Abstract
Introduction
While trials comparing antiplatelet strategies after coronary intervention report average risks of bleeding and ischemia in a population, there is limited information to guide choices based on individual patient risks, particularly beyond one year after treatment.
Methods
Patient-level data from PROTECT, a broadly inclusive trial enrolling 8709 subjects treated with drug-eluting stents (sirolimus vs. zotarolimus-eluting stent), and PROTECT US, a single arm study including 1018 subjects treated with a zotarolimus-eluting stent were combined. The risk of ischemic events, cardiovascular death/non-periprocedural myocardial infarction (MI)/definite or probable stent thrombosis and the risk of bleeding events, GUSTO moderate or severe bleed were predicted using logistic regression, and the correlation between predicted ischemic and bleeding risks within individual patients was estimated.
Results
At median follow-up of 4.1 years, major bleeding occurred in 260 subjects (2.8%), and ischemic events in 595 (6.3%). Multivariate predictors of bleeding were: older age, smoking, diabetes mellitus, congestive heart failure, and chronic kidney disease (all p<0.05). Ischemic events shared all of the same predictors with bleeding events as well as: sex, BMI, prior MI, prior CABG, STEMI on presentation, stent length and sirolimus-eluting stent use (all p<0.05). Within individual subjects, bleeding and ischemic risks were strongly correlated (ρ=0.76, p<0.001). 97% of subjects had a greater risk of ischemic events than bleeding.
Conclusions
Individual patient risks of ischemia and bleeding are related to many common risk factors, yet the predicted risks of ischemic events are greater than those of major bleeding in the large majority of patients in long-term follow-up.
Introduction
While clinical studies generally summarize average treatment effects, in clinical practice, treatment choices are made for individual patients according to their perceived risks for benefit and harm based on their unique clinical presentation and characteristics. Such choices may be particularly complex when risks of late events may be affected differently by treatment choices and when reported outcomes were influenced predominantly by early events. The traditional way to assess treatment heterogeneity in clinical trials is usually restricted to subgroup and interaction analyses based on prespecified baseline characteristics.1, 2
Both ischemic and bleeding events may occur in patients following percutaneous coronary intervention (PCI), and medication choices and treatment duration are areas of uncertainty regarding their impact on individual risks. For example, since the first reports of an increase in very late stent thrombosis (ST) with drug-eluting stents (DES), the appropriate antiplatelet regimen has been a matter of debate.3 The American College of Cardiology, American Heart Association and the Society for Cardiovascular Angiography and Interventions recommend dual antiplatelet therapy (DAPT) [e.g. aspirin with a P2Y12 receptor antagonist] for at least 12 months for patients receiving a DES based on observational evidence while the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery advocate DAPT for at least 6 months after DES implantation and ideally 12 months in patients deemed not at high bleeding risk.4, 5 Large randomized trials are ongoing to examine varying duration of dual antiplatelet therapy, and smaller studies suggest an increased risk of bleeding with longer treatment.6–12 However, uncertainty still persists given the limited power of completed studies to detect ischemic events.13 In patients with acute coronary syndrome (ACS), clinical treatment guidelines recommend treatment for 12 months regardless of stent type despite some increase in bleeding risk, based on multiple large randomized trials where 1 year of treatment is associated with reduction in risk of subsequent death and myocardial infarction (MI) by 20% compared with one month.4, 14–17
However, some patients may be at great risk of ischemic events based on their characteristics while others may face more bleeding risk. Determining this balance requires understanding the correlation between both risks for individual patients. We sought to estimate individual patient risks of long-term ischemic and bleeding complications after PCI, and to compare the magnitude of these risks in individual patients and subgroups using data from an inclusive prospective study of subjects treated with DES. To focus on late ischemic risks, we excluded periprocedural ischemic events and included late follow up to 4 years after DES implantation.
Methods
The study population consisted of 9727 subjects enrolled in PROTECT (Patient Related OuTcomes with Endeavor versus Cypher stenting Trial) or in the PROTECT US Study.18, 19 The PROTECT trial was a large, broadly inclusive, multicenter randomized controlled trial comparing the long-term safety of 2 different DES, the Endeavor zotarolimus-eluting stent (E-ZES) and the Cypher sirolimus-eluting stent (C-SES). 8709 subjects were randomized between May 2007 and December 2008, and have reached at least 4 years follow-up. The PROTECT US Study was a single-arm study following the PROTECT trial inclusion/exclusion criteria of 1018 patients who received an E-ZES and were followed for at least 3 years. Both included patients undergoing PCI with DES implantation and excluded patients with previous bare-metal stent (BMS) in the last 12 months, previous DES, prior brachytherapy or need for oral anticoagulation. Long-term use of DAPT consisting of aspirin and a thienopyridine was recommended for a minimum of 3 months up to 12 months or longer according to guidelines and treating physicians.
The primary ischemic endpoint for the current analysis was a composite of cardiovascular death, MI and Academic Research Consortium (ARC) definite/probable ST. Periprocedural MI (occurring within 48 hours from index PCI) was excluded to evaluate more precisely the long-term ischemic risk. The primary bleeding endpoint was the occurrence of a moderate/severe bleeding event as defined per GUSTO (Global Use of Strategies to Open Occluded Arteries) criteria. All endpoints were adjudicated by an independent clinical event committee.
Detailed baseline characteristics were available for analysis such as demographic factors (age, gender and body-mass index), risk factors for coronary artery disease (hypertension, diabetes, dyslipidemia and smoking status), cardiovascular history (prior PCI, prior coronary artery bypass graft surgery, prior MI, prior stroke, peripheral arterial disease, heart failure and chronic kidney disease), presentation for index procedure (stable angina/non-ST elevation MI [NSTEMI]/ST-elevation MI [STEMI]) and angiographic/procedural data (unprotected left main intervention, multivessel PCI, lesion length, total stent length, vessel diameter, bifurcation lesion, saphenous vein graft intervention, in-stent restenosis and type of stent implanted (E-ZES vs C-SES).
Baseline characteristics are presented as mean and standard deviation for continuous covariates, and proportion for categorical covariates, and they were compared using Student t tests and chi-square tests respectively. Temporal distribution of the primary endpoints and their individual components was assessed by reporting crude rates (number of events in patients at risk) for the time periods: 0–30 days, 1–12 months and beyond 12 months. In estimating these rates, we allowed subjects with an event in more than one time period to be considered as having an event in each time period to avoid underestimation of late risks. To examine the magnitude of risk from periods of variable length, rates were annualized for purpose of comparison.
Patients who completed the 4-year follow-up in PROTECT and the 3-year follow-up in PROTECT US were analyzed by logistic regression. Using the same candidate list of covariates described above, a separate multivariable model was built for each primary outcome. A backward selection method was used to select covariates with a p-value < 0.05 to stay in the final model. Internal validation and model calibration were done by the bootstrapping method described by Harrell (1000 bootstrap samples) using the rms package in R statistical software.20 Intercepts, beta coefficients and C-statistics were corrected for optimism. Goodness-of-fit was assessed by the Hosmer and Lemeshow test. Individual predicted probabilities of long-term ischemic and bleeding events were calculated using the optimism-corrected multivariate models and were presented on a scatter plot for purpose of comparison. Correlation between both risks was also assessed by means of the Pearson correlation coefficient. Finally, the subgroup of patients at higher bleeding than ischemic risk was identified and its characteristics were described. Sensitivity analyses were performed to evaluate robustness of the bleeding primary endpoint by censoring events occurring within 48 hours of index PCI and by using the TIMI major bleeding criteria.
All analyses were performed using SAS 9.2 (Cary, NC, USA) and R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance level was a p-value < 0.05 for all analyses.
Results
Complete follow-up was available for 9410 patients (96.7% of 9727 patients). Baseline characteristics of patients according to the occurrence of each primary endpoint are shown in Table 1. Median follow-up duration was 4.1 years (IQR 4.0 – 5.0). Use of dual antiplatelet therapy was assessed at 1, 6, 12, 24, 36 and 48 months, and was 96%, 94%, 87%, 39%, 32% and 27% respectively.
Table 1.
Baseline characteristics according to clinical outcomes.
| No ischemic event (n=8815) |
Ischemic event (n=595) |
No bleeding event (n=9150) |
Bleeding event (n=260) |
|
|---|---|---|---|---|
| Age, mean ± SD | 62.1 ± 10.5 | 66.2 ± 11.2* | 62.2 ± 10.6 | 67.3 ± 10.1† |
| Male | 6705/8815 (76.1%) |
458/595 (77.0%) |
6691/9150 (76.4%) |
172/260 (66.2%)† |
| BMI (kg/m2), mean ± SD | 28.0 ± 4.6 | 28.4 ±5.7 | 28.1 ± 4.7 | 27.9 ± 5.6 |
| Active smoking | 2170/8814 (24.6%) |
151/595 (25.4%) |
2261/9149 (24.7%) |
60/260 (23.1%) |
| Hypertension | 5719/8815 (64.9%) |
441/595 (74.1%)* |
5967/9150 (65.2%) |
193/260 (74.2%)† |
| Diabetes | 2378/8815 (27.0%) |
262/595 (44.0%)* |
2548/9150 (27.9%) |
92/260 (35.4%)† |
| Prior MI | 1695/8815 (19.2%) |
175/595 (29.4%)* |
1820/9150 (19.9%) |
50/260 (19.2%) |
| Prior PCI | 1096/8815 (12.4%) |
96/595 (16.1%)* | 1161/9150 (12.7%) |
31/260 (11.9%) |
| Known PVD | 414/8815 (4.7%) | 53/595 (8.9%)* | 442/9150 (4.8%) |
25/260 (9.6%)† |
| Prior stroke | 261/8815 (3.0%) | 34/595 (5.7%)* | 283/9150 (3.1%) |
12/260 (4.6%) |
| Prior CABG | 446/8815 (5.1%) | 55/595 (9.2%)* | 484/9150 (5.3%) |
17/260 (6.5%) |
| Prior CHF | 260/8815 (3.0%) | 49/595 (8.2%)* | 289/9150 (3.2%) |
20/260 (7.7%)† |
| Creatinine clearance, mL/min ≥60 30–59 <30 Missing |
7099/8815(80.5%) 1120/8815 (12.7%) 44/8815 (0.5%) 552/8815 (6.3%) |
379/595 (63.7%)* 151/595 (25.4%)* 20/595 (3.4%)* 45/595 (7.6%)* |
7308/9150 (79.9%) 1196/9150 (13.1%) 56/9150 (0.6%) 590/9150 (6.4%) |
170/260 (65.4%)† 75/260 (28.9%)† 8/260 (3.1%)† 7/260 (2.7%)† |
| Presentation Stable angina NSTEACS STEMI |
5073/8815 (57.6%) 3077/8815 (34.9%) 665/8815 (7.5%) |
317/595 (53.3%)* 218/595 (36.6%)* 60/595 (10.1%)* |
5252/9150 (57.4%) 3194/9150 (34.9%) 704/9150 (7.7%) |
138/260 (53.1%) 101/260 (38.9%) 21/260 (8.1%) |
| Stent C-SES E-ZES |
3934/8815 (44.6%) 4881/8815 (55.4%) |
301/595 (50.6%)* 294/595 (49.4%)* |
4126/9150 (45.1%) 5024/9150 (54.9%) |
109/260 (41.9%) 151/260 (58.1%) |
| Left main PCI | 103/8815 (1.2%) | 13/595 (2.2%)* | 110/9150 (1.2%) |
6/260 (2.3%) |
| Lesion length > 18 mm |
3823/8809 (43.4%) |
299/595 (50.3%)* |
4007/9150 (43.8%) |
115/259 (44.4%) |
| Stent length | 31.1 ± 20.6 | 35.4 ± 24.2* | 31.3 ± 20.8 | 32.1 ± 23.3 |
| Vessel diameter ≤2.75 mm |
3500/8808 (39.7%) |
278/595 (46.7%)* |
3677/9144 (40.2%) |
101/259 (39.0%) |
| Bifurcation | 1854/8814 (21.0%) |
140/595 (23.5%) |
1939/9150 (21.2%) |
55/259 (21.2%) |
| Multivessel PCI | 1648/8815 (18.7%) |
145/595 (24.4%)* |
1751/9150 (19.1%) |
42/260 (16.2%) |
| SVG PCI | 27/8815 (0.3%) | 7/595 (1.2%)* | 33/9150 (0.4%) | 1/260 (0.4%) |
| In-stent restenosis | 124/8815 (1.4%) | 6/595 (1.0%) | 124/9150 (1.4%) | 6/260 (2.3%) |
| Stent number 0 1 2 ≥3 |
84/8815 (0.9%) 5191/8815 (58.9%) 2270/8815 (25.8%) 1270/8815 (14.4%) |
9/595 (1.5%)* 286/595 (48.1%)* 185/595 (31.1%)* 115/595 (19.3%)* |
86/9150 (0.9%) 5333/9150 (58.3%) 2386/9150 (26.1%) 1345/9150 (14.7%) |
7/260 (2.7%)† 144/260 (55.4%)† 69/260 (26.5%)† 40/260 (15.4%)† |
p<0.05 for the comparison of no ischemic event vs. ischemic event
p<0.05 for the comparison of no bleeding event vs. bleeding event
BMI, body-mass index; MI, myocardial infarction; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; CABG, coronary artery bypass graft surgery; CHF, congestive heart failure; NSTEACS, non-ST elevation acute coronary syndrome; STEMI, ST elevation myocardial infarction; SVG, saphenous vein graft; C-SES, Cypher sirolimus eluting stent; E-ZES, Endeavor zotarolimus eluting stent.
The long-term event rate was 6.3% (N=595) for the composite ischemic outcome and 2.8% (N=260) for the bleeding outcome. Of these 75 patients (0.8%) had both an ischemic and a bleeding event. Temporal trends for both combined endpoints and their individual components are shown in Table 2 and Figure 1.
Table 2.
Clinical outcomes and temporal trends.
| Event Rate | ||||
|---|---|---|---|---|
| Event | Overall | 0–30 days | Month 1–12 | 12 month – 4 year |
| ISCHEMIC ENDPOINT | ||||
| Cardiovascular death, non-periprocedural myocardial infarction or definite/probable stent thrombosis | 1.64%/yr 0.14%/mo (6.51%)* |
10.76%/yr 0.90%/mo (0.89%)* |
1.42%/yr 0.12%/mo (1.29%)* |
1.44%/yr 0.12%/mo (4.40%)* |
| Cardiovascular death | 0.91%/yr 0.08%/mo (3.69%) |
5.24%/yr 0.44%/mo (0.44%)* |
0.82%/yr 0.07%/mo (0.75%)* |
0.88%/yr 0.07%/mo (2.72%)* |
| Non-periprocedural myocardial infarction | 0.87%/yr 0.07%/mo (3.52%)* |
5.24%/yr 0.44%/mo (0.44%)* |
1.10%/yr 0.09%/mo (1.00%)* |
0.69%/yr 0.06%/mo (2.11%)* |
| Definite/probable stent thrombosis | 0.51%/yr 0.04%/mo (2.06%)* |
|||
| Acute | 34.45%/yr 2.87%/mo (0.10%)* |
N/A | N/A | |
| Subacute | 8.47%/yr 0.71%/mo (0.68%)* |
N/A | N/A | |
| Late | N/A | 0.31%/yr 0.03%/mo (0.29%)* |
N/A | |
| Very late | N/A | N/A | 0.37%/yr 0.03%/mo (1.14%)* |
|
| BLEEDING ENDPOINT | ||||
| GUSTO Moderate/Severe bleeding | 0.75%/yr 0.06%/mo (3.03%)* |
8.19%/yr 0.68%/mo (0.68%)* |
1.08%/yr 0.09%/mo (0.98%)* |
0.44%/yr 0.04%/mo (1.36%)* |
| Moderate | 0.40%/yr 0.03%/mo (1.64%)* |
2.94%/yr 0.24%/mo (0.24%)* |
0.64%/yr 0.05%/mo (0.59%)* |
0.26%/yr 0.02%/mo (0.82%)* |
| Severe | 0.34%/yr 0.03%/mo (1.39%)* |
5.24%/yr 0.44%/mo (0.44%)* |
0.57%/yr 0.05%/mo (0.52%)* |
0.17%/yr 0.01%/mo (0.54%)* |
Absolute rate
Figure 1.
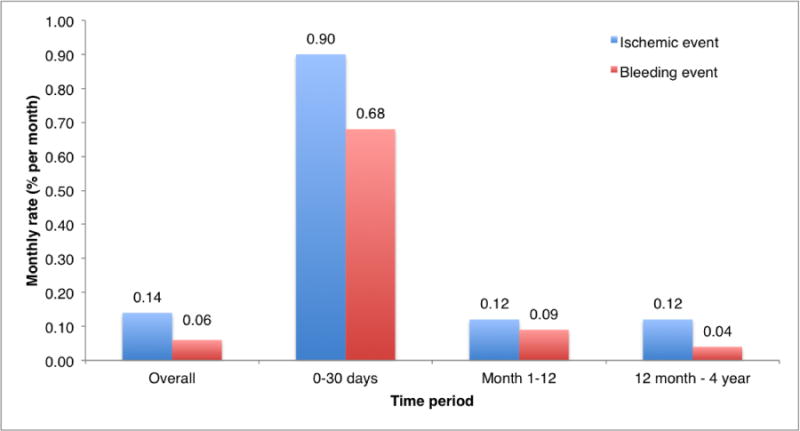
Monthly rate of the combined primary ischemic (cardiovascular death, non-periprocedural MI or definite/probable ST) and primary bleeding endpoint (GUSTO moderate/severe bleeding event) over specified time periods.
Temporal distribution of the ischemic and bleeding endpoints.
Multivariable predictors of long-term ischemic and bleeding complications are presented in Table 3. The strongest predictors of ischemic risk were diabetes, prior congestive heart failure, chronic kidney disease and diagnosis at presentation. All 5 independent predictor of bleeding events (age, smoking, diabtetes, prior congestive heart failure and chronic kidney disease) were also predictors in the final ischemic model. Both multivariable models had acceptable discriminative power with optimism-corrected c-statistic 0.68 for the ischemic model and 0.64 for the bleeding model. Model fit for both endpoints was good with Hosmer-Lemeshow p-values of 0.48 and 0.85 respectively.
Table 3.
Multivariable predictors for ischemic and bleeding endpoints
| ISCHEMIC ENDPOINT | BLEEDING ENDPOINT | |||
|---|---|---|---|---|
|
| ||||
| Optimism-corrected odds ratio | 95% confidence interval | Optimism-corrected odds ratio | 95% confidence interval | |
|
| ||||
| Age, per 10 years | 1.37 | 1.25 – 1.50 | 1.38 | 1.22 – 1.56 |
|
| ||||
| Male | 1.36 | 1.12 – 1.66 | – | – |
|
| ||||
| BMI, per kg/m2 | 1.03 | 1.01 – 1.04 | – | – |
|
| ||||
| Active smoking | 1.49 | 1.22 – 1.81 | 1.34 | 1.03 – 1.75 |
|
| ||||
| Diabetes mellitus | 1.77 | 1.50 – 2.09 | 1.24 | 1.00 – 1.55 |
|
| ||||
| Prior MI | 1.47 | 1.23 – 1.76 | – | – |
|
| ||||
| Prior CABG | 1.41 | 1.06 – 1.87 | – | – |
|
| ||||
| Prior CHF | 1.75 | 1.27 – 2.39 | 1.73 | 1.15 – 2.60 |
|
| ||||
| Creatinine clearance, mL/min | ||||
| ≥ 60 | Reference | Reference | Reference | Reference |
| 30–59 | 1.93 | 1.55 – 2.41 | 1.61 | 1.23 – 2.11 |
| < 30 | 5.41 | 3.14 – 9.32 | 2.79 | 1.43 – 5.41 |
| Missing | 1.61 | 1.19 – 2.18 | 0.56 | 0.30 – 1.07 |
|
| ||||
| Presentation | ||||
| Stable angina | Reference | Reference | – | – |
| NSTEACS | 1.14 | 0.96 – 1.35 | ||
| STEMI | 1.71 | 1.29 – 2.26 | ||
|
| ||||
| Zotarolimus-eluting stent | 0.80 | 0.68 – 0.93 | – | – |
|
| ||||
| Total stent length, per 10 mm | 1.07 | 1.03 – 1.10 | – | – |
BMI, body-mass index; MI, myocardial infarction; CABG, coronary artery bypass graft surgery; CHF, congestive heart failure; NSTEACS, non-ST elevation acute coronary syndrome; STEMI, ST elevation myocardial infarction
The relationship between predicted long-term probabilities of ischemic and bleeding outcomes within individual subjects is depicted in the Figure 2A. Only 3.1% of patients had a higher predicted bleeding risk than ischemic risk, and when this was the case, the absolute difference between both risks was small (largest absolute risk difference was 0.9%)[Figure 2B]. The predicted probabilities of ischemic and bleeding outcomes were strongly correlated (ρ=0.76). Patients in whom the bleeding risk was greater than the ischemic risk are compared with those with higher ischemic risk in Table 4. No subjects with prior MI, prior coronary artery bypass graft (CABG) surgery or STEMI presentation and very few with diabetes mellitus had a higher bleeding risk than ischemic risk. While female sex and chronic kidney disease were predictors of higher bleeding risk, 87.2% of females in the study had a higher ischemic risk than bleeding, and 97.8% of those with chronic kidney disease.
Figure 2.
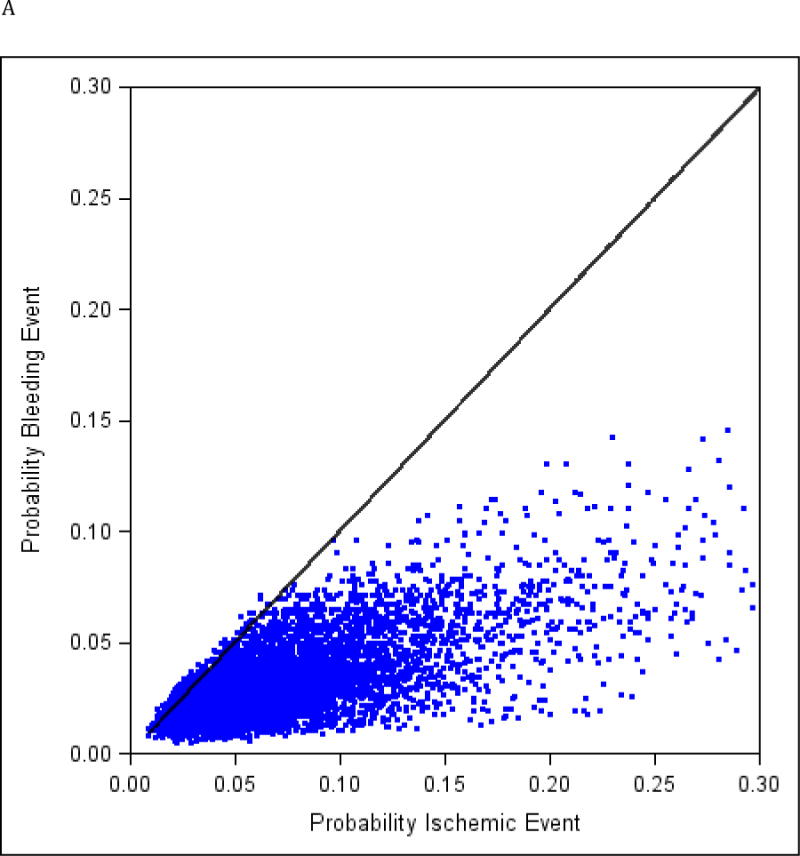
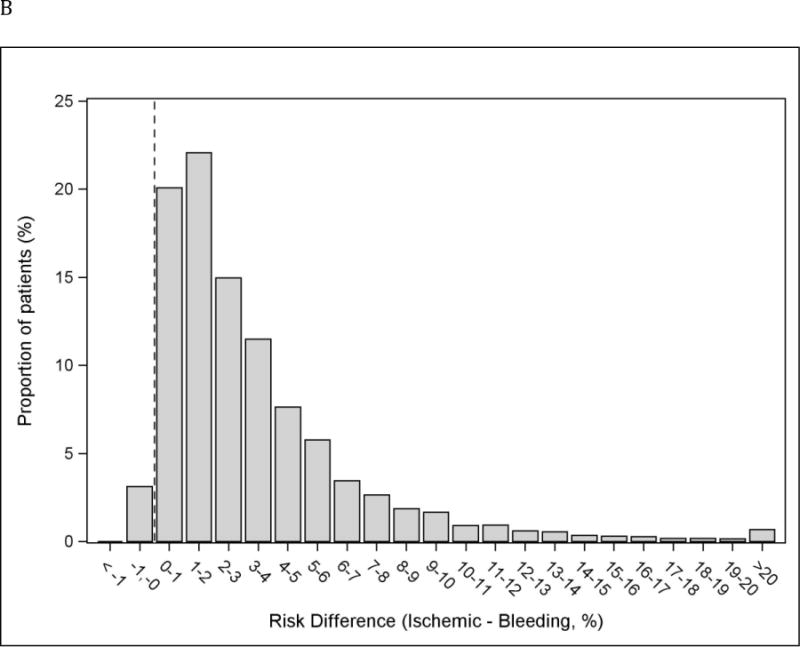
Individual predicted probabilities of long-term ischemic and bleeding events. Black line represents identical ischemic and bleeding risk within an individual subject. ρ=0.76; p < 0.001 (A). Distribution of the absolute difference in risk of ischemic and bleeding endpoints. Dotted vertical line represents identical ischemic and bleeding risk within an individual subject. Bars on the right side of the line correspond to subjects with greater ischemic risk than bleeding risk. 3.1% of subjects had ischemic risk exceeding bleeding risk. (B).
*Negative risk difference represents a higher bleeding risk than ischemic risk.
Table 4.
Characteristics of subgroup with greater bleeding than ischemic risk.
| Characetristics | Bleeding Risk >Ischemic Risk (n = 294) |
Ischemic Risk ≥ Bleeding Risk (n = 9112) |
|---|---|---|
|
| ||
| Age (years), mean±SD | 64.5±10.1 | 62.3±10.6 |
|
| ||
| BMI (kg/m2), mean±SD | 24.8±3.5 | 28.2±4.7 |
|
| ||
| Male | 2.4% | 78.5% |
|
| ||
| Diabetes | 0.3% | 29.0% |
|
| ||
| Smoking | 10.9% | 25.1% |
|
| ||
| Prior MI | 0% | 20.5% |
|
| ||
| Prior CABG | 0% | 5.5% |
|
| ||
| Prior CHF | 0.7% | 3.4% |
|
| ||
| Creatinine clearance | ||
| ≥ 60 mL/min | 85.7% | 79.3% |
| 30–59 mL/min | 14.3% | 13.5% |
| < 30 mL/min | 0% | 0.7% |
| Missing | 0% | 6.5% |
|
| ||
| Presentation | ||
| Stable | 79.6% | 56.6% |
| NSTEACS | 20.4% | 35.5% |
| STEMI | 0% | 7.9% |
| Total stent length (mm), mean±SD | 19.4±8.1 | 31.7±19.4 |
|
| ||
| Drug-eluting stent used | ||
|
| ||
| E-ZES | 91.2% | 53.8% |
BMI, body-mass index; MI, myocardial infarction; CABG, coronary artery bypass graft surgery; CHF, congestive heart failure; NSTEACS, non-ST elevation acute coronary syndrome; STEMI, ST elevation myocardial infarction, E-ZES, Endeavor zotarolimus eluting stent
Only 14.2% of the bleeding events occurred within 48 hours of the index PCI (37 subjects). Excluding these events from the primary analysis yielded similar results with all predictors of bleeding events remaining predictors of ischemic events. Using the TIMI major criteria as bleeding endpoint (206 events [2.2%]) also gave similar results regarding the primary analysis, the correlation between risks was 0.63, p<0.001, and the subjects at greater risk of bleeding than ischemic outcomes represented 1.6% of the sample.
Discussion
The current analysis performed on the data derived from two large prospective cohorts with broad inclusion criteria reflecting contemporary interventional cardiology practice, and with clinical event adjudication, showed that the predicted long-term risk of ischemic events (cardiovascular death/non-periprocedural MI/definite or probable ST) was greater than the risk of bleeding events (moderate/severe bleeding) in the large majority of patients. In the small subset of patients that are at greater bleeding than ischemic risk, the absolute risk difference was small. Each of the significant predictors of long-term bleeding events were also predictors of ischemic outcomes, and there was a strong correlation between individual predicted ischemic and bleeding events among patients.
Treatment strategies, such as prescription of dual antiplatelet therapy after DES, require clinicians to weigh the individual patient’s risk of bleeding and recurrent ischemic events based on his or her unique clinical presentation and characteristics. Furthermore, prior studies of adverse events after PCI have been limited to or strongly influenced by early periprocedural events, making conclusion regarding later risks challenging.21–25 Mehran and colleagues built a model to predict 30-day non-CABG-related TIMI major bleeding after PCI and they showed that early bleeding events were associated with worse prognosis at 1 year.26 Only a few studies reported long-term predictors (> 1 year) of ischemic events following PCI with DES implantation. The logistic clinical SYNTAX score was designed to predict mortality at one year by combining the components of the age, creatinine, and left ventricular ejection fraction (ACEF) score, initially developed for elective cardiac surgery, and the Syntax score in data from 7 coronary stent trials, and has been validated at 3 years.27–29 The New York database risk score was developed to predict mortality up to 5 years from patients treated in 2003.30 Data from the CathPCI Registry in 2004–2007 were linked to Center for Medicare and Medicaid Services, and therefore limited to subjects 65 and older, to provide median follow-up 15 months and predict survival.30 All of the above studies have included periprocedural events, which for both myocardial infarction and bleeding are high frequency compared with later events, yet not modified by late treatment choices of adjunctive therapy. Our rich dataset included prospectively collected site-reported angiographic and procedural characteristics and central adjudication of events allowed us to predict both the ischemic and bleeding risks over a 4-year period. Furthermore, by excluding periprocedural myocardial infarction and in sensitiviy analysis, periprocedural bleeding, we were able to focus on late risks which are impacted by post procedure treatment choices.
In our population of patients eligible for DES and subsequent dual antiplatelet therapy, the long-term rate of non-periprocedural ischemic cardiovascular endpoints was only 6.3% at 4 years. As reported in PROTECT, the sirolimus-eluting stent was associated with a higher rate of stent thrombosis than the zotarolimus-eluting stent and especially in patients not on dual antiplatelet therapy, suggesting that late ischemic events are at least partially stent related, and we adjusted for this effect in our models.19, 31 However, beyond the stented segment, global ischemic risk of recurrent myocardial infarction in other vascular territories and cardiac death attributable to plaque rupture represent another important target of long-term DAPT.32 Prolonging DAPT is known to be associated with an increase in bleeding events.12 Our study showed that the GUSTO moderate/severe bleeding risk beyond 1 year was 0.4% per year, 3–4 times smaller than late ischemic risk overall (1.4% per year) and that in individual subjects ischemic risk exceeded bleeding risk in only 3% of subjects. Because of the degree of overlap between ischemic and bleeding risk factors, these subjects were not easily distinguishable from the remaining patients, yet they were significantly more likely to be female or have chronic kidney disease, and less likely to have markers of chronic cardiovascular risk such diabetes, or prior myocardial infarction or revascularization.
Some limitations to this study need to be acknowledged. We compared the risk of moderate/severe bleeding to the composite risk of cardiovascular death/non-periprocedural MI/definite or probable ST. The clinical weight of each event may not be equivalent and this is not accounted for in our analyses. By being more restrictive for ischemic events, e.g. excluding smaller periprocedural events, the conclusion that the risk of ischemic complications is usually greater than the risk of bleeding events in our dataset is, therefore, conservative. Second, the use of moderate/severe GUSTO criteria to define bleeding events could lead to an underestimation of bleeding complications as these criteria require either an intracranial event, a hemodynamic compromise or the need for blood transfusion. However, these criteria represent meaningful clinical events on par with similarly important ischemic events. Using bleeding criteria that are more sensitive for clinically less important events could influence the conclusion regarding the balance between ischemic and bleeding risks. A possible limitation to the generalizability of our observations is that while the PROTECT Study sought to be broadly inclusive, it is possible that patients at very high risk of bleeding complications were excluded. Third, the only thienopyridine used in this study was clopidogrel. Newer oral P2Y12 antagonists such as ticagrelor and prasugrel are available for clinical use since the realization of this study and with their more potent antiplatelet effect; they could alter the balance between ischemic and bleeding risks. How varying duration of antiplatelet therapy would impact the relative balance between bleeding and ischemia is also unknown. Several large randomized studies will provide more insight on this important clinical dilemma.10, 11
In summary, for patients eligible to receive a DES, characteristics predicting long-term bleeding events were also associated with ischemic complications. In the large majority of patients, long term risks of ischemic events were greater than those of bleeding events, particularly beyond 12 months of follow up. While we identified a group of patients whose individual long-term bleeding risk was larger than ischemic risk, this group represented less than 5% of study subjects. These findings imply that to better understand individual patients risks, accounting for the combination of risk factors in each individual, and for the impact of therapies on both benefit and risk, further study with adequately-powered studies will be beneficial.
Footnotes
Disclosures
AM: None, RWY: Salary (Harvard Clinical Research Institute[HCRI]), Grant (HCRI), EC: none, PGS: Research Support (NYU School of Medicine, Sanofi-Aventis, Servier), Consulting fees (Ablynx, Amarin, Astellas, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo-Lilly, GlaxoSmithKline, Medtronic, MSD, Novartis, Pfizer, Roche, Sanofi-Aventis, Servier and The Medicines Company), Stockholding (Aterovax), WW: Grant (to institution: Medtronic, Cordis), Stockholding (Argonauts, Genae, Cardio3BioSciences), Consultant fees (to institution: Medtronic, Cordis, St Jude), JM: none:, AG: Speaker fees (Abbott Vascular, Boston Scientific, Eli Lilly, Daiichi Snkyo Inc., The Medicines Company, Boehringer Ingelheim, Medtronic), MdB: None, GD: Speaker fees (Astra Zeneca, Eli Lilly), Consultant fees (Astra Zeneca, Eli Lilly), LM: Grant Support (Abbott Vascular, Cordis, Boston Scientific, Medtronic, Eli Lilly, Daiichi Sankyo Inc., Bristol Myers Squibb, Sanofi Aventis), Consultant fees (Biotronik, St Jude, Medtronic).
References
- 1.Burke JF, Hayward RA, Nelson JP, Kent DM. Using internally developed risk models to assess heterogeneity in treatment effects in clinical trials. Circ Cardiovasc Qual Outcomes. 2014;7:163–9. doi: 10.1161/CIRCOUTCOMES.113.000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varadhan R, Segal JB, Boyd CM, Wu AW, Weiss CO. A framework for the analysis of heterogeneity of treatment effect in patient-centered outcomes research. J Clin Epidemiol. 2013;66:818–25. doi: 10.1016/j.jclinepi.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisenstein EL, Anstrom KJ, Kong DF, Shaw LK, Tuttle RH, Mark DB, Kramer JM, Harrington RA, Matchar DB, Kandzari DE, Peterson ED, Schulman KA, Califf RM. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. JAMA. 2007;297:159–68. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 4.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 5.Task Force on Myocardial Revascularization of the European Society of C, the European Association for Cardio-Thoracic S, European Association for Percutaneous Cardiovascular I. Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez-Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–55. [Google Scholar]
- 6.Park SJ, Park DW, Kim YH, Kang SJ, Lee SW, Lee CW, Han KH, Park SW, Yun SC, Lee SG, Rha SW, Seong IW, Jeong MH, Hur SH, Lee NH, Yoon J, Yang JY, Lee BK, Choi YJ, Chung WS, Lim DS, Cheong SS, Kim KS, Chae JK, Nah DY, Jeon DS, Seung KB, Jang JS, Park HS, Lee K. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med. 2010;362:1374–82. doi: 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- 7.Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, Han KR, Choi JH, Choi SH, Kang HJ, Koo BK, Ahn T, Yoon JH, Jeong MH, Hong TJ, Chung WY, Choi YJ, Hur SH, Kwon HM, Jeon DW, Kim BO, Park SH, Lee NH, Jeon HK, Jang Y, Kim HS. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125:505–13. doi: 10.1161/CIRCULATIONAHA.111.059022. [DOI] [PubMed] [Google Scholar]
- 8.Valgimigli M, Campo G, Monti M, Vranckx P, Percoco G, Tumscitz C, Castriota F, Colombo F, Tebaldi M, Fuca G, Kubbajeh M, Cangiano E, Minarelli M, Scalone A, Cavazza C, Frangione A, Borghesi M, Marchesini J, Parrinello G, Ferrari R, Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study I Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–26. doi: 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- 9.Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, King SB, 3rd, Negoita M, Liu M, de Paula JE, Mangione JA, Meireles GX, Castello HJ, Jr, Nicolela EL, Jr, Perin MA, Devito FS, Labrunie A, Salvadori D, Jr, Gusmao M, Staico R, Costa JR, Jr, de Castro JP, Abizaid AS, Bhatt DL, Investigators OT Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. 2013;310:2510–22. doi: 10.1001/jama.2013.282183. [DOI] [PubMed] [Google Scholar]
- 10.Byrne RA, Schulz S, Mehilli J, Iijima R, Massberg S, Neumann FJ, ten Berg JM, Schomig A, Kastrati A, Intracoronary S, Antithrombotic Regimen S and Investigators EFoSMDATAD-ES Rationale and design of a randomized, double-blind, placebo-controlled trial of 6 versus 12 months clopidogrel therapy after implantation of a drug-eluting stent: The Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) study. Am Heart J. 2009;157:620–4 e2. doi: 10.1016/j.ahj.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Mauri L, Kereiakes DJ, Normand SL, Wiviott SD, Cohen DJ, Holmes DR, Bangalore S, Cutlip DE, Pencina M, Massaro JM. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J. 2010;160:1035–41. 1041 e1. doi: 10.1016/j.ahj.2010.07.038. [DOI] [PubMed] [Google Scholar]
- 12.Pandit A, Giri S, Hakim FA, Fortuin FD. Shorter (</=6 months) versus longer (>/=12 months) duration dual antiplatelet therapy after drug eluting stents: A meta-analysis of randomized clinical trials. Catheter Cardiovasc Interv. 2014 doi: 10.1002/ccd.25520. [DOI] [PubMed] [Google Scholar]
- 13.Galper BZ, Mauri L. Antiplatelet therapy after coronary stenting. Curr Treat Options Cardiovasc Med. 2013;15:1–10. doi: 10.1007/s11936-012-0223-4. [DOI] [PubMed] [Google Scholar]
- 14.Writing Committee M. Jneid H, Anderson JL, Wright RS, Adams CD, Bridges CR, Casey DE, Jr, Ettinger SM, Fesmire FM, Ganiats TG, Lincoff AM, Peterson ED, Philippides GJ, Theroux P, Wenger NK, Zidar JP, Anderson JL, American College of Cardiology F and American Heart Association Task Force on Practice G 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/Non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2012;126:875–910. doi: 10.1161/CIR.0b013e318256f1e0. [DOI] [PubMed] [Google Scholar]
- 15.O’Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW, American College of Cardiology Foundation/American Heart Association Task Force on Practice G 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial I Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 17.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D, Guidelines ESCCfP ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 18.Camenzind E, Wijns W, Mauri L, Boersma E, Parikh K, Kurowski V, Gao R, Bode C, Greenwood JP, Gershlick A, O’Neill W, Serruys PW, Jorissen B, Steg PG, Committee PS and Investigators Rationale and design of the Patient Related OuTcomes with Endeavor versus Cypher stenting Trial (PROTECT): randomized controlled trial comparing the incidence of stent thrombosis and clinical events after sirolimus or zotarolimus drug-eluting stent implantation. Am Heart J. 2009;158:902–909 e5. doi: 10.1016/j.ahj.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Camenzind E, Wijns W, Mauri L, Kurowski V, Parikh K, Gao R, Bode C, Greenwood JP, Boersma E, Vranckx P, McFadden E, Serruys PW, O’Neil WW, Jorissen B, Van Leeuwen F, Steg PG, Committee PS and Investigators Stent thrombosis and major clinical events at 3 years after zotarolimus-eluting or sirolimus-eluting coronary stent implantation: a randomised, multicentre, open-label, controlled trial. Lancet. 2012;380:1396–405. doi: 10.1016/S0140-6736(12)61336-1. [DOI] [PubMed] [Google Scholar]
- 20.Steyerberg EW, Harrell FE, Jr, Borsboom GJ, Eijkemans MJ, Vergouwe Y, Habbema JD. Internal validation of predictive models: efficiency of some procedures for logistic regression analysis. J Clin Epidemiol. 2001;54:774–81. doi: 10.1016/s0895-4356(01)00341-9. [DOI] [PubMed] [Google Scholar]
- 21.Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, Mautner B, Corbalan R, Radley D, Braunwald E. The TIMI risk score for unstable angina/non-ST elevation MI: A method for prognostication and therapeutic decision making. JAMA. 2000;284:835–42. doi: 10.1001/jama.284.7.835. [DOI] [PubMed] [Google Scholar]
- 22.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA, Investigators G A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291:2727–33. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 23.Sianos G, Morel MA, Kappetein AP, Morice MC, Colombo A, Dawkins K, van den Brand M, Van Dyck N, Russell ME, Mohr FW, Serruys PW. The SYNTAX Score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–27. [PubMed] [Google Scholar]
- 24.Chen SL, Chen JP, Mintz G, Xu B, Kan J, Ye F, Zhang J, Sun X, Xu Y, Jiang Q, Zhang A, Stone GW. Comparison between the NERS (New Risk Stratification) score and the SYNTAX (Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery) score in outcome prediction for unprotected left main stenting. JACC Cardiovasc Interv. 2010;3:632–41. doi: 10.1016/j.jcin.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Garg S, Sarno G, Garcia-Garcia HM, Girasis C, Wykrzykowska J, Dawkins KD, Serruys PW, Investigators A-I A new tool for the risk stratification of patients with complex coronary artery disease: the Clinical SYNTAX Score. Circ Cardiovasc Interv. 2010;3:317–26. doi: 10.1161/CIRCINTERVENTIONS.109.914051. [DOI] [PubMed] [Google Scholar]
- 26.Mehran R, Pocock S, Nikolsky E, Dangas GD, Clayton T, Claessen BE, Caixeta A, Feit F, Manoukian SV, White H, Bertrand M, Ohman EM, Parise H, Lansky AJ, Lincoff AM, Stone GW. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC Cardiovasc Interv. 2011;4:654–64. doi: 10.1016/j.jcin.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Farooq V, Vergouwe Y, Raber L, Vranckx P, Garcia-Garcia H, Diletti R, Kappetein AP, Morel MA, de Vries T, Swart M, Valgimigli M, Dawkins KD, Windecker S, Steyerberg EW, Serruys PW. Combined anatomical and clinical factors for the long-term risk stratification of patients undergoing percutaneous coronary intervention: the Logistic Clinical SYNTAX score. Eur Heart J. 2012;33:3098–104. doi: 10.1093/eurheartj/ehs295. [DOI] [PubMed] [Google Scholar]
- 28.Ranucci M, Castelvecchio S, Menicanti L, Frigiola A, Pelissero G. Risk of assessing mortality risk in elective cardiac operations: age, creatinine, ejection fraction, and the law of parsimony. Circulation. 2009;119:3053–61. doi: 10.1161/CIRCULATIONAHA.108.842393. [DOI] [PubMed] [Google Scholar]
- 29.Iqbal J, Vergouwe Y, Bourantas CV, Klaveren DV, Zhang YJ, Campos CM, Garcia-Garcia HM, Morel MA, Valgimigli M, Windecker S, Steyerberg EW, Serruys PW. Predicting 3-Year Mortality After Percutaneous Coronary Intervention: Updated Logistic Clinical SYNTAX Score Based on Patient-Level Data From 7 Contemporary Stent Trials. JACC Cardiovasc Interv. 2014;7:464–70. doi: 10.1016/j.jcin.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Wu C, Camacho FT, King SB, 3rd, Walford G, Holmes DR, Jr, Stamato NJ, Berger PB, Sharma S, Curtis JP, Venditti FJ, Jacobs AK, Hannan EL. Risk stratification for long-term mortality after percutaneous coronary intervention. Circulation Cardiovascular interventions. 2014;7:80–7. doi: 10.1161/CIRCINTERVENTIONS.113.000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camenzind E, Boersma E, Wijns W, Mauri L, Rademaker-Havinga T, Ordoubadi FF, Suttorp MJ, Kurdi MA, Steg PG, on behalf of the PSC and Investigators Modifying effect of dual antiplatelet therapy on incidence of stent thrombosis according to implanted drug-eluting stent type. Eur Heart J. 2014 doi: 10.1093/eurheartj/ehu084. [DOI] [PubMed] [Google Scholar]
- 32.Bhatt DL, Flather MD, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Fabry-Ribaudo L, Hu T, Topol EJ, Fox KA, Investigators C Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–8. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]


