Abstract
Low HDL is an independent risk factor for myocardial infarction. This paper reviews our current understanding of HDL, HDL structure and function, HDL subclasses, the relationship of low HDL with myocardial infarction, HDL targeted therapy, and clinical trials and studies. Furthermore potential new agents, such as alirocumab (praluent) and evolocumab (repatha) are discussed.
Keywords: dyslipidemia, review, hyperlipidemia, cholesterol, HDL, HDL-C, LDL, monoclonal antibody, PCSK, PCSK9, proprotein, convertase, sublisin, kexin, statin, heart attack, myocardial infarction, low HDL, high density lipoprotein, HDL subclass, Biosynthesis, storage, elimination, paraoxonase-1, PON1, platelet activating factor, acetylhydrolase, PAF-AH, apolipoprotein, Apo, ApoA-I, ApoA-II, CETP, niacin, TRIUMPH, AIM-HIGH, HP2-THRIVE, endothelial lipase gene, LIPG, atheroprotective, alirocumab, praluent, evolocumab, repatha, ODYSSEY, OSLER
Background
Myocardial infarction (MI) remains a leading cause of death worldwide. An acute MI occurs when myocardial ischemia exceeds a critical threshold, usually due to an acute plaque rupture in the coronary arteries, and the cellular cascade of events overwhelms myocardial cellular repair mechanisms leading to myocardial cell damage.1 Myocardial ischemia occurs as a result of plaque buildup in the coronary arteries, a disease formally known as atherosclerosis or coronary artery disease (CAD). Rupturing of vulnerable atherosclerotic plaque follows a period of continual plaque destabilization and/or plaque growth due to various pathobiological processes.2 Plaque contents are enclosed within a stabilizing fibrous cap that prevents exposure of the thrombogenic core to the bloodstream, and weakening of this cap can therefore lead to plaque rupture and MI.3 There is substantial evidence illustrating a positive relationship between low-density lipoprotein cholesterol (LDL-C) and elevated triglyceride (TG) to CAD progression. Oxidized LDL particles damage endothelium and promote plaque rupture. Although LDL-C is well studied, there is also a significant inverse relationship between high-density lipoprotein cholesterol (HDL-C), the “good cholesterol,” and CAD progression. Studies show that the strongest independent risk factor for CAD is a low serum concentration of HDL, resulting in an increased risk of MI and stroke, although many patients with MI have normal HDL-C levels.4 In this review, we discuss the potential role of low HDL in accelerated CAD and examine the need for patients in this subset to be treated with appropriate medication to help prevent MI.
Importance of low HDL in clinical associations
Numerous factors influence the reduction of HDL-C levels, including smoking and elevated nonfasting TG levels. In a study by Xenophontos et al analyzing the relationship between low HDL-C levels (<40 mg/dL), smoking, and polymorphism and MI in Greek Cypriot males, it was shown that smoking reduces HDL-C and apolipoprotein concentrations and increases LDL-C, plasma TGs, and very-low-density lipoprotein TGs, which all contribute to an increased risk of MI.5 When TG levels are elevated, TGs saturate HDL particles, leading to an increased exchange of cholesterol esters by cholesteryl esterase transfer protein.6 Furthermore, a study by Virmani et al showed that thin-cap fibroatheromas, a type of vulnerable plaque and the major precursor lesion associated with plaque rupture, are the most common in patients with low HDL-C levels and a high total cholesterol/HDL ratio.7,8
HDL: Brief Overview of Structure and Function
HDLs in the blood serum represent heterogeneous lipoproteins with a density >1.063 g/mL and a small size between 5 and 12 nm.9 HDL-C is composed of proteins – apolipoprotein A-I (ApoA-I) and apolipoprotein A-II (ApoA-II) – and other molecules, including phospholipids, cholesterol esters, unesterified cholesterol, and TGs. Recent studies have examined numerous HDL subclasses that differ in the quality and quantity of lipids and apolipoproteins and can be distinguished by shape, charge, density, and size through various methods.10 The varying methods used to determine HDL subclasses are inconsistent in their findings, each method obtaining various sets of subclasses in comparison to another. In a recent review of the HDL subfractions, Rizzo et al discussed a unified and integrated HDL nomenclature that defined five HDL subclasses for simplicity: very large, large, medium, small, and very small HDL particles. However, the two major subclasses discussed in literature are the HDL2 and HDL3, which are large and small, dense HDL particles, respectively. HDL particle structures consist of an outer layer composed of free cholesterol, phospholipids, and varied apolipoptroteins and a hydrophobic center composed of TGs and the esters of cholesterol.10,11
HDLs can contribute to the maintenance of endothelial cell homeostasis and have potent antioxidant properties via the following enzymes: paraoxonase-1 (PON1), platelet-activating factor acetylhydrolase (PAF-AH), glutathione selenoperoxidase, lecithin-cholesterol acyltransferase (LCAT), and phospholipid transfer protein.10 HDL particles also display antithrombotic and antiaggregating properties via the protection of the endothelium through different mechanisms: by stimulating endothelial cell nitric oxide and prostacyclin production, HDL particles are able to promote better regulation of vascular structure and tone.10,12,13 The capacity of HDL to inhibit endothelial cell apoptosis has, therefore, been suggested as an important potential antiatherogenic property, and interruptions to this function can be deleterious. Another important function of HDL particles is the protection of LDL particles from oxidation through the activity of associative enzymes such as PAF-AH and PON.10
Perhaps the most relevant atheroprotective function of HDL is to promote the removal of intracellular cholesterol by a process called reverse cholesterol transport (RCT). As defined by Vergeer et al.14, RCT is the uptake of cholesterol from peripheral cells by lipid-poor apoA-I and HDL that is mediated by lipid transporter molecules such as ATP-binding cassette transporter A1 and G1 and scavenger receptor B-I, and the subsequent delivery to the liver for ultimate excretion into the feces as neutral sterols or bile acids. The whole RCT process is physiologically important as it allows removal of excess cholesterol from the artery wall and from atherosclerotic plaques, thereby reducing plaque buildup in the arteries that can lead to an MI.15
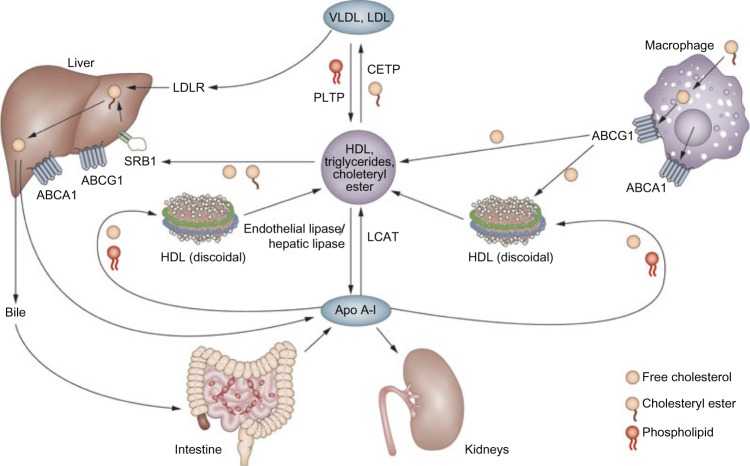
In the Tromsø study, a 7-year, prospective, population-based observational study of carotid plaque progression was measured using ultrasound in 1,952 subjects with evidence of carotid atherosclerosis at baseline; it was shown that more dense, echogenic atherosclerotic plaques were associated with higher levels of HDL-C. The stability and density of these plaques can be attributed to the removal of the lipid within the plaque via RCT.16,17 Figure 1 illustrates the various roles of HDL.18
Figure 1.
HDL biosynthesis, storage, and elimination.18
HDL subpopulations and MI
The major subclasses of HDL, HDL2, and HDL3 vary in their composition and activities of lipids and apolipoproteins.19 In HDL3 populations, for example, increased activities of antioxidative enzymes such as PON1, PAF-AH, and LCAT, as well as a composition dominated by apoJ, apoL-1, and apoF proteins have been noted. In HDL2 populations, different proteins such as apoE, apoC-1, apoC-11, and apoC-111 have been seen to dominate in these larger, less dense particles.10 The importance of the differing compositions and functionalities remains ambiguous. Numerous studies on HDL functionality propose the value of obtaining more information on HDL subfractions such as lipids and apolipoproteins as a means of better understanding the relationship between HDL-C and cardiovascular diseases. Moreover, there are equivocal data in studies analyzing these two subclasses and a direct relationship with CAD and MI remains unclear.
As discussed by Rizzo et al, the two major subclasses, HDL2 and HDL3, possess different functionalities, yet most studies support the view that larger HDL2 particles are more atheroprotective.10 However, Stampfer et al analyzed the cholesterol and apolipoprotein contents of 246 male patients with and without MIs and found that although HDL2 was indeed associated with lower MI risk, it is HDL3 that had the strongest correlation with MI and was therefore the strongest MI predictor. Meanwhile, Salonen et al.20 studied 1,799 randomly selected male subjects who were 43, 48, 54, or 60 years old and found that a total serum HDL-C level of <1.09 mmol/L was associated with a 3.3-fold risk of acute MI, serum HDL2 cholesterol of <0.65 mmol/L was associated with a 4.0-fold risk of acute MI, and serum HDL3 cholesterol of <0.4 mm/L was associated with a 2.0-fold risk of acute MI. The authors concluded that both total HDL and HDL2 levels were inversely related to MI risk, possibly conferring atheroprotection against ischemic heart disease, while the role of HDL3 remains ambiguous. Contrastingly, Martin et al.21 investigated the two major HDL subclasses, HDL2 and HDL3, in secondary prevention and found that increased risk for long-term hard clinical events is associated with low HDL3, but not HDL2 or total HDL. Evidence from analysis of the TRIUMPH study of 2,465 acute MI patients, and the IHCS study of 2,414 patients who underwent coronary angiography, determined that HDL3 was independently associated with a 50% greater risk for MI in each study. Thus, the individual atheroprotective roles of HDL2 and HDL3 remain controversial.
HDL dysfunction
As with the vast majority of biological molecules, the function of HDL-C particles is dependent on their structure. Therefore, even the slightest modifications to their structure may prevent appropriate. Infection/inflammation and oxidative stresses have been shown to be causative factors in the alteration of lipoprotein particles.22 The duration of infection/inflammation induces numerous changes in the apolipoproteins, enzymes, and transfer proteins associated with HDL-C, as well as a reduction in HDL-C levels.23 Hypothetically, the alterations of associative molecules inexorably result in functional changes of the HDL-C particles themselves. The known molecules affected are lecithin (LCAT), cholesteryl esterase transfer protein, hepatic lipase, and phospholipid transfer protein, which play important roles in HDL metabolism and RCT.24–27 A study in 1995 comparing HDL before and during an acute phase response (APR) – systemic reaction to infectious and noninfectious tissue destructive processes – in both human beings and rabbit model produced the following results: “in rabbits, from the onset of APR the protective effect of HDL progressively decreased and was completely lost by day three. As serum amyloid A (SAA) levels in acute phase HDL (AP-HDL) increased, apo A-I levels decreased 73%. Concurrently, PON and PAF-AH levels in HDL declined 71 and 90%, respectively, from days one to three. After day three, there was some recovery of the protective effect of HDL.” These results indicate that during bodily responses to infection and inflammation, changes to HDL and its associative proteins disarm its ability to protect LDL from oxidation in the aortic cell wall – a key function of HDL in the prevention of CAD – and can become pro-inflammatory agents.24–28
Riwanto et al compared the effects of HDL particles in mouse cohorts with CAD (HDL-CAD) vs HDL particles in healthy subjects (HDL-Healthy) for the activation of endothelial anti- and proapoptotic signaling pathways to determine which changes to the lipoprotein were relevant, thereby illustrating how structural changes to HDL proteomics may be harmful.29 According to this study, HDL-Healthy reduced endothelial apoptosis in the aorta, whereas HDL-CAD did not reduce endothelial apoptosis. Proteomic analysis identified significantly reduced clusterin levels and an increase in the apoC-III content in HDL-CAD relative to HDL-Healthy, which contribute to this discrepancy in function. Clusterin is a protein that, when bound to HDL, increases its endothelial antiapoptotic effects. Conversely, apoC-III has been found to diminish the capacity of HDL to lessen endothelial apoptosis and has been shown to transfer between lipoproteins.29,30 This study also shows that the “apoC-III content was not only increased in the HDL fraction, but also in the serum and the LDL/very-low-density lipoprotein fraction of patients with CAD, suggesting an increased synthesis or a reduced clearance of apoC-III in patients with CAD.” Moreover, elevated apoC-III levels have been linked to other factors associated with atherosclerotic plaque vulnerability and progression such as hypertriglyceridemia, metabolic syndrome, and diabetes mellitus.31–33 ApoC-III also inhibits the clearance of TG-rich lipoproteins, a key process in the prevention of CAD, and when bound to LDL is independently associated with an increased risk of CAD.34
Taken together, these adverse effects of dysfunctional HDL may offer an explanation for how low serum levels of HDL-C contribute to the destabilization and increased vulnerability of atherosclerotic plaque, potential rupture, and MI.
Treatment: Targeting HDL Levels
The well-known “HDL hypothesis” suggests that therapies aimed at raising HDL-C concentrations will lower the risk of CAD and MI. In a widely cited meta-analysis of four large studies (total number of individuals studied: 15,252), a 1 mg/dL increase of HDL-C levels was reported to be associated with a 2%–3% decreased CVD risk.14,35 Niacin, presently prescribed with a statin, is one of the most commonly used pharmacological therapy aimed at raising HDL-C concentrations in patients with such risks. At a pharmacological dose of ~1.5–2 g per day, Niacin is one of the most potent agents available for this purpose. Niacin also reduces all proatherogenic lipids and lipoproteins, including total cholesterol, TGs, very-low-density lipoprotein, LDL, and lipoprotein(a).15 Despite its popularity, the efficacy of niacin has come into question in recent studies.36 Two distinct studies, Atherosclerosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) and Heart Protection Study 2 – Treatment of High-density Lipoprotein to Reduce the Incidence of Vascular Events (HPS2-THRIVE) were aimed at evaluating whether adding the modern, extended-release niacin formulations to statin therapy provides incremental benefit over statin therapy alone in terms of cardiovascular primary events in patients with established CAD.15 These clinical trials studied specific populations of stable ischemic heart disease patients, excluding patients with MI or those with significant residual mixed dyslipidemia not treated with optimal doses of intensive statin therapy.37 Both the AIM-HIGH and HPS2-THRIVE clinical trials were stopped prematurely due to a lack of beneficial effects and an inability to meet primary endpoints of reduced cardiovascular disease and MI risk.38
A genetic study conducted by Voight et al failed to show a causal association between HDL-C elevation and reduced risk of MI. Using two mendelian randomization analyses, they tested the hypothesis of this causal association. Voight et al identified the endothelial lipase gene (LIPG) variant that has a serine substituted for asparagine at amino acid 396 (LIPG 396Ser) that only affects HDL-C without changing other lipid/nonlipid MI risk factors. Carriers of LIPG 396Ser display significant increases in HDL-C as compared to noncarriers. To determine whether the carriers of this LIPG 396Ser are protected from MI, Voight et al studied the association of LIPG Asn396Ser – a single-nucleotide polymorphism in the LIPG – with incident MI in 50,763 participants from six prospective cohort studies. They found that this LIPG variant did not protect against MI, challenging the idea that higher levels of HDL-C confer protection.39 Data from this study show no direct correlation between HDL-C levels and risk of MI from a genetic standpoint, suggesting that increasing HDL-C medically is not sufficient to prevent MI.
However, one caveat to the above study, Rizzo et al suggest that total HDL-C may not accurately reflect the true atheroprotective properties in particular patients. More studies are warranted with the HDL subfractions.
There have been a number of recent studies on the novel monoclonal antibody drugs, evolocumab (Amgen) and alirocumab (Sanofi Aventis/Regeneron). These drugs comprise a monoclonal antibody targeting a liver protein: proprotein convertase subtilisin/kexin type 9 (PCSK9) that inactivates it [reviewed in Ref. 40]. These studies, ODYSSEY41 and OSLER,42 suggest PCSK inhibitors may be highly effective LDL-C lowering drugs for patients who are statin intolerant, and therefore potentially lower the risk for CAD, MI, and stroke. Although there is sufficient data supporting its effect on LDL, more data are warranted to discuss its effects on HDL and patients with dyslipidemia.
Conclusion
In this review, we have established that HDL is decreased in many patients with acute MI. We have analyzed its role in accelerated CAD mildly. Brief analysis of its subparticles show that increasing total concentration may not be sufficient to protect MI. However, blood levels of HDL <40 mg/dL may be an effective warning sign for atherosclerotic development. Therefore, in these patients, statin treatment should be used to prevent accelerated CAD and MI. In the future, more data from the new PCSK9 inhibitors will help ascertain their role in this process.
Acknowledgments
A.R. thanks her parents Carlos and Cindy Ramirez for all their hard work and support to help her all these years. P.P.H. thanks his parents and brother for their unwavering support.
Footnotes
ACADEMIC EDITOR: Thomas E. Vanhecke, Editor in Chief
PEER REVIEW: Three peer reviewers contributed to the peer review report. Reviewers’ reports totaled 532 words, excluding any confidential comments to the academic editor.
FUNDING: Authors disclose no funding sources.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived the concepts: AR, PH. Analyzed the data: AR, PH. Wrote the first draft of the manuscript: AR, PH. Contributed to the writing of the manuscript: AR, PH. Agree with manuscript results and conclusions: AR, PH. Jointly developed the structure and arguments for the paper: AR, PH. Made critical revisions and approved final version: AR, PH. Both authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Cotran RS, Kumar V, Robbins SL, editors. Robbins Pathologic Basis of Disease. 5th ed. Philadelphia: WB Saunders; 1994. [Google Scholar]
- 2.Andreou I, Antoniadis AP, Shishido K, et al. How do we prevent the vulnerable atherosclerotic plaque from rupturing? Insights from in vivo assessments of plaque, vascular remodeling, and local endothelial shear stress. J Cardiovasc Pharmacol Ther. 2015;20(3):261–75. doi: 10.1177/1074248414555005. [DOI] [PubMed] [Google Scholar]
- 3.Kullo IJ, Edwards WD, Schwartz RS. Vulnerable plaque: pathobiology and clinical implications. Ann Intern Med. 1998;129:1050–60. doi: 10.7326/0003-4819-129-12-199812150-00010. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Méndez Ó, Pacheco HG, Martínez-Sánchez C, Franco M. HDL-cholesterol in coronary artery disease risk: function or structure? Clin Chim Acta. 2014;429:111–22. doi: 10.1016/j.cca.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Xenophontos S, Hadjivassiliou M, Karagrigoriou A, et al. Low HDL cholesterol, smoking and polymorphism are associated with myocardial infarction in Greek Cypriot males. A pilot study. Open Cardiovasc Med J. 2008;2:52–9. doi: 10.2174/1874192400802010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egeland GM, Igland J, Sulo G, Nygard O, Ebbing M, Tell GS. Non-Fasting Triglycerides Predict Incident Acute Myocardial Infarction Among Those with Favorable HDL-Cholesterol: Cohort Norway. Bergen, Norway: Division of Epidemiology, Norwegian Institute of Public Health; [DOI] [PubMed] [Google Scholar]
- 7.Burke AP, Farb A, Malcom GT. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–82. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 8.Virmani R, Burke AP, Farb A, Kolodgie FD. Pathology of the vulnerable plaque. J Am Coll Cardiol. 2006;47(8 s1):C13–8. doi: 10.1016/j.jacc.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 9.Calabresi L, Gomaraschi M, Simonelli S, Bernini F, Franceschini G. HDL and atherosclerosis: insights from inherited HDL disorders. Biochim Biophys Acta. 2015;1851(1):13–8. doi: 10.1016/j.bbalip.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo M, Otvos J, Nikolic D, Montalto G, Toth PP, Banach M. Subfractions and subpopulations of HDL: an update. Curr Med Chem. 2014;2I:2881–91. doi: 10.2174/0929867321666140414103455. [DOI] [PubMed] [Google Scholar]
- 11.Asztalos BF, Schaefer EJ. High-density lipoprotein subpopulations in pathologic conditions. Am J Cardiol. 2003;91(7 A):12E–7E. doi: 10.1016/s0002-9149(02)03383-0. [DOI] [PubMed] [Google Scholar]
- 12.Besler C, Luscher TF, Landmesser U. Molecular mechanisms of vascular effects of high-density lipoprotein: alterations in cardiovascular disease. EMBO Mol Med. 2012;4:251–68. doi: 10.1002/emmm.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161:1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 14.Vergeer M, Holleboom AG, Kastelein JJP, Kuivenhoven JA. The HDL hypothesis: does high-density lipoprotein protect from atherosclerosis? J Lipid Res. 2010;51(8):2058–73. doi: 10.1194/jlr.R001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdier C, Martinez LO, Ferrières J, Elbaz M, Genoux A, Perret B. Targeting high-density lipoproteins: update on a promising therapy. Arch Cardiovasc Dis. 2013;106(11):601–11. doi: 10.1016/j.acvd.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 16.Tschoepe D, Stratmann B. Plaque stability and plaque regression: new insights. Eur Heart J Suppl. 2006;8(suppl F):F34–9. [Google Scholar]
- 17.Johnsen SH, Mathiesen EB, Fosse E, et al. Elevated high-density lipoprotein cholesterol levels are protective against plaque progression: a follow-up study of 1952 persons with carotid atherosclerosis the Tromsø study. Circulation. 2005;112:498–504. doi: 10.1161/CIRCULATIONAHA.104.522706. [DOI] [PubMed] [Google Scholar]
- 18.Navab M, Reddy ST, Van Lenten BJ, Fogelman AM. HDL and cardiovascular disease: atherogenic and atheroprotective mechanisms. Nat Rev Cardiol. 2011;8:222–32. doi: 10.1038/nrcardio.2010.222. [DOI] [PubMed] [Google Scholar]
- 19.Tian L, Fu M. The relationship between high density lipoprotein subclass profile and plasma lipids concentrations. Lipids Health Dis. 2010;9:118. doi: 10.1186/1476-511X-9-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salonen JTI, Salonen R, Seppanen K, Rauramaa R, Tuomilehto J. A Prospective Population Study in Eastern Finnish Men. Kuopio, Finland: Department of Community Health and General Practice, University of Kuopio; 1991. HDL, HDL2, and HDL3 Subfractions, and the Risk of Acute Myocardial Infarction. [DOI] [PubMed] [Google Scholar]
- 21.Martin SS, Khokhar AA, May HT, et al. Lipoprotein Investigators Collaborative (LIC) HDL cholesterol subclasses, myocardial infarction, and mortality in secondary prevention: the Lipoprotein Investigators Collaborative. Eur Heart J. 2015;36(1):22–30. doi: 10.1093/eurheartj/ehu264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khovidhunkit W, Memon RA, Feingold KR, Grunfeld C. Infection and inflammation-induced proatherogenic changes of lipoproteins. J Infect Dis. 2000;181(suppl 3):S462–72. doi: 10.1086/315611. [DOI] [PubMed] [Google Scholar]
- 23.Sammalkorpi K, Valtonen V, Kerttula Y, Nikkila E, Taskinen MR. Changes in serum lipoprotein pattern induced by acute infections. Metabolism. 1988;37:859–65. doi: 10.1016/0026-0495(88)90120-5. [DOI] [PubMed] [Google Scholar]
- 24.Ly H, Francone OL, Fielding CJ, et al. Endotoxin and TNF lead to reduced plasma LCAT activity and decreased hepatic LCAT mRNA levels in Syrian hamsters. J Lipid Res. 1995;36:1254–63. [PubMed] [Google Scholar]
- 25.Hardardóttir I, Moser AH, Fuller J, Fielding C, Feingold K, Grünfeld C. Endo-toxin and cytokines decrease serum levels and extra hepatic protein and mRNA levels of cholesteryl ester transfer protein in syrian hamsters. J Clin Invest. 1996;97:2585–92. doi: 10.1172/JCI118707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feingold KR, Memon RA, Moser AH, Shigenaga JK, Grunfeld C. Endo-toxin and interleukin-1 decrease hepatic lipase mRNA levels. Atherosclerosis. 1999;142:379–87. doi: 10.1016/s0021-9150(98)00265-2. [DOI] [PubMed] [Google Scholar]
- 27.Jiang XC, Bruce C. Regulation of murine plasma phospholipid transfer protein activity and mRNA levels by lipopolysaccharide and high cholesterol diet. J Biol Chem. 1995;270:17133–8. doi: 10.1074/jbc.270.29.17133. [DOI] [PubMed] [Google Scholar]
- 28.Van Lenten BJ, Hama SY, de Beer FC, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell co-cultures. J Clin Invest. 1995;96:2758–67. doi: 10.1172/JCI118345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riwanto M, Rohrer L, Roschitzki B, et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127(8):891–904. doi: 10.1161/CIRCULATIONAHA.112.108753. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen MN, Chan DC, Dwyer KP, Bolitho P, Watts GF, Barrett PH. Use of intralipid for kinetic analysis of HDL apoC-III: evidence for a homogeneous kinetic pool of apoC-III in plasma. J Lipid Res. 2006;47:1274–80. doi: 10.1194/jlr.M600018-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Olivieri O, Bassi A, Stranieri C, et al. Apolipoprotein C-III, metabolic syndrome, and risk of coronary artery disease. J Lipid Res. 2003;44:2374–81. doi: 10.1194/jlr.M300253-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Cohn JS, Patterson BW, Uffelman KD, Davignon J, Steiner G. Rate of production of plasma and very-low-density lipoprotein (VLDL) apolipoprotein C-III is strongly related to the concentration and level of production of VLDL triglyceride in male subjects with different body weights and levels of insulin sensitivity. J Clin Endocrinol Metab. 2004;89:3949–55. doi: 10.1210/jc.2003-032056. [DOI] [PubMed] [Google Scholar]
- 33.Lee SJ, Moye LA, Campos H, Williams GH, Sacks FM. Hypertriglyceridemia but not diabetes status is associated with VLDL containing apolipoprotein CIII in patients with coronary heart disease. Atherosclerosis. 2003;167:293–302. doi: 10.1016/s0021-9150(03)00007-8. [DOI] [PubMed] [Google Scholar]
- 34.Mendivil CO, Rimm EB, Furtado J, Chiuve SE, Sacks FM. Low-density lipoproteins containing apolipoprotein C-III and the risk of coronary heart disease. Circulation. 2011;124:2065–72. doi: 10.1161/CIRCULATIONAHA.111.056986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 36.Farnier M, Chen E, Johnson-Levonas AO, McCrary Sisk C, Mitchel YB. Effects of extended-release niacin/laropiprant, simvastatin, and the combination on correlations between apolipoprotein B, LDL cholesterol, and non-HDL cholesterol in patients with dyslipidemia. Vasc Health Risk Manag. 2014;10:279–90. doi: 10.2147/VHRM.S58694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tariq SM, Sidhu MS, Toth PP, Boden WE. HDL hypothesis: where do we stand now? Curr Atheroscler Rep. 2014;16(4):398. doi: 10.1007/s11883-014-0398-0. [DOI] [PubMed] [Google Scholar]
- 38.Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. HPS2-THRIVE Collaborative Group. N Engl J Med. 2014;371(3):203–12. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 39.Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380(9841):572–80. doi: 10.1016/S0140-6736(12)60312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sahebkar A, Watts GF. New LDL-cholesterol lowering therapies: pharmacology, clinical trials, and relevance to acute coronary syndromes. Clin Ther. 2013;35(8):1082–98. doi: 10.1016/j.clinthera.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 41.Robinson JG, Farnier M, Krempf M, et al. ODYSSEY LONG TERM Investigators Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–99. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 42.Sabatine MS, Giugliano RP, WIviott SD, et al. Open-Label Study of Long-Term Evaluation against LDL Cholesterol (OSLER) Investigators Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–9. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]