Abstract
Background & Aims
Substance P (SP), a neuropeptide member of the tachykinin family, plays a critical role in colitis. MicroRNAs (miRNAs) are small noncoding RNAs that negatively regulate gene expression. We examined whether SP modulates expression of microRNAs in human colonic epithelial cells.
Methods
We performed microRNA profiling analysis of SP-stimulated human colonic epithelial NCM460 cells overexpressing neurokinin-1 receptor (NCM460-NK-1R). Targets of SP-regulated microRNAs were validated by real-time polymerase chain reaction (RT-PCR). Functions of miRNAs were tested in NCM460-NK-1R cells and the trinitrobenzene sulfonic acid (TNBS) and dextran sulfate sodium (DSS) models of colitis.
Results
SP stimulated differential expression of 29 microRNAs, including miR-221-5p, the highest up-regulated miR (by 12.6-fold) upon SP stimulation. Bioinformatic and luciferase reporter analyses identified interleukin-6 receptor (IL-6R) mRNA as a direct target of miR-221-5p in NCM460 cells. Accordingly, SP exposure of NCM460-NK-1R cells increased IL-6R mRNA expression, and overexpression of miR-221-5p reduced IL-6R expression. Nuclear factor κB and c-Jun N-terminal kinase inhibition decreased SP-induced miR-221-5p expression. MiR-221-5p expression was increased in both TNBS- and DSS-induced colitis and in colonic biopsy samples from ulcerative colitis but not Crohn’s disease patients compared with controls. In mice, intracolonic administration of a miR-221-5p chemical inhibitor exacerbated TNBS- and DSS-induced colitis and increased colonic tumor necrosis factor-α, C-X-C motif chemokine 10 (Cxcl10), and collagen, type II, α 1 (Col2α1) mRNA expression. In situ hybridization in TNBS- and DSS-exposed colons revealed increased miR-221-5p expression primarily in colonocytes.
Conclusions
Our results reveal a novel NK-1R-miR-221-5p-IL-6R network that protects from colitis. The use of miR-221-5p mimics may be a promising approach for colitis treatment.
Keywords: Colitis, Inflammation, MicroRNA, Substance P
Abbreviations used in this paper: as, anti-sense; CAPE, caffeic acid phenethyl ester; COL2α1, collagen, type II, α 1; CXCL10, C-X-C motif chemokine 10; DSS, dextran sulfate sodium; IBD, inflammatory bowel disease; IL, interleukin; IL-6R, interleukin-6 receptor; JAK-STAT, Janus kinase/signal transducer and activator of transcription; JNK, c-Jun N-terminal kinase; miRNA, microRNA; NF-κB, nuclear factor-κB; NK-1R, neurokinin-1 receptor; RT-PCR, real-time polymerase chain reaction; siRNA, small-interfering RNA; SP, substance P; TNBS, trinitrobenzene sulfonic acid; TNFα, tumor necrosis factor-α; UC, ulcerative colitis; UTR, untranslated region
Summary.
Substance P–neurokinin-1 (NK-1R) microRNA-221-5p (miR-221-5p) network regulates inflammation in human colonic epithelial cells through inhibition of interleukin-6R expression. Because silencing of miR-221-5p exacerbates experimental colitis, the use of miR-221-5p mimics may be a promising approach for colitis treatment.
Substance P (SP), an 11-amino-acid peptide member of the tachykinin family, is expressed in many organs including the intestine.1 SP plays a critical role in colitis pathophysiology by interacting with its high-affinity neurokinin-1 receptor (NK-1R)2, 3, 4 and activating signaling pathways related to nuclear factor-κB (NF-κB)5, 6, 7 and c-Jun N-terminal kinase (JNK)8 in different cell types, including colonocytes.5, 8, 9 NK-1R expression is increased in the colon of inflammatory bowel disease (IBD) patients,10, 11 further suggesting an important role in IBD pathogenesis.
MicroRNAs (miRNA, MiR) represent a class of small noncoding single-stranded RNAs that control translation and mRNA degradation by binding to target mRNAs through complementary sequences.12 Functional studies have demonstrated that miRNAs play critical roles in many physiologic and pathologic conditions, including inflammation13 and colitis pathogenesis.14, 15, 16 There is limited evidence, however, on the interaction between the SP/NK-1R system and miRNAs. Both miR-130a and miR-206 were found to regulate SP synthesis and release in neuronal cells,17 and miR-449b and miR-500 modulate NK-1R expression in human astrocytoma cells.18 Moreover, a miR-203 mimic blocked SP-mediated increased phospholipase-A2 activating protein expression in keratinocytes.19 However, whether SP modulates miRs in colonic epithelial cells and whether this response is related to the ability of SP to regulate colitis is not known. We performed a miRNA expression analysis to detect the miRNA signature upon SP stimulation of human colonic NCM460 epithelial cells.
Materials and Methods
Cell Studies and Reagents
NCM460 human colonic epithelial cells overexpressing NK-1R (NCM460-NK-1R), cultured as previously described elsewhere,5 were starved in serum-free medium overnight and then stimulated with 0.1 μM SP at specific times. CAPE (caffeic acid phenethyl ester; cat. no. C8221), a specific inhibitor of NF-κB, was obtained from Sigma-Aldrich (St. Louis, MO), and the JNK inhibitor SP600125 (cat no. 8177) was obtained from Cell Signaling Technology (Beverly, MA). SP was purchased from Sigma-Aldrich (cat. no. S6883). Rabbit anti-interleukin-6 receptor (anti-IL6R; SC-661) was purchased from Santa Cruz Biotechnology (Dallas, TX). Mouse anti-β-actin was obtained from Sigma-Aldrich.
Transfection Experiments
Inhibitors of miR-221-5p (cat. no. 4464084), negative anti-miRNAs controls (cat. no. 4464076), a miR-221-5p mimic (cat. no. 4464067), and mimic miRNA controls (cat. no. 4464058) were purchased from Life Technologies (Carlsbad, CA). Mouse anti-miR-221-5p and negative control were purchased from Exiqon (Vedbæk, Denmark); the target sequence of anti-miR-221-5p is TGTAACATACGGTCC, and the target sequence of anti-miR-control is ACGTCTATACGCCCA. Lipid-based siPORTNeoFX Transfection Agent was purchased from Ambion (AM4511; Ambion/Life Technologies, Austin, TX), Lipofectamine 2000 was purchased from Life Technologies (cat. no. 52758). NF-κB p65 small-interfering RNA (siRNA; sc-29140), c-Jun siRNA (sc-29223), and control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology.
NCM460-NK-1R cells were transfected with siRNA using Lipofectamine RNAiMAX (Life Technologies). For miR-221-5p silencing or overexpression, the cells were transfected with antisense-miR-221-5p (as-miR-221-5p) or miR-221-5p mimic, respectively. Cells transfected with siRNA-control, antisense-control miR, or miR-mimic control served as controls.
Microarray Analysis of miRNA Expression
The miRNA microarray experiments were performed using the TaqMan low-density array human miRNA v1.0 system, which contains 365 microRNAs. The high-capacity reverse transcription reagent for cDNA was from Applied Biosystems (cat. no. 4368813; Foster City, CA). The real-time polymerase chain reaction (RT-PCR) primers were purchased from Life Technologies, except the miR-221-5p primers which were from Exiqon (cat. no. 204302). The total RNA of the NCM460-NK-1R cells were isolated by using TRIzol reagents, and the RNA concentration were determined by Nanodrop. Data were collected and normalized to nonfunctional small RNA internal controls. The results were validated using quantitative reverse-transcription PCR. The miRNA template for RT-PCR analysis was prepared using Exiqon reagents. RNU1A1 (cat. no. 203909; Exiqon) expression was used as the internal control. The threshold cycle (Ct) value formula was used to calculate the relative expression of selected miRNAs, as we previously reported elsewhere.20
Human Inflammatory Bowel Disease Biopsy Specimens
Total RNAs from the colon tissues of patients with active ulcerative colitis (UC) (n = 14), active Crohn’s disease (n = 15), and healthy individuals (n = 9–10) were purchased from OriGene (Rockville, MD). These biopsy samples were obtained through strict institutional review board protocols and with full, documented patient consent, all from accredited U.S.-based medical institutions (www.origene.com). Conversion of the cDNA of RNA samples was performed as described earlier, and the levels of NK-1R, IL-6R and miR-221-5p were determined by quantitative RT-PCR analysis.
Luciferase Assays
IL-6R 3′-UTR (untranslated region) containing the two predicted binding sites and mutated sequences were chemically synthesized by GENEWIZ (South Plainfield, NJ). The wild-type and mutants of the IL-6R 3′-UTR sequence were then subcloned into the luciferase reporter vector from SwitchGear Genomics (cat. no. 32011; Carlsbad, CA). NCM460-NK-1R cells were transfected using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) with luciferase reporter constructs containing the wild-type or mutant of 3′-UTR of IL-6R and the miR-221-5p mimic. Cell lysates were prepared 24 hours after transfection, and luciferase activity was measured using the LightSwitch Luciferase Assay Kit from SwitchGear Genomics (cat no. LS100) according to the manufacturer’s instructions.
Immunoblot Analyses
NCM460-NK-1R cells were washed with ice-cold phosphate-buffered saline and incubated with radiolabeled immunoprecipitation assay buffer containing the protease inhibitors and sodium orthovanadate (Santa Cruz Biotechnology) for 5 minutes on ice. Equal amount of cell lysates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes. The membranes were blocked (phosphate-buffered saline, 5% nonfat dry milk, 0.05% Tween-20) and probed with antibodies followed by corresponding horseradish peroxidase-labeled secondary antibodies. Blots were developed with an enhanced chemiluminescence reagent (cat. no. 34080; Thermo Fisher Scientific, Waltham, MA).
In Situ Hybridization
In situ hybridization was performed on mice colon tissue from C57BL/6J mice after treatement with trinitrobenzene sulfonic acid (TNBS) (7 days) or dextran sodium sulfate(DSS) (6 days), as we previously reported elsewhere21 and as described herein. The sequence of the probe specific for mouse miR-221-5p (cat no. 99999-15; Exiqon) was as follows: 5′-ACAGAAATCTACATTGTATGCCA. The experiments were performed according to the manufacturer’s instructions using miRCURY LNA microRNA ISH optimization Kit (FFPE; Exiqon).
Experimental Colitis
We induced colitis via TNBS and DSS treatment with modifications of previously described protocols.21 All animal studies were approved by the institutional animal care and use committee. Total RNA from the colonic tissues of the TNBS model was purified by use of the miReasy Mini Kit (Qiagen, Valencia, CA) and from the DSS model by use of the lithium chloride precipitation method.22 Tissue sections were scored for histopathology analyses in a double-blinded manner, as previously reported elsewhere.21
Trinitrobenzene Sulfonic Acid-Induced Colitis
Animals were maintained at University of California–Los Angeles animal research facility and received standard pelleted chow and water ad libitum. The 6- to 8-week-old male C57BL/6J mice (n = 5–8 per group) received a 50-μL intracolonic injection of 40 mg/kg TNBS (Fluka, Ronkonkoma, NY) in 30% ethanol, using a 1-mL syringe (Becton Dickinson, Laguna Hills, CA) fitted with a polyethylene cannula (Intramedic PE-20 tubing; Becton Dickinson). The control groups were injected with 50 μL of 30% ethanol intracolonically. The mice were held head down for 1 minute after the enema administration to ensure the containment of the TNBS solution into the colon. The mice returned to their cages; they were sacrificed after 7 days by cervical dislocation, and the colonic tissues were collected.
To assess the effect of miR-221-5p in TNBS-induced colitis, mice were intracolonically administered 40 mg/kg TNBS; then, on days 1, 3 and 5, they were intracolonically injected with 40 μg of miRCURY LNA Inhibitor probe in vivo against mmu-miR-221-5p (Exiqon). Briefly, the appropriate amount of oligonucleotides against mmu-miR-221-5p and its respective control were resuspended in 100 μL of Opti-MEM with 2 μL of lipofectamine 2000 and administered intracolonically. On day 7 the mice were sacrificed, and their colon tissue was collected for H&E staining and RNA expression analysis.
DSS-Induced Colitis
To assess the miRNA expression, 1% DSS was dissolved in the drinking water and administered to C57BL/6J mice for 5 days (n = 5–8 mice per group). To test the effect of miR-221-5p in DSS-induced colitis, mice were administered 1% DSS, then, on days 1, 3, and 5, the mice were intracolonically injected with 40 μg anti-miR-221-5p or anti-miR-control, as described earlier. On day 6 the mice were sacrificed, and their colon tissue was collected for H&E staining and RNA expression analysis.
Statistical Analysis
Student t test for two-group comparisons and analysis of variance for multiple-group comparisons were performed to determine any statistically significant differences between the experimental groups. P < .05 was considered statistically significant. All results are expressed as mean ± standard deviation.
Results
Substance P Regulates Expression of MicroRNAs in Human Colonic Epithelial Cells
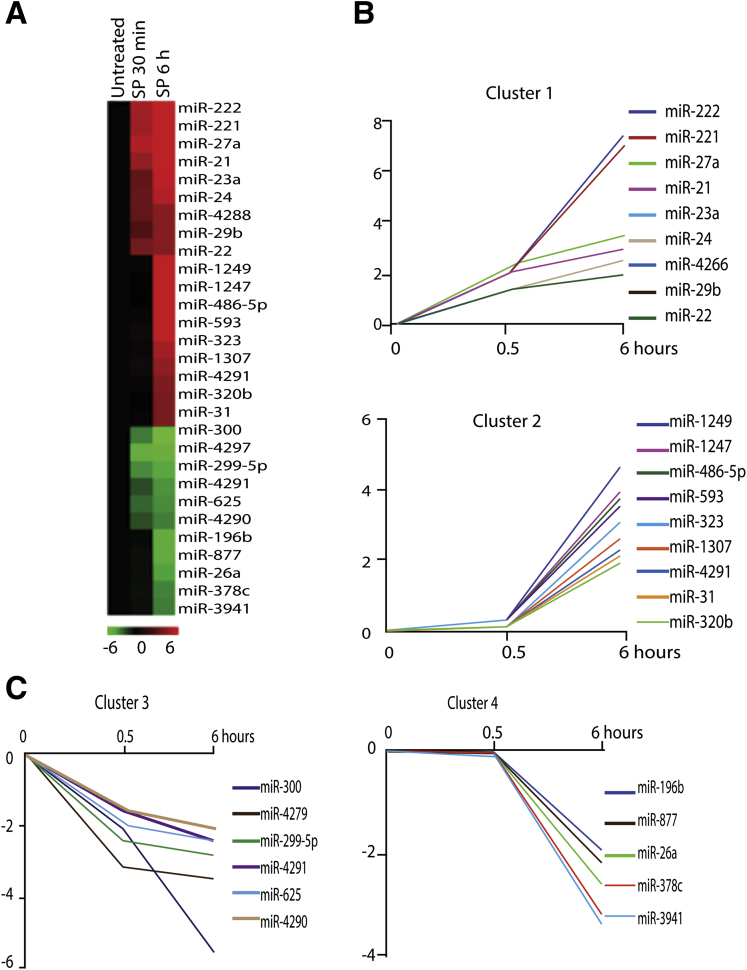
To investigate the effect of SP on miRNA expression in human colonic epithelial cells, we used 0.1 μM SP to stimulate NCM460-NK-1R cells in serum-free medium at 0.5 and 6 hours. This SP concentration was used in previous studies to stimulate NCM460-NK-1R cells.9, 23 Total RNA was isolated, and the miRNA array analysis was performed as we previously reported.24 We found that 29 miRNAs (18 up-regulated and 11 down-regulated) had altered expression upon SP stimulation (Figure 1A).
Figure 1.
Substance P (SP)-regulated microRNAs (miRNA) in colon epithelial cells. (A) Heat map represents differentially expressed miRNAs after 0.1 μM SP treatment (0.5 and 6 hours) in NCM460-NK-1R cells. Red indicates up-regulated miRNAs, and green indicates down-regulated miRNAs. Cluster of (B) SP-induced and (C) SP-suppressed miRNAs according to their response dynamics.
Hierarchical clustering analysis revealed that four different clusters of miRNAs were affected by SP. Specifically, the up-regulated miRNAs were divided into miRNAs that showed an early and stable response (expression was increased at 30 minutes of stimulation) and a late response (expression was no different at 30 minutes of stimulation but was altered after 6 hours of stimulation) (see Figure 1B). Similarly, the down-regulated miRNAs were divided into early (cluster 3) or late (cluster 4) response clusters (see Figure 1C). Among the up-regulated miRNAs, miR-221 and miR-222 showed the highest increase.
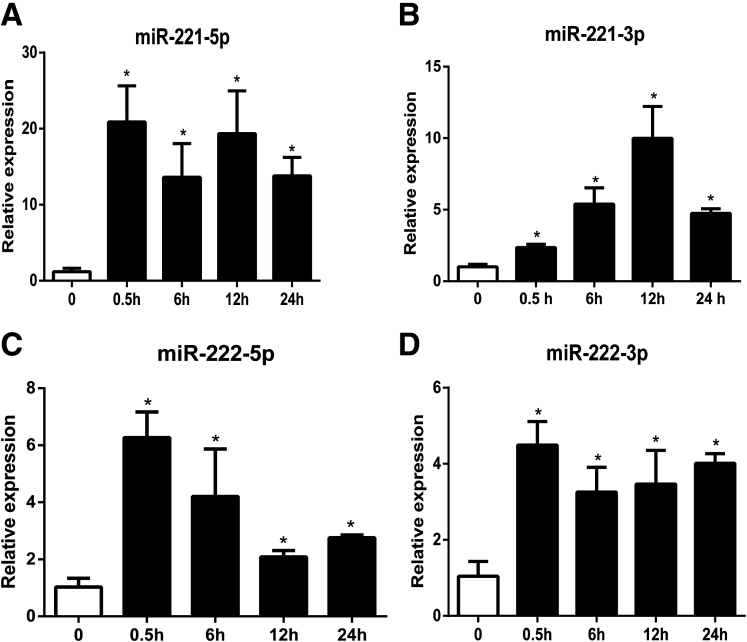
MiR-221 and miR-222 are encoded in a cluster on the X chromosome. Pre-miR-221 is processed in the cytoplasm and produces two forms of mature miRNA: miR-221-5p (also named as miR-221*) and miR-221-3p (normally referred as miR-221). RT-PCR analysis validated that miR-221-5p, miR-221-3p, miR-222-5p, and miR-222-3p were substantially increased by SP at 30 minutes, with a sustained increase over controls at 6, 12, and 24 hours (Figure 2). Among those four mature miRNAs, miR-221-5p showed a more substantial increase compared with other three mature miRNAs. Thus, we next focused on SP-miR-221-5p interactions.
Figure 2.
Quantitative real-time polymerase chain reaction (RT-PCR) detection of substance P (SP) induced mature microRNA (miRNA) expression. Mature miRNAs expression quantification was performed after 0.1 μM SP treatment (0.5, 6, 12, and 24 hours) of NCM460-NK-1R cells. Expression levels of miR-221-5p, miR-221-3p, miR-222-5p, and miR-222-3p were assessed by RT-PCR analysis. *P < .05, data show mean ± standard deviation of triplicate samples per experimental condition.
MicroRNA-221-5p and Neurokinin-1 Receptor Are Up-Regulated in Human Ulcerative Colitis Tissues
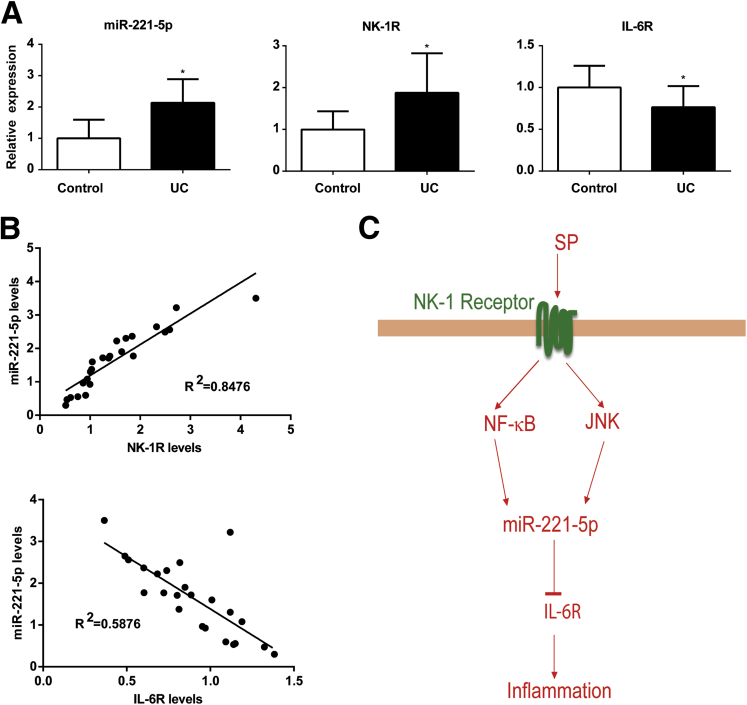
Our results indicated that NK-1R signaling is linked to increased miR-221-5p expression in human colonic epithelial cells. Previous results had demonstrated increased NK-1R expression in the colon of IBD patients.10, 25 Therefore, we next examined whether miR-221-5p expression is also increased in human IBD samples. Our results showed increased expression of miR-221-5p in UC biopsy samples (n = 14) compared with biopsy samples from controls (n = 10, P = .01; Figure 3A). However, we found no statistically significant differential expression of miR-221-3p, miR-222-5p, or miR-221-3p in those samples. The expression of miR-221-5p was not statistically significantly different in the Crohn’s disease tissues (n = 15) compared with the control samples (n = 9, P = .28). Examination of the same UC tissues also revealed increased NK-1R mRNA expression when compared with controls (see Figure 3A).
Figure 3.
Level of miR-221-5p in inflammatory bowel disease tissues. (A) Expression of microRNA (miR)-221-5p, neurokinin-1 receptor (NK-1R), and interleukin-6 receptor (IL-6R) in human ulcerative colitis (UC) tissue samples (n = 14) compared with control tissue samples (n = 10). *P < .05, data show mean ± standard deviation. (B) Correlation of miR-221-5p levels with NK-1R and IL-6R. The correlation coefficient was calculated using Prism6 (GraphPad Software, San Diego, CA). (C) Schematic representation of proposed substance P (SP)–miRNA signaling pathway in human colonic epithelial cells. After binding with the NK-1R receptor, SP stimulates miR-221-5p expression via nuclear factor-κB (NF-κB) and c-Jun N-terminal kinase (JNK) activation in human colonic epithelial cells. MiR-221-5p regulates inflammation through down-regulation of IL-6R expression.
Substance P Regulates MicroRNA-221-5p Expression Through Nuclear Factor-κB and c-Jun N-Terminal Kinase Pathways
Because NK-1R coupling activates the transcription factor NF-κB and JNK5, 6, 7, 8 and these pathways are involved in experimental colitis,26, 27 we examined their involvement in SP-regulated miR-221-5p expression. Bioinformatics analysis by using the online transcription factor search software TFSearch (Computational Biology Research Consortium, Tokyo, Japan) indicated the presence of NF-κB binding sites (The sequence is 5′-GGAACGTCCC-3′, from 45750897bp to 45750906 bp of X chromosome, localized in 4740 bp upstream of miR-221, confirming a recent report.28)
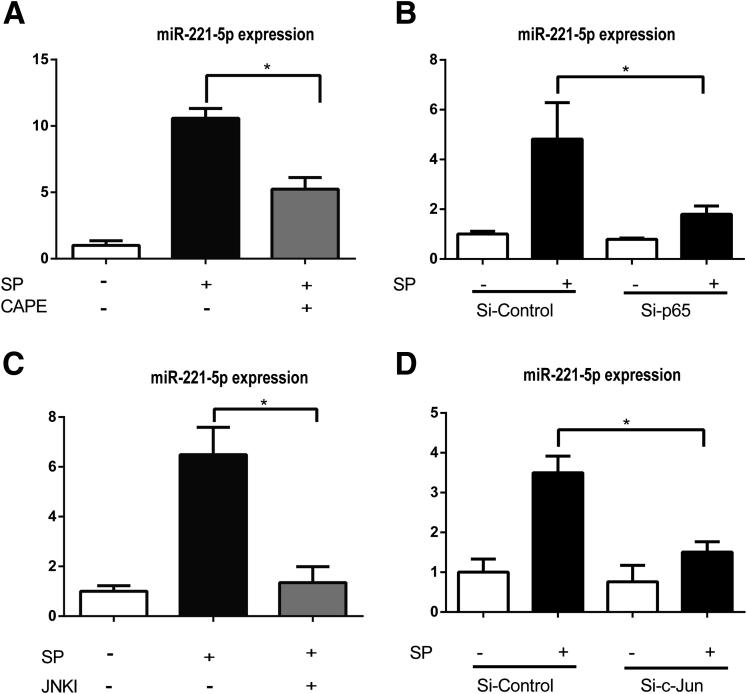
To examine the functional role of NF-κB on miR-221 expression levels, we used the NF-κB inhibitor CAPE, which prevents translocation of the p65 subunit of NF-κB to the nucleus.29 NCM460-NK-1R cells were treated with 10 μM CAPE for 30 minutes before SP (0.1 μM) stimulation for 6 hours. Our results showed that CAPE significantly reduced miR-221-5p expression (Figure 4A). Moreover, silencing of the p65 subunit of NF-κB using siRNA substantially decreased SP-induced miR-221-5p expression in NCM460-NK-1R cells compared with control siRNA (see Figure 4B).
Figure 4.
Substance P (SP) regulates microRNA (miR)-221-5p expression through nuclear factor-κB (NF-κB) and c-Jun N-terminal kinase (JNK) pathways. MiR-221-5p expression was assessed by real-time polymerase chain reaction (RT-PCR). (A) NCM460-NK-1R (neurokinin-1 receptor) cells were pretreated with the NF-κB inhibitor caffeic acid phenethyl ester (CAPE, 10 μM) 30 minutes before SP exposure for 6 hours. (B) MiR-221-5p levels in cells transfected with siRNA-p65 for 48 hours before SP exposure of 6 hours. (C) NCM460-NK-1R cells were pretreated with the JNK inhibitor SP600125 30 minutes before SP exposure for 6 hours. (D) MiR-221-5p levels in cells transfected with siRNA-c-Jun for 48 hours before SP exposure for 6 hours. *P < .05, data show mean ± standard deviation of triplicate.
Previous results indicated that c-Jun regulates miR-221 expression in lung, liver, and prostate cancer cells.28, 30 To address the involvement of c-Jun in SP-increased miR-221-5p expression, we pretreated NCM460-NK-1R cells with 50 μM of the JNK inhibitor SP60012531 and found that SP-stimulated miR-221-5p expression was almost normalized by SP600125 (see Figure 4C). To confirm this result, we silenced c-Jun using a siRNA approach as described in Materials and Methods. As shown in Figure 4D, c-Jun silencing reduced SP-induced increased miR-221-5p expression. Taken together, these results indicate that SP regulates miR-221-5p expression in human colonic epithelial cells through activation of NF-κB and JNK.
MicroRNA-221-5p Regulates Directly Interleukin-6 Receptor Expression in Human Colonic Epithelial Cells
MicroRNAs negatively regulate gene expression by binding to complementary sequences in the 3′ untranslated region (3′-UTR) of their target genes. Because SP regulates expression of several proinflammatory genes, we next determined whether miR-221-5p regulates directly inflammation-related genes using bioinformatics.
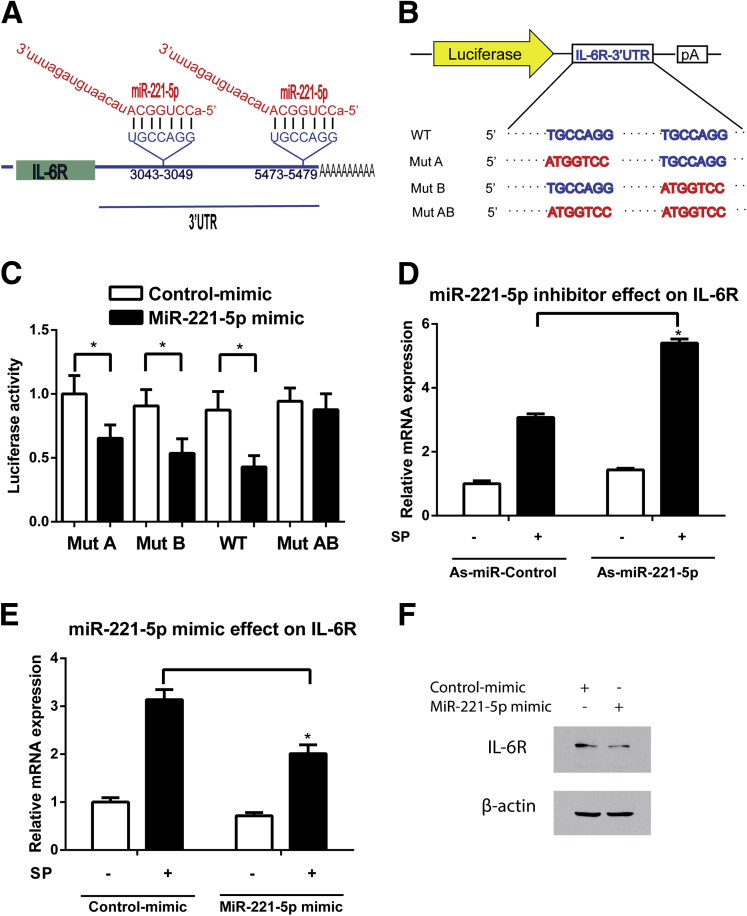
Our bioinformatics analysis, using a miRNA target prediction program from DIANA laboratory,32 showed the presence of two binding sites for miR-221-5p in the 3′-UTR of human IL-6R mRNA sequence (NM_00565.3) (Figure 5A). A luciferase activity assay using plasmids containing the miR-221-5p binding sites in the 3′-UTR of IL-6R indicated that cells transfected with a miR-221-5p mimic had lower luciferase activity compared with control-miR-mimic-treated NCM460-NK-1R cells (n = 3, P < .05; see Figure 5C), suggesting that IL-6R 3′-UTR is sufficient to confer miR-221-5p regulation (see Figure 5B and C). Importantly, when both the miR-221-5p seed regions in the IL-6R 3′-UTR were mutated, miR-221-5p regulation was abolished (see Figure 5B and C).
Figure 5.
MicroRNA (MiR)-221-5p regulates interleukin-6 receptor (IL-6R) expression. (A) Human IL-6R mRNA contains two predicted miR-221-5p binding sites. (B) A diagram of IL-6R 3′–untranslated region reporters. (C) Wild-type or mutant IL-6R 3′-UTR luciferase constructs were cotransfected with miR-221-5p mimic or control mimic in NCM460-NK-1R cells. Luciferase activity was determined 24 hours after transfection, and normalized luciferase activity is shown. *P < .05, data show mean ± standard deviation of triplicate. (D). MiR-221-5p down-regulation increased IL-6R mRNA expression in response to substance P (SP) stimulation. *P < .05, data show mean ± standard deviation of triplicate (E). Up-regulation of miR-221-5p decrease IL-6R mRNA expression in response to SP stimulation. *P < .05, data show mean ± standard deviation of triplicate. (F) IL-6R and β-actin protein levels are assessed by Western blot in miR-221-5p mimic or control-mimic treated NCM460-NK-1R cells.
To elucidate the effects of miR-221-5p on IL-6R mRNA expression levels, we transfected NCM460-NK-1R cells with 0.1 μM of antisense miR-221-5p (as-miR-221-5p), or miR-221-5p mimic, or their controls using siPORT NeoFX (Life Technologies). After 48 hours, the cells were placed in serum-free medium overnight, and then they were stimulated with 0.1 μM SP. The total RNA was isolated for RT-PCR analysis. As shown in Figure 5D, miR-221-5p silencing increased the IL-6R levels. Conversely, miR-221-5p mimic treatment reduced the IL-6R levels compared with the mimic control-treated cells (see Figure 5E). Thus, miR-221-5p inhibits IL-6R mRNA expression in human colonic epithelial cells. Moreover, treatment of cells with miR-221-5p mimic reduces IL-6R protein level (see Figure 5F). Together, these results indicate that IL-6R is a downstream target of miR-221-5p in human colonocytes.
Based on the negative correlation of miR-221-5p to IL-6R expression shown here, we examined IL-6R mRNA levels in the same colon samples. As shown in Figure 3A, the IL-6R expression levels were statistically significantly decreased (by 23%, P < .05) compared with controls. NK-1R levels were correlated positively with miR-221-5p (R2 = 0.8476), whereas miR-221-5p levels correlated negatively with IL-6R (R2 = 0.5876) (see Figure 3B). These results indicate that NK-1R-miR-221-5p signaling is activated and IL-6R expression is decreased in the colon during active UC.
MicroRNA-221-5p Is Up-Regulated in Experimental Colitis
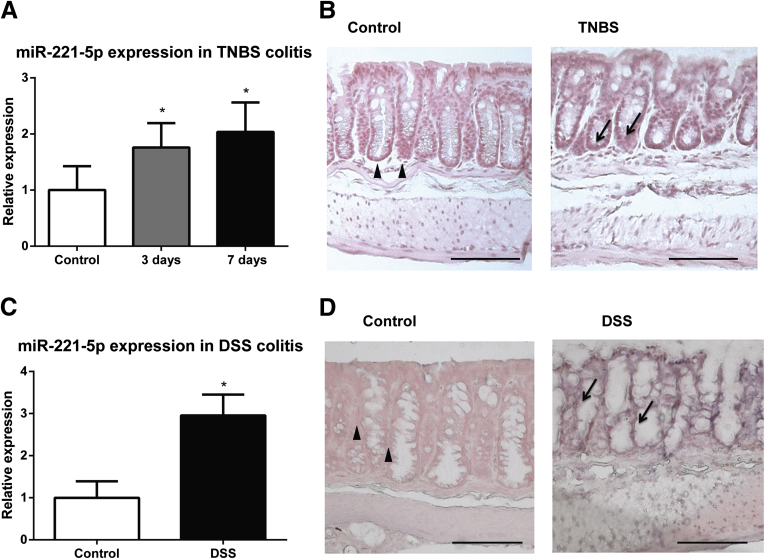
To begin to address a possible functional role for miR-221-5p in the pathophysiology of colitis, we first examined colonic expression of miR-221-5p in two chemical models of experimental colitis. As shown in Figure 6A, colonic expression of miR-221-5p was significantly up-regulated 3 and 7 days after intracolonic administration of TNBS. Moreover, expression of miR-221-5p was also increased by twofold 5 days after administration of 1% DSS into the drinking water of C57BL/6J mice (see Figure 6C).
Figure 6.
MiR-221-5p expression is increased in experimental colitis. Expression levels of miR-221-5p are assessed by real-time polymerase chain reaction (RT-PCR). (A) MiR-221-5p is increased in the colonic mucosa of trinitrobenzene sulfonic acid (TNBS)-exposed mice. *P < .05 versus control (n = 8). (B) Representative images of in situ hybridization of miR-221-5p of colon tissues from TNBS-treated C57BL6/J mice and their control counterparts. (C) MiR-221-5p is increased in the colonic mucosa of dextran sulfate sodium (DSS)-treated mice. *P < .05 versus control (n = 7) and DSS-treated mice (n = 5). (D) Representative images of in situ hybridization of miR-221-5p of colon tissues from DSS-treated C57BL6/J mice and their control counterparts. Scale bar: 100 μm.
To examine the cellular localization of miR-221-5p, we performed in situ hybridization using a miR-221-5p probe in colon tissue sections obtained from control and TNBS- or DSS-treated mice as described in Materials and Methods. Our results showed that miR-221-5p expression is increased in the colonic mucosa, primarily in colonic epithelial cells in TNBS- or DSS-exposed mice, compared with controls (see Figure 6B and D).
Intracolonic Silencing of MicroRNA-221-5p Enhances Trinitrobenzene Sulfonic Acid–Induced Colitis
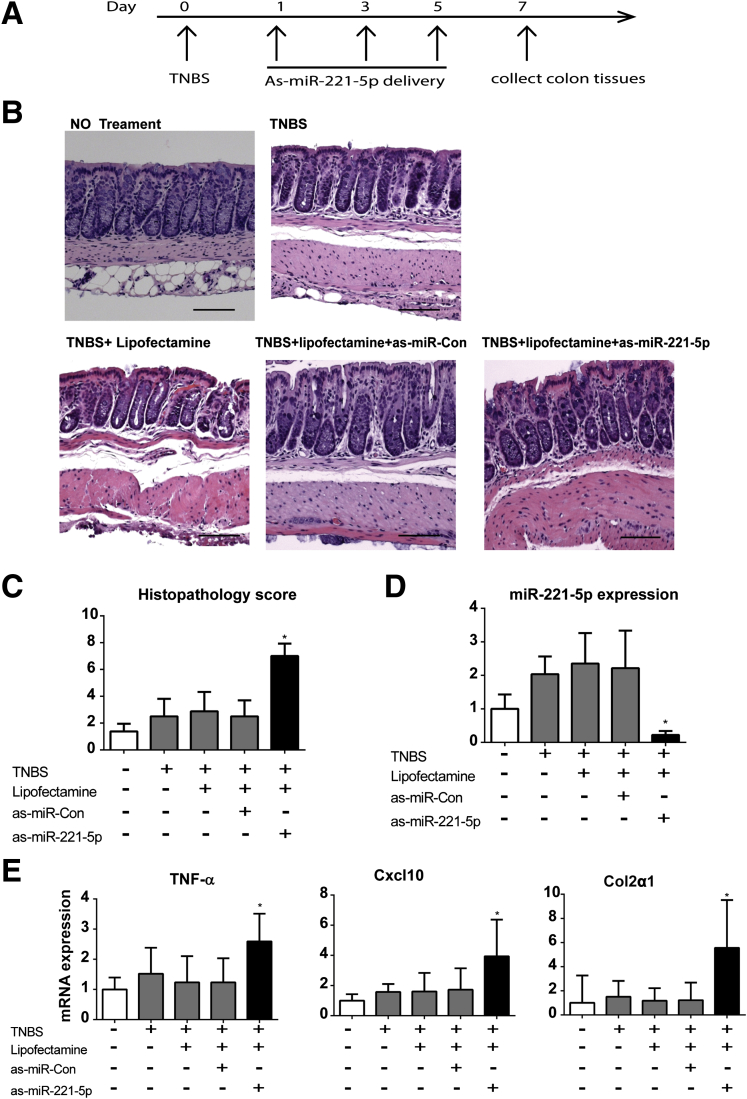
We next tested the importance of miR-221-5p in colitis pathogenesis in the TNBS colitis model. Mice were intracolonically administered 40 mg/kg of TNBS on day 0, then on days 1, 3, and 5 they were intracolonically administered anti-sense (as) miR-221-5p or as-miRNA-control (40 μg/mouse). On day 7 the colon tissues were collected for further analysis (see the experimental design diagram in Figure 7A).
Figure 7.
Anti-sense microRNA (as-miR)-221-5p exacerbates trinitrobenzene sulfonic acid (TNBS)-induced colitis. (A) Timeline of as-miR-221-5p treatment in TNBS-induced colitis. (B) TNBS-induced histologic changes in colons of control or as-miR-221-5p-treated C57BL6/J mice. Scale bar: 100 μm. (C) Histopathology score of as-miR-221-5p treated mice with TNBS-induced colitis. (D) Expression level of miR-221-5p was assessed by real-time polymerase chain reaction (PCR) after intracolonic administration of as-miR-221-5p. *P < .05 compared with controls (n = 8). (E) Expression of tumor necrosis factor-α (TNFα), C-X-C motif chemokine 10 (Cxcl10), and collagen, type II, α 1 (Col2α1) in the colon tissue of as-miR-221-5p or as-miR-control treated TNBS mice colitis models. *P < .05 compared with controls (n = 8).
To verify the efficient silencing of endogenous miR-221-5p, we measured colonic miR-221-5p expression in the different mouse groups and found reduced endogenous miR-221-5p levels (by 80%) in as-miR-221-5p-exposed mice compared with those exposed to as-control miR (see Figure 7D). Mice treated with TNBS showed epithelial destruction and inflammation and an increased histopathology score compared with mice who received an intracolonic administration of vehicle (see Figure 7C). We found no difference in the histopathology score and levels of miR-221-5p expression in the mice treated with TNBS alone, or TNBS with control as-miR, or TNBS with Lipofectamine vehicle (see Figure 7C and D). However, the TNBS-exposed mice treated with as-miR-221-5p demonstrated more convoluted crypts and epithelial cell damage than the as-miRNA control-treated mice (see Figure 7B). These changes are also depicted in the increased histopathology score in TNBS-exposed mice after as-miR-221-5p treatment (see Figure 7C).
We also found a marked increase in the levels of tumor necrosis factor-α (TNFα) expression after silencing miR-221-5p expression in TNBS-exposed mice. The expression of the neutrophil chemoattractant C-X-C motif chemokine 10 (Cxcl10) was also significantly increased (see Figure 7E). Our results also demonstrate increased expression of collagen, type II, α1 (Col2α1) mRNA (see Figure 7E), an important molecule involved in tissue remodeling and fibrosis,33, 34 and a trend for increased expression of CXCL1 mRNA (by twofold), although it was not statistically significant (n = 8 per group, P > .05). We did not find any significant further increase in myeloperoxidase mRNA expression after si-miR-221-5p treatment (data not shown). Moreover, there was no increase in colonic IL-6R levels in TNBS-exposed as-miR-221-5p-treated mice (data not shown). These results indicated that miR-221-5p regulates experimental colitis by modulating expression of proinflammatory and fibrosis-related genes.
Anti-MicroRNA-221-5p Exacerbates Dextran Sulfate Sodium–Induced Colitis
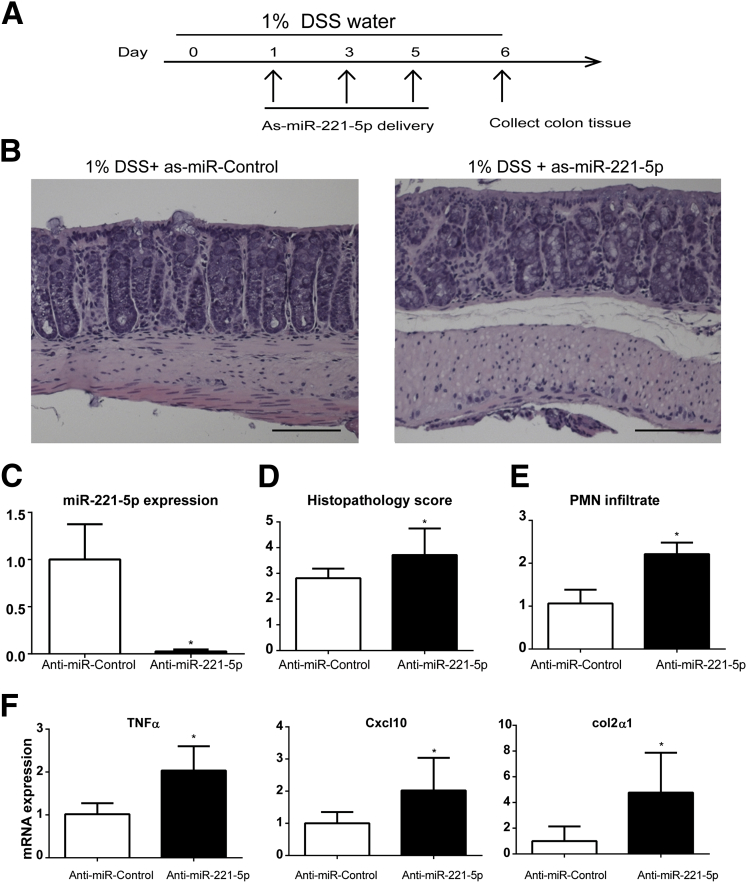
Currently, there is no animal colitis model that can simulate every aspect of human IBD, and each model has its own limits.35 To further confirm the anti-inflammatory effect of miR-221-5p in colitis, we also used the DSS colitis model. Mice were provided with 1% DSS in their drinking water on day 0. Next, as-miR-221-5p or control as-miR was injected intracolonically on days 1, 3, and 5; the mice were sacrificed on day 6, and their colons were harvested for further analysis.
Treatment with anti-miR-221-5p worsened the histologic damage and colitis (Figure 8B), the histopathology score (see Figure 8D), and the mucosal polymorphonuclear neutrophil infiltrates (see Figure 8E) compared with the colon of DSS-exposed animals exposed to control as-miR. Consistent with results from the TNBS colitis model, anti-miR-221-5p treatment greatly reduced miR-221-5p expression in the colon tissue (see Figure 8C) and increased the expression of TNFα, Cxcl10, and Col2 α 1 (see Figure 8F), and CXCL1 mRNA (n = 8, P > .05) in the DSS model of colitis with si-miR-221-5p treatment. These data further confirm an anti-inflammatory role for miR-221-5p in vivo.
Figure 8.
Anti-sense microRNA (as-miR)-221-5p regulates inflammation in dextran sulfate sodium (DSS)-induced colitis models. (A) Timeline of as-miR-221-5p treatment in 1% DSS-induced colitis. (B) Histologic changes in colons of with controls or as-miR-221-5p-treated C57BL6/J mice in DSS colitis model. Scale bar: 100 μm. (C) Expression level of miR-221-5p after intracolonic administration of as-MiR-221-5p. *P < .05 compared with controls (n = 6). (D) Histopathology sore of as-miR-221-5p-treated mice with DSS-induced colitis. (E) The polymorphonuclear neutrophil infiltration sore of as-miR-221-5p-treated mice with DSS-induced colitis. (F) Expression of TNFα, C-X-C motif chemokine 10 (Cxcl10), and collagen, type II, α 1 (Col2α1) in the colon tissue of as-miR-221-5p or anti-miR-control treated DSS-induced colitis models. *P < .05 compared with controls (n = 6).
Discussion
MiRNA expression is deregulated across a broad spectrum of inflammatory disorders,13 including IBD.15, 16 Levels of SP are elevated in IBD tissues.1 SP and its high-affinity receptor NK-1R have been also implicated in the pathophysiology of both acute and chronic colitis because they regulate several genes involved in the promotion of colitis as well mucosal healing after colitis.1, 36, 37 However, the contribution of miRs and miR-regulated pathways involved in the intestinal inflammatory mechanisms of SP has not been studied. Our results indicate that coupling of SP to NK-1R in human colonic epithelial cells regulates differential expression of 29 miRNAs (see Figure 1), and among them miR-21 has been implicated in the pathogenesis of colitis and IBD.38, 39, 40 We also show that miR-221 and miR-222 represent the highest up-regulated miRs in response to SP (see Figure 1A and B) and that miR-221-5p affects the pathophysiology of colitis through stimulation of an anti-inflammatory feedback network (see Figure 3C). Most importantly, our results indicate that this SP-NK-1R-dependent miR-221-5p-IL-6R circuit is activated in human colonic epithelial cells and UC specimens (see Figure 3), suggesting an important role for NK-1R-dependent miRNA regulation in colitis.
We demonstrate that silencing of endogenous colonic miR-221-5p enhances experimental colitis in two different mouse chemical models. Mucosal histologic damage worsened, and colonic mRNA levels of TNFα, Cxcl10, and Col2α1 were increased after intracolonic silencing of miR-221-5p in both TNBS- and DSS-induced colitis (see Figures 7 and 8). Interestingly, TNFα, CXCL10, and Col2α1 have been associated with the pathophysiology of IBD. Neutralization of TNFα with monoclonal antibodies represents one of the most promising recent therapies in IBD.41 CXCL10 is increased in inflamed colons of IBD patients and stimulates monocyte, natural killer, and T-cell migration42, 43 whereas Col2α1 is important in tissue remodeling and fibrosis.33, 34
We also present direct molecular and biochemical evidence that IL-6R is a novel downstream target of miR-221-5p that may mediate intestinal anti-inflammatory signaling after SP-miR-221-5p interactions in human colonocytes. IL-6R is implicated in cytokine-cytokine receptor signaling that involves the Janus kinase/signal transducer and activator of transcription (JAK-STAT) signaling pathways, known to be dysregulated in T-cell-mediated, and DSS- and TNBS-induced colitis.20, 44, 45 Additionally, the JAK-STAT pathway is involved in the pathogenesis of UC,46 whereas treatment with antibodies against IL-6R attenuates immune-mediated and chemically induced colitis.47
The ability of SP to activate IL-6R expression and the identification of IL-6R as a downstream target of miR-221-5p in human epithelial cells has not previously recognized. Interestingly, our results indicate that SP induces IL-6R expression and that exposure of NCM460-NK-1R cells to a miR-221-5p mimic inhibits IL-6R expression (see Figure 5E and F). This contradictory response is likely due to multiple signaling pathways regulated by SP-NK-1R interactions.1 Thus, SP–NK-1R signaling may regulate IL-6R expression not only through miR-221-5p but also via other transcription factors activated via NK-1R signaling that can affect IL-6R expression1 while, as shown here, miR-221-5p directly regulates IL-6R expression through binding IL-6R 3′-UTR. In addition, our bioinformatics analysis indicates that mouse IL-6R mRNA has no binding sites for miR-221-5p, suggesting that in the mouse other miR-221-5p downstream targets may be involved in the effects of this miR in amelioration of colitis suggested by our in vivo results with miR-221-5p silencing.
A shown in Figure 4, the mechanism by which SP-NK-1R interactions regulate expression of miR-221-5p involves activation of NF-κB and JNK, important signaling pathways known to be regulated by NK-1R activation.1 Our results are in line with previous reports demonstrating that NF-κB induces the expression of miR-221 in prostate carcinoma, glioblastoma, and colorectal cancer cells.48 Importantly, our finding demonstrates that miR-221-5p act as an anti-inflammatory miRNA by controlling IL-6R expression in human epithelial cells. IL-6R is implicated in cytokine-cytokine receptor interactions and in the JAK-STAT signaling pathways, known to be dysregulated in colitis induced by T-cells,20 DSS,45 and TNBS.44
Compared with controls, IL-6R expression is decreased in inflamed the colon biopsy tissues from UC patients; in the same samples, the expression of NK-1R and miR-221-5p are increased (see Figure 3A and B). These findings combined with our in vitro analysis (see Figures 4 and 5) demonstrate a positive correlation between miR-221-5p and NK-1R and an inverse correlation with IL-6R in UC. This NK-1R-miR-221-5p-dependent pathway, its association with the NF-κB and JNK signaling pathways, and IL-6R as a downstream target of this miR are summarized in the diagram under Figure 3C. Previous studies, however, reported increased soluble IL-6R in human IBD serum,49 and another study found no differences in the relative expression of IL-6R in blood T cells and lamina propria T cells among Crohn’s disease, UC and control patients.47 These differences in IL-6R expression levels comparing our study and the studies of Atreya et al47 and Mitsuyama et al49 may be due to different IL-6R measurement methods (ELISA, FACS, vs. quantitative reverse-transcription PCR) and/or materials used (serum and lamina propria T cells versus mucosal biopsies).
Our results show increased expression of miR-221-5p in colonic biopsies from UC patients (see Figure 3A), a disease state highly associated with colon cancer,50 and in the colonic mucosa of mice with experimental colitis (see Figure 6A and C). MiR-221-5p is also up-regulated in cancer-associated fibroblasts compared with normal fibroblast cells, in line with a role for miR-221-5p in tumorigenesis.51 Yuan et al52 found that miR-221-5p expression levels correlate negatively with colorectal cancer-associated metastasis by inhibiting MBD2 expression. Interestingly, Rokavec et al53 found that IL-6R/STAT3 pathways promote epithelial-to-mesenchymal transition–mediated colorectal cancer invasion and metastasis. These results,51, 52, 53 together with our findings, suggest that miR-221-5p may regulate colon cancer metastasis through IL-6R/STAT3-related pathways.
In summary, we have identified miR-221-5p as a SP-responsive miRNA that regulates IL-6R mRNA and protein expression in human colonic epithelial cells in vitro and regulates experimental colitis in vivo. Our studies support that the possibility that miR-221-5p may serve as an important anti-“inflamiR” by controlling IL-6R signaling pathways under pathologic conditions. Strategies that activate miR-221-5p expression may represent a novel therapeutic approach for IBD treatment.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was funded by the U.S. Public Health Service [grants R01 DK47343, R01 DK60729], the Neuroendocrine Assay Core [P50 DK64539] (to C.P.), a Crohn’s and Colitis Foundation Fellowship Award (to K.B.), and a CURE:DDRC P30 DK 41301 Pilot and Feasibility study (to K.F.), and Animal Core (to C.P. and K.F.).
References
- 1.Steinhoff M.S., von Mentzer B., Geppetti P. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94:265–301. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pothoulakis C., Castagliuolo I., LaMont J.T. CP-96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc Natl Acad Sci USA. 1994;91:947–951. doi: 10.1073/pnas.91.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stucchi A.F., Shebani K.O., Leeman S.E. A neurokinin 1 receptor antagonist reduces an ongoing ileal pouch inflammation and the response to a subsequent inflammatory stimulus. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1259–G1267. doi: 10.1152/ajpgi.00063.2003. [DOI] [PubMed] [Google Scholar]
- 4.Castagliuolo I., Riegler M., Pasha A. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J Clin Invest. 1998;101:1547–1550. doi: 10.1172/JCI2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao D., Kuhnt-Moore S., Zeng H. Substance P-stimulated interleukin-8 expression in human colonic epithelial cells involves Rho family small GTPases. Biochem J. 2002;368:665–672. doi: 10.1042/BJ20020950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karagiannides I., Kokkotou E., Tansky M. Induction of colitis causes inflammatory responses in fat depots: evidence for substance P pathways in human mesenteric preadipocytes. Proc Natl Acad Sci USA. 2006;103:5207–5212. doi: 10.1073/pnas.0600821103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieb K., Fiebich B.L., Berger M. The neuropeptide substance P activates transcription factor NF-kappa B and kappa B-dependent gene expression in human astrocytoma cells. J Immunol. 1997;159:4952–4958. [PubMed] [Google Scholar]
- 8.Tansky M.F., Pothoulakis C., Leeman S.E. Functional consequences of alteration of N-linked glycosylation sites on the neurokinin 1 receptor. Proc Natl Acad Sci USA. 2007;104:10691–10696. doi: 10.1073/pnas.0703394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koon H.W., Zhao D., Zhan Y. Substance P mediates antiapoptotic responses in human colonocytes by Akt activation. Proc Natl Acad Sci USA. 2007;104:2013–2018. doi: 10.1073/pnas.0610664104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goode T., O’Connell J., Anton P. Neurokinin-1 receptor expression in inflammatory bowel disease: molecular quantitation and localisation. Gut. 2000;47:387–396. doi: 10.1136/gut.47.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koon H.W., Zhao D., Xu H. Substance P-mediated expression of the pro-angiogenic factor CCN1 modulates the course of colitis. Am J Pathol. 2008;173:400–410. doi: 10.2353/ajpath.2008.080222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valencia-Sanchez M.A., Liu J., Hannon G.J. Control of translation and mRNA degradation by miRNAs and siRNAs. Genes Dev. 2006;20:515–524. doi: 10.1101/gad.1399806. [DOI] [PubMed] [Google Scholar]
- 13.Iliopoulos D. MicroRNA circuits regulate the cancer-inflammation link. Sci Signal. 2014;7:pe8. doi: 10.1126/scisignal.2005053. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen H.T., Dalmasso G., Muller S. Crohn’s disease-associated adherent invasive Escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy. Gastroenterology. 2014;146:508–519. doi: 10.1053/j.gastro.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Koukos G., Polytarchou C., Kaplan J.L. MicroRNA-124 regulates STAT3 expression and is down-regulated in colon tissues of pediatric patients with ulcerative colitis. Gastroenterology. 2013;145:842–852.e2. doi: 10.1053/j.gastro.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu F., Zikusoka M., Trindade A. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635.e24. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 17.Greco S.J., Rameshwar P. MicroRNAs regulate synthesis of the neurotransmitter substance P in human mesenchymal stem cell-derived neuronal cells. Proc Natl Acad Sci USA. 2007;104:15484–15489. doi: 10.1073/pnas.0703037104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez Freire V., Burkhard F.C., Kessler T.M. MicroRNAs may mediate the down-regulation of neurokinin-1 receptor in chronic bladder pain syndrome. Am J Pathol. 2010;176:288–303. doi: 10.2353/ajpath.2010.090552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Y., Li X.Q., Sahbaie P. miR-203 regulates nociceptive sensitization after incision by controlling phospholipase A2 activating protein expression. Anesthesiology. 2012;117:626–638. doi: 10.1097/ALN.0b013e31826571aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang K., Zhang S., Glawe J. Temporal genome expression profile analysis during t-cell-mediated colitis: identification of novel targets and pathways. Inflamm Bowel Dis. 2012;18:1411–1423. doi: 10.1002/ibd.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law I.K., Bakirtzi K., Polytarchou C. Neurotensin–regulated miR-133α is involved in proinflammatory signalling in human colonic epithelial cells and in experimental colitis. Gut. 2015;64:1095–1110. doi: 10.1136/gutjnl-2014-307329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viennois E., Chen F., Laroui H. Dextran sodium sulfate inhibits the activities of both polymerase and reverse transcriptase: lithium chloride purification, a rapid and efficient technique to purify RNA. BMC Res Notes. 2013;6:360. doi: 10.1186/1756-0500-6-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koon H.W., Zhao D., Na X. Metalloproteinases and transforming growth factor-alpha mediate substance P-induced mitogen-activated protein kinase activation and proliferation in human colonocytes. J Biol Chem. 2004;279:45519–45527. doi: 10.1074/jbc.M408523200. [DOI] [PubMed] [Google Scholar]
- 24.Bakirtzi K., Hatziapostolou M., Karagiannides I. Neurotensin signaling activates microRNAs-21 and -155 and Akt, promotes tumor growth in mice, and is increased in human colon tumors. Gastroenterology. 2011;141:1749–1761.e1. doi: 10.1053/j.gastro.2011.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renzi D., Pellegrini B., Tonelli F. Substance P (neurokinin-1) and neurokinin A (neurokinin-2) receptor gene and protein expression in the healthy and inflamed human intestine. Am J Pathol. 2000;157:1511–1522. doi: 10.1016/S0002-9440(10)64789-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun P., Zhou K., Wang S. Involvement of MAPK/NF-kappaB signaling in the activation of the cholinergic anti-inflammatory pathway in experimental colitis by chronic vagus nerve stimulation. PloS One. 2013;8:e69424. doi: 10.1371/journal.pone.0069424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assi K., Pillai R., Gomez-Munoz A. The specific JNK inhibitor SP600125 targets tumour necrosis factor-alpha production and epithelial cell apoptosis in acute murine colitis. Immunology. 2006;118:112–121. doi: 10.1111/j.1365-2567.2006.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galardi S., Mercatelli N., Farace M.G. NF-kB and c-Jun induce the expression of the oncogenic miR-221 and miR-222 in prostate carcinoma and glioblastoma cells. Nucleic Acids Res. 2011;39:3892–3902. doi: 10.1093/nar/gkr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Natarajan K., Singh S., Burke T.R., Jr. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci USA. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garofalo M., Di Leva G., Romano G. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16:498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Bennett B.L., Sasaki D.T., Murray B.W. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maragkakis M., Reczko M., Simossis V.A. DIANA-microT web server: elucidating microRNA functions through target prediction. Nucleic Acids Res. 2009;37:W273–W276. doi: 10.1093/nar/gkp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanley K.P., Oakley F., Sugden S. Ectopic SOX9 mediates extracellular matrix deposition characteristic of organ fibrosis. J Biol Chem. 2008;283:14063–14071. doi: 10.1074/jbc.M707390200. [DOI] [PubMed] [Google Scholar]
- 34.Nakken K.E., Nygard S., Haaland T. Multiple inflammatory-, tissue remodelling- and fibrosis genes are differentially transcribed in the livers of Abcb4−/− mice harbouring chronic cholangitis. Scand J Gastroenterol. 2007;42:1245–1255. doi: 10.1080/00365520701320521. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann J.C., Pawlowski N.N., Kuhl A.A. Animal models of inflammatory bowel disease: an overview. Pathobiology. 2002;70:121–130. doi: 10.1159/000068143. [DOI] [PubMed] [Google Scholar]
- 36.Koon H.W., Pothoulakis C. Immunomodulatory properties of substance P: the gastrointestinal system as a model. Ann NY Acad Sci. 2006;1088:23–40. doi: 10.1196/annals.1366.024. [DOI] [PubMed] [Google Scholar]
- 37.Gross K.J., Pothoulakis C. Role of neuropeptides in inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:918–932. doi: 10.1002/ibd.20129. [DOI] [PubMed] [Google Scholar]
- 38.Paraskevi A., Theodoropoulos G., Papaconstantinou I. Circulating MicroRNA in inflammatory bowel disease. J Crohns Colitis. 2012;6:900–904. doi: 10.1016/j.crohns.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y., Ma Y., Shi C. Overexpression of miR-21 in patients with ulcerative colitis impairs intestinal epithelial barrier function through targeting the Rho GTPase RhoB. Biochem Biophys Res Commun. 2013;434:746–752. doi: 10.1016/j.bbrc.2013.03.122. [DOI] [PubMed] [Google Scholar]
- 40.Shi C., Liang Y., Yang J. MicroRNA-21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PloS One. 2013;8:e66814. doi: 10.1371/journal.pone.0066814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen O.H., Ainsworth M.A. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369:754–762. doi: 10.1056/NEJMct1209614. [DOI] [PubMed] [Google Scholar]
- 42.Mendez Samperio P., Trejo A., Miranda E. Role of type I interferon in the bacillus Calmette-Guerin-induced expression of CXCL10 from human monocytes. Mediators Inflamm. 2004;13:343–348. doi: 10.1155/S096293510400050X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas S., Baumgart D.C. Targeting leukocyte migration and adhesion in Crohn’s disease and ulcerative colitis. Inflammopharmacology. 2012;20:1–18. doi: 10.1007/s10787-011-0104-6. [DOI] [PubMed] [Google Scholar]
- 44.Kretzmann N.A., Fillmann H., Mauriz J.L. Effects of glutamine on proinflammatory gene expression and activation of nuclear factor kappa B and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm Bowel Dis. 2008;14:1504–1513. doi: 10.1002/ibd.20543. [DOI] [PubMed] [Google Scholar]
- 45.Fang K., Bruce M., Pattillo C.B. Temporal genomewide expression profiling of DSS colitis reveals novel inflammatory and angiogenesis genes similar to ulcerative colitis. Physiol Genomics. 2011;43:43–56. doi: 10.1152/physiolgenomics.00138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sugimoto K. Role of STAT3 in inflammatory bowel disease. World J Gastroenterol. 2008;14:5110–5114. doi: 10.3748/wjg.14.5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atreya R., Mudter J., Finotto S. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–588. doi: 10.1038/75068. [DOI] [PubMed] [Google Scholar]
- 48.Liu S., Sun X., Wang M. A microRNA 221- and 222-mediated feedback loop, via PDLIM2, maintains constitutive activation of NFκB and STAT3 in colorectal cancer cells. Gastroenterology. 2014;147:847–859.e11. doi: 10.1053/j.gastro.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitsuyama K., Toyonaga A., Sasaki E. Soluble interleukin-6 receptors in inflammatory bowel disease: relation to circulating interleukin-6. Gut. 1995;36:45–49. doi: 10.1136/gut.36.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rutter M.D., Saunders B.P., Wilkinson K.H. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology. 2006;130:1030–1038. doi: 10.1053/j.gastro.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 51.Zhao L., Sun Y., Hou Y. MiRNA expression analysis of cancer-associated fibroblasts and normal fibroblasts in breast cancer. Int J Biochem Cell Biol. 2012;44:2051–2059. doi: 10.1016/j.biocel.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 52.Yuan K., Xie K., Fox J. Decreased levels of miR-224 and the passenger strand of miR-221 increase MBD2, suppressing maspin and promoting colorectal tumor growth and metastasis in mice. Gastroenterology. 2013;145:853–864.e9. doi: 10.1053/j.gastro.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rokavec M., Oner M.G., Li H. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2014;124:1853–1867. doi: 10.1172/JCI73531. [DOI] [PMC free article] [PubMed] [Google Scholar]