early childhood memories results in a new perspective on the characteristic distribution of autobiographical memories across the life span. As depicted in Figure 3, Panel a, tradition views on changes in the corpus suggest a gradually increasing number of autobiographical memories across the first decade of life. From the first 3 to 4 years, there are few (if any) memories in the corpus. Between the ages of 3 or 4 to 5 or 7, the number of memories increases, but is smaller than the number that would be expected by chance. The adult distribution is achieved in the latter part of the first decade, after age 7 years. As noted earlier, this distribution had been tested empirically (Wetzler & Sweeney, 1986). It is the perspective on the distribution of autobiographical memories that informs traditional theories of childhood amnesia (e.g., Nelson & Fivush, 2004; Pillemer & White, 1989).
Figure 3.
Schematic depiction of the distribution of memories across the first decade of life from a traditional perspective, suggesting a gradually increasing number of memories with age (Panel a); and from the complementary processes perspective, suggesting a residual number of early memories remaining after forgetting (Panel b).
Recognition that autobiographical memories are formed beginning early in life, and of the powerful forces of forgetting to which those early memories are subjected, results in a dramatic change in perspective. As illustrated in Figure 3, Panel b, the suggestion advanced here and developed in Bauer (2015) is that, rather than gradually increasing over time, the corpus of autobiographical memories actually shrinks over time. That is, events that once were remembered fail to fully consolidate, and thus remain vulnerable to decay and interference, until eventually, many are forgotten. From this perspective, the resulting distribution does not depict a gradual increase, but rather, the residual number of memories left in the corpus, after forgetting. In other words, from this perspective, the tail of the distribution apparent on the right of the figure is not the start of remembering, but the number of memories left in the corpus after forgetting. Moreover, because the rate of forgetting does not slow down, the pool of memories is ever-shrinking, contributing to the appearance of a “childhood amnesia component” (Pillemer & White, 1989)—a smaller number of memories than expected by normal forgetting (i.e., with “normal” forgetting equated with an adult rate, characterized by the power function). We may think of the process as one that reduces “pools” of memories to isolated “puddles” of memories. The result is a sparser representation. An additional consequence is that the representational structures become more difficult to retrieve, because they are more isolated from one another.
As evidenced in Bauer and Larkina (2014a; see also Bauer et al., 2007), accelerated forgetting is the norm throughout the first decade of life. Yet even during this period, there is a gradual approximation of normal forgetting. That is, between 7 and 11 years of age, the rate of forgetting, represented by the b parameter of the power function (e.g., Wixted & Ebbesen, 1997), decreased from 2.21 to 1.62; there was a further decrease to 1.01 by the college years. Though direct evidence of structure-process-function relations is not yet available, it is reasonable to attribute the change in the rate of loss of information from memory to development of the neural substrate responsible for encoding, consolidation, and retrieval of memories (see above). Neural developments would herald inceases in the efficiency and thus the efficacy of mnemonic processes. That is, with more efficient processes, fewer elements of experience would be lost, both early in the transformation of experience into a memory trace, and as the trace is being incorporated into long-term storage. Stronger, better integrated traces are more readily retrieved. The consequence of improvements in memory processes would be improved function, in this case, a reduction in the rate of forgetting. Over the course of development, the rate of forgetting gradually approximates that seen in adults.
Conclusions
The argument put forth in this review is that understanding of developmental changes in episodic and autobiographical memory requires consideration of both sides of the mnemonic coin: the complementary processes of improvements in the quality of memory representation and decreases in the vulnerability of those traces to forgetting (Bauer, 2015). Much of the developmental literature has focused only (or certainly, primarily) on improvements in memory with development. As a result of the attention, we have well documented evidence of increases in the fidelity with which events and experiences are encoded, in the precision with which the events in memory can be located in place and time, and in the robustness and degree of autonomy with which memory traces are retrieved, for example. Although these positive changes account for the waxing of the corpus of memories formed over the first decade of life, they fail to account for the waning of the corpus. A more complete understanding of the shape of the distribution of memories is achieved when we consider not only developmental changes that contribute to formation of memories that are of higher quality, but also developmental changes in the vulnerability of memories to the ravages of forgetting.
The primary focus of the present review has been on changes in forgetting. In the review, I documented age-related differences in the rate of forgetting, as evidenced by differences in the length of time over which infants maintain memories of specific past events (e.g., Bauer et al., 2000). The differences are apparent even when infants of different ages are well matched for their levels of initial encoding (Bauer, 2005), or when encoding-related variability is controlled statistically (Bauer et al., 1999), or by bringing all children to a criterion level of learning, thus eliminating encoding-related variability entirely (Bauer et al., 2011; Howe & Courage, 1997). The patterns strongly implicate age-related differences in the processes that take place after encoding, and which are responsible for the transformation of fleeting patterns of experience into enduring memory traces (a.k.a. consolidation). Critically, the amount of forgetting shortly after encoding explains significant unique variance in long-term recall both in infancy (e.g., Pathman & Bauer, 2013) and in the preschool years (Bauer et al., 2012).
A role for forgetting in shaping the distribution of what is remembered also is apparent in the literature on autobiographical or personal memory, and the specific phenomenon of childhood amnesia. Adults have few if any memories from the first years of life, and for much of their childhoods, a smaller number of memories than would be expected by normal forgetting alone (with adult rates of forgetting characterized by the power function; see Bauer, 2014, for a review). Traditional developmental accounts of the phenomenon explain the apparent increase in the number of memories over developmental time in terms of improvements in the quality of memory traces. Yet missing from these explanations is a role for forgetting, the complement of remembering (Bauer, 2015). Forgetting is essential to explanation of why events that once clearly were remembered later cannot be retrieved. Both retrospective (Bauer & Larkina, 2014a) and prospective (Morris et al., 2010) studies of autobiographical memory in childhood indicate that children forget more rapidly than adults, and that younger children forget more rapidly than older children (respectively). It is as rates of forgetting slow to adult levels that the adult distribution of autobiographical memories is achieved.
In the review, I also put forth an explanation for why youth is a risk factor for memory. The explanation is in terms of the processes that are necessary for the transformation of fleeting patterns of activation we call “experiences” into enduring memory representations. The transformation is demanding of neural structures and networks that undergo a protracted course of development. It is logical to assume that until they are fully developed, the structures and networks will operate with less than full efficiency and thus relatively ineffectively. The result is that memory traces are more vulnerable to forgetting. As the neural substrate develops, the rate of loss of information from memory approximates that seen in adults. The result is stabilization of the corpus of memories: memories that survived the “storm and stress” of their infancy and early childhood remain accessible to recollection. This helps to explain the stability of the corpus of earliest memories among adults (Bauer et al., 2014), and the absence of cohort effects even when comparing the age of earliest memory by 20-year-olds and 60- to 70-year-olds (Rubin & Schulkind, 1997).
When we entertain the argument that forgetting has a role to play in shaping the distribution of autobiographical memories over the first decade of life, we must take a new perspective on the traditional graph of that distribution. Rather than a depiction of a gradual increase in the number of memories over childhood (Figure 3, Panel a), the graph can be seen as representing the residual in the corpus of memories that remains after forgetting (Figure 3, Panel b). That is, when a 10-year-old looks back on her or his lifetime of memories, recent events are well represented—many memories are accessible to recollection. Yet when called upon to retrieve memories from earlier and earlier in life, fewer and fewer of the memories remain. The younger the child at the time of the experience, the more rapidly they were forgotten. This complementary processes account helps us to achieve a better understanding of the classic phenomenon of childhood amnesia as experienced by children as well as adults.
Highlights.
This review highlights the role of forgetting as an important variable in understanding the development of episodic and autobiographical memory.
Forgetting processes are implicated as a source of variability in long-term recall.
Forgetting processes are implicated due to the protracted course of development of the neural substrate for memory.
A complete account of how memory develops must consider changes in forgetting.
Acknowledgments
Support for much of the research from the author’s laboratory was provided by HD28425, HD42486, and Emory College of Arts and Sciences. The author extends a special note of gratitude to the many collaborators who contributed to the empirical work that forms that basis of this review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahám H, Vincze A, Jewgenow I, Veszprémi B, Kravják A, Gömöri E, Seress L. Myelination in the human hippocampal formation from midgestation to adulthood. International Journal of Developmental Neuroscience. 2010;28(5):401–410. doi: 10.1016/j.ijdevneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Holding it all together: How enabling relations facilitate young children’s event recall. Cognitive Development. 1992;7:1–28. [Google Scholar]
- Bauer PJ. Developments in declarative memory: Decreasing susceptibility to storage failure over the second year of life. Psychological Science. 2005;16:41–47. doi: 10.1111/j.0956-7976.2005.00778.x. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Remembering the times of our lives: Memory in infancy and beyond. Mahwah, NJ: Lawrence Erlbaum Associates; 2007. [Google Scholar]
- Bauer PJ. Infantile amnesia. In: Haith MM, Benson JB, editors. Encyclopedia of infant and early childhood development. San Diego, CA: Academic Press; 2008. pp. 51–61. [Google Scholar]
- Bauer PJ. The cognitive neuroscience of the development of memory. In: Courage ML, Cowan N, editors. The Development of Memory in Infancy and Childhood. Second Edition. New York, NY: Psychology Press; 2009. pp. 115–144. [Google Scholar]
- Bauer PJ. The life I once remembered: The waxing and waning of early memories. In: Berntsen D, Rubin DC, editors. Understanding autobiographical memory: Theories and approaches. Cambridge, UK: Cambridge University Press; 2012. pp. 205–225. [Google Scholar]
- Bauer PJ. Memory. In: Zelazo PD, editor. Oxford handbook of developmental psychology, Volume 1: Body and Mind. New York, NY: Oxford University Press; 2013. pp. 505–541. [Google Scholar]
- Bauer PJ. The development of forgetting: Childhood amnesia. In: Bauer PJ, Fivush R, editors. The Wiley-Blackwell Handbook on the Development of Children’s Memory. West Sussex, UK: Wiley-Blackwell; 2014. pp. 519–544. [Google Scholar]
- Bauer PJ. A complementary processes account of the development of childhood amnesia and a personal past. Psychological Review. 2015;2:204–231. doi: 10.1037/a0038939. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Burch MM, Scholin SE, Guler O. Using cue words to investigate the distribution of autobiographical memories in childhood. Psychological Science. 2007 doi: 10.1111/j.1467-9280.2007.01999.x. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Dow GAA. Episodic memory in 16- and 20-month-old children: Specifics are generalized, but not forgotten. Developmental Psychology. 1994;30:403–417. [Google Scholar]
- Bauer PJ, Doydum AO, Pathman T, Larkina M, Güler OE, Burch M. It’s all about location, location, location: Children’s memory for the “where” of personally experienced events. Journal of Experimental Child Psychology. 2012;113:510–522. doi: 10.1016/j.jecp.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Fivush R. The development of memory: Multiple levels and perspectives. In: Bauer PJ, Fivush R, editors. The Wiley-Blackwell Handbook on the Development of Children’s Memory. West Sussex, UK: Wiley-Blackwell; 2014. pp. 1–13. [Google Scholar]
- Bauer PJ, Güler OE, Starr RM, Pathman T. Equal learning does not result in equal remembering: The importance of post-encoding processes. Infancy. 2011;16:557–586. doi: 10.1111/j.1532-7078.2010.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Larkina M. Childhood amnesia in the making: Different distributions of autobiographical memories in children and adults. Journal of Experimental Psychology: General. 2014a;143(2):597–611. doi: 10.1037/a0033307. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Larkina M. The onset of childhood amnesia in childhood: A prospective investigation of the course and determinants of forgetting of early-life events. Memory. 2014b;22:907–924. doi: 10.1080/09658211.2013.854806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Larkina M, Doydum AO. Explaining variance in long-term recall in 3- and 4-year-old children: The importance of post-encoding processes. Journal of Experimental Child Psychology. 2012;113:195–210. doi: 10.1016/j.jecp.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Bauer PJ, Leventon JS. Memory for one-time experiences in the second year of life: Implications for the status of episodic memory. Infancy. 2013;18:755–781. [Google Scholar]
- Bauer PJ, Lukowski AF. The memory is in the details: Relations between memory for the specific features of events and long-term recall in infancy. Journal of Experimental Child Psychology. 2010;107:1–14. doi: 10.1016/j.jecp.2010.04.004. (Ms. ID# NIHMS196588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Mandler JM. One thing follows another: Effects of temporal structure on one- to two-year-olds’ recall of events. Developmental Psychology. 1989;25:197–206. [Google Scholar]
- Bauer PJ, Shore CM. Making a memorable event: Effects of familiarity and organization on young children’s recall of action sequences. Cognitive Development. 1987;2:327–338. [Google Scholar]
- Bauer PJ, Stewart R, White EA, Larkina M. A place for every event and every event in its place: Memory for locations and activities by 4-year-old children. Journal of Cognition and Development. (in press). [Google Scholar]
- Bauer PJ, Tasdemir-Ozdes A, Larkina M. Adults’ reports of their earliest memories: Consistency in events, ages, and narrative characteristics over time. Consciousness and Cognition. 2014;27:76–88. doi: 10.1016/j.concog.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer PJ, Van Abbema DL, de Haan M. In for the short haul: Immediate and short-term remembering and forgetting by 20-month-old children. Infant Behavior and Development. 1999;22:321–343. [Google Scholar]
- Bauer PJ, Wenner JA, Dropik PL, Wewerka SS. Parameters of remembering and forgetting in the transition from infancy to early childhood. Monographs of the Society for Research in Child Development. 2000;65 (4, Serial No. 263). [PubMed] [Google Scholar]
- Bauer PJ, Wewerka SS. Saying is revealing: Verbal expression of event memory in the transition from infancy to early childhood. In: van den Broek P, Bauer PJ, Bourg T, editors. Developmental spans in event representation and comprehension: Bridging fictional and actual events. Mahwah, NJ: Erlbaum; 1997. pp. 139–168. [Google Scholar]
- Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Archives of General Psychiatry. 1994;51(6):477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- Brainerd CJ, Reyna VF, Howe ML, Kingma J. The development of forgetting and reminiscence. Monographs of the Society for Research in Child Development. 1990;53:2–3. (Whole No. 222). [PubMed] [Google Scholar]
- Carver LJ, Bauer PJ. When the event is more than the sum of its parts: Nine-month-olds’ long-term ordered recall. Memory. 1999;7:147–174. doi: 10.1080/741944070. [DOI] [PubMed] [Google Scholar]
- Carver LJ, Bauer PJ. The dawning of a past: The emergence of long-term explicit memory in infancy. Journal of Experimental Psychology: General. 2001;130:726–745. [PubMed] [Google Scholar]
- Cleveland ES, Reese E. Children remember early childhood: Long-term recall across the offset of childhood amnesia. Applied Cognitive Psychology. 2008;22:127–142. [Google Scholar]
- Crovitz HF, Schiffman H. Frequency of episodic memories as a function of their age. Bulletin of the Psychonomic Society. 1974;4:517–518. [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cerebral Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Dudycha GJ, Dudycha MM. Adolescents’ memories of preschool experiences. Journal of Genetic Psychology. 1933a;42:468–480. [Google Scholar]
- Dudycha GJ, Dudycha MM. Some factors and characteristics of childhood memories. Child Development. 1933b;4:265–278. [Google Scholar]
- Ebbinghaus H. In: On memory. Ruger HA, Bussenius CE, translators. New York: Teachers’ College; 1885. 1913. Paperback edition, New York: Dover, 1964. [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. New York, NY, US: Oxford University Press; 2001. [Google Scholar]
- Fivush R. Subjective perspective and personal timeline in the development of autobiographical memory. In: Berntsen D, Rubin DC, editors. Understanding autobiographical memory: Theories and approaches. New York, NY: Cambridge University Press; 2012. pp. 226–245. [Google Scholar]
- Fivush R, Schwarzmueller A. Children remember childhood: Implications for childhood amnesia. Applied Cognitive Psychology. 1998;12:455–473. [Google Scholar]
- Fivush R, Zaman W. Gender, subjective perspective, and autobiographical consciousness. 2014:586–604. [Google Scholar]
- Freud S. Childhood and concealing memories. In: Brill AA, translator. The basic writings of Sigmund Freud. New York: The Modern Library; 1905/1953. [Google Scholar]
- Freud S. In: A general introduction to psycho-analysis. Riviere J, translator. Garden City, NY: Garden City Publishing Company, Inc.; 1920/1935. [Google Scholar]
- Friedman WJ. The development of memory for the times of past events. In: Bauer PJ, Fivush R, editors. The Wiley-Blackwell Handbook on the Development of Children’s Memory. West Sussex, UK: Wiley-Blackwell; 2014. pp. 394–407. [Google Scholar]
- Friedman WJ, Reese E, Dai J. Children’s memory for the times of events from the past years. Applied Cognitive Psychology. 2010;25:156–165. [Google Scholar]
- Ghetti S, Lee JK. The development of recollection and familiarity during childhood: Insight from studies of behavior and brain. In: Bauer PJ, Fivush R, editors. The Wiley-Blackwell Handbook on the Development of Children’s Memory. West Sussex, UK: Wiley-Blackwell; 2014. pp. 309–335. [Google Scholar]
- Gilboa A. Autobiographical and episodic memory - one and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42:1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Shi F, Woolson SL, Knickmeyer RC, Short SJ, Lin W, Zhu H, et al. Longitudinal Development of Cortical and Subcortical Gray Matter from Birth to 2 Years. Cerebral Cortex. 2011 doi: 10.1093/cercor/bhr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, Herman DH, Ordonez A, Greenstein D, Hayashi KM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Hamond NR, Fivush R. Memories of Mickey Mouse: Young children recount their trip to Disneyworld. Cognitive Development. 1991;6:433–448. [Google Scholar]
- Henri V, Henri C. On our earliest recollections of childhood. Psychological Review. 1895;2:215–216. [Google Scholar]
- Henri V, Henri C. Enquete sur les premiers souvenirs de l’enfance. Annee Psychology. 1896;3:184–198. [Google Scholar]
- Henri V, Henri C. Earliest recollections. Popular Science Monthly. 1898;53:108–115. [Google Scholar]
- Howe ML. The co-emergence of the self and autobiographical memory: An adaptive view of early memory. In: Bauer PJ, Fivush R, editors. The Wiley-Blackwell Handbook on the Development of Children’s Memory. West Sussex, UK: Wiley-Blackwell; 2014. pp. 545–567. [Google Scholar]
- Howe ML, Courage ML. On resolving the enigma of infantile amnesia. Psychological Bulletin. 1993;113:305–326. doi: 10.1037/0033-2909.113.2.305. [DOI] [PubMed] [Google Scholar]
- Howe ML, Courage ML. Independent paths in the development of infant learning and forgetting. Journal of Experimental Child Psychology. 1997;67:131–163. doi: 10.1006/jecp.1997.2395. [DOI] [PubMed] [Google Scholar]
- Howe ML, O’Sullivan JT. What children’s memoriestell us about recalling our childhoods: A review of storage and retrieval processes in the development of long-term retention. Developmental Review. 1997;17:148–204. [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex: Developmental changes and effects of aging. Brain Research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Squire LR. Neuroscience: Breaking down scientific barriers to the study of brain and mind. Science. 2000;290:1113–1120. doi: 10.1126/science.290.5494.1113. [DOI] [PubMed] [Google Scholar]
- Kihlstrom JF, Harackiewicz JM. The earliest recollection: A new survey. Journal of Personality. 1982;50:134–148. [Google Scholar]
- Lechuga MT, Marcos-Ruiz R, Bauer PJ. Episodic recall of specifics and generalisation coexist in 25-month-old children. Memory. 2001;9:117–132. doi: 10.1080/09658210042000111. [DOI] [PubMed] [Google Scholar]
- Lourenco SF, Frick A. Remembering where: The origins and early development of spatial memory. In: Bauer PJ, Fivush R, editors. The Wiley-Blackwell Handbook on the Development of Children’s Memory. West Sussex, UK: Wiley-Blackwell; 2014. pp. 367–393. [Google Scholar]
- Lukowski AF, Bauer PJ. Long-term memory in infancy and early childhood. In: Bauer PJ, Fivush R, editors. The Wiley-Blackwell Handbook on the Development of Children’s Memory. West Sussex, UK: Wiley-Blackwell; 2014. pp. 230–254. [Google Scholar]
- Lukowski AF, Lechuga MT, Bauer PJ. Memory for events and locations obtained in the context of elicited imitation: Evidence for differential retention in the scond year of life. Infant Behavior and Development. 2011;34:55–62. doi: 10.1016/j.infbeh.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. Philosophical Transactions of the Royal Society of London. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- McCormick C, St-Laurent M, Ty A, Valiante TA, McAndrews MP. Functional and effective hippocampal-neocortical connectivity during construction and elaboration of autobiographical memory retrieval. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory—A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Eichenbaum H. Consolidation and reconsolidation: Two lives of memories? Neuron. 2011;28:224–233. doi: 10.1016/j.neuron.2011.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzoff AN. Immediate and deferred imitation in fourteen- and twenty-four-month-old infants. Child Development. 1985;56:62–72. [Google Scholar]
- Miles C. A study of individual psychology. American Journal of Psychology. 1893;6:534–558. [Google Scholar]
- Morris G, Baker-Ward L, Bauer PJ. What remains of that day: The survival of children’s autobiographical memories across time. Applied Cognitive Psychology. 2010;24:527–544. [Google Scholar]
- Nelson CA, de Haan M, Thomas K. Neural bases of cognitive development. In: Kuhn D, Siegler R, Damon W, Lerner RM, editors. Handbook of child psychology. 6th ed. Hoboken, NJ: John Wiley & Sons, Inc; 2006. pp. 3–57. (Volume Editors: Volume 2 – Cognition, perception, and language) (Editors-in-Chief). [Google Scholar]
- Nelson K, Fivush R. The emergence of autobiographical memory: A social cultural developmental theory. Psychological Review. 2004;111:486–511. doi: 10.1037/0033-295X.111.2.486. [DOI] [PubMed] [Google Scholar]
- Ornstein PA, Baker-Ward L, Naus MJ. The development of mnemonic skill. In: Weinert FW, Perlmutter M, editors. Memory development: Universal changes and individual differences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. pp. 31–49. [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Walhovd KB. Morphometry and connectivity of the fronto-parietal verbal working memory network in development. Neuropsychologia. 2011;49(14):3854–3862. doi: 10.1016/j.neuropsychologia.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Østby Y, Tamnes CK, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of neuroscience. 2009;29(38):11772–11782. doi: 10.1523/JNEUROSCI.1242-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathman T, Bauer PJ. Beyond initial encoding: Measures of the post-encoding status of memory traces predict long-term recall in infancy. Journal of Experimental Child Psychology. 2013;114:321–338. doi: 10.1016/j.jecp.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathman T, Larkina M, Burch MM, Bauer PJ. Young children’s memory for the times of personal past events. Journal of Cognition and Development. 2013;14:120–140. doi: 10.1080/15248372.2011.641185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perner J, Ruffman T. Episodic memory and autonoetic consciousness: Developmental evidence and a theory of childhood amnesia. Journal of Experimental Child Psychology. 1995;59:516–548. doi: 10.1006/jecp.1995.1024. [DOI] [PubMed] [Google Scholar]
- Peterson C, Grant VV, Boland LD. Childhood amnesia in children and adolescents: Their earliest memories. Memory. 2005;13:622–637. doi: 10.1080/09658210444000278. [DOI] [PubMed] [Google Scholar]
- Peterson C, Warren KL, Short MM. Infantile amnesia across the years: A 2-year follow-up of children’s earliest memories. Child Development. 2011;82(4):1092–1105. doi: 10.1111/j.1467-8624.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- Piaget J. Play, dreams and imitation in childhood. New York: W. W. Norton & Co.; 1962. [Google Scholar]
- Pillemer DB, White SH. Childhood events recalled by children and adults. In: Reese HW, editor. Advances in child development and behavior, Volume. Vol. 21. Orlando, FL: Academic Press; 1989. pp. 297–340. [DOI] [PubMed] [Google Scholar]
- Reese E, Jack F, White N. Origins of adolescents’ autobiographical memories. Cognitive Development. 2010;25:352–367. [Google Scholar]
- Riggins T. Longitudinal investigation of source memory reveals qualitative differences between item memory and binding. Developmental Psychology. 2014;50:449–459. doi: 10.1037/a0033622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebers C. Children’s deliberate memory development: The contribution of strategies and metacognitive processes. In: Bauer PJ, Fivush R, editors. The Wiley-Blackwell Handbook on the Development of Children’s Memory. West Sussex, UK: Wiley-Blackwell; 2014. pp. 865–894. [Google Scholar]
- Rubin DC. On the retention function for autobiographical memory. Journal of Verbal Learning and Verbal Behavior. 1982;21:21–38. [Google Scholar]
- Rubin DC. The distribution of early childhood memories. Memory. 2000;8:265–269. doi: 10.1080/096582100406810. [DOI] [PubMed] [Google Scholar]
- Rubin DC. A basis systems approach to autobiographical memory. Current Directions in Psychological Science. 2005;14:79–83. [Google Scholar]
- Rubin DC. The basic-systems model of episodic memory. Perspectives on Psychological Science. 2006;1:277–311. doi: 10.1111/j.1745-6916.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Schulkind MD. The distribution of important and word-cued autobiographical memories in 20-, 35-, and 70-year-old adults. Psychology and Aging. 1997;12:524–535. doi: 10.1037//0882-7974.12.3.524. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Wenzel AE. One hundred years of forgetting: A quantitative description of retention. Psychological Review. 1996;103:734–760. [Google Scholar]
- Rubin DC, Wetzler SE, Nebes RD. Autobiographical memory across the adult lifespan. In: Rubin DC, editor. Autobiographical memory. Cambridge: Cambridge University Press; 1986. pp. 202–221. [Google Scholar]
- Schneider JFL, Il’yasov KA, Hennig J, Martin E. Fast quantitative diffusion-tensor imaging of cerebral white matter from the neonatal period to adolescence. Neuroradiology. 2004;46:258–266. doi: 10.1007/s00234-003-1154-2. [DOI] [PubMed] [Google Scholar]
- Seress L, Ábrahám . Pre- and postnatal morphological development of the human hippocampal formation. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. 2nd ed. Cambridge, MA: The MIT Press; 2008. pp. 187–212. [Google Scholar]
- Sheingold K, Tenney YJ. Memory for a salient childhood event. In: Neisser U, editor. Memory observed: Remembering in natural contexts. New York: W. H. Freeman and Company; 1982. pp. 201–212. [Google Scholar]
- St. Jacques PL, Kragel PA, Rubin DC. Dynamic neural networks supporting memory retrieval. NeuroImage. 2011;57:608–616. doi: 10.1016/j.neuroimage.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suddendorf T, Nielson M, van Gehlen R. Children’s capacity to remember a novel problem and to secure its future solution. Developmental Science. 2011;14:26–33. doi: 10.1111/j.1467-7687.2010.00950.x. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Tulving E. Episodic memory and autonoesis: Uniquely human? In: Terrace HS, Metcalfe J, editors. The missing link in cognition. Oxford: Oxford University Press; 2005. pp. 3–56. [Google Scholar]
- Usher J, Neisser U. Childhood amnesia and the beginnings of memory for four early life events. Journal of Experimental Psychology: General. 1993;122:155–165. doi: 10.1037/0096-3445.122.2.155. [DOI] [PubMed] [Google Scholar]
- Van Abbema DL, Bauer PJ. Autobiographical memory in middle childhood: Recollections of the recent and distant past. Memory. 2005;13:829–845. doi: 10.1080/09658210444000430. [DOI] [PubMed] [Google Scholar]
- Waldfogel S. The frequency and affective character of childhood memories. Psychological Monographs. 1948;62 (Whole No. 291). [Google Scholar]
- Wang Q. Earliest recollections of self and others in European American and Taiwanese young adults. Psychological Science. 2006;17(8):708–714. doi: 10.1111/j.1467-9280.2006.01770.x. [DOI] [PubMed] [Google Scholar]
- Wang Q. The cultured self and remembering. In: Bauer PJ, Fivush R, editors. Wiley-Blackwell Handbook on the Development of Children’s Memory. 2014. [Google Scholar]
- Wang Q, Conway M, Hou Y. Infantile amnesia: A crosscultural investigation. Cognitive Sciences. 2004;1:123–135. [Google Scholar]
- Weigle TW, Bauer PJ. Deaf and hearing adults’ recollections of childhood and beyond. Memory. 2000;8:293–309. doi: 10.1080/09658210050117726. [DOI] [PubMed] [Google Scholar]
- West TA, Bauer PJ. Assumptions of infantile amnesia: Are there differences between early and later memories? Memory. 1999;7:257–278. doi: 10.1080/096582199387913. [DOI] [PubMed] [Google Scholar]
- Wetzler SE, Sweeney JA. Childhood amnesia: An empirical demonstration. In: Rubin DC, editor. Autobiographical memory. New York: Cambridge University Press; 1986. pp. 191–201. [Google Scholar]
- Wheeler MA. Episodic memory and autonoetic awareness. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. New York: Oxford University Press; 2000. pp. 597–608. [Google Scholar]
- Wickelgren WA. Single-trace fragility theory of memory dynamics. Memory and Cognition. 1974;2:775–780. doi: 10.3758/BF03198154. [DOI] [PubMed] [Google Scholar]
- Wickelgren WA. Alcoholic intoxication and memory storage dynamics. Memory and Cognition. 1975;3:385–389. doi: 10.3758/BF03212929. [DOI] [PubMed] [Google Scholar]
- Wiebe SA, Bauer PJ. Interference from additional props in an elicited imitation task: When in sight, firmly in mind. Journal of Cognition and Development. 2005;6:325–363. [Google Scholar]
- Winocur G, Moscovitch M. Memory transformation and systems consolidation. Journal of the International Neuropsychological Society. 2011;17:766–780. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- Wixted JT. On common ground: Jost’s (1897) law of forgetting and Ribot’s (1881) law of retrograde amnesia. Psychological Review. 2004;111:864–879. doi: 10.1037/0033-295X.111.4.864. [DOI] [PubMed] [Google Scholar]
- Wixted JT, Ebbesen EB. Genuine power curves in forgetting: A quantitative analysis of individual subject forgetting functions. Memory & Cognition. 1997;25:731–739. doi: 10.3758/bf03211316. [DOI] [PubMed] [Google Scholar]
- Zola SM, Squire LR. The medial temporal lobe and the hippocampus. In: Tulving E, Craik FIM, editors. The Oxford handbook of memory. New York: Oxford University Press; 2000. pp. 485–500. [Google Scholar]