Abstract
Objective
Participation of minority populations in clinical trials is paramount to understanding and overcoming cancer racial disparities. The goal of this project is to evaluate minority participation in published GOG clinical trials.
Methods
GOG publications from years 1985 to 2013 were reviewed. Minority enrollment was stratified by tumor site, type of study, and year published. Based on Centers of Disease Control and Prevention (CDC) age-adjusted incidence for race, expected and observed ratios of racial participation were calculated.
Results
445 GOG publications involving 67,568 patients were reviewed. Racial breakdown was provided in 170 studies (38%) for a total of 45,259 patients: 83% White (n=37,617); 8% Black (n=3,686), and 9% Other (n= 3,956). The majority of studies were Ovarian (n=202) and Phase 2 (n=290). When evaluating the quartiles of publication year, a steady decline in proportion of Black patients enrolled was seen. Race was not reported in any publication prior to 1994. Compared to years 1994–2002, a 2.8-fold lower proportion of black enrollment was noted in years 2009–2013 (16% & 5.8%, respectively; p<0.01). Utilizing CDC age-adjusted incidence, observed enrollment of Black patients onto GOG clinical trials was significantly less than expected enrollment. Observed Black enrollment was 15-fold lower than expected for Ovarian trials, 10-fold lower for Endometrial, 4.5-fold for Cervix, and 5.2-fold for Sarcoma (each p <0.001).
Conclusions
Based on age-adjusted incidence, observed enrollment of Black patients is lower than expected enrollment onto GOG studies. Despite national emphasis on minority enrollment on clinical trials, fewer Black patients were enrolled over time.
INTRODUCTION
Since the 1990's, cancer mortality rates have declined significantly in the United States (US) with an approximate 10% reduction in death in women alone. Despite this overall reduction, 5-year overall survival rates remain significantly lower for Black patients compared to Whites at each stage of diagnosis. [1] In fact, the racial disparity gap in mortality from all cancers have actually widened over the past few decades. [2] The etiology for this racial disparity and increasing death rates due to cancer is an area of intense research. The etiology of cancer racial disparities are likely multifactorial and encompass clinical features, environmental factors, socioeconomic factors and differences in tumor biology. Socioeconomic factors such as poverty, inadequate education, and lack of health insurance have been argued to be more important than biological differences leading to some declaring “poverty as a carcinogen” [3]
Racial differences in incidence, mortality and survival have been reported in endometrial, uterine sarcoma, ovarian, and cervical cancers. In a review of advanced/recurrent endometrial cancer, Black women were 60–80% more likely to die from endometrial cancer when controlling for other variables such as performance status, disease stage, tumor histology, tumor grade and treatment rendered. [4] In the US, Black patients have the highest death rate from cervical cancer compared to all racial or ethnic groups [2] and racial disparity in ovarian cancer not only exists for Black patients, but has worsened over the past few years. [5] The specific etiologies underlying racial/ethnic disparities in these cancers lack clarity. Causation is likely multifactorial incorporating biological aspects of the cancer plus consequences of social, economical or cultural environments. [6]
The NCI Strategic Plan for Leading the Nation, Objective #8 calls to not only identify but to overcome cancer health disparities. This is echoed by the American Cancer Society 2015 Challenge Goals to eliminate the disparity in the burden of cancer. The first step in this journey is to clarify the etiology of these racial disparities. Racial disparities exist across a spectrum from primary prevention, early detection, incidence, treatment received, and survival/mortality. Considering this temporal and multifactorial process, inspecting cancer racial disparity in a relatively homogenous population can assist in identifying truly causative factors. As such evaluating patients who participate in clinical trials provides a relatively homogenous cancer population that received same treatments in a prospective fashion. Therefore, the participation of minority populations in clinical trials is paramount to understanding and overcoming cancer racial disparities and should be the initial resource to study.
METHODS
Study Design
After IRB approval was obtained, citations from Gynecologic Oncology Group (GOG) publications were obtained from the GOG website. All publications from years 1985 to 2013 were reviewed for data abstraction. For GOG studies that published multiple manuscripts, each manuscript was reviewed separately to capture the differences in reporting of racial breakdown. Data variables included patient demographics, surgicopathologic variables, tumor type, study type, and year published. When provided in the publication, minority enrollment was stratified by:
-
-
Tumor type (Ovary, Endometrial, Cervix, and Sarcoma)
-
-
Study type (Phase I, II, III, Translational, and Observational/QOL studies)
-
-
Quartile of Year published (≤ 1993, 1994–2002, 2003–2008, and 2009–2013)
Statistical analysis
Comparisons between 2 groups (Black versus White) were performed to determine differences in enrollment characteristics. Data were analyzed using SPSS 21.0 with t-tests, chi-square, and ANOVA tests were performed where appropriate. Utilizing 2013 CDC age-adjusted incidence for each cancer studied, the expected enrollment for White and Black patients were calculated and set to ratios of White:Black (W:B). These expected enrollment ratios were then compared to the observed enrollment ratios of racial participation in GOG studies.
RESULTS
Trial Breakdown
A total of 445 GOG publications from years 1985 to 2013 involving 67,568 patients were reviewed. Ovarian trials were the most common accounting for 45% of publications reviewed (n=202) and involving 35,800 patients (57%) of all patients enrolled. The remaining study breakdown was Cervix 29% (n= 128), Endometrial 16% (n=70), and Sarcoma 10% (n=45). (Table 1)
Table 1.
GOG Studies stratified by tumor site
| Ovary | Endometrial | Cervix | Sarcoma | Totals | |
|---|---|---|---|---|---|
| Number of studies | 202 (45%) | 70 (16%) | 128 (29%) | 45 (10%) | 445 (100%) |
| Number of patients | 35,800 (57%) | 14,516 (21%) | 14,848 (22%) | 2,404 (4%) | 67,568 (100%) |
| Type of Study | |||||
| Phase I | 28 (13.9%) | 4 (5.8%) | 11 (8.6%) | 0 (0%) | 43 (9.7%) |
| Phase II | 118 (58.4%) | 49 (70%) | 83 (64.8%) | 40 (88.9%) | 290 (65%) |
| Phase III | 36 (17.8%) | 13 (18.6%) | 26 (20.3%) | 5 (11.1%) | 80 (18%) |
| Observational | 12 (5.9%) | 0 (0%) | 6 (4.7%) | 0 (0%) | 18 (4%) |
| Translational | 8 (3.9%) | 3 (4.3%) | 2 (1.6%) | 0 (0%) | 13 (2.9%) |
| Publication Years | |||||
| ≤ 1993 | 43 (21.3%) | 13 (18.6%) | 46 (35.9%) | 11 (24.4%) | 113 (25.4%) |
| 1994 to 2002 | 44 (21.8%) | 15 (21.4%) | 42 (32.8%) | 16 (35.6%) | 117 (26.3%) |
| 2003 to 2008 | 45 (22.3%) | 25 (35.7%) | 27 (21.1%) | 11 (24.4%) | 108 (24.3%) |
| 2009 to 2013 | 70 (34.7%) | 17 (24.3%) | 13 (10.2%) | 7 (15.6%) | 107 (24%) |
Phase II studies were the most common type of trials published comprising 65% of all studies (n= 290; p 0.015). This was followed by Phase III trials at 18% (n=80 trials), Phase I at 9.7% (n=43), Observational at 4% (n=18), and Translational at 2.9% (n= 13). (Table 1) When stratifying by year of publication, more studies were published prior to year 2003 (230 trials, 52%; p= ns), however these were grouped by quartiles.
Racial Breakdown
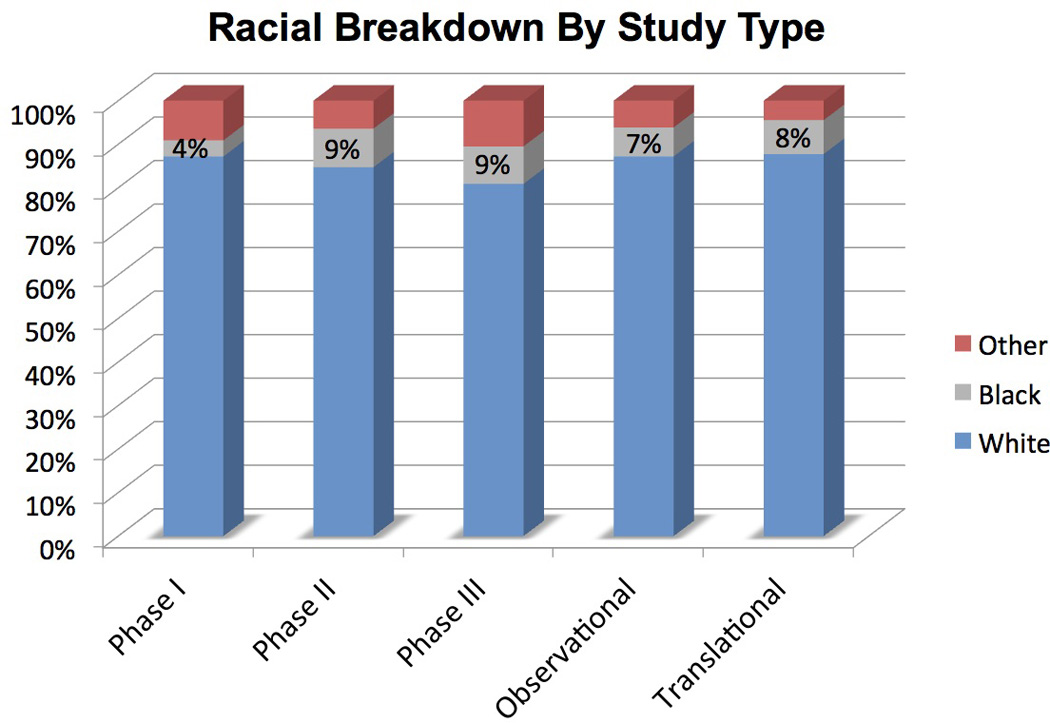
Of the 445 studies, racial breakdown was provided in only 38% (n=170) of publications. Prior to 1994 (years 1985 to 1993), inclusion of racial breakdown was not reported (0 of 113 publications; 0%). However, racial breakdown reporting increased significantly over the next 3 quartiles: 15.4% (18 of 117 publications from 1994–2002); 56.5% (61 of 108 publications from 2003–2008); and 85% (91 of 107 publications from 2009–2013). Across all publications that reported race (n=170), White patients comprised 83% of all patients enrolled (n= 37,617), with 8% Black (n=3,686) and 9% Other (n=3,956). Overall enrollment of Black patients ranged from 4–9% onto all publications evaluated. The number of patients in the Other race category exceeded Black patients in 67 of the 170 publications (40%) that included racial breakdown. In all tumor types, White patients significantly outnumbered the minority patients of Black and Other; p values ranged from <0.01 to <0.001 across tumor types. (Table 2, Figure 1)
Table 2.
GOG Studies stratified by race
| White N=37,617(%) |
Black N= 3,686(%) |
Other N=3,956(%) |
p value | |
|---|---|---|---|---|
| Number of patients | ||||
| Tumor Site | ||||
| Ovary | 23,269 (87%) | 1,258 (5%) | 2,290 (8%) | <0.001 |
| Endometrial | 9,289 (87%) | 850 (8%) | 590 (5%) | <0.001 |
| Cervix | 4,421 (65%) | 1,340 (20%) | 1,038 (15%) | <0.001 |
| Sarcoma | 638 (70%) | 238 (26%) | 38 (4%) | <0.01 |
| Type of Study | ||||
| Phase I | 449 (87%) | 19 (4%) | 47 (9%) | |
| Phase II | 3,277 (85%) | 343 (9%) | 249 (6%) | |
| Phase III | 22,038 (81%) | 2,342 (9%) | 2,865 (10%) | |
| Observational | 5,322 (87%) | 405 (7%) | 376 (6%) | |
| Translational | 4,115 (88%) | 365 (8%) | 210 (4%) | |
| Publication Years | ||||
| ≤ 1993 | 0 (0) | 0 (0) | 0 (0) | |
| 1994 to 2002 | 2,818 (76.0) | 593 (16.0) | 295 (8.0) | |
| 2003 to 2008 | 11,206 (80.2) | 1,483 (10.8) | 1,063 (7.7) | |
| 2009 to 2013 | 23,593 (84.9) | 1,610 (5.8) | 2,598 (9.3) | |
Figure 1.
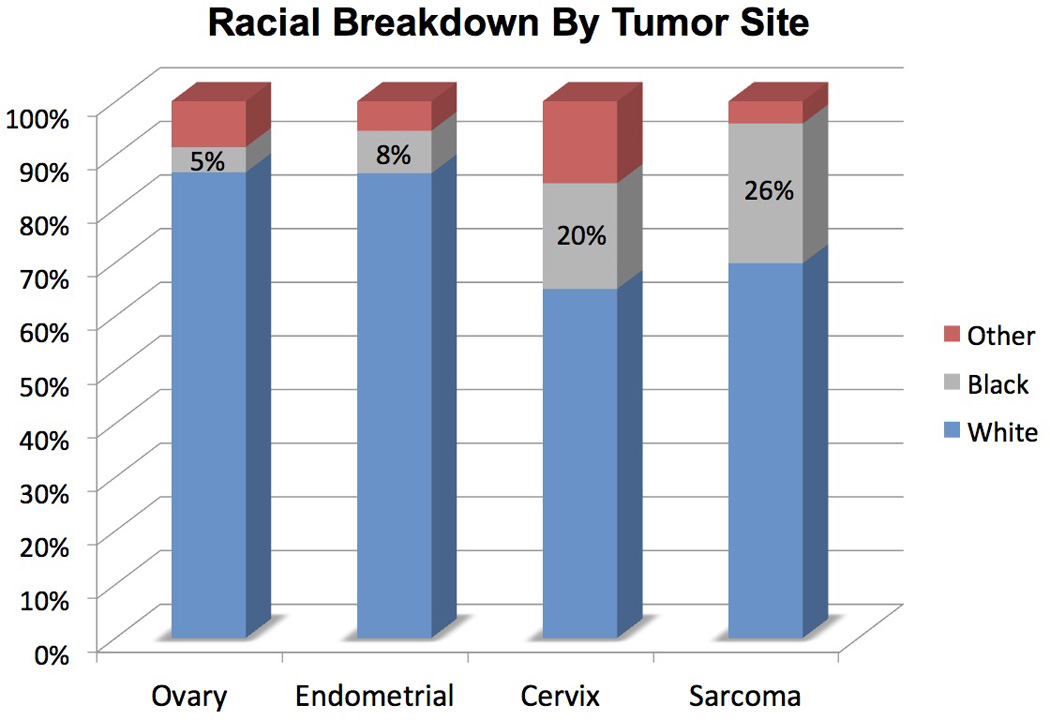
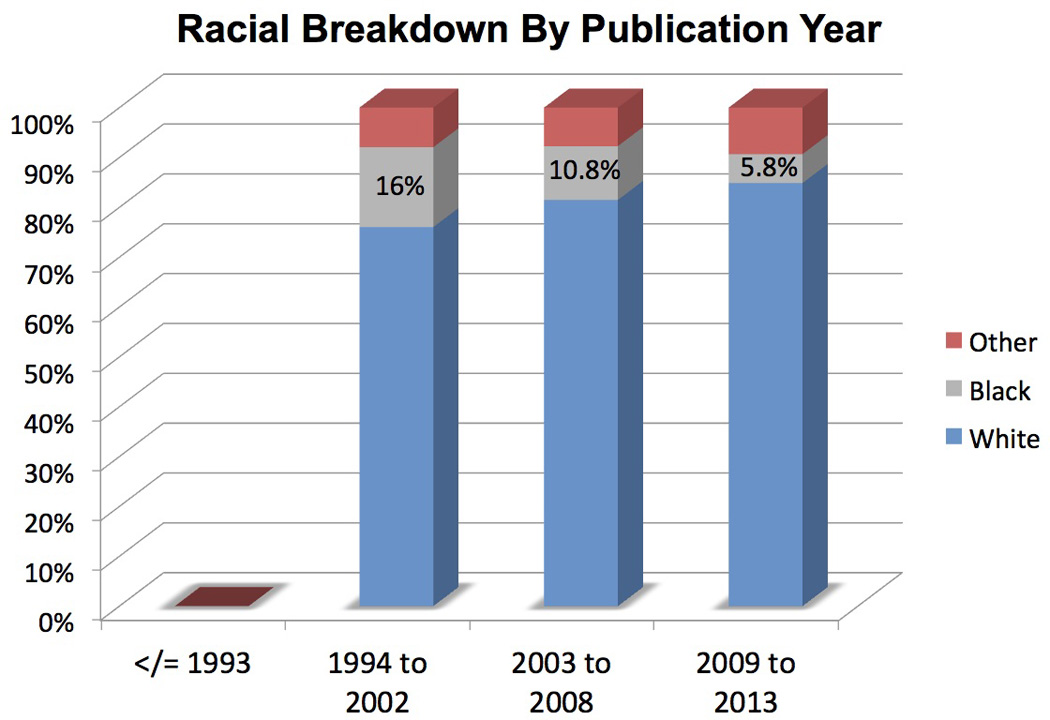
Black patients were more likely to be enrolled onto Sarcoma (26% of total enrollment) or Cervix (20%) trials compared to Ovary (5%) or Endometrial (8%) trials. Despite the increase of racial breakdown in publications over time, a 2.8-fold reduction in Black enrollment was seen with 16% in 1994–2002 compared to 5.8% from 2009 to 2013. (p< 0.001) (Table 2, Figure 1)
Utilizing 2013 CDC age-adjusted incidence for each cancer studied, the expected enrollment for white and black patients were calculated and set to ratios of White:Black (W:B). These expected enrollment ratios were then compared to the observed enrollment ratios. By these calculations the observed enrollment of Black patients onto GOG clinical trials were significantly less than expected if accrual rates were consistent with age-adjusted incidence. Ovarian trials demonstrated a near 15-fold lower than expected enrollment of Black patients. Other tumor types were similar with Endometrial, Cervical, and Sarcoma demonstrating a 9.8-fold, 4.5-fold, and 5.2-fold lower enrollment than expected, respectively. (Table 3).
Table 3.
Expected vs. Observed Enrollment
| White Incidence | Black Incidence |
Expected W:B ratio |
|
| Expected Incidence Per 100,000 | |||
| Ovary | 12.9 | 9.5 | 1 to 0.74 |
| Endometrial | 24.8 | 22.4 | 1 to 0.90 |
| Cervical | 7.7 | 10.3 | 1 to 1.34 |
| Sarcoma | 3.6 | 7 | 1 to 1.94 |
| Observed White | Observed Black |
Observed W:B ratio |
|
| Observed Incidence | |||
| Ovary | 23,269 | 1,258 | 1 to 0.05 |
| Endometrial | 9,289 | 850 | 1 to 0.09 |
| Cervical | 4,421 | 1,340 | 1 to 0.30 |
| Sarcoma | 638 | 238 | 1 to 0.37 |
|
Expected W:B ratio |
Observed W:B ratio |
Fold Difference in Ratios |
|
| Ovary | 1 to 0.74 | 1 to 0.05 | 14.8 fold |
| Endometrial | 1 to 0.90 | 1 to 0.09 | 9.8 fold |
| Cervical | 1 to 1.34 | 1 to 0.30 | 4.5 fold |
| Sarcoma | 1 to 1.94 | 1 to 0.37 | 5.2 fold |
CONCLUSIONS
In this study, enrollment of Black patients onto GOG studies over nearly 30 years were significantly less than expected irrespective of tumor type, study type and year published. Despite a national focus on addressing healthcare disparities [7] and enhancing minority participation on clinical trials, surprisingly a decrease in percentage of Black patients were noted from 16% from years 1994 to 2002 to 6% from years 2009 to 2013. Assuming an ideal equal accrual rates across all races, age-adjusted incidence from the CDC was utilized to calculate expected enrollment to observed enrollment. Observed enrollment of black patients onto GOG clinical trials was significantly less than expected ranging from 4.5-fold to 15-fold difference between different tumor types.
Some have suggested that enrollment of minority populations onto clinical trials is vital for adequately describing the true racial disparity in gynecologic malignancies. However, without adequately studying this population, it is difficult to gain significant insight into the potential factors that contribute to well-described disparities.
Similar results were recently seen in regards to minority access to novel agents. Evaluating pivotal clinical trial participants for newly approved FDA agents from 2010 to 2012, minority racial/ethnic groups had lower participation rates in the study population than would be representative of the US racial group populations. [8]
A limitation of this report is the manner in which race/ethnicity is obtained varies greatly. Depending on study, this could range from self-reporting of race to abstraction from medical records of data managers. Considering the intermixing of ancestry, the definition of race also varies over time. Some authors have suggested that utilization of genetic ancestry-informative markers could be a more accurate method to estimate race/ethnicity. [9] The differences between expected and observed enrollment of Black patients onto GOG trials could be a product of GOG member sites having disproportionally lower minority catchment area. Unfortunately, this possible confounder could not be evaluated in this analysis. Future analysis of raw data from specific GOG member sites and their corresponding catchment area would be important to address this potential issue.
Despite racial and ethnic minorities having disproportionately higher rates of health care disparities, underrepresentation in clinical trials remains. A recent review of the literature evaluating barriers to clinical trials for racial and ethnic minorities demonstrated that key barriers to recruitment included clinical trial awareness, opportunities to participate and acceptance to enrollment. They concluded that strategies that specifically targeted the providers, the participants within the community are needed. [10]
In a recent publication based on US cancer center interviews, barriers to minority enrollment onto clinical trials included minority skepticism, not being offered trial participation, lack of encouragement to enroll, and lack of referral procedures to clinical trial staff. [11] Although understanding barriers is important to describing these deficiencies, specific interventions should be employed to direct and facilitate minority enrollment.
Strategies that have been shown to be successful include a lay navigator program to enhance minority participation in clinical trials [12, 13], recruitment letters directed towards minorities [14], and incorporating programmatic changes to the evaluation and referral to clinical trial mechanism [15] Quite simply, it’s impossible to improve or eliminate a disparity if you fail to enroll the patients that stand the most to benefit.
Although all trials have room for improvement, priority should be given to tumor types that have a well-documented racial disparity. Increasing minority enrollment will provide important information on racial differences in tumor biology, response to therapy and survival but does not equate overcoming these disparities. In addition to obtaining valuable clinical descriptions of the disparity, clinical trials that involve basic science endpoints could provide insights into potential etiologies. The clinical and scientific descriptions of racial disparities is paramount to enhancing our understanding of the burden of gynecologic cancer in minority patients and coincides with the goals of the National Cancer Institute and American Cancer Society.
REFERENCES
- 1.DeSantis C, Naishadham D, Jemal A. Cancer statistics for African Americans, 2013. CA Cancer J Clin. 2013 May;63(3):151–166. doi: 10.3322/caac.21173. [DOI] [PubMed] [Google Scholar]
- 2.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004 Mar-Apr;54(2):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 3.Broder s. Progress and Challenges in the National Cancer Program. In: Brugge J, Curran T, Harlow E, McCormick F, editors. Origins of Human Cancer: A Comprehensive Review. Plainview, NY: Cold Spring Harbor Laboratory Press; 1991. pp. 27–33. [Google Scholar]
- 4.Maxwell GL, Tian C, Risinger J, et al. Racial disparity in survival among patients with advanced/recurrent endometrial adenocaqrcinoma: a Gynecologic Oncology Group study. Cancer. 2006;107:2197–2205. doi: 10.1002/cncr.22232. [DOI] [PubMed] [Google Scholar]
- 5.Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecol Oncol. 2013 Apr;129(1):258–264. doi: 10.1016/j.ygyno.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill HA, Eley JW, Harlan LC, et al. Racial differences in endometrial cancer survival: the black/white cancer survival study. Obstet.Gynecol. 1996;88:919–926. doi: 10.1016/s0029-7844(96)00341-9. [DOI] [PubMed] [Google Scholar]
- 7.National Institutes of Health. Healthy People 2020. [Accessed March 11, 2015]; https://www.healthypeople.gov.
- 8.Eshera N, Itana H, Zhang L, Soon G, Fadiran EO. Demographics of Clinical Trials Participants in Pivotal Clinical Trials for New Molecular Entity Drugs and Biologics Approved by FDA From 2010 to 2012. Am J Ther. 2015 Jan 23; doi: 10.1097/MJT.0000000000000177. [Epub ahead of print] PMID:25621972. [DOI] [PubMed] [Google Scholar]
- 9.Rocconi RP, Fernandez J, Brady WE, Darcy KM, Goodfellow P, Lankes H, Tritchler D, Ramirez N, Creasman W, Alvarez RD. The role of racial genetic admixture with endometrial cancer outcomes: A Gynecologic Oncology Group study. Gynecologic Oncology. 2013;130(1):e168–e169. doi: 10.1016/j.ygyno.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heller C, Balls-Berry JE, Nery JD, Erwin PJ, Littleton D, Kim M, Kuo WP. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review. Contemp Clin Trials. 2014 Nov;39(2):169–182. doi: 10.1016/j.cct.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durant RW, Wenzel JA, Scarinci IC, Paterniti DA, Fouad MN, Hurd TC, Martin MY. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: enhancing minority participation in clinical trials (EMPaCT) Cancer. 2014 Apr 1;120(Suppl 7):1097–1105. doi: 10.1002/cncr.28574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghebre RG, Jones LA, Wenzel JA, Martin MY, Durant RW, Ford JG. State-of-the-science of patient navigation as a strategy for enhancing minority clinical trial accrual. Cancer. 2014 Apr 1;120(Suppl 7):1122–1130. doi: 10.1002/cncr.28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fouad MN, Partridge E, Dignan M, Holt C, Johnson R, Nagy C, Parham G, Person S, Scarinci I, Wynn T. A community-driven action plan to eliminate breast and cervical cancer disparity: successes and limitations. J Cancer Educ. 2006 Spring;21(1 Suppl):S91–S100. doi: 10.1207/s15430154jce2101s_16. [DOI] [PubMed] [Google Scholar]
- 14.Brown SD, Partee PN, Feng J, Quesenberry CP, Hedderson MM, Ehrlich SF, Kiernan M, Ferrara A. Outreach to diversify clinical trial participation: A randomized recruitment study. Clin Trials. 2015 Feb;Feb; doi: 10.1177/1740774514568125. Pii: 1740774514568125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anwuri VV, Hall LE, Mathews K, Springer BC, Tappenden JR, Farria DM, Jackson S, Goodman MS, Eberlein TJ, Colditz GA. An institutional strategy to increase minority recruitment to therapeutic trials. Cancer Causes Control. 2013 Oct;24(10):1797–1809. doi: 10.1007/s10552-013-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


