Abstract
Analysis of an invasive species' niche shift between native and introduced ranges, along with potential distribution maps, can provide valuable information about its invasive potential. The tawny crazy ant, Nylanderia fulva, is a rapidly emerging and economically important invasive species in the southern United States. It is originally from east‐central South America and has also invaded Colombia and the Caribbean Islands. Our objectives were to generate a global potential distribution map for N. fulva, identify important climatic drivers associated with its current distribution, and test whether N. fulva's realized climatic niche has shifted across its invasive range. We used MaxEnt niche model to map the potential distribution of N. fulva using its native and invaded range occurrences and climatic variables. We used principal component analysis methods for investigating potential shifts in the realized climatic niche of N. fulva during invasion. We found strong evidence for a shift in the realized climatic niche of N. fulva across its invasive range. Our models predicted potentially suitable habitat for N. fulva in the United States and other parts of the world. Our analyses suggest that the majority of observed occurrences of N. fulva in the United States represent stabilizing populations. Mean diurnal range in temperature, degree days at ≥10°C, and precipitation of driest quarter were the most important variables associated with N. fulva distribution. The climatic niche expansion demonstrated in our study may suggest significant plasticity in the ability of N. fulva to survive in areas with diverse temperature ranges shown by its tolerance for environmental conditions in the southern United States, Caribbean Islands, and Colombia. The risk maps produced in this study can be useful in preventing N. fulva's future spread, and in managing and monitoring currently infested areas.
Keywords: Biological invasions, biotic homogenization, ecological niche models, invasion stages, MaxEnt, niche expansion, risk analysis, species distribution modeling
Introduction
Rapidly increasing global trade and human movement have accelerated the rate of species introductions and establishment into novel areas across the world (Mack et al. 2000). Invasive species can negatively affect native ecosystems, agriculture, forestry, animal, and human health and cause enormous economic losses (Pimentel et al. 2005). They can also cause local extinction of rare and unique native species resulting in biotic homogenization and are considered as one of the greatest threats to biodiversity worldwide (Sax et al. 2002). Maps of species' current and potential distributions are valuable tools for resource managers for preventing the introduction or establishment of invasive alien species, and for designing an effective early detection and rapid response system (Peterson 2003; Jimenez‐Valverde et al. 2011). Non‐native species, when introduced to new geographic areas, may establish in environmental conditions different from their native range because of absence of natural enemies or local adaptation. Therefore, analysis of how a species' niche may have changed between native and introduced ranges may be useful in understanding range expansion and invasion potential (González‐Moreno et al. 2015).
Several invasive ant species around the world have caused economic losses, affected human health, decreased agricultural production, damaged infrastructure, and reduced the diversity of local ant and arthropod assemblages (Holway et al. 2002a; Gutrich et al. 2007). An alien ant species, the tawny crazy ant, Nylanderia fulva (Mayr) (Hymenoptera: Formicidae), previously Paratrechina fulva (LaPolla et al. 2010), and originally referred to as Paratrechina nr. pubens, is invading the southern United States (Gotzek et al. 2012). Its occurrence in the United States (US) was first documented in Houston in 2002 (Meyers and Gold 2008) and may have arrived in Florida earlier (Klotz et al. 1995; Deyrup et al. 2000), but collections of the very similar P. pubens from Florida dating back to the 1950s (Trager 1984) prevent an accurate identification of early pest populations. At present, populations of this species occur in 27 counties in Texas, 27 counties in Florida, and several counties in southern Mississippi (MacGown and Layton 2010) and southern Louisiana (Hooper‐Bui et al. 2010; Fig. 1). Introduction of N. fulva in Colombia caused extensive ecological and agricultural damage (Zenner de Polania 1990). In the Southern United States, N. fulva displaces red imported fire ants (Solenopsis invicta), and regionally distributed native species, thereby reducing both biological and functional diversity (LeBrun et al. 2013). Nylanderia fulva can also transport pathogens of plants, humans, and other animals (McDonald 2012). Nests within populations contain multiple queens (Zenner de Polania 1990). Interconnected nests of these ants form extraordinarily dense populations that greatly exceed the combined densities of all ants in adjacent uninvaded assemblages (LeBrun et al. 2013). They feed on small insects and vertebrates, and honeydew secreted by aphids (Zenner de Polania and Bolaños 1985). They invade people's homes, nest in crawl spaces and walls, and damage electrical equipment resulting in millions of dollars of losses (Blackwell 2014). Populations spread about 200 m per year as a result of nest fission at the invasion front (Meyers and Gold 2008). Female reproductives of N. fulva have not been observed to engage in alate flights, so long‐distance dispersal occurs largely as a result of human transport of nesting ants. Despite N. fulva being a potentially devastating invasive species, no information currently exists on its potential distribution in the United States. There is an acute need for climate‐based projection of the invasion potential of N. fulva in the near‐term to guide conservation (e.g., potential biocontrol; Waltari and Perkins 2010).
Figure 1.
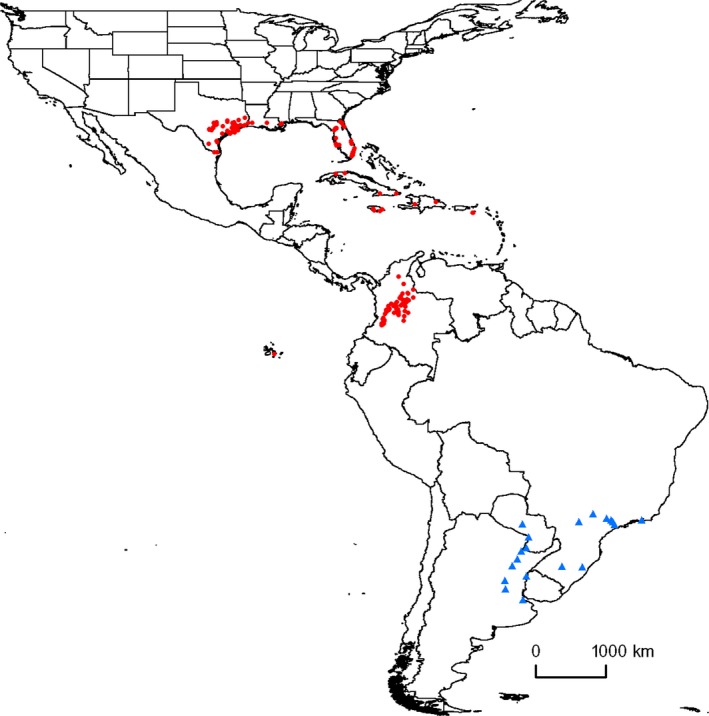
Known native (blue triangles) and invasive (red circles) occurrences of Nylanderia fulva in South America, Caribbean islands, and southern United States.
Availability of suitable environmental conditions is a prerequisite for population establishment. Species environment matching models, also called habitat models, ecological niche models (ENM), and species distribution models (SDM), quantify the range of environmental conditions to assure viable populations. These models are based on the “niche” concept (hereafter niche models), which can be defined as the multivariate environmental space within which a species can live indefinitely without immigrational subsidy (Grinnell 1917; Hutchinson 1957). The fundamental niche represents the conditions where a species can live indefinitely, whereas a species' realized niche is where a species actually lives; species do not occupy all portions of their fundamental niche because of biotic constraints (e.g., competition or lack of host species) or dispersal limitations (Peterson et al. 2011). Niche models can be broadly classified as correlative models or process‐based/mechanistic models (Dormann et al. 2012). The correlative niche models map the realized or potential distribution of a species by associating occurrence data with environmental data (Jimenez‐Valverde et al. 2011) and are widely used tools for assessing the risk of invasive species (e.g., Peterson 2003; Kumar et al. 2009, 2014; Menke et al. 2009; Roura‐Pascual et al. 2009; Stohlgren et al. 2010).
Numerous correlative niche modeling techniques are available for mapping the risk of invasive species (Peterson et al. 2011). These can be broadly categorized into presence‐only (e.g., BIOCLIM, DOMAIN), presence‐background (e.g., MaxEnt, GARP), and presence–absence (GLM, GAM, CART) models. Presence‐only and presence‐background (i.e., randomly selected absences from areas that have been accessible to the species) niche models are better suited for modeling potential distributions of invasive species because absence data for such species may not be reliable; a species may go undetected or it may not have had enough time to disperse to new locations yet (Jimenez‐Valverde et al. 2011). The presence–absence models are more suitable for estimating actual distributions of a species.
The use of presence‐only or presence‐background correlative niche models for mapping the potential distributions of introduced invasive species can be challenging because the species may not yet have reached equilibrium within its invaded environment (Vaclavik and Meentemeyer 2009). Therefore, a model trained only with invaded range occurrences may highly underestimate areas where a species may potentially exist (Jimenez‐Valverde et al. 2008), thus providing inaccurate information for management actions and policy development. This problem can be overcome by developing a model using species occurrence data from native and invaded ranges (e.g., Fitzpatrick et al. 2007; Broennimann and Guisan 2008). The presence‐only data, if not collected using statistically designed field surveys, may also have locational and taxonomic uncertainties, environmental and spatial bias, and may come from sink populations (Wolmarans et al. 2010). Therefore, care must be taken in using data from a variety of sources, and models must be corrected for potential biases for accurate predictions.
Despite the high economic and ecological importance of N. fulva, very little information currently exists on its global distribution or the potential environmental factors that constrain its distribution. Land managers urgently need such information for managing currently infested areas and planning for prevention of future invasions. Our objectives were to: (1) map the global potential distribution of N. fulva; (2) identify climatic drivers associated with N. fulva distribution; (3) test whether the climatic niche of N. fulva has shifted during invasion; and (4) make inferences about the invasion stages of N. fulva in the United States.
Methods
Species occurrence data
Occurrence data for N. fulva were compiled from specimens at natural history museums, personal collections, scientific literature, and field surveys (Fig. 1; see Table S1 in Appendix S1 in Supporting Information). Taxonomic details on how we defined native and invasive ranges of N. fulva, and acceptance criteria for occurrence records are provided in Appendix S1.
Twenty‐seven presence records were collected from N. fulva's native range including Argentina, Brazil, and Paraguay (Fig. 1; Table S1 in Appendix S1). We collected 311 records from the invaded range outside the continental United States (CONUS), and 1061 records from within CONUS (Florida, Louisiana, Mississippi, and Texas). Colombian data were provided by A. Arcila. Thus, a total of 1399 occurrence records covering South America, Caribbean islands, and southern United States were available for modeling. The total number of presence records was reduced to 307 after removing duplicate data points (i.e., more than 1 point within a ~1 km2 grid cell) and applying spatial filtering to reduce the effect of spatial autocorrelation on models (Boria et al. 2014).
Environmental data
A total of 20 bioclimatic variables were considered in developing N. fulva potential distribution models (Table S2 in Appendix S1). These included 19 bioclim variables from the WorldClim dataset at ~1‐km resolution (Hijmans et al. 2005). These bioclim variables were derived using monthly temperature and precipitation data covering a period from ~1950 to 2000, and represent average temperature and precipitation, seasonal variables, and climatic extreme indices (Hijmans et al. 2005). They are considered biologically meaningful as they aggregate climate information that influences biological processes. Additionally, “degree days with average temperature ≥10°C” variable was generated in Arc Map (ESRI, Redlands, CA) using average monthly temperature data based on N. fulva's responses to different temperatures (Arcila et al. 2002a,b; McDonald 2012). These variables were chosen based on our knowledge of N. fulva ecology (McDonald 2012; LeBrun et al. 2013), and their use in previous invasive ant species modeling studies (Menke et al. 2009; Roura‐Pascual et al. 2009). Highly collinear variables (Pearson correlation coefficient, ¦r¦ ≥ 0.80) were removed, and only one variable from a set of highly correlated variables was included in the same model (Table S3 in Appendix S1). All geographical information system (GIS) layers were projected to an equal area projection (World Cylindrical Equal Area Conic projection, Datum WGS1984).
Model calibration and validation
Maximum entropy model, MaxEnt (version 3.3.3k; Phillips et al. 2006), was used for mapping potential distribution of N. fulva. The MaxEnt model was chosen because (1) it uses presence‐background data; species true absences are not required; (2) generally performs better than other niche modeling algorithms (Evangelista et al. 2008; Kumar et al. 2009); and (3) is relatively robust to small sample sizes (Guisan et al. 2007a,b; Kumar and Stohlgren 2009). MaxEnt uses species occurrence data and spatial environmental variables and produces an index of relative suitability that varies from 0 (unsuitable or most dissimilar to presence locations) to 1 (most suitable or most similar to presence locations). Background points (50,000 for the MaxEnt model) were randomly selected from areas that have been accessible to N. fulva using the “Biotic‐Abiotic‐Mobility” (BAM) framework (Soberon and Peterson 2005). We suspected a sampling bias because the occurrence data were not collected randomly and came from multiple sources. Thus, we generated a bias surface using a kernel density estimate using SDMToolbox (Brown 2014). The bias surface was used in MaxEnt to weight the selection of background points to account for sampling intensity and potential sampling bias (Elith et al. 2010; Syfert et al. 2013). Three models were fitted: (1) invasive range model calibrated using only the continental US occurrence data (IRM‐CONUS); (2) native and invasive range model calibrated using occurrence data from the Americas (NIRM‐Americas); and (3) all occurrence data with the global background (NIRM‐Global; Table 1). Background points for NIRM‐Global model were randomly drawn from all terrestrial areas of the world assuming unlimited dispersal (Table 1).
Table 1.
Areas of calibration and performance statistics for different models
| Model | Area of calibration/background extent | MaxEnt settings | Test AUCcv | pAUC | Test sensitivity | |
|---|---|---|---|---|---|---|
| 0% OR | 10% OR | |||||
| IRM‐CONUS | Continental United States of America (USA) | Linear, Quadratic and Hinge features; β = 1.5 | 0.961 (±0.01) | 1.96 (±0.01) | 0.006 | 0.110 |
| NIRM‐Americas | Continental USA, Caribbean islands, and South America | Linear, Quadratic and Product features; β = 2.5 | 0.937 (±0.02) | 1.82 (±0.06) | 0.006 | 0.114 |
| NIRM‐Global | Global (all terrestrial areas of the world) | Linear, Quadratic, Product and Hinge features; β = 2.5 | 0.966 (±0.01) | 1.91 (±0.03) | 0.003 | 0.105 |
Note: β is regularization multiplier; OR is training omission rate. Test AUCcv is MaxEnt generated 10‐fold cross‐validation area under the ROC curve; pAUC is partial AUC ratio calculated at 0% omission rate (Peterson et al. 2008). The AUCcv and pAUC values are not comparable across models because models were calibrated at different extents.
As default settings in MaxEnt do not always produce the best predictions (Merow et al. 2013; Kumar et al. 2014), it was run with combinations of different feature types and regularization multiplier values (ranging from 1 to 3). The ENMTools (Warren et al. 2010) was used to calculate Akaike's information criterion (AIC) values for MaxEnt models with different settings at different extents of calibration, and models with optimal complexity were retained for further evaluation (Table S4 in Appendix S2). The 10‐fold cross‐validation was used in MaxEnt, and the area under the ROC (receiver‐operating characteristic) curve (AUCcv; Fielding and Bell 1997) values were reported. In addition, the partial area under the ROC curve (pAUC) ratio was used for evaluating model performance (Peterson et al. 2008). The pAUC ratio values were calculated using a Visual Basic program (Barve 2008). A pAUC ratio >1.0 indicates better than random model performance. The sensitivity index (i.e., number of correctly classified presences) was also used as an additional metric to evaluate model performance. Test sensitivity was calculated at 0% and 10% training omission rates (see Liu et al. 2013; Kumar et al. 2014). The best models for each extent of calibration were selected based on AIC, AUCcv, pAUC values, and omission rates. In addition, the response curves generated by MaxEnt were evaluated for their biological relevance to N. fulva, and models that resulted in biologically nonsensical (i.e., highly jagged or multimodal) curves were eliminated or ranked low (Table S4 in Appendix S2).
Realized niche shift and invasion stage analyses
The principal component analysis (PCA) approach proposed by Broennimann et al. (2012) was used to test any potential niche shift by quantifying climatic niche space for N. fulva at different extents. This method compares the environmental conditions available for a species within a defined study extent (background) with its observed occurrences and calculates the available environmental space defined by the first two axes from the PCA. The same 20 climatic variables, as used in MaxEnt, were used for the PCA. This method automatically corrects for sampling bias using a smooth kernel density function (Broennimann et al. 2012). The niche overlap score for each comparison was calculated using Schoener's D index (Schoener 1970), which varies from 0 (no overlap between niches) to 1 (complete overlap). The statistical significance of niche overlap index (D) was tested against chance using 100 randomizations (alpha = 0.05). The R code for the PCA was modified from Broennimann et al. (2012). We compared native and invasive niche spaces for N. fulva using three regional contrasts: (1) native vs. invasive_CONUS (invasive occurrences from the continental US); (2) native vs. invasive_Non‐CONUS (invasive occurrences from outside CONUS); (3) invasive_CONUS vs. invasive_Non‐CONUS; and (4) native vs. invasive (all invasive range occurrences).
We adopted a theoretical framework suggested by Gallien et al. (2012) to identify stages of N. fulva invasion in the continental United States by plotting predicted probabilities from the “invasive_CONUS” model (regional) against the native and invasive occurrences combined model (NIRM‐Americas). This framework helps in making inferences about the stages of invasion for different populations of a species in the ecological niche space (Gallien et al. 2012). In the niche space, a species would be at quasi‐equilibrium if the regional and global models predict higher probabilities (e.g., >0.5) for the species' presence (stabilizing populations); regional colonization occurs when the regional model predicts low probability of presence, but the global model predicts high probability. However, if the regional and global models predict low probability of presence, these occurrences may represent population sinks. In contrast, evidence that some form of regional adaptation may be occurring arises when the regional model predicts high probabilities of presence for some set of occurrences, but the global model predicts low probabilities (Gallien et al. 2012).
All GIS analyses were performed using ArcGIS version 10.2.2 (ESRI). All statistical analyses were conducted in R (R Development Core Team, 2013).
Results
Model performance and variable importance
All three models (IRM‐CONUS, NIRM‐Americas, and NIRM‐Global) performed better than random with AUCcv values ranging from 0.94 to 0.97, and pAUC values from 1.82 to 1.96 (Table 1). The models had low omission rates; test sensitivity at 0% training omission rate varied from 0.003 to 0.006, and at 10% training omission rate varied from 0.105 to 0.114 (Table 1). The best model for the continental United States (IRM‐CONUS) included four climatic variables, whereas NIRM‐Americas and NIRM‐Global models each included six variables (Table 2). The best IRM‐CONUS model included Linear, Quadratic, and Hinge features (regularization multiplier [RM] = 1.5), whereas the NIRM‐Americas model included Linear, Quadratic, and Product features (RM = 2.5). The NIRM‐Global model included Linear, Quadratic, Product and Hinge features (RM = 2.5; Table 1). The NIRM‐Global model with moderate level of complexity ranked highest compared to other models with lower or higher levels of complexity (Table S4 in Appendix S2).
Table 2.
Average percent contribution of environmental variables to different Nylanderia fulva models; values were averaged across 10 replicate runs
| Variable | IRM‐CONUS | NIRM‐ Americas | NIRM‐Global |
|---|---|---|---|
| Degree days with average temp. ≥10°C (degdays10)a | 90.1 | 25.3 | 24.5 |
| Precipitation of driest quarter (bio17; mm) | 6.5 | 5.2 | 65.0 |
| Mean temperature of wettest quarter (bio8; °C)a | 1.9 | – | – |
| Temperature seasonality (SD × 100) (bio4)b | 1.4 | 17.8 | – |
| Mean diurnal range in temp. (bio2; °C) | – | 26.2 | 0.4 |
| Precipitation seasonality (CV) (bio15) | – | 16.8 | 3.1 |
| Precipitation of wettest quarter (bio16; mm) | – | 8.6 | 2.6 |
| Isothermality (bio3)b | – | – | 4.5 |
Note: IRM‐CONUS is the invasive range model using occurrence data from only continental United States; NIRM‐America is the native and invasive range model using data from Americas; NIRM‐Global is the native and invasive range model using data from Americas but calibrated using global extent background data.
Variables highly correlated at NIRM‐Americas and NIRM‐Global extents (Pearson's correlation coefficient |r| ≥ 0.80).
Variables highly correlated at all three extents.
The mean diurnal range in temperature, degree days with average temperature ≥10°C, and precipitation of driest quarter were the most important climatic variables associated with N. fulva distribution (Table 2). The importance of variables slightly changed with the calibration extent (Table 2). For example, degree days at ≥10°C was the top most important predictor in the IRM‐CONUS model, but it ranked second in NIRM‐Americas and NIRM‐Global models (Table 2). The jackknife test of variable importance showed that the degree days at ≥10°C had the most information that was not present in other variables (NIRM‐Americas model; Figure S1 in Appendix S3). The probability of N. fulva presence was highest when mean diurnal range in temperature was between 6 to 11°C, and degree days at ≥10°C was between 3000 and 5000 (Figure S2A, B in Appendix S3). The probability of N. fulva presence was higher at lower levels of temperature and precipitation seasonality (Figure S2C, D in Appendix S3).
Predicted potential distribution of Nylanderia fulva in the United States
The predicted potential distribution of N. fulva closely matched observed occurrences (Figs. 2 and 3 vs. Fig. 1). Both IRM‐CONUS and NIRM‐Americas models predicted highly suitable areas for N. fulva in southeastern Texas, southern Mississippi, southern Louisiana, and most of Florida (Fig. 2). The NIRM‐Americas model predicted low‐to‐medium suitability in southeastern parts of California, southern Nevada, and southwestern Arizona, whereas the IRM‐CONUS model predicted very low suitability in these areas (Fig. 2). The NIRM‐Americas and NIRM‐Global models predicted low suitability for N. fulva in northwestern Washington and northern Oregon (Figs. 2 and 3). The partial model using N. fulva invaded range occurrences from the southern United States (IRM‐CONUS; Fig. 2A) predicted less expansive regions of the suitable habitat compared to a full model using all native and invaded range occurrences (NIRM‐Americas; Fig. 2B). The full model predicted lower climatic suitability for N. fulva as far north as southern Missouri, Illinois, and Indiana (Fig. 2B). However, both models largely agree on areas of high probability (>0.5) of suitable conditions, these were largely restricted to the Gulf and Southern Atlantic Coast regions, plus coastal and Central Texas. The NIRM‐Global model predicted highly suitable areas for N. fulva in all the Hawaiian Islands (see inset in Fig. 3).
Figure 2.
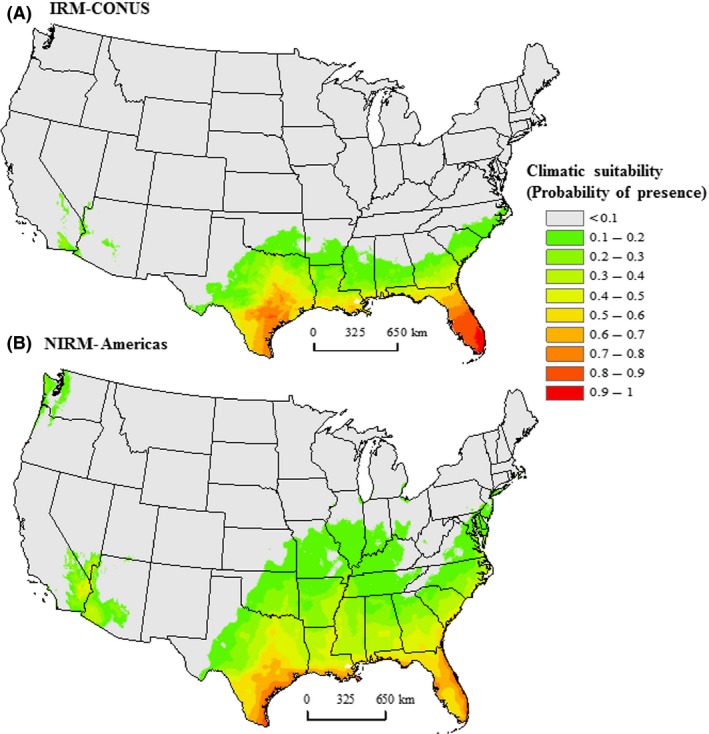
Predicted potential distribution of Nylanderia fulva in the continental United States based on occurrences from (A) invaded range in southern United States (IRM‐CONUS), and (B) native and invaded range combined (NIRM‐Americas).
Figure 3.
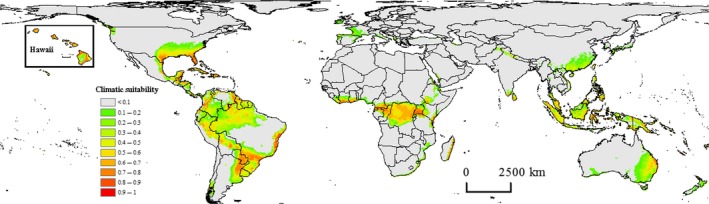
Global potential distribution of Nylanderia fulva based on native and invasive range global (NIRM‐Global) model.
Global potential distribution of Nylanderia fulva
The NIRM‐Global model predicted highly suitable areas for N. fulva in eastern Mexico, the Caribbean islands, and Central America (Fig. 3). The model predicted highly suitable areas in western Colombia, western, southern and eastern coastal Brazil, Ecuador (including the Galapagos Islands), northern Peru, northern Bolivia, eastern Paraguay, Uruguay, and western Argentina (Fig. 3). The model predicted suitable areas in central parts of Africa, eastern Madagascar, lower Himalayas in India and Pakistan, southern India and Sri Lanka, southeastern China (including Taiwan), southeastern parts of Asia, eastern Australia, and northern parts of New Zealand (Fig. 3). Based on observed presences in the Americas (Fig. 1), N. fulva currently occurs in areas with an average annual temperature between 13 and 29°C, and an average annual precipitation between 378 and 4900 mm (Table S2 in Appendix S1).
Niche shift and stages of invasion
The principal component analysis (PCA) showed that the realized climatic niche of N. fulva may have shifted and expanded in the invasive regions examined; the center of the realized climatic niche moved toward warmer temperatures and higher temperature seasonality, and there was only 24% niche overlap between native and invaded ranges (Schoener's D = 0.24; Fig. 4A). The PCA showed a similar realized niche shift and expansion in the southern United States occurrences with only 24% niche overlap (Fig. 4B); the climate space in the United States that is currently “unfilled” (green shaded areas within solid red contour line) by N. fulva. This may be representing geographic areas where N. fulva is currently undetected or absent. The amount of niche overlap was lower between N. fulva's native and invaded ranges outside the continental United States (Schoener's D = 0.16; Fig. 4C), although the niche expansion was higher (i.e., red shaded areas within solid red contour line; Fig. 4C). There was little niche overlap (8%) between N. fulva's invaded ranges inside or outside the CONUS (Fig. 4D) suggesting a more extreme shift in the realized niche during invasion of the continental United States compared to Colombia and the Caribbean islands.
Figure 4.
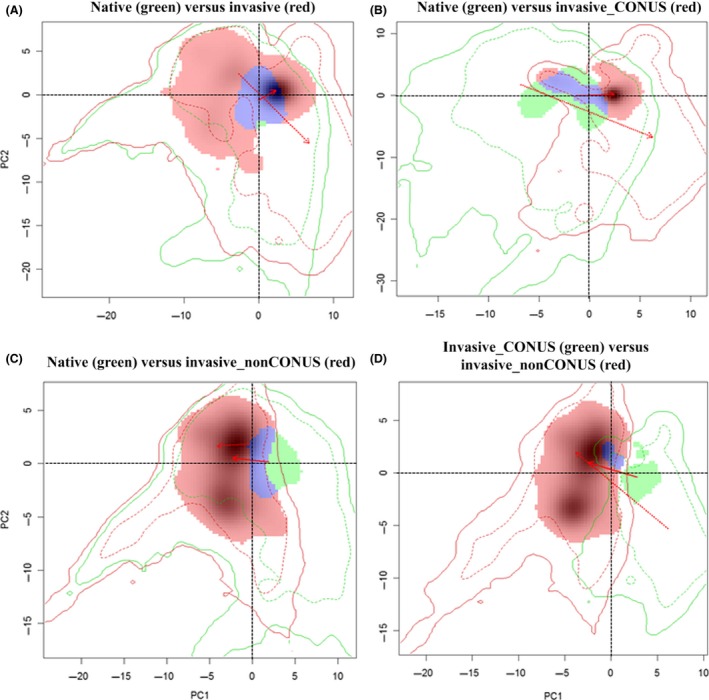
Native and invasive niches of Nylanderia fulva in different regions; multivariate climatic space was calculated using PCA‐env method. PC1 and PC2 represent the first two axes of the principal competent analysis (PCA). The green and red shadings represent density of species occurrences in different regions; blue represents overlap. Solid and dashed lines show 100% and 50% of the available (background) environment. The red arrows show how the center of the climatic niche for N. fulva (solid) and background extent (dotted) has moved between two ranges.
The analysis of current stages of N. fulva invasion in the United States based on regional (IRM‐CONUS) and global model (NIRM‐Americas) predictions revealed that the majority of observed N. fulva occurrences are stabilizing populations; one population (Miami, Dade County, Florida) may be at colonization stage, two at regional adaptation, and three represent sink populations (Fig. 5A). The majority of southern occurrences are in the stabilizing zone, whereas colonization and adaptation zones were predicted for northern populations toward the leading edge of N. fulva invasion (Fig. 5B).
Figure 5.
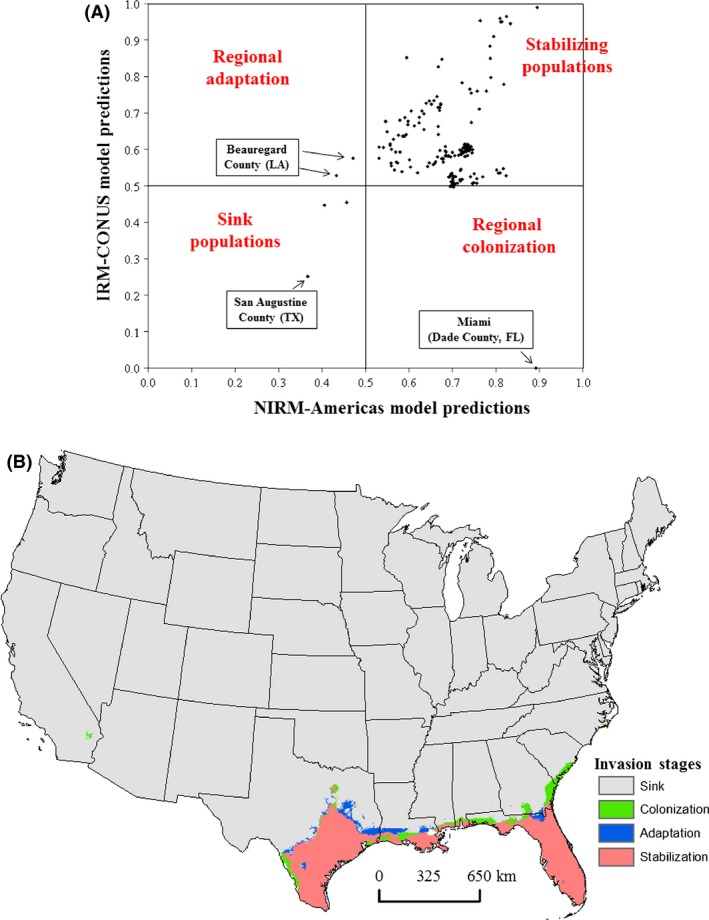
(A) Nylanderia fulva observed occurrences at different stages of invasion based on global and regional model predictions, and (B) mapped areas showing potential (hypothesized) for population stabilization, adaptation, colonization, and sink.
Discussion
Our study provides strong evidence of a shift in the realized climatic niche of N. fulva during its spread through Colombia, Caribbean islands, and southern United States. We showed that a partial model using invaded range occurrences from southern United States underestimated the potential risk of N. fulva invasion in the United States. Our models predicted potentially suitable habitat for N. fulva in southern parts of the United States, Hawaii, and other parts of the world. The predicted potential distribution of N. fulva conforms well to currently known occurrences in the southern United States and southeastern South America (Figs. 1 and 3), indicating that it meets biological expectations. The model predicted large amounts of climatically suitable areas in South America outside of the native range region occupied by this species. The absence of N. fulva from these areas suggests the existence of currently unappreciated biological constraints on its realized niche, potentially in the form of closely allied competitors or natural enemies. However, it may change with time with the arrival of new propagules through human transportation.
By applying the Gallien et al. (2012) theoretical framework, we found that the majority of observed occurrences of N. fulva in the United States are likely stabilizing populations. We quantified unfilled climatic niche space for N. fulva in the United States, where range expansion and colonization could occur. Indeed, new reports of N. fulva in Albany, Georgia (Dowdy 2013) and Mobile, Alabama (Carroll 2014) fall within this expansion zone. We identified the mean diurnal range in temperature, the number of degree days at ≥10°C, and precipitation of driest quarter as the most important variables associated with N. fulva distribution.
Climatic niche shift
The niche shift and expansion quantified here shows N. fulva's potential to invade novel areas. Our models indicated that the realized niche of N. fulva has shifted to warmer and drier conditions between the native and invasive ranges (Fig. 4A), likely due to a release from interspecific competition across the invasive range. Nylanderia fulva seems to occupy the majority of the climate space in its introduced range, matching its native range climatic space (Fig. 4A). However, the same results were not observed across the continental United States (Fig. 4B). This could be because N. fulva's realized climatic niches in its invaded range are different within and outside the continental United States (Fig. 4D); the magnitude of the shift in realized niche was greater during N. fulva invasion into Colombia and the Caribbean Islands compared to continental United States (Fig. 4D).
The niche shift showed here is not unique to N. fulva. Several studies have documented climate niche shifts for other invasive ants and plant species (e.g., Broennimann et al., 2007; Fitzpatrick et al. 2007; Petitpierre et al., 2012; Guisan et al. 2014). Positive species interactions can expand the fundamental niche and range of a species, especially when species experience physical and biological stresses (He and Bertness 2014). Local adaptation of an introduced species in new geographic areas can occur because of its ability to exploit empty niches, or the frequency and magnitude of local disturbances creating new habitats, and the absence of its natural enemies (Sax et al. 2005).
Is the climatic niche of N. fulva evolving rapidly over time? We do not know. The issue of rate of niche evolution is a subject of ongoing debate; several studies suggest rapid niche evolution for some species (e.g., Holt and Gaines 1992; Sexton et al. 2009; Guisan et al. 2014), whereas other studies show niche conservatism over time (e.g., Peterson et al. 1999; Peterson 2011). Further research is needed to understand the changes and rates of N. fulva's fundamental niche shifts.
Caveats and uncertainties
Results from correlative niche models such as MaxEnt should be interpreted with caution because of inherent uncertainties and model specific assumptions. Niche model predictions may be affected by sampling bias, number of samples, incomplete species occurrence data, failure to account for biotic processes (e.g., presence of natural enemies), choice and spatial resolution of abiotic variables, multicollinearity, and species characteristics (Guisan et al. 2007a,b; Dormann et al., 2013; Syfert et al. 2013). A mismatch between the time period of species occurrences and climatic data might also influence niche estimates. Our models also do not account for microclimates available to the species because of the coarser spatial resolution (~1 km2) of climate dataset used in model calibration. Finer‐scale climate data (e.g., at a scale of a few square meters) were not available at the global level; the generation of such a dataset was beyond the scope of this study and would be impractical (Bennie et al. 2014). Thus, our results are applicable to population‐level responses of N. fulva to macroclimate rather than individual responses. Our models may have overestimated the potential suitable areas because not all predicted areas have suitable habitat for N. fulva (e.g., water bodies). Additionally, occurrences in urban areas where the species may be buffered from the natural climate envelope due to human habitat alterations (e.g., irrigation and structures) may cause the models to over predict suitability in nonurbanized areas.
Nylanderia fulva is a member of a taxonomically difficult group. Because of similarity in the worker caste among members of this genus, misidentifications in museum collections, and the literature occur (Gotzek et al. 2012). Due to the co‐occurrence of the morphologically similar, closely related species, N. pubens, in the Caribbean region records re‐reported herein from that region should be viewed as provisional and in need of additional collection to verify. Examination of the climatic values associated with these records from the Caribbean indicates that they cover conditions from within the climatic envelope for N. fulva (Appendix S1). Whether N. fulva as defined represents a single coherent biological entity has been questioned (Trager 1984; Kallal and Lapolla 2012). Additional studies of species boundaries within this group and population genetic studies of the source localities for invasive populations are needed because introduction history might determine the genetic diversity and structure of a species in invaded range (Ascunce et al. 2011; Le Roux et al. 2011); subspecies may have distinct climatic niches (e.g., Thompson et al. 2011).
The effects of environmental variables on species distributions are scale‐dependent. Environmental factors such as climate are generally associated with species' distributions at regional or continental scales, whereas biotic factors such as presence of a competitor or a host plant species control species distributions at local scale (Austin 2002). At the local scale, factors such as soil moisture and temperature can influence ant distribution and abundance (e.g., Holway et al. 2002b; Menke et al. 2009). Roura‐Pascual et al. (2004) found that the distribution of Argentine ants (Linepithema humile Mayr) may be limited by cooler temperatures and decreased humidity levels in the northern latitudes. The introduction and establishment of an alien insect species can also be affected by its behavior (e.g., nesting biology and social organization) and life history traits (Holway and Suarez 1999). Little is known about the abiotic, biotic, and phenological constraints that limit N. fulva distribution in its native and invasive range; our study generated hypotheses about these unknown factors, which could be experimentally tested. For example, our study showed that N. fulva is highly influenced by degree days at ≥10°C, and does quite well between 3000 and 5000 degree days (Figures S1 and S2 in Appendix S3). This finding can be tested in the laboratory.
Fine‐scale environmental heterogeneity may affect distribution and abundance of ant species (Savage et al. 2014), a factor not considered in our models because of the focus on climatic niche of N. fulva. Incorporating variables representing the fine‐scale heterogeneity at a finer spatial resolution might improve local and regional models. For example, remotely sensed indices such as Normalized Difference Vegetation Index (NDVI), and Enhanced Vegetation Index (EVI), soil moisture, and anthropogenic factors (e.g., Human Footprint Index) could be used to develop finer resolution local or regional models of N. fulva distribution. Future research should also investigate N. fulva's response to climate change. Given the strong association of climatic factors with the distribution of N. fulva, it is highly likely that its future distribution may be affected by climate change.
The eradication of N. fulva from its invasive range appears to be an unachievable goal given its current levels of infestation. Therefore, prevention and control would be a better strategy for managing N. fulva invasion (Hoffmann et al. 2010). Prioritizing the prevention of further spread of N. fulva, especially to at‐risk areas (i.e., climatically suitable; Figs. 2 and 3), might be the best way to contain its future invasion (e.g., Bromberg et al., 2011). The information on climatic niche expansion and risk maps produced in our study can be useful tools in managing and monitoring N. fulva future spread and currently infested areas. For example, land managers in at‐risk areas can design policies and take appropriate steps (e.g., quarantine measures) to stop movement of N. fulva propagules to their regions, and thus, reduce management costs due to N. fulva invasion.
Conflict of Interest
None declared.
Supporting information
Appendix S1. Occurrence data, climatic variables and cross‐correlation Tables.
Appendix S2. Model selection summary Table.
Appendix S3. Variable importance and species response curves.
Acknowledgments
We thank the Natural Resource Ecology Laboratory at Colorado State University for providing the logistical support. S. Kumar was partially supported by U.S. Geological Survey and the USDA UV‐B Monitoring and Research Program, NREL, Colorado State University (USDA‐NRI, 2008‐35615‐04666). T. J. Stohlgren was partially supported by USDA CSREES/NRI 2008‐35615‐04666. We thank A. M. Arcila (Universidad del Vaiie, Cali. Valle, Colombia) for providing occurrence data. E. G. LeBrun was supported by a grant from the Lee and Ramona Bass Foundation. We are grateful to four anonymous reviewers whose comments improved the manuscript.
Ecology and Evolution 2015; 5(20): 4628–4641
References
- Arcila, A. M. , Gomez L. A., and Ulloa‐Chacon P.. 2002a. Immature development and colony growth of crazy ant Paratrechina fulva under laboratory conditions (Hymenoptera: Formicidae). Sociobiology 39:307–321. [Google Scholar]
- Arcila, A. M. , Ulloa‐Chacon P., and Gomez L. A.. 2002b. Factors that influence individual fecundity of queens and queen production in crazy ant Paratrechina fulva (Hymenoptera: Formicidae). Sociobiology 39:323–334. [Google Scholar]
- Ascunce, M. S. , Yang C.‐C., Oakey J., Calcaterra L., Wu W.‐J., Shih C.‐J., et al. 2011. Global invasion history of the fire ant Solenopsis invicta . Science 331:1066–1068. [DOI] [PubMed] [Google Scholar]
- Austin, M. P. 2002. Spatial prediction of species distribution: an interface between ecological theory and statistical modelling. Ecol. Model. 157:101–118. [Google Scholar]
- Barve, N. 2008. Tool for Partial‐ROC (Biodiversity Institute, Lawrence, Kansas), ver 1.0; available at http://biodiversity-informatics-training.org (accessed on August 8, 2014).
- Bennie, J. , Wilson R. J., Maclean I. M. D., and Suggitt A. J.. 2014. Seeing the woods for the trees – when is microclimate important in species distribution models? Glob. Change Biol. 20:2699–2700. [DOI] [PubMed] [Google Scholar]
- Blackwell, T. 2014. The rise of crazy ants and how to fight back. Property Management Insider, available at http://www.propertymanagementinsider.com/the-rise-of-crazy-ants (accessed on February 5, 2015).
- Boria, R. A. , Olson L. E., Goodman S. M., and Anderson R. P.. 2014. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 275:73–77. [Google Scholar]
- Broennimann, O. , and Guisan A.. 2008. Predicting current and future biological invasions: both native and invaded ranges matter. Biol. Lett. 4:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broennimann, O. , Treier U. A., Muller‐Scharer H., Thuiller W., Peterson A. T., and Guisan A.. 2007. Evidence of climatic niche shift during biological invasion. Ecol. Lett. 10:701–709. [DOI] [PubMed] [Google Scholar]
- Broennimann, O. , Fitzpatrick M. C., Pearman P. B., Petitpierre B., Pellissier L., Yoccoz N. G., et al. 2012. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 21:481–497. [Google Scholar]
- Brown, J. L. 2014. SDMtoolbox: a python‐based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 5:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg, J. E. , Kumar S., Brown C. S., and Stohlgren T. J.. 2011. Distributional changes and range predictions of downy brome in Rocky Mountain National Park. Invasive Plant Sci. Manage. 4:173–182. [Google Scholar]
- Carroll, D. 2014. Extension corner: Tawny crazy ants now in Alabama. The Gadsden Times, available at http://www.gadsdentimes.com/article/20140813/NEWS/140819931 (accessed February 16, 2015).
- Deyrup, M. , Davis L., and Cover S.. 2000. Exotic ants in Florida. Trans. Am. Entomol. Soc. 126:293–326. [Google Scholar]
- Dormann, C. F. , Schymanski S. J., Cabral J., Chuine I., Graham C., Hartig F., et al. 2012. Correlation and process in species distribution models: bridging a dichotomy. J. Biogeogr. 39:2119–2131. [Google Scholar]
- Dormann, C. F. , Elith J., Bacher S., Buchmann C., Carl G., Carre G., et al. 2013. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46. [Google Scholar]
- Dowdy, S. 2013. Invasive tawny crazy ant found in Georgia. University of Georgia Today, available at http://news.uga.edu/releases/article/invasive-tawny-crazy-ant-found-in-georgia-0913/ (accessed February 16, 2015).
- Elith, J. , Kearney M., and Phillips S.. 2010. The art of modelling range‐shifting species. Methods Ecol. Evol. 1:330–342. [Google Scholar]
- Evangelista, P. H. , Kumar S., Stohlgren T. J., Jarnevich C. S., Crall A. W., Norman J. B., et al. 2008. Modelling invasion for a habitat generalist and a specialist plant species. Divers. Distrib. 14:808–817. [Google Scholar]
- Fielding, A. H. , and Bell J. F.. 1997. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24:38–49. [Google Scholar]
- Fitzpatrick, M. C. , Weltzin J. F., Sanders N. J., and Dunn R. R.. 2007. The biogeography of prediction error: why does the introduced range of the fire ant over‐predict its native range? Glob. Ecol. Biogeogr. 16:24–33. [Google Scholar]
- Gallien, L. , Douzet R., Pratte S., Zimmermann N. E., and Thuiller W.. 2012. Invasive species distribution models ‐ how violating the equilibrium assumption can create new insights. Glob. Ecol. Biogeogr. 21:1126–1136. [Google Scholar]
- González‐Moreno, P. , Diez J. M., Richardson D. M., and Vilà M.. 2015. Beyond climate: disturbance niche shifts in invasive species. Glob. Ecol. Biogeogr. 24:360–370. [Google Scholar]
- Gotzek, D. , Brady S. G., Kallal R. J., and LaPolla J. S.. 2012. The importance of using multiple approaches for identifying emerging invasive species: the case of the Rasberry Crazy Ant in the United States. PLoS One 7:e45314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell, J. 1917. The niche‐relationships of the California Thrasher. Auk 34:427–433. [Google Scholar]
- Guisan, A. , Zimmermann N. E., Elith J., Graham C. H., Phillips S., and Peterson A. T.. 2007a. What matters for predicting the occurrences of trees: techniques, data, or species' characteristics? Ecol. Monogr. 77:615–630. [Google Scholar]
- Guisan, A. , Graham C. H., Elith J., Huettmann F., and Group N.S.D.M. 2007b. Sensitivity of predictive species distribution models to change in grain size. Divers. Distrib., 13:332–340. [Google Scholar]
- Guisan, A. , Petitpierre B., Broennimann O., Daehler C., and Kueffer C.. 2014. Unifying niche shift studies: insights from biological invasions. Trends Ecol. Evol. 29:260–269. [DOI] [PubMed] [Google Scholar]
- Gutrich, J. J. , VanGelder E., and Loope L.. 2007. Potential economic impact of introduction and spread of the red imported fire ant, Solenopsis invicta, in Hawaii. Environ. Sci. Policy 10:685–696. [Google Scholar]
- He, Q. , and Bertness M. D.. 2014. Extreme stresses, niches, and positive species interactions along stress gradients. Ecology 95:1437–1443. [DOI] [PubMed] [Google Scholar]
- Hijmans, R. J. , Cameron S. E., Parra J. L., Jones P. G., and Jarvis A.. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25:1965–1978. [Google Scholar]
- Hoffmann, B. D. , Abbott K. L., and Davis P. D.. 2010. Invasive ant management Pp. 287–304 in Lach L., Parr C. L., Abbott K. L., eds. Ant ecology. Oxford Univ. Press, Oxford, U.K.. [Google Scholar]
- Holt, R. D. , and Gaines M. S.. 1992. Analysis of adaptation in heterogeneous landscapes: implications for the evolution of fundamental niches. Evol. Ecol. 6:433–447. [Google Scholar]
- Holway, D. A. , and Suarez A. V.. 1999. Animal behavior: an essential component of invasion biology. Trends Ecol. Evol. 14:328–330. [DOI] [PubMed] [Google Scholar]
- Holway, D. A. , Lach L., Suarez A. V., Tsutsui N. D., and Case T. J.. 2002a. The causes and consequences of ant invasions. Annu. Rev. Ecol. Syst. 33:181–233. [Google Scholar]
- Holway, D. A. , Suarez A. V., and Case T. J.. 2002b. Role of abiotic factors in governing susceptibility to invasion: a test with argentine ants. Ecology 83:1610–1619. [Google Scholar]
- Hooper‐Bui, L. M. , Strecker R., Chen X., Aguillard D., and Miller A.. 2010. Super‐colonies of crazy ants in Louisiana Pp 13–16 in Hopkins J. D., ed. Proceedings of the imported fire ant and invasive ant conference, Division of Agriculture Cooperative Extension Service, University of Arkansas, Little Rock, Arkansas, USA. [Google Scholar]
- Hutchinson, G. E. 1957. Population studies ‐ animal ecology and demography ‐ concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 22:415–427. [Google Scholar]
- Jimenez‐Valverde, A. , Lobo J. M., and Hortal J.. 2008. Not as good as they seem: the importance of concepts in species distribution modelling. Divers. Distrib. 14:885–890. [Google Scholar]
- Jimenez‐Valverde, A. , Peterson A. T., Soberon J., Overton J. M., Aragon P., and Lobo J. M.. 2011. Use of niche models in invasive species risk assessments. Biol. Invasions 13:2785–2797. [Google Scholar]
- Kallal, R. J. , and LaPolla J. S.. 2012. Monograph of Nylanderia (Hymenoptera: Formicidae) of the World, Part II: Nylanderia in the Nearctic. Zootaxa, 3508:1–64. [Google Scholar]
- Klotz, J. H. , Mangold J. R., Vail K. M., Davis L. R., and Patterson R. S.. 1995. A survey of the urban pest ants (Hymenoptera: Formicidae) of peninsular Florida. Fla. Entomol. 78:109–118. [Google Scholar]
- Kumar, S. , and Stohlgren T. J.. 2009. Maxent modeling for predicting suitable habitat for threatened and endangered tree Canacomyrica monticola in New Caledonia. J. Ecol. Nat. Environ. 1:94–98. [Google Scholar]
- Kumar, S. , Spaulding S. A., Stohlgren T. J., Hermann K. A., Schmidt T. S., and Bahls L. L.. 2009. Potential habitat distribution for the freshwater diatom Didymosphenia geminata in the continental US. Front. Ecol. Environ. 7:415–420. [Google Scholar]
- Kumar, S. , Neven L. G., and Yee W. L.. 2014. Evaluating correlative and mechanistic niche models for assessing the risk of pest establishment. Ecosphere 5:1–23. [Google Scholar]
- LaPolla, J. S. , Brady S. G., and Shattuck S. O.. 2010. Phylogeny and taxonomy of the Prenolepis genus‐group of ants (Hymenoptera: Formicidae). Syst. Entomol. 35:118–131. [Google Scholar]
- Le Roux, J. J. , Brown G. K., Byrne M., Ndlovu J., Richardson D. M., Thompson G. D., et al. 2011. Phylogeographic consequences of different introduction histories of invasive Australian Acacia species and Paraserianthes lophantha (Fabaceae) in South Africa. Divers. Distrib. 17:861–871. [Google Scholar]
- LeBrun, E. G. , Abbott J., and Gilbert L. E.. 2013. Imported crazy ant displaces imported fire ant, reduces and homogenizes grassland ant and arthropod assemblages. Biol. Invasions 15:2429–2442. [Google Scholar]
- Liu, C. , White M., and Newell G.. 2013. Selecting thresholds for the prediction of species occurrence with presence‐only data. J. Biogeogr. 40:778–789. [Google Scholar]
- MacGown, J. , and Layton B.. 2010. The invasive Rasberry crazy ant, Nylanderia sp. nr. pubens (Hymenoptera: Formicidae), reported from Mississippi. Midsouth Entomol. 3:44–47. [Google Scholar]
- Mack, R. N. , Simberloff D., Lonsdale W. M., Evans H., Clout M., and Bazzaz F. A.. 2000. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol. Appl. 10:689–710. [Google Scholar]
- McDonald, D. L. 2012. Investigation of an invasive ant species: Nylanderia fulva colony extraction, management, diet preference, fecundity, and mechanical vector potential. Ph.D. Dissertation. Texas A&M University, College Station, TX. [Google Scholar]
- Menke, S. B. , Holway D. A., Fisher R. N., and Jetz W.. 2009. Characterizing and predicting species distributions across environments and scales: Argentine ant occurrences in the eye of the beholder. Glob. Ecol. Biogeogr. 18:50–63. [Google Scholar]
- Merow, C. , Smith M. J., and Silander J. A.. 2013. A practical guide to MaxEnt for modeling species' distributions: what it does, and why inputs and settings matter. Ecography 36:1058–1069. [Google Scholar]
- Meyers, J. M. , and Gold R. E.. 2008. Identification of an exotic pest ant, Paratrechina sp.nr. pubens (Hymenoptera: Formicidae), in Texas. Sociobiology 52:589–604. [Google Scholar]
- Petitpierre, B. , Kueffer C., Broennimann O., Randin C., Daehler C., and Guisan A.. 2012. Climatic niche shifts are rare among terrestrial plant invaders. Science 335:1344–1348. [DOI] [PubMed] [Google Scholar]
- Peterson, A. T. 2003. Predicting the geography of species' invasions via ecological niche modeling. Q. Rev. Biol. 78:419–433. [DOI] [PubMed] [Google Scholar]
- Peterson, A. T. 2011. Ecological niche conservatism: a time‐structured review of evidence. J. Biogeogr. 38:817–827. [Google Scholar]
- Peterson, A. T. , Soberon J., and Sanchez‐Cordero V.. 1999. Conservatism of ecological niches in evolutionary time. Science 285:1265–1267. [DOI] [PubMed] [Google Scholar]
- Peterson, A. T. , Papes M., and Soberon J.. 2008. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model., 213:63–72. [Google Scholar]
- Peterson, A. T. , Soberon J., Pearson R. G., Anderson R. P., Martinez‐Meyer E., Nakamura M., et al. 2011. Ecological niches and geographic distributions. Princeton Univ. Press, Princeton, NJ. [Google Scholar]
- Phillips, S. J. , Anderson R. P., and Schapire R. E.. 2006. Maximum entropy modeling of species geographic distributions. Ecol. Model. 190:231–259. [Google Scholar]
- Pimentel, D. , Zuniga R., and Morrison D.. 2005. Update on the environmental and economic costs associated with alien‐invasive species in the United States. Ecol. Econ. 52:273–288. [Google Scholar]
- R Development Core Team . 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: URL http://www.R-project.org/. [Google Scholar]
- Roura‐Pascual, N. , Suarez A. V., Gomez C., Pons P., Touyama Y., Wild A. L., et al. 2004. Geographical potential of Argentine ants (Linepithema humile Mayr) in the face of global climate change. Proc. R. Soc. B Biol. Sci. 271:2527–2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura‐Pascual, N. , Brotons L., Peterson A. T., and Thuiller W.. 2009. Consensual predictions of potential distributional areas for invasive species: a case study of Argentine ants in the Iberian Peninsula. Biol. Invasions 11:1017–1031. [Google Scholar]
- Savage, A. M. , Hackett B., Guénard B., Youngsteadt E. K., and Dunn R. R.. 2014. Fine‐scale heterogeneity across Manhattan's urban habitat mosaic is associated with variation in ant composition and richness. Insect Conserv. Divers.. doi:10.1111/icad.12098. [Google Scholar]
- Sax, D. F. , Gaines S. D., and Brown J. H.. 2002. Species invasions exceed extinctions on islands worldwide: a comparative study of plants and birds. Am. Nat. 160:766–783. [DOI] [PubMed] [Google Scholar]
- Sax, D. F. , Stachowicz J. J., and Gaines S. D.. 2005. Species invasions insights into ecology, evolution, and biogeography. Sinauer Associates, Sunderland, MA. [Google Scholar]
- Schoener, T. W. 1970. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 51:408–418. [Google Scholar]
- Sexton, J. P. , McIntyre P. J., Angert A. L., and Rice K. J.. 2009. Evolution and ecology of species range limits. Annu. Rev. Ecol. Evol. Syst. 40:415–436. [Google Scholar]
- Soberon, J. , and Peterson A. T.. 2005. Interpretation of models of fundamental ecological niches and species' distributional areas. Biodivers. Inform. 2:1–10. [Google Scholar]
- Stohlgren, T. J. , Ma P., Kumar S., Rocca M., Morisette J. T., Jarnevich C. S., et al. 2010. Ensemble habitat mapping of invasive plant species. Risk Anal. 30:224–235. [DOI] [PubMed] [Google Scholar]
- Syfert, M. M. , Smith M. J., and Coomes D. A.. 2013. The effects of sampling bias and model complexity on the predictive performance of MaxEnt species distribution models. PLoS One 8:e55158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, G. D. , Robertson M. P., Webber B. L., Richardson D. M., Le Roux J. J., and Wilson J. R. U.. 2011. Predicting the subspecific identity of invasive species using distribution models: Acacia saligna as an example. Divers. Distrib. 17:1001–1014. [Google Scholar]
- Trager, J. C. 1984. A revision of the genus Paratrechina (Hymenoptera, Formicidae) of the continental United States. Sociobiology 9:51–162. [Google Scholar]
- Vaclavik, T. , and Meentemeyer R. K.. 2009. Invasive species distribution modeling (iSDM): are absence data and dispersal constraints needed to predict actual distributions? Ecol. Model. 220:3248–3258. [Google Scholar]
- Waltari, E. , and Perkins S. L.. 2010. In the hosts' footsteps? Ecological niche modelling and its utility in predicting parasite distributions Pp. 145–157 in Morand S., Krasnov B. R., eds. The biogeography of host‐parasite interactions. Oxford Univ. Press, Oxford, U.K. [Google Scholar]
- Warren, D. L. , Glor R. E., and Turelli M.. 2010. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33:607–611. [Google Scholar]
- Wolmarans, R. , Robertson M. P., and van Rensburg B. J.. 2010. Predicting invasive alien plant distributions: how geographical bias in occurrence records influences model performance. J. Biogeogr. 37:1797–1810. [Google Scholar]
- Zenner de Polania, I. 1990. Biological aspects of the “Hormiga Loca,” Paratrechina (Nylanderia) fulva (Mayr), in Colombia Pp. 290–297 in Vander Meer R. K., Jaffe K., Cedeno A., eds. Applied myrmecology, a world perspective. Westview Press, Boulder, CO. [Google Scholar]
- Zenner de Polania, I. , and Bolaños R. N.. 1985. Habitos alimenticios y relaciones simbioticas de la “hormiga loca” Nylanderia fulva con otros artropodos. Rev. Colomb. Entomol. 11:3–10. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Occurrence data, climatic variables and cross‐correlation Tables.
Appendix S2. Model selection summary Table.
Appendix S3. Variable importance and species response curves.


