Abstract
Background
Oncology drug shortage is associated with increased patient adverse events and decreased enrollment on clinical trials for adult patients; however, the impact of oncology drug shortages has not been well studied in children with cancer.
Procedure
The Children’s Oncology Group (COG) distributed a 5-item survey to 226 COG site-specific principal investigators (PI’s) and 14-item survey to 161 COG pharmacists to gather data the impact of chemotherapeutic shortages on clinical trials and patient care.
Results
The response rate was 66.4% (150/226) for PI’s and 29.8% (48/161) for pharmacists. COG PI’s reported daunorubicin (73%), methotrexate (56%), asparaginase/PEG-asparaginase (42%), doxorubicin (26%), thiotepa (21%), and cytarabine (20%) were most commonly in shortage, while COG pharmacists reported daunorubicin (80%), methotrexate (66%), vincristine (21%), thiotepa (41%), asparaginase/PEG-asparaginase (34%), and cytarabine (34%) were most commonly in shortage over the past two years. Pharmacists were twice as likely to report a shortage compared with PI’s (OR 2.1, 95% CI: 1.6–2.7, P<0.0001). Fifty percent (74/147) of COG PI’s reported at least one patient enrolled on a clinical trial was impacted by drug shortage, and 66% (98/148) of COG PI’s reported at least one patient had clinical care impacted by drug shortage.
Conclusions
Chemotherapy shortages remain widespread across institutions, hinder clinical trials, and may contribute to adverse events in children with cancer. The increased frequency of chemotherapy shortages reported by pharmacists suggests that pharmacist efforts may mitigate negative impact chemotherapy shortages. Over half of pediatric institutions are implementing recommendations to address shortages, such as cross-institutional collaboration and center-level guidelines.
Keywords: Chemotherapy, Pediatric Hematology/Oncology, Hematology/Oncology general, FDA, Ethics
INTRODUCTION
Drug shortages represent a growing challenge for the American healthcare system. Generic sterile injectable drugs, including chemotherapeutic agents and intravenous fluids, have been particularly susceptible to these shortages, with shortages more than quadrupling from 2005 to 2011.[1] The factors causing such shortages include shortage of raw materials, manufacturing problems due to aging facilities, limited profit margins for generic drugs, emerging drugs with increased profitability, and long delays for FDA approval; these factors have been previously discussed in detail elsewhere.[2–4] Numerous surveys have described how shortages of generic sterile injectable drugs place substantial burden on the healthcare system by increasing cost of care, labor needed to manage shortages, and patient adverse events.[5–10] Chemotherapeutics represent one of the largest drug classes impacted by recent drug shortages.[11]
Three recent surveys have assessed the impact of recent drug shortages in adult oncology practice.[7, 8, 12] In 2011, the Hematology/Oncology Pharmacy Association surveyed 243 pharmacists, assessing the impact of oncology drug shortages on resources, patient safety and care, and research.[7] Respondents noted increased cost of care, reimbursement challenges, and labor to manage shortages. Additionally, 16% of respondents reported near miss errors, and 6% reported one or more actual medication errors. Forty-four percent of institutions found that drug shortages impacted clinical trials. This impact was primarily manifest as delays or stops in patient enrollment (44% and 67% respectively). The second study surveyed 214 oncologists from September 2012 to March 2013; it found that chemotherapy shortages prevented physicians from prescribing desired medications and led to substantive regimen changes, treatment delays, as well as increased cost of care. The most recent study surveyed 398 pharmacy directors from March 2013 to April 2013 and was conducted after the reauthorization of the Prescription Drug User Fee Act, the Generic Drug User Fee Amendments of 2012, and the Food and Drug Administration Safety and Innovation Act of 2012. This study confirmed prior findings regarding the widespread nature of drug shortages in adult oncology practice and its negative impact on patient care.[12] However, none of these studies evaluated the impact of drug shortages on pediatric oncology practice or pediatric oncology clinical trials.[8] Although drug shortages are known to lead to protocol deviation in clinical trials that can cause significant complications for data interpretation, these effects have not been studied in a systematic way.[13]
In 2013, a Working Group on Chemotherapy Drug Shortages in Pediatric Oncology was formed to provide recommendations for oncology care providers and institutions treating children with cancer.[14] This group recommended optimizing drug usage to mitigate the effects of current shortages, creating policies that give equal priority to evidence-based use of drugs whether inside or outside of clinical trials, developing improved methods for sharing information about drug shortages, and sharing drugs in shortage among institutions, supporting national measure to prevent drug shortages, and developing a strategy for stakeholder engagement in managing drug shortages.[14]
In order to further assess the current impact of chemotherapy shortages in pediatric oncology, a survey was created and distributed to Children’s Oncology Group (COG) principal investigators (PI’s) and COG pharmacists. This survey was designed to assess the impact of the chemotherapy shortage on clinical trial and patient care and safety in pediatric oncology.
METHODS
A 5-item survey for U.S.-based COG principal investigators (PI’s) and a 14-item survey for U.S.-based COG pharmacists were developed and administered by the Children’s Oncology Group (COG) (supplemental materials). The authors developed and pilot tested the electronic survey. The survey was deployed using the Web-based survey tool SurveyMonkey (SurveyMonkey, Palo Alto, CA). Requests to participate were sent via e-mail to the primary PI at each U.S. COG site (226 site-specific PI’s). Survey requests were all sent to one responsible-designated investigator pharmacist at each U.S. COG site; 161 pharmacists requests were sent as a responsible investigator pharmacist as not all COG institutions had this role identified.
The electronic invitation included the purpose of the survey and a link to the survey instrument. The invitation stated that the survey was voluntary and confidential, and that the data collected would only be reported in aggregate so that individual participants and institutions could not be specifically identified. Each survey requested one participant per institution. Data collection began on March 12, 2014 and ended on April 14, 2014. Two electronic reminders were sent out prior to the closing date to encourage survey completion.
The pharmacist-targeted survey contained demographic and facility information including participation in a group purchasing organization (GPO), wholesaler use, and facility type. The survey asked participants to identify medications from a list of chemotherapy drugs that have been difficult to obtain at their institution over the past two years. Additionally, participants were asked to identify medications whose shortage has limited enrollment on COG and non-COG clinical trials. The survey also allowed for identification of non-chemotherapy supportive care medications in shortage. To evaluate the impact of drug shortages on patient care, the survey asked participants to estimate the number of medication errors due to drug shortage over the past 2 years. The survey also assessed how institutions specifically addressed drug shortages and whether they had administered center-level guidelines to address these issues.
The PI-targeted survey was designed to focus on the impact of drug shortages on clinical trials. It contained an identical question from the pharmacist survey, which asked participants to identify medications from a list of chemotherapy drugs known to be at risk for shortage. Additionally, it asked investigators to estimate the number of patients who had their clinical trial or clinical care impacted by drug shortages over the past 2 year and to estimate whether drug shortages had lead to an increased incidence of adverse events at his or her institution.
Univariate statistics were used to summarize response data and logistic regression models were used to determine whether pharmacists or principal investigators had differential reporting of drug shortages, overall and then for each individual medication.
This study was deemed exempt from Institutional Review Board (IRB) approval by The Children’s Hospital of Philadelphia IRB.
RESULTS
A total of 203 survey responses were received. Two pharmacist survey responses and 3 PI survey responses were excluded due to data indicating that the centers described were outside of the U.S. The response rate was 29.8% (48/161) for the pharmacist survey and 66.4% (150/226) for the PI survey.
Table 1 presents the facility characteristics as reported from the pharmacist survey. The majority of institutions represented in the pharmacist survey were university medical centers (57%). Seventy-four percent of respondents (35/47) stated that their organization was part of a group purchasing organization. Most common wholesalers used by participants were AmeriSourceBergen (33%, n=15), Cardinal (35%, n=16), and McKesson (30%, n=14).
Table 1.
Institutional characteristics as determined from pharmacist survey.
| No. (%) Respondents (n = 47) | |
|---|---|
| Institution Type | |
| University Medical Centers | 27 (57%) |
| Community Hospital | 3 (6%) |
| Freestanding Children’s Hospital | 17 (36%) |
| Children’s Hospital within an Adult Hospital | 13 (28%) |
| Other | 7 (15%) |
| Group Purchasing Organization | No. (%) Respondents (n = 47) |
| Yes | 35 (74%) |
| No | 12 (26%) |
| Wholesalers Used | No. (%) Respondents (n = 46) |
| AmeriSourceBergen | 15 (33%) |
| Cardinal | 16 (35%) |
| McKesson | 14 (30%) |
| Morris and Dickson | 4 (9%) |
| Other | 2 (4%) |
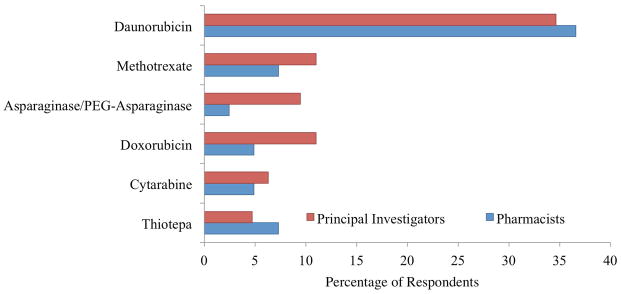
The drug shortages reported most commonly to impact clinical trials were daunorubicin (37% or 15/41 pharmacists, 35% or 44/127 PI’s), methotrexate (7% or 3/41 pharmacists, 11% or 14/127 PI’s), asparaginase/PEG-asparaginase (2% or 2/41 pharmacists, 9% or 12/127 PI’s), doxorubicin (5% or 2/41 pharmacists, 11% or 14/127 PI’s), cytarabine (5% or 2/41 pharmacists, 6% or 8/127 PI’s), and thiotepa (7% or 3/41 pharmacists, 2 % or 2/127 PI’s) (Figure 1). One-third of pharmacist respondents (15/45) reported a shortage of non-chemotherapy supportive agent including fluids and electrolytes, such as D5 ½ normal saline and sodium bicarbonate, as well as other supportive care agents, such as leucovorin and dexrazoxane.
Figure 1.
Percentage of pharmacist and principal investigator respondents that indicated that shortage of chemotherapy agent impacted clinical trials over the past two years.
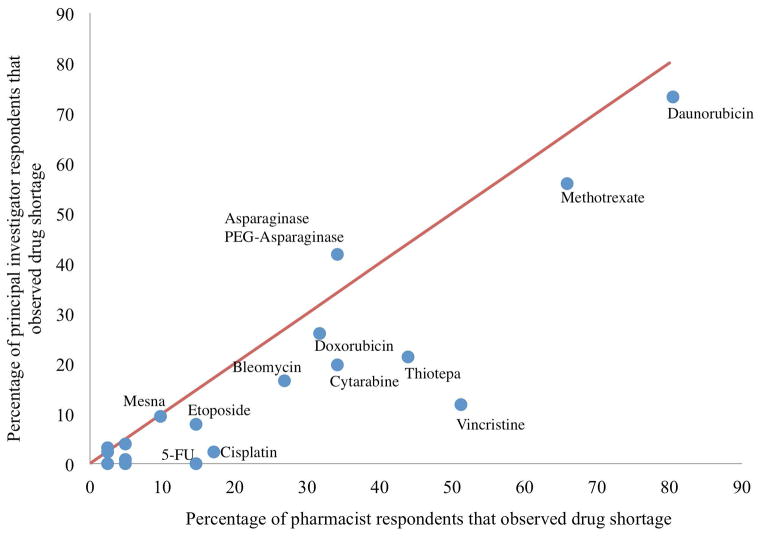
The concordance between pharmacist and PI responses regarding the most common chemotherapy shortages was reasonably strong (Figure 2). Of note, pharmacists were twice as likely to report a chemotherapy drug shortage in comparison with principal investigators (OR 2.1, 95% CI: 1.6–2.7, P<0.0001). In the models for individual medications, the increased reporting of shortage for pharmacists vs. principal investigators remained statistically significant, for the drugs vincristine (OR 9.2, 95% CI: 4.0–21.3, P<0.0001), thiotepa (OR 3.2, 95% CI: 1.5–6.7, P=0.0023), 5-Fu (OR 10.7 95% CI: 2.1–55.4, P=0.0047) and cisplatin (OR 7.1, 95% CI: 1.7–29.8, P=0.0075).
Figure 2.
Concordance between pharmacist (n=41) and principal investigator (n=127) reported drug shortages
Fifty percent (74/147) of COG PI’s reported that at least one patient enrolled on a clinical trial was impacted by drug shortage, and 66% (98/148) of COG PI’s reported that at least one patient had clinical care impacted by drug shortage. Thirty four percent (14/41) of pharmacist respondents reported at least one near miss or actual medication errors due to drug shortage.
The most common institutional methods of addressing drug shortages included obtaining drugs from an outside institution (66% or 25/38), converting to another form of drug (50% or 19/38), cohorting patients (47% or 18/38), changing regimens (39% or 15/38), preventing enrollment on a clinical trials (26% or 10/38), and reducing drug dosage for treatment (16% or 6/38). Seventy-three percent of pharmacist respondents (30/41) reported that center level guidelines to address drug shortages had been issued by their institution.
DISCUSSION
Here we report an initial assessment of the national impact of drug shortages on clinical trials and clinical care in the pediatric oncology community. Drug shortages remain common and affect two-thirds of institutions treating children with cancer, impact clinical trials, and may contribute to adverse events. The higher reporting rate of drug shortages by pharmacists than physicians suggests that pharmacists’ efforts may help avert certain local drug shortages. Importantly, more than half of pediatric institutions are implementing recommendations to address shortages, such as collaborating with other institutions and issuing center-level guidelines.
Both pharmacist and PI survey responses suggested that a large number of chemotherapy drugs remain in shortage, and that these shortages negatively impact clinical care and clinical trials. Consistent with prior studies at adult oncology institutions that identified frequent shortages in daunorubicin, liposomal doxorubicin, cytarabine, bleomycin, fluorouracil, and paclitaxel, we found that daunorubicin, doxorubicin, cytarabine, bleomycin, methotrexate, asparaginase/PEG-asparaginase, and thiotepa were most commonly reported to be in shortage over the past two years. [7, 8] More granular data are needed to investigate the impact of drug shortages on protocol deviations and clinical trial outcomes.[13] Our study also demonstrated that two-thirds of institutions have one or more patients with clinical care impacted by drug shortage.
Pharmacists were twice as likely to report chemotherapy shortages than physicians. This difference may reflect success in pharmacists’ efforts to mitigate certain drug shortages by obtaining drug supply from an outside institution or by other methods that may compensate for shortages. For example, pharmacists may address a medication shortage by purchasing an alternative vial size. While such compensatory mechanisms are not evident to the physician or patient, these mechanisms may create complexities within the pharmacy regarding safety and training of personnel on the new vial size, physical stocking modifications, and required changes within the institutional electronic medical record for the alternate vial size. Moreover, complex ordering requirements for agents in shortage, such as thiotepa are more evident to pharmacists than physicians.[15] The increase in complexity and resulting costs of care given the additional time required to address the shortages have previously been discussed.[7]
The recent Working Group on Chemotherapy Drug Shortages in Pediatric Oncology specifically recommended optimizing drug usage to mitigate the effects of current shortages, developing improved methods for sharing information about drug shortages, and sharing drugs in shortage among institutions.[14] Our study suggested that methods to optimize drug usage, such as cohorting or scheduling patients to receive a scarce drug on the same day to minimize waste, are implemented in approximately one-half of institutions. The reasons for the incomplete implementation of this recommendation are not known. Some centers may find cohorting of patients neither necessary nor feasible. Other institutions may be responding to billing requirements of third-party payors for partial vial use by not cohorting patients. Although we did not investigate this in detail, it is possible that perceived or real billing requirements of third-party payors may contribute to the limited use of this approach. Understanding the role of these potential reasons will likely be important if chemotherapy shortages persist or worsen.
While previous studies reported that approximately 30% of adult hospitals have center level guidelines to address shortages, 73% of pharmacist respondents reported center level guidelines. The substantially higher percentage of pediatric centers with pharmacy guidelines may be due to the collaborative nature of pediatric oncology and participation in COG clinical trials that provide specific guidelines for chemotherapy adjustment for medication shortages. Of note, 66% of pharmacist respondents reported that they obtained a drug in shortage from another institution whereas only 7% of adult respondents reported being able to do so.[7, 8] Although the administrative mechanisms necessary for sharing of drugs in shortage varies between institutions, it is encouraging that the majority of pediatric institutions caring for children with cancer are already engaging in this process.
This study has several limitations. While the sample size provided a basic characterization of drug shortages in pediatric oncology in the United States, the sample size is insufficient to quantify precisely the specific number of patients impacted by drug shortages. Further, the response rate amongst pharmacists was relatively low. Since the survey was administered anonymously, differences in responding and non-responding institutions cannot be characterized. In addition, since the survey did not request extensive details on shortage related adverse events, the survey is unable to characterize the shortage related adverse events in detail. As with any retrospective survey, respondents may be susceptible to recall bias for both more recent shortages and more severe shortage effects. Finally, the one pharmacist and principal investigator respondent per an institution may not represent the complete impact of drug shortages at their institution.
Despite these limitations, this survey highlights a range of issues related to generic medication shortages for children with cancer. Specifically, these data clearly demonstrate that medication shortages are an important problem facing a majority of institutions treating children with cancer and hindering ongoing clinical trials. The collaborative response of centers to these shortages suggests that certain institutional strategies to mitigate these shortages, notably increased pharmacist efforts, establishment of institutional guidelines, and a collaborative approach to medication procurement, may be effective. Despite implementation of some of these efforts, two thirds of institutions treating children with cancer have reported adverse effects on patient care by chemotherapy and supportive care drug shortages. The widespread impact of these shortages across institutions provides an impetus for a coordinated national effort to prevent future shortages. Such an effort will require the engagement at a national level of patients and patient advocacy groups, the pharmaceutical industry, health systems, and the federal government.
Supplementary Material
Acknowledgments
Grant sponsor: Hematologic Research Malignancies Fund at the Children’s Hospital of Philadelphia.
Footnotes
CONFLICTS OF INTEREST STATEMENT
There are no conflicts of interest to report.
References
- 1.Dill S, Anne M. Overview of US Drug Shortages. U.S. Food and Drug Administration; [Accessed May 16, 2014]. website. http://www.fda.gov/downloads/Drugs/UCM327108.pdf. Published November 6, 2012. [Google Scholar]
- 2.Woodcock J, Wosinska M. Economic and technological drivers of generic sterile injectable drug shortages. Clinical Pharmacology and Therapeutics. 2013;93(2):170–176. doi: 10.1038/clpt.2012.220. [DOI] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services Services. Economic Analysis of the Causes of Drug Shortages. [Accessed May 16, 2014];Office of the Assistant Secretary for Planning and Evaluation Issue Brief website. http://aspe.hhs.gov/sp/reports/2011/drugshortages/ib.shtml. Published October 2011.
- 4.Link MP, Hagerty K, Kantarjian HM. Chemotherapy drug shortages in the United States: genesis and potential solutions. Journal of Clinical Oncology. 2012;30(7):692–694. doi: 10.1200/JCO.2011.41.0936. [DOI] [PubMed] [Google Scholar]
- 5.Baumer AM, Clark AM, Witmer DR, Geize SB, Vermeulen LC, Deffenbaugh JH. National survey of the impact of drug shortages in acute care hospitals. American Journal of Health-system Pharmacy. 2004;61(19):2015–2022. doi: 10.1093/ajhp/61.19.2015. [DOI] [PubMed] [Google Scholar]
- 6.Kaakeh R, Sweet BV, Reilly C, Bush C, DeLoach S, Higgins B, Clark AM, Stevenson J. Impact of drug shortages on U.S. health systems. American Journal of Health-system Pharmacy. 2011;68(19):1811–1819. doi: 10.2146/ajhp110210. [DOI] [PubMed] [Google Scholar]
- 7.McBride A, Holle LM, Westendorf C, Sidebottom M, Griffith N, Muller RJ, Hoffman JM. National survey on the effect of oncology drug shortages on cancer care. American Journal of Health-system Pharmacy. 2013;70(7):609–617. doi: 10.2146/ajhp120563. [DOI] [PubMed] [Google Scholar]
- 8.Gogineni K, Shuman KL, Emanuel EJ. Survey of oncologists about shortages of cancer drugs. The New England Journal of Medicine. 2013;369(25):2463–2464. doi: 10.1056/NEJMc1307379. [DOI] [PubMed] [Google Scholar]
- 9.Institute for Safe Medication Practices. Drug shortages: national survery reveals high level of frustration, low level of safety. Institute for safe medication practices; [Accessed May 16, 2014]. website. https://www.ismp.org/newsletters/acutecare/articles/20100923.asp. Published September 23, 2010. [Google Scholar]
- 10.American Hospital Association. American hospital association survey on drug shortages. American Hospital Association; [Accessed May 16, 2014]. website. http://www.aha.org/content/11/drugshortagesurvey.pdf. Published July 12, 2011. [Google Scholar]
- 11.IMS Institute for Healthcare Informatics. Drug shortages: a closer look at products, suppliers, and volume volatility, executive summary. [Accessed May 16, 2014];IMS drug shortages report website. http://www.imshealth.com/deployedfiles/ims/Global/Content/Insights/IMS%20Institute%20for%20Healthcare%20Informatics/Static%20Files/IIHI_Drug_Shortage_Media_ExecSumm.pdf. Published October 7, 2011.
- 12.Goldsack JC, Reilly C, Bush C, McElligott S, Bristol MN, Motanya UN, Field R, Vozniak JM, Wong YN, Schwartz JS, Domchek S. Impact of shortages of injectable oncology drugs on patient care. American Journal of Health-system Pharmacy. 2014;71(7):571–578. doi: 10.2146/ajhp130569. [DOI] [PubMed] [Google Scholar]
- 13.Goozner M. Drug shortages delay cancer clinical trials. Journal of the National Cancer Institute. 2012;104(12):891–892. doi: 10.1093/jnci/djs293. [DOI] [PubMed] [Google Scholar]
- 14.Decamp M, Joffe S, Fernandez CV, Faden RR, Unguru Y Working Group on Chemotherapy Drug Shortages in Pediatric Oncology. Chemotherapy drug shortages in pediatric oncology: a consensus statement. Pediatrics. 2014;133(3):e716–724. doi: 10.1542/peds.2013-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Food and Drug Administration. US Food and Drug Administration Drug Shortage Update: Thiotepa (Thioplex) for Injection Drug Shortages 2014. U.S. Food and Drug Administration Drug Shortage; [Accessed June 3, 2014]. website. http://www.fda.gov/downloads/Drugs/DrugSafety/DrugShortages/UCM251666.pdf. Published July 25, 2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.