Abstract
The following study deals with the chemical composition, antioxidant and antimicrobial activity of essential oils of Wedelia prostrata and their main constituents in vitro. A total of 70 components representing 99.26 % of the total oil were identified. The main compounds in the oil were limonene (11.38 %) and α-pinene (10.74 %). Antioxidant assays (1,1-diphenyl-2-picrylhydrazyl, superoxide anion radical, and reducing power test) demonstrate moderate activities for the essential oil and its main components (limonene and α-pinene). The essential oil (1000 μg/disc) exhibited promising antimicrobial activity against 10 strains of test microorganisms as a diameter of zones of inhibition (20.8 to 22.2 mm) and MIC values (125 to 250 µg/ml). The activities of limonene and α-pinene were also determined as main components of the oil. α-Pinene showed higher antimicrobial activity than the essential oil with a diameter of zones of inhibition (20.7 to 22.3 mm) and MIC values (62.5 to 125 µg/ml). The antioxidant and antimicrobial properties of the essential oil may be attributed to the synergistic effects of its diverse major and minor components.
Keywords: Wedelia prostrata, chemical composition, essential oil, antioxidant activity, antimicrobial activity
Introduction
Microorganisms and oxidation are the major causes of food deterioration. In particular, lipid peroxidation of food lipid components produced during the manufacturing process and food storage is the main cause food quality deterioration, leading to rancidity and changes in the taste, smell, and colour, and eventually the loss of food quality (Mau et al., 2004[15]). The subsistence and growth of microorganisms in food may also lead to spoilage, toxin formation, and quality deterioration of food products (Celiktas et al., 2007[4]). Moreover, the consumption of spoiled food encompass a wide spectrum of illnesses and is a growing public health problem worldwide.
For many years, different synthetic preservatives have been widely used as antioxidants and antimicrobial agents in the food industry to increase the storage and marketing shelf life of food. However, although synthetic preservatives have been proven highly effective and less expensive than natural substances, these compounds exhibit mutagenic activity against non-target organisms (Tripathi et al., 2007[25]) and cause environmental pollution (Misra and Pavlostathis, 1997[17]). The growing interest in the substitution of synthetic food preservatives has fostered research on the screening of new antioxidants and antimicrobial preservatives from natural sources (Bajpai et al., 2008[1]). At present, interest in the effective use of essential oils from plants in food preservation has been increasing.
The genus Wedelia comprises approximately 60 species that are distributed in tropical and warm temperate regions, including India, Burma, Ceylon, China, and Japan (Li et al., 2007[13]). Several species are used as folk medicine in many countries to treat a variety of diseases, such as headaches, fevers, infections, and pathologies of the respiratory tract (Li et al., 2007[13]; Miles et al., 1990[16]). Five of these species, namely, Wedelia biflora, W. urticifolia, W. wallichii, W. prostrata, and W. chinensis, are found and used as folk medicines in the southern provinces of China. W. prostrata is mainly distributed in tropical and subtropical areas in Asia. In traditional Chinese medicine, W. prostrata is used for the treatment of inflammation and ulcer.
A series of studies has demonstrated the potential medicinal effect of essential oils from various Wedelia species. For example, essential oil from W. trilobata leaves exhibited antibacterial activity against Bacillus subtilis and Staphylococcus aureus (Nirmal et al., 2005[18]); essential oil from W. chinensis was found effective against gram positive bacteria and fungi; and essential oils from W. chinensis and W. biflora exhibited significant antibacterial and antifungal activities when compared to that of standard ciproflacin (Sureshkumar et al., 2007[24]).
To the best of our knowledge, no report on the phytochemical and biological studies of W. prostrata has been published so far. The aim of the current study is to determine the chemical composition of the essential oil of W. prostrata via gas chromatography-flame ionisation detection (GC-FID) and gas chromatography-mass spectrometry (GC-MS), evaluate its antimicrobial activity against pathogens and the antioxidant activity.
Materials and Methods
Plant material
W. prostrata plants were collected in the Danxia Mountains, Guangdong Province, China on June 2009, and identified by Dr. Xun Gong. The plants were dried in the shade (at room temperature). The voucher specimen (No. 499993) was deposited in the Kuming Institute of Botany, Chinese Academy of Sciences.
Chemicals
2,2-Diphenyl-1-picrylhydrazyl (DPPH, 95 %), limonene, α-pinene, α-tocopherol, streptomycin, tetracycline, riboflavin, methionine, and nitro blue tetrazolium were purchased from Sigma-Aldrich (St. Louis, MI, USA). Potassium ferricyanide, trichloroacetic acid, methanol, and all other reagents were of analytical grade and were obtained from Jinhuada Chemical Reagent Co. (Guangzhou, China).
Isolation of the essential oil
The air-dried plant materials (500 g) of W. prostrata was chopped and subjected to hydrodistillation for 3 h using a Clevenger type apparatus. The obtained oils were dried over sodium sulphate for 24 h, filtered, and then stored at 4° C in sealed brown glass vials until tested.
GC-FID analysis
An Agilent HP-6890 gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) with a HP-5 5 % phenylmethylsiloxane capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness) and equipped with an FID detector was used for the GC-FID analysis. Helium gas at a constant flow rate of 1 ml/min was used as the carrier gas. The injector and mass transfer line temperatures were set at 250 and 280 °C, respectively. The essential oil solution (1 μL) in hexane was injected and analysed under the following column conditions: initial column temperature at 40 °C for 1 min, which was then increased to 250 °C at a 3 °C/min heating ramp, and then subsequently kept at 250 °C for 20 min.
GC-MS analysis
Quantitative and qualitative analysis of the essential oil was performed using a GC-MS 6890-5975 system (Agilent Technologies, Palo Alto, CA, USA) equipped with a HP-5 MS fused silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness). For GC-MS detection, an electron ionisation system with a 70 eV ionisation energy was used. Helium gas was used as the carrier gas at a constant flow rate of 1 ml/min. The injector and mass transfer line temperatures were set at 250 and 280 °C, respectively. Essential oil solution (1 μL) in hexane was injected and then analysed under the following column conditions: initial column temperature at 40 °C for 1 min, which was then increased to 250 °C at a 3 °C/min heating ramp, and then subsequently kept at 250 °C for 20 min. The Kovats indices were calculated for all volatile components using a homologous series of n-alkanes (C8-C25) on the HP-5 MS column. The major oil components were identified via coinjection with standards (whenever possible) and confirmed through the Kovats indices using the Wiley (V.7.0) and National Institute of Standards and Technology (NIST) V.2.0 GC-MS library. The relative concentration of each compound in the essential oil was quantified based on the peak area integrated in the analysis program.
Antioxidant activity determination
1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity
0.1 ml of 25, 50, 75 and 100 μg/ml essential oil and its main components (limonene and α-pinene) were each mixed with 1 ml of 0.2 mM DPPH (dissolved in methanol). The reaction mixture was incubated for 20 min at 28 °C in a dark environment. The control solution, which contained all the reagents except the sample, was used as a blank. The DPPH radical scavenging activity was determined by measuring the absorbance at 517 nm using a spectrophotometer and calculating using the following equation:
DPPH scavenging effect % = (Acontrol-Asample/Acontrol)×100
where Acontrol and Asample are the absorbance of the control sample and the test compound, respectively. The DPPH radical scavenging activity of α-tocopherol was also assayed for comparison.
Superoxide anion radical scavenging activity
All solutions were prepared in a 0.2 M phosphate buffer (pH 7.4). 0.1 ml of 25, 50, 75 and 100 μg/ml samples were each mixed with 3 ml of the reaction buffer solution (pH 7.4) containing 1.3 μM riboflavin, 0.02 M methionine, and 5.1 μM nitro blue tetrazolium. The reaction solution was illuminated by exposing them to two 30 W fluorescent lamps for 20 min, and the absorbance was measured at 560 nm. The reaction mixture without any test sample was used as the control. The superoxide anion radical scavenging activity (%) was calculated using the equation:
Superoxide anion radical scavenging activity %=(Acontrol-Asample/Acontrol)×100
The superoxide anion radical scavenging activity of α-tocopherol was also assayed for comparison.
Determination of the reducing power
0.1 ml of 25, 50, 75 and 100 μg/ml samples were each mixed with a phosphate buffer (2.5 ml, 0.2 M, pH 6.6), and potassium ferricyanide (2.5 ml, 1 %), and the resulting mixtures were incubated at 50 °C for 20 min. Trichloroacetic acid (2.5 ml, 10 %) was added to each sample, and the mixtures were centrifuged at 3000 r/min for 10 min. A 5 ml aliquot of the upper layer was mixed with distilled water (5 ml), followed by the addition of ferric chloride (1 ml, 0.1 %). The absorbance was then measured at 700 nm against a control that consisted of all the reagents without the test sample. A higher absorbance would indicate higher reducing power. The reducing power of α-tocopherol was also determined for comparison.
Antimicrobial activity
Test microorganisms
The in vitro antimicrobial activities of the essential oil and its main components were evaluated against a panel that included laboratory control strains obtained from the China Centre for Type Culture Collection (CCTCC). These strains are
two Gram-negative bacteria (Pseudomonas aeruginosa CCTCC AB93066 and Escherichia coli CCTCC AB91112),
two Gram-positive bacteria (Bacillus subtilis CCTCC AB92068 and Staphylococcus aureus CCTCC AB91053),
two yeast strains (Hansenula anomala CCTCC AY92046 and Saccharomy cescerevisiae CCTCC AY92042), and
four moulds (Aspergillus niger CCTCC AF91004, Chaetomium globosum CCTCC AF 200039, Mucor racemosus CCTCC AF 93209, and Monascus anka CCTCC AF93208).
All strains were maintained on an agar slant at 4 °C. The bacterial strains were cultured in a Muller-Hinton broth (MHB) at 37 °C for 24 h, whereas the yeast strains were cultured on a Sabouraud dextrose agar (SDA) at 28 °C for 48 h. The fungal strains were cultured on SDA at 28 °C for 120 h prior to testing.
Inhibitory effect via the disc diffusion method
The disc diffusion method was used to determine the antimicrobial activities of the essential oils. Petri plates were prepared by pouring 20 ml MBH or SDA and allowing the solution to solidify. The plates were then dried, and 0.1 ml of the standardised inoculum containing 106 to 107 CFU/ml of the bacterial suspension was poured, uniformly spread, and allowed to dry for 5 min. A Whatman No. 1 sterile filter paper disc (6 mm diameter) was impregnated with 1000 μg/disc of the essential oils. Negative controls were prepared using the same solvent employed to dissolve the samples. The standard reference antibiotics, namely, streptomycin and tetracycline (10 μg/disc) were used as the positive controls for the test bacteria. The plates were incubated for bacteria at 37 °C for 24 h, for yeasts at 28 °C for 48 h, and for fungi at 28 °C for 120 h. The antimicrobial activity was evaluated by measuring the diameter of the zones of inhibition against the test organisms. The experiments were repeated in triplicate and the results are expressed as average values.
Determination of the minimuminhibitory concentration (MIC)
The MICs of the essential oils against the test microorganisms were determined using the broth microdilution method. Dilutions of the essential oils, ranging from 0.25 to 1000 µg/ml, were prepared in MHB or SDA. Exactly 0.5 MacFarland standard suspensions of the test microorganisms were inoculated in the tubes. A control test was also performed using inoculated broth or agar supplemented only with dimethyl sulphoxide under identical conditions, with streptomycin as the reference. The bacteria were incubated at 37 °C for 24 h, the yeast strains at 28 °C for 48 h, and fungi at 28 °C for 120 h.
Statistical analysis
All tests were performed in triplicate, and the results were calculated as the mean ± SD.
Results and Discussion
Chemical composition of the essential oil
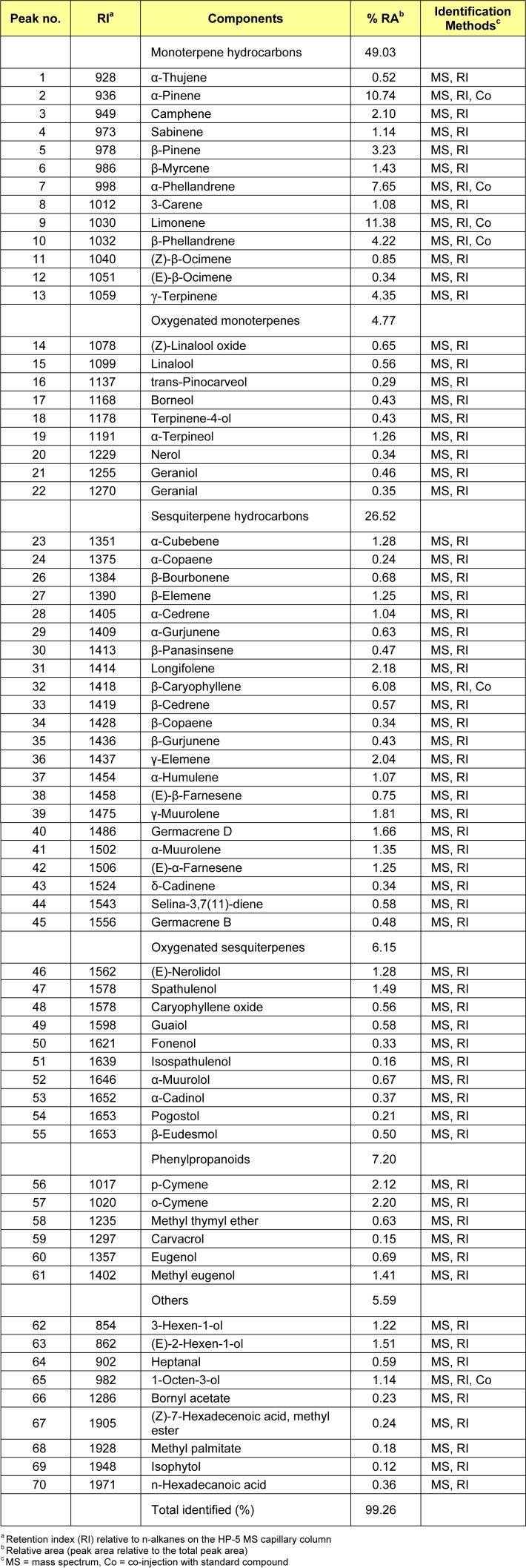
The steam distillation of 500 g dried plant material yielded 2.8 ml (0.56 % v/w) greenish oil with a distinct smell. The oil sample was analysed via GC-FID and GC-MS, and the components were identified on the basis of their RI values as well as by comparison of their mass spectra with those reported in literature. The GC-MS analysis of the essential oil of W. prostrata indicated 70 components representing 99.26 % of the oil (Table 1(Tab. 1)). The composition of the essential oil was as follows: 49.03 % monoterpene hydrocarbon fraction, 26.52 % sesquiterpene hydrocarbon fraction, 4.77 % oxygenated monoterpene fraction, 6.15 % oxygenated sesquiterpenoid fraction, 7.20 % phenylpropanoids fraction and 5.59 % others. The main components in the oil were d-limonene (11.38 %) and α-pinene (10.74 %).
Table 1. Chemical composition of the essential oil of Wedelia prostrata.
Previous reports (Craveiro et al., 1993[6]; Koheil, 2000[12]) on the Wedelia species showed that monoterpene hydrocarbons are the major compounds in their essential oils. The W. paludosa oils contain β-pinene (10.3 %), limonene (21.3 %), and γ-muurolene (11.8 %), whereas the major components of the W. trilobata oils are α-phellandrene (17.4 %) and limonene (16.3 %) (Craveiro et al., 1993[6]). The major components of the essential oils obtained from the flowerheads of W. trilobata are β-phellandrene (25.65 %), limonene (8.93 %), γ-terpinene (5.90 %), trans-β-caryophyllene (4.83 %) and α-pinene (4.72 %) (Koheil, 2000[12]). The current results indicate that the essential oil of W. prostrata contains components relatively similar to those of other Wedelia species.
Antioxidant activity
Free radical-scavenging is one of the known mechanisms by which antioxidants inhibit lipid oxidation (Hatano et al., 1989[9]). In addition, scavenging activity on DPPH radicals has been widely used to determine the free radical-scavenging activity. The DPPH radical scavenging activity can be reduced by the hydrogen donating ability (Prasad et al., 2005[19]). Under oxidative stress, the concentration of superoxide radical can dramatically increase in all cells, thereby inducing several pathophysiological processes, because of its transformation into a more reactive species (Wickens, 2001[29]). Therefore, the measurement of the comparative interceptive ability of antioxidant extracts by determining their ability to scavenge the superoxide radical has been proposed (Vani et al., 1997[27]). The superoxide anion scavenging activity may be due to the action of a free hydroxyl group (Siddhuraju et al., 2002[20]). The reducing power, which is associated with and may be a major indicator of antioxidant activity, is widely used to evaluate the antioxidant activity of polyphenols (Hsu et al., 2006[10]). Most nonenzymatic antioxidant activity, such as the scavenging of free radicals and the inhibition of peroxidation, is mediated by redox reactions (Zhu et al., 2002[30]).
The essential oil of W. prostrata and its main components (limonene and α-pinene) were screened for possible antioxidant activities using three different test systems, namely, the DPPH, superoxide anion, and reducing power assays. The essential oil and its main components exhibited moderate antioxidant activity at all the concentrations tested (Figures 1-3(Fig. 1)(Fig. 2)(Fig. 3)). The highest percentage of DPPH radical scavenging activity (88.1 %) and superoxide anion scavenging activity (86.2 %) and the highest absorbance of reducing power (0.92) were exhibited by the 100 μg/ml essential oil. The order of antioxidant activity was determined as α-tocopherol > limonene > essential oil > α-pinene.
Figure 1. DPPH radical scavenging activity of W. prostrata essential oil and its main components.
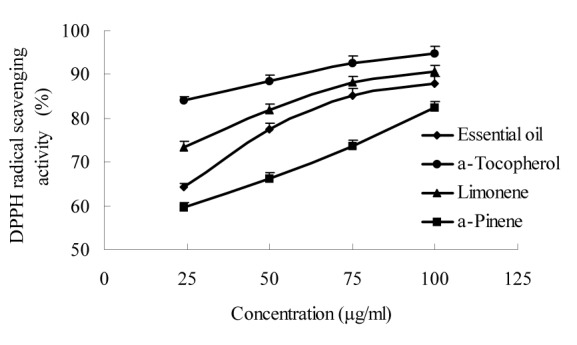
Figure 2. Superoxide radical scavenging activity of W. prostrata essential oil and its main components.
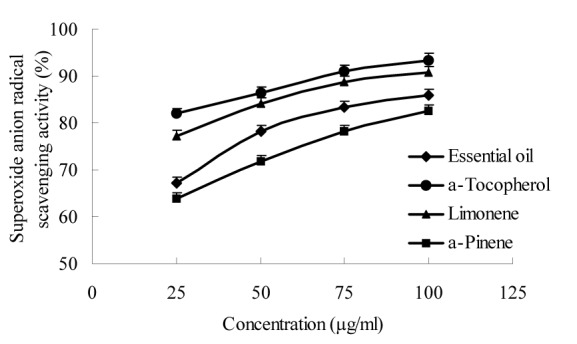
Figure 3. Reducing power of W. prostrata essential oil and its main components.
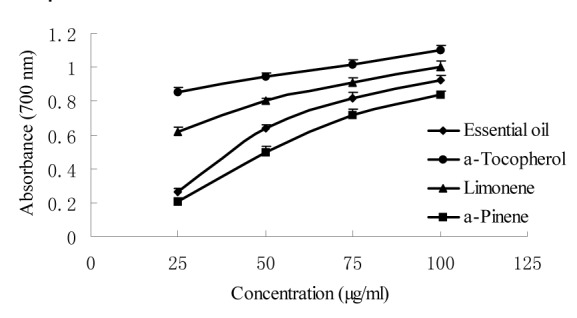
Previous studies also revealed that α-pinene (Wang et al., 2008[28]) and limonene (Maróstica et al., 2009[14]) possess antioxidant activities, which were confirmed by our results. The current results further show that the antioxidant activity of essential oil can be attributed to the synergistic activities of multiform unsaturated compounds such as limonene and α-pinene.
Antimicrobial activity
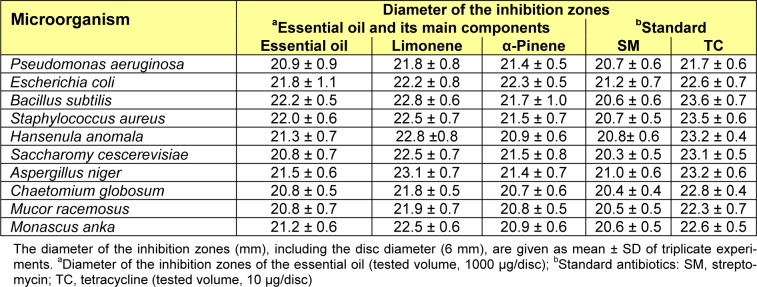
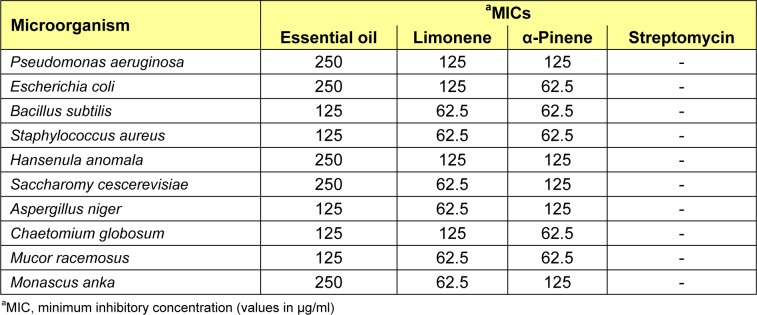
The antimicrobial activity of W. prostrata essential oil and its main components were evaluated against a set of 10 microorganisms, and their potency were qualitatively and quantitatively assessed by the presence or absence of inhibition zones, zone diameters (Table 2(Tab. 2)), and MIC values (Table 3(Tab. 3)).
Table 2. Zones of growth inhibition (mm) showing antimicrobial activity for W. prostrata essential oil and its main components.
Table 3. Minimum inhibitory concentrations (MIC) of the W. prostrata essential oil and its main components against the growth of microorganisms.
Table 2(Tab. 2) shows that the oil has a definite antimicrobial activity against all the test organisms. Limonene and α-pinene also showed considerable antimicrobial activities. In each case, tetracycline showed the highest antimicrobial effect, whereas the essential oil, limonene, and α-pinene were more effective compared to streptomycin. As for the negative control, the concentration of the solvent used in the current study did not affect the growth of the sample strains.
As shown in Table 3(Tab. 3), the essential oil exhibited moderate to high antimicrobial effect against all test microorganisms, with MIC values ranging from 125 to 250 μg/ml. Limonene and α-pinene exhibited high antimicrobial effect, with MIC values ranging from 62.5 to 125 μg/ml.
Earlier papers on the analysis and antibacterial properties of the essential oils of W. trilobata have shown that they have varying degrees of growth inhibitory effects against some bacteria because of their chemical constituents, including limonene, β-phellandrene, α-phellandrene, γ-terpinene, β-caryophyllene, and α-pinene (Nirmal et al., 2005[18]; Craveiro et al., 1993[6]; Koheil, 2000[12]). The current study shows that the antimicrobial activity of the oils from W. prostrata could, in part, be associated with its major components (limonene, α-pinene, α-phellandrene, and β-caryophyllene). Limonene has been demonstrated to have bacteriostatic activity against several microorganisms (Bakkali et al., 2008[2]; Sokovic and van Griensven, 2006[23]; Donsì et al., 2011[7]; Singh et al., 2010[22]). Pinene has been previously shown active against many organisms (Bakkali et al., 2008[2]; Sokovic and van Griensven, 2006[23]; Jiang et al., 2011[11]). Pinene can destroy the cellular integrity, thereby inhibit the respiration and ion transport processes. Moreover, pinene can also increase the membrane permeability in yeast cells and isolated mitochondria (Uribe et al., 1985[26]). The antimicrobial activities of α-phellandrene and β-caryophyllene have also been reported (Simic et al., 2002[21]). Our results on the antimicrobial activity of limonene and α-pinene are similar to these reports.
In addition, the components with lower concentrations, such as γ-terpinene, β-phellandrene, β-pinene, camphene, p-cymene, o-cymene, longifolene, and γ-elemene, may also be contributing to the antimicrobial activity of the oil. Therefore, the synergistic effects of the diverse major and minor components of the essential oils should be taken into consideration to account for the oil biological activity (Burt, 2004[3]).
The mechanism of action of this class of compounds has not been completely elucidated to date; however, these chemical components may be exerting their toxic effects against these microorganisms through the disruption of bacterial or fungal membrane integrity (Filipowicz et al., 2003[8]). Conner and Beuchat (1984[5]) suggested that the antimicrobial activity of the essential oils of herbs and spices or their components could be the result of damage to or disturbance of several enzymatic cell systems, including energy production and synthesis of structural components.
Conclusion
The antioxidative and antimicrobial properties of the essential oils from many plants are of great interest to both the academe and the food, cosmetic, and pharmaceutical industries because of their possible use as natural additives to replace synthetic antimicrobial agents. For the first time, we demonstrate that the essential oil of W. prostrata exhibits antioxidant activity and successfully inhibits the growth of different pathogens that can cause food spoiling as well as health problems. The results obtained in this study show that the essential oil of W. prostrata may be a new potential source of natural antioxidants and antimicrobial agents for the food industry. However, further studies need to be conducted to understand the mechanism of the activity and obtain more information on the safety and toxicity of the oil.
Acknowledgements
The authors are grateful for financially supported by Open Project Program of Guangdong Province Key Laboratory for Green Processing of Natural Products and Product Safety (201206) and Changshan Municipal Science and Technology Plan (K1203002-21).
References
- 1.Bajpai VK, Rahman A, Dung NT, Huh MK, Kang SC. In vitro inhibition of food spoilage and foodborne pathogenic bacteria by essential oil and leaf extracts of Magnolia liliflora Desr. J Food Sci. 2008;73:M314–M320. doi: 10.1111/j.1750-3841.2008.00841.x. [DOI] [PubMed] [Google Scholar]
- 2.Bakkali F, Averbeck S, Averbeck D, Idaomar M. Biological effects of essential oils – A review. Food Chem Toxicol. 2008;46:446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 3.Burt S. Essential oil: their antibacterial properties and potential applications in foods-a review. Int J Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Celiktas OY, Kocabas EEH, Bedir E, Sukan FV, Ozek T, Baser KHC. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007;100:553–559. [Google Scholar]
- 5.Conner DE, Beuchat LR. Effects of essential oils from plants on growth of food spoilage yeasts. J Food Sci. 1984;49:429–434. [Google Scholar]
- 6.Craveiro AA, Matos FJA, Alencar JW, Machado MIL, Krush A, Silva MGV. Volatile constituents of two Wedelia species. J Essent Oil Res. 1993;5:439–41. [Google Scholar]
- 7.Donsì F, Annunziata M, Sessa M, Ferrari G. Nanoencapsulation of essential oils to enhance their antimicrobial activity in foods. LWT - Food Sci Technol. 2011;44:1908–1914. [Google Scholar]
- 8.Filipowicz N, Kaminski M, Kurlenda J, Asztemborska M. Antibacterial and antifungal activity of juniper berry oil and its selected components. Phytother Res. 2003;17:227–231. doi: 10.1002/ptr.1110. [DOI] [PubMed] [Google Scholar]
- 9.Hatano T, Edamatsu R, Hiramatsu M, Mori A, Fujita Y, Yasuhara T, et al. Effects of the interaction of tannins with coexisting substances. VI. Effect of tannins and related polyphenols on superoxide anion radicals and on DPPH. Chem Pharm Bull. 1989;37:2016–2021. [Google Scholar]
- 10.Hsu B, Coupar IM, Ng K. Antioxidant activity of hot water extract from the fruit of the Doum palm, Hyphaene thebaica. Food Chem. 2006;98:317–328. [Google Scholar]
- 11.Jiang Y, Wu N, Fu YJ, Wang W, Luo M, Zhao CJ, et al. Chemical composition and antimicrobial activity of the essential oil of Rosemary. Environ Toxicol Pharmacol. 2011;32:63–68. doi: 10.1016/j.etap.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Koheil MA. Study of the essential oil of the flower-heads of Wedelia trilobata (L.) Hitch. J Pharm Sci. 2000;26:288–293. [Google Scholar]
- 13.Li X, Dong M, Liu Y, Shi QW, Kiyota H. Structures and biological properties of the chemical constituents from the genus Wedelia. Chem Biodivers. 2007;4:823–836. doi: 10.1002/cbdv.200790070. [DOI] [PubMed] [Google Scholar]
- 14.Maróstica MR, Silva TAARE, Franchi GC, Nowill A, Pastore GM, Hyslop S. Antioxidant potential of aroma compounds obtained by limonene biotransformation of orange essential oil. Food Chem. 2009;116:8–12. [Google Scholar]
- 15.Mau JL, Huang PN, Huang SJ, Chen CC. Antioxidant properties of methanolic extracts from two kinds of Antrodia camphorata mycelia. Food Chem. 2004;86:25–31. [Google Scholar]
- 16.Miles DH, Chittawong V, Payne AM. Cotton boll weevil antifeedant activity and antifungal activity (Rhizoctonia solani and Pythium ultimum) of extracts of the stems of Wedelia biflora. J Agr Food Chem. 1990;38:1591–1594. [Google Scholar]
- 17.Misra G, Pavlostathis SG. Biodegradation kinetics of monoterpenes in liquid and soil-slurry systems. Appl Microbiol Biot. 1997;47:572–577. [Google Scholar]
- 18.Nirmal SA, Chavan MJ, Tambe VD, Jadhav RS, Ghogare PB, Bhalke RD, et al. Chemical composition and antimicrobial activity of essential oil of Wedelia trilobata leaves. Indian J Nat Prod. 2005;21(3):33–35. [Google Scholar]
- 19.Prasad NK, Divakar S, Shivamurthy GR, Aradhya SM. Isolation of a free radical scavenging antioxidant from water spinach (Ipomoea aquatica Forsk) J Sci Food Agr. 2005;85:1461–1468. [Google Scholar]
- 20.Siddhuraju P, Mohan PS, Becker K. Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem. 2002;79:61–67. [Google Scholar]
- 21.Simic N, Palic R, Vajs V, Milosavljevic S, Djokovic D. Composition and antibacterial activity of Achillea asplenifolia essential oil. J Essent Oil Res. 2002;14:76–78. [Google Scholar]
- 22.Singh P, Shukla R, Prakash B, Kumar A, Singh S, Mishra PK, et al. Chemical profile, antifungal, antiaflatoxigenic and antioxidant activity of Citrus maxima Burm. and Citrus sinensis (L.) Osbeck essential oils and their cyclic monoterpene, DL-limonene. Food Chem Toxicol. 2010;48:1734–1740. doi: 10.1016/j.fct.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 23.Sokovic M, van Griensven LJLD. Antimicrobial activity of essential oils and their components against the three major pathogens of cultivated button mushroom, Agaricus bisporus. Eur J Plant Pathol. 2006;116:211–24. [Google Scholar]
- 24.Sureshkumar S, Kanagasabail R, Sivakumar T, Chandrasekar MJN, Thiruvenkatasubramaniam R, Thenmozhi S. Antimicrobiological studies on different essential oils of Wedelia species (W. chinensis, W. trilobata and W. biflora) and Eclipta alba (Asteraceae) Asian J Chem. 2007;19:4674–4678. [Google Scholar]
- 25.Tripathi R, Mohan H, Kamat JP. Modulation of oxidative damage by natural products. Food Chem. 2007;100:81–90. [Google Scholar]
- 26.Uribe S, Ramirez J, Peña A. Effects beta-pinene on yeast membrane functions. J Bacteriol. 1985;161:1195–200. doi: 10.1128/jb.161.3.1195-1200.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vani T, Rajani M, Sarkar S, Shishoo CJ. Antioxidant properties of the ayurvedic formulation triphala and its constituents. Int J Pharmacog. 1997;35:313–317. [Google Scholar]
- 28.Wang W, Wu N, Zu YG, Fu YJ. Antioxidative activity of Rosmarinus officinalis L. essential oil compared to its main components. Food Chem. 2008;108:1019–1022. doi: 10.1016/j.foodchem.2007.11.046. [DOI] [PubMed] [Google Scholar]
- 29.Wickens AP. Aging and the free radical theory. Respir Physiol. 2001;128:379–391. doi: 10.1016/s0034-5687(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 30.Zhu QT, Hackman RM, Ensunsa JL, Holt RR, Keen CL. Antioxidative activities of oolong tea. J Agric Food Chem. 2002;50:6929–6934. doi: 10.1021/jf0206163. [DOI] [PubMed] [Google Scholar]