Abstract
Manganese Superoxide Dismutase (MnSOD) expression has been found to be low in human pancreatic ductal adenocarcinoma (PDAC). Previously, we have reported that microRNA-301a (miR-301a) was found being upregulated via nuclear factor-κB (NF-κB) feedback loop in human PDAC. In this study, we investigate whether the miR-301a expression level is associated with MnSOD expression in human PDAC. We established a xenograft PDAC mouse model using transfected PanC-1 cells (miR-301a antisense or scrambled control) to investigate tumor growth and the interaction between MnSOD and miR-301a. The animal study indicated that miR-301a antisense transfection could significantly decrease the growth rate of inoculated PDAC cells, and this decrease in tumor growth rate is associated with increased MnSOD expression. To evaluate the MnSOD-miR-301a correlation in human PDAC, we have analyzed a total of 60 PDAC specimens, along with 20 normal pancreatic tissue (NPT) specimens. Human specimens confirmed a significant decrease of MnSOD expression in PDAC specimens (0.88 ± 0.38) compared with NPT control (2.45 ± 0.76; P<0.05), while there was a significant increase in miR-301a levels in PDAC specimens (0.89 ± 0.28) compared with NPT control (0.25 ± 0.41; P<0.05). We conclude that MnSOD expression is negatively associated with miR-301a levels in PDAC tissues, and lower miR-301a levels are associated with increased MnSOD expression and inhibition of PDAC growth.
Keywords: Pancreatic cancer, Manganese Superoxide Dismutase, microRNA-301a, MnSOD, PDAC
Introduction
Pancreatic cancer is the fifth most deadly cancer in the United States, according to the Centers for Disease Control and Prevention (CDC).1 As reported in 2013 SEER report, the age-adjusted death rate for pancreatic cancer was 10.7% and the 5-year survival rate was 4%.2 Advancement in pancreatic cancer research with an objective to understand the potential mechanism is needed to improve patients’ life for this challenging malignancy. Pancreatic Ductal Adenocarcinoma (PDAC) is the most common and lethal cancer of pancreas among all subtypes.3 Chronic inflammation and oxidative stress have been suggested as a driving force in most cancers including PDAC, whereas the antioxidants such as Manganese Superoxide Dismutase (MnSOD) has been well reported as a protective player in PDAC carcinogenesis.4–6
MnSOD is an essential mitochondrial antioxidant enzyme required for the reactive oxygen species (ROS) mediated response in the mammalian cell.5 Antioxidant MnSOD is important for the protective role in oxidative stress by converting the superoxide radical to hydrogen peroxide in mitochondria.5 Increased ROS level is suggested as a mitogenic factor, and is capable of inducing cell proliferation.7 Decrease in MnSOD expression has been reported in human pancreatic cancer, and adenovirus mediated overexpression of MnSOD triggered decreased growth rates in rapidly growing PDAC cells.4 Ough et al.6 further confirmed that MnSOD overexpression could inhibit cell growth in human pancreatic carcinoma as a tumor suppressor in pancreatic cancer.5 Some studies have also reported the role of microRNAs in the dysregulation of MnSOD, which is found to be associated with tumor metastasis.8–10
MicroRNAs (miRNAs) are functional noncoding RNAs with 22–26 nucleotides in length, which negatively regulate target gene expression.11 MiRNAs are crucial regulators of gene expression in numerous biological contexts,11,12 and changes in miRNA levels have been reported during altered gene expressions, which lead to the development of cancers including pancreatic cancer.12–17 Aberrant microRNA-301a (miR-301a) expression has been reported in many types of cancers.18–22 In pancreatic cancer, specifically, increase in miR-301a expression has been found in expression profiling.23 Also, miR-301a directly targets Bim (Bcl-2 family pro-apoptotic protein) and downregulates its expression.24 In the previous study, we found that miR-301a activates nuclear factor-κB (NF-κB) by targeting NF-κB-repressing factor (Nkrf) in pancreatic cancer.25
Altogether, in human PDAC, MnSOD was considered as a tumor suppressor, whereas miR-301a could be considered as an oncogene.6,18,22,25 However, each of these two events was found to be independently associated with human PDAC, and a possible correlation between MnSOD and miR-301a has not been studied. In this study, we therefore investigated the correlation between MnSOD expression and miR-301a levels in human PDAC. We established an animal model by inoculated PanC-1 cells with stable silencing of miR-301a expression within the PDAC tumor. MnSOD expression and miR-301a levels were studied in the PDAC tumor tissues. We have also analyzed PDAC human tissue specimens for MnSOD expression and miR-301a levels. Our finding suggests a negative correlation between MnSOD expression and miR-301a levels in human PDAC.
Materials and methods
Cell culture
Human pancreatic cancer cell-line PanC-1 (CRL-1469) was obtained directly from American Type Cell Culture (ATCC, Manassas, VA, USA). ATCC authenticates human cell-lines in their collection using short tandem repeat analysis (STR profiling). All the experiments were performed within six months after receiving new PanC-1 cells from ATCC. All PanC-1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum, containing 1x antibiotic–antimycotic (Invitrogen), at 37 °C in a humidified atmosphere of 5% CO2.
Animals and materials
Nude BALB/C mice were purchased from Jackson laboratories (Bar Harbor, ME, USA). DAKO EnVision+ system kits (K4007, K4011) were purchased from Agilent technologies (Carpinteria, CA, USA). All animal studies were conducted in accordance with National Institutes of Health animal use guidelines, and a protocol approved by the University of Louisville’s Institutional Animal Care and Use Committee (IACUC).
Rabbit polyclonal anti-MnSOD (06-984) was purchased from Upstate antibodies (Millipore, Temecula, CA, USA). Mouse monoclonal anti-NF-κB (sc-71677) was purchased from Santa Cruz biotechnology (Dallas, TX, USA). Rabbit polyclonal anti-Nkrf (HPA001476) was purchased from Sigma Aldrich (St. Louis, MO, USA).
Stable transfection knockdown and xenograft study
We have used tough decoy RNA (TuD) with lentivirus delivery technology to generate stable knockdown of miR-301a in PanC-1 cells, as developed and tested in our earlier study.25 In summary, for stable miR-301a inhibition, PanC-1 cells were infected with lentiviruses carrying TuD:anti-miR-301a. Vector map and miR-301a antisense sequence is provided in the Supplementary Data. The vector pSIF contained green fluorescent protein (GFP) marker (Supplementary Figure S1), and GFP-positive PanC-1 cells were sorted by flow cytometry. Sorted GFP-positive PanC-1 cells were then cultured again. Exponentially growing cells (PanC-1 cells transfected with miR-301a antisense and control) were harvested, and injected subcutaneously (5.0 million cells per animal) into 6-week-old nude BALB/C mice (n = 5 per group, animals were randomly assigned to each group by a technician). Tumor bearing animals were maintained for 8 weeks and tumor size was monitored once a week for 8 weeks before killing, and Student’s t-test (two-tailed) was used to analyze the tumor volumes (TVs). TV was determined using the standard formula, TV = (L ×W2)/2. Tumors were harvested, weighed and frozen immediately (−80 °C).
Histopathology
At the end of experiment, the animals were euthanized and tumor tissues were isolated and weighted. A piece of tissue was taken from each lobe and fixed in 10% buffered formalin for 24 h and transferred to 80% ethanol. The formalin-fixed liver tissue was processed and embedded in paraffin. Serial 5-μm sections were mounted onto glass slides. These slides were used for different staining purposes. Hematoxylin- and eosin-stained slides were obtained for each animal. All pathology findings for the specimens were scored by two pathologists who were both blinded to the subjects. The gradation was scored from 0 to 3 according to the intensity of staining (0, negative; 1, weak; 2, moderate; 3, strong). We analyzed the data using Student’s t-test (two-tailed) and mean values were accompanied with s.d. Sigmaplot statistical software (San Jose, CA, USA) is used for calculation and analysis.
Human specimens
To evaluate MnSOD and miR-301a expression in human, a total of 60 PDAC specimens, along with 20 normal pancreatic tissue (NPT) specimens, were obtained from cancer patients in the UofL clinics (IRB-approved protocol) for immunohistochemistry (IHC) and in situ hybridization (ISH) studies. All pathology findings of the specimens were scored by two pathologists who were blinded to the subjects. The gradation was scored from 0 to 3 according to the intensity of staining (0, negative; 1, weak; 2, moderate; 3, strong). We analyzed data using Student’s t-test (two-tailed). Box/whisker plot is used for representing data including outliers. Sigmaplot statistical software is used for calculation and data analysis.
Immunohistochemistry assay (IHC)
Immunohistochemical staining was carried out on the paraffin-embedded material using the DAKO EnVision+ System Kits. In brief, the sections were deparaffinized and hydrated. The slides were washed with a TRIS buffer, and peroxidase blocking was performed for 5 min. After rewashing, the anti-MnSOD (1:100), anti-Nkrf (1:100) or anti-NF-κB (1:100) was applied for 30 min. The slides were rinsed, and the specimens were incubated with labeled polymer for 30 min at room temperature. The substrate chromogen solution (diaminobenzidine) was added as a visualization reagent. Finally, the slides were counterstained with methyl green. A negative control was included in each run. The digital images of anti-MnSOD, anti-Nkrf or anti-NF-κB staining was acquired with the microscope at × 20 magnification using the spot camera via the MetaMorph imaging system (Universal Imaging Corporation, Downingtown, PA, USA) and stored as JPEG data files (the resolutions were fixed as 200 pixels per inch).
In situ hybridization (ISH)
ISH was performed using antisense oligonucleotide probes for miR-301a (Exiqon, Woburn, MA, USA), with scrambled-miR (5′-GTGTAACACGTCTATACGCCCA-3′) serving as a negative control. After the sections were deparaffinized, hydrated and deproteinated, prehybridization was performed in hybridization buffer for 2 h in a humidified chamber at 55 °C. Hybridization was then performed by applying 20 nM of probe in hybridization buffer to the slides covered with nescofilm overnight at 55 °C in a humidified chamber. Hybridized probes were detected by incubation with anti-digoxigenin–alkaline phosphatase conjugate at 37 °C for 30 min, followed by substrate 3,3′-diaminobenzidine to develop a brown color. Finally, the cells were counterstained with methyl green for 3–5 min and mounted on slides.
Results
Previously, we have shown specifically increased miR-301a level in PDAC, and possible NF-κB mediated tumor growth mechanism.25 Like other miRNAs, miR-301a may have multiple mechanisms contributing to the tumor growth in PDAC. Here, we studied the correlation between MnSOD and miR-301a in PDAC.
SOD2 (MnSOD gene) is predicted target of miR-301a
By using bioinformatics prediction search (www.targetscan.org), we have found that miR-301a targets 3′-UTR of longest transcript variant of SOD2 mRNA [GenBank: NM_000636.2]. Although there is no published study confirming this relationship with biochemical assays, these in-silico analysis results serve as a possible mechanism to support our hypothesis that MnSOD expression is associated with miR-301a level in PDAC (Figure 1).
Figure 1. Bioinformatics — in silico analysis.
A snapshot of search result, suggesting that miR-301a is a 7-mer-8 match at the 3′-untranslated region (3′-UTR) of SOD2 (manganese superoxide dismutase (MnSOD) gene), and may be involved in SOD2 regulation by direct targeting.
miR-301a knockdown is associated with decreased tumor growth rate in Xenograft PDAC mouse model
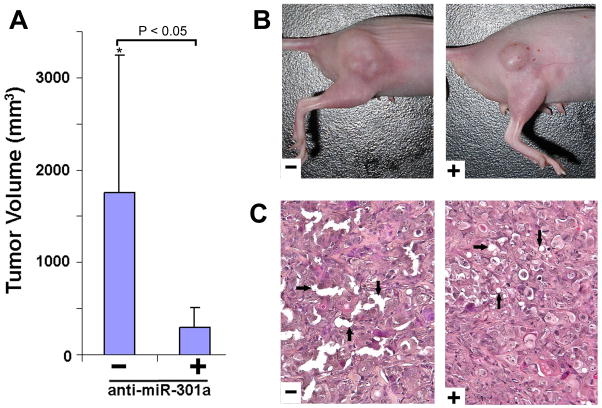
We used PanC-1 cells transfected with anti-miR-301a (anti-miRNA) or scrambled miRNA (negative control) to generate stable knockdown using tough decoy RNA (TuD) lentivirus vector system, as established in the earlier study (Supplementary Figure S1).25 This vector also contains GFP marker to identify successful transfection, and facilitate cell sorting. Following transfection confirmation and GFP positive cell sorting, transfected PanC-1 cells were inoculated subcutaneously in BALB/C nude mice for the formation of PDAC in the right hindlimb. Tumor growth was monitored and tumor size was recorded by calculation of the TV in tumor bearing animals for 8 weeks. We found that the mouse transfected with TuD:anti-miR-301a developed smaller tumors, and TV was significantly lower compared with the TuD-negative control (P<0.05, n = 5) (Figure 2A and B). Hematoxylin and eosin staining (H&E) performed in the tumor tissue xenografts showed that anti-miR-301a-containing tumor (+) had less dilated pancreatic ducts and less intraductal mucin, indicating reduced malignancy, thus confirming the difference in the histological changes between anti-miR-301a-containing tumor (+) and scramble miRNA containing tumor (−) (Figure 2C).
Figure 2. PDAC mouse model: evaluation of tumor volume and pathology in a xenograft.
(A) Comparison of tumor volume in control and miR-301a-knockdown groups. Tumor volume in mir-301a-knockdown group was significantly lower compared with tumor volume in the control animal group (*P<0.05, n=5 animals in each group, error bar=S.D.). (B) The representative xenografted tumor in the control group (−) and miR-301a-knockdown group (+). (C) Representative histology of the xenografted tumor in the control (−) group and miR-301a-knockdown group (+). Anti-miR-301a-containing tumor (+) had less dilated pancreatic ducts and less intraductal mucin, indicating that the malignancy of these tumors was reduced (black arrows).
Decreased MnSOD expression is associated with miR-301a knockdown in Xenograft PDAC mouse model
As discussed, SOD2 (MnSOD gene) is a predicted target of miR-301a (Figure 1). Studies have shown decreased MnSOD expression in PDAC. MiR-301a was found to be upregulated in PDAC.23 We hypothesized that miR-301a knockdown could affect MnSOD expression in PDAC. To test this hypothesis, MnSOD protein levels were determined in the tumor tissues of miR-301a knockdown (antisense) as well as the scrambled miRNA control by IHC staining. We found a significant increase in MnSOD expression in the miR-301a-knockdown group compared with the scrambled control (P<0.05, n=5) (Figure 3A and B). Because miR-301a downregulates Nkrf and elevates NF-κB activation,25 we further studied the NF-κB and Nkrf expressions. Our results indicated that in the miR-301a-knockdown group, Nkrf expression was significantly upregulated and NF-κB expression was significantly downregulated, compared with the scrambled controls (Figure 3A and B).
Figure 3. Pancreatic ductal adenocarcinoma (PDAC) mouse model: manganese superoxide dismutase (MnSOD) expression is increased by miR-301a knockdown (Anti-miRNA) in a xenograft mouse model.
(A) representative slides of immunohistochemistry (IHC) staining of MnSOD, nuclear factor-κB (NF-κB) and NF-κB-repressing factor (Nkrf) in tumor sections from PanC-1 mouse xenografts with scrambled control or TuD:anti-miR-301a (Anti-miRNA). Magnification, × 20. (B) Expression levels of MnSOD, NF-κB and Nkrf in scrambled control (control) and anti-miR-301a (Anti-miRNA) groups. Scoring scale was 0–3 according to the intensity of staining (0, negative; 1, weak; 2, moderate; 3, strong). MnSOD expression was found significantly higher in the miR-301a-knockdown group (Anti-miRNA) (*P<0.05). Changes in NF-κB (indirect target of miR-301a) and Nkrf (direct target of miR-301a) expressions in corresponding groups confirm effective knockdown of miR-301a. Error bar indicate S.D., n=5 animals per group (L, low level; H, high level).
Increased miR-301a expression is associated with decreased MnSOD expression in human PDAC specimens
Our xenograft PDAC mouse model data suggested that miR-301a silencing could be able to contribute to the increased MnSOD level and decreased PDAC tumor growth rate. Confident with the in-vivo data in animal model, we further studied the correlation in human samples. A total of 60 PDAC specimens, along with 20 normal pancreatic tissues (NPT) specimens were analyzed for MnSOD, NF-κB, and Nkrf levels by IHC, and miR-301a levels by ISH. We chose specifically Nkrf and NF-κB because we have already shown in our previous study that Nkrf is a direct target of miR-301a, and miR-301a up-regulation increases NF-κB levels by decreasing Nkrf, a validated repressor of NF-κB, in PDAC.
We found a significant decrease of MnSOD expression in PDAC specimens (0.88±0.38 (mean±S.D.)) compared with NPT controls (2.45±0.76; P= 1e−8) (Figure 4B and C). As expected, miR-301a expression was significantly increased in PDAC specimens (0.89±0.28) compared with NPT controls (0.25±0.41; P=8.9e−7) (Figure 4B and C). An increase in miR-301a expression in the PDAC group showed consensus with its downstream targets, Nkrf and NF-κB. Decreased expression of Nkrf (1.81±0.59) but increased NF-κB expression (2.55±0.86) was found in human PDAC specimens (Figure 4C) compared with NPT control (Nkrf: 2.85±0.37; P=1.24e−12) (NF-κB: 1.55±0.76; P=1.86e−05). These human specimens data were consistent with the data from the xenograft mouse model.
Figure 4. In human pancreatic ductal adenocarcinoma (PDAC), low manganese superoxide dismutase (MnSOD) expression is associated with higher miR-301 levels.
(A) Hematoxylin and eosin (HE) staining representative histology. (B) Representative slides of immunohistochemistry (IHC) staining of MnSOD, nuclear factor-κB (NF-κB), NF-κB-repressing factor (Nkrf) and in situ hybridization (ISH) probing of miR-301a in PDAC and normal pancreatic tissue (NPT) tissues. Images were captured at × 20 magnification. A scrambled probe for miR-301a was used as a negative control that did not stain any tissue sections (image not shown). (C) Expression levels of MnSOD, NF-κB, Nkrf, and miR-301a were scored in PDAC (n=60) and NPT (n=20). The gradation was scored from 0 to 3 according to the intensity of staining (0, negative; 1, weak; 2, moderate; 3, strong). Whisker box plot with boxes indicate 25th and 75th percentile; thick lines, the mean values; whisker caps, 10th and 90th percentile; filled circle, outliers. P-values of two-tailed Student’s t-test are provided. We found significant decreases in MnSOD expression in PDAC specimens (0.88±0.38 (mean±s.d.)) compared with NPT control specimens (2.45±0.76; P=1e−8). MiR-301a expression was significantly increased in PDAC specimens (0.89±0.28) compared with NPT control specimens (0.25±0.41; P=8.9e−7). In PDAC specimens, Nkrf (direct target of miR-301a) was found to be decreased, and NF-κB expression was subsequently increased compared with NPT specimens.
Discussion
In this study, we found that MnSOD expression is negatively associated with miR-301a expression, an important regulator miRNA in pancreatic cancer. Bioinformatics search suggests that miR-301a may directly target 3′ UTR of longest SOD2 transcript variant (MnSOD coding gene). Our data suggest that silencing miR-301a can significantly increase MnSOD expression, while decrease PDAC tumor growth rate in a PDAC mouse model. Our finding was further confirmed by analyzing known downstream targets of miR-301a i.e. Nkrf and NF-κB expressions in the tumor tissues. The human specimen analysis corroborated our finding in a PDAC mouse model that miR-301a levels and MnSOD expression are negatively associated.
PDAC is a severe malignancy, however the detailed mechanism is largely unknown, especially at the molecular level. Our results indicate that MnSOD down-regulation and miR-301a up-regulation are associated with greater PDAC tumor growth. In the current study, we have shown for the first time that possible direct relationship between MnSOD and miR-301a in context of regulation of PDAC growth. MicroRNA mediated regulation of MnSOD expression has been identified in a few studies and is not a new mechanism or concept.8–10 Also, recent studies in other cancers support emerging role of miR-301a in carcinogenesis, i.e. tumor proliferation and invasion,26 inflammation,27 and metastasis. 19 In Hepatocellular Carcinoma (HCC) miR-301a has been suggested as oncogene.22 Yet, no study has yet confirmed the role of miR-301a in tumor initiation.
In the previous study, we have shown that miR-301a targets Nkrf and activates NF-κB activity in pancreatic cancer.25 Investigators reported a direct relationship between NF-κB and MnSOD in normal and cancer tissues, and shown either can activate the second in different physiological conditions.5 Oncogenic or tumor suppressor function of MnSOD is still an active debate in the scientific community because of the fact that MnSOD can interact with multiple transcription factors including NF-κB.5 Our study, which suggests that miR-301a can affect MnSOD level, added one more brick in the available knowledgebase regarding MnSOD mediated regulations. Very importantly, there could be two possibilities – either, (1) miR-301a directly down-regulate MnSOD activity by acting on 3′-UTR of longest SOD2 transcript, or (2) miR-301a could be associated with MnSOD through NF-κB mediated route. Undoubtedly, further investigation in this direction is required.
To summarize, we have shown that in human PDAC, low MnSOD levels and high miR-301a expression are associated with the PDAC tumor state. Our animal model confirmed that silencing miR-301a could restore/increase MnSOD level in pancreatic tissue which may be a reason for the decrease in tumor growth. MnSOD is an important enzyme for oxidative stress management within cancer cells, and lower levels of MnSOD have been shown in PDAC.5, 6 In conclusion, our study suggests that the protective function of tumor-suppressor MnSOD in PDAC may be under the regulation of miR-301a and demands further investigation to understand this relationship at the molecular level.
Supplementary Material
Supplementary figure S1 (Vector construction map, and miR-301a antisense sequence)
Acknowledgments
Funding:
This project is supported by The Clinical and Translational Science Pilot Grant Program’s Basic Award and Innovative Award at The University of Louisville, and partly by Award Number R03CA137801 from the National Cancer Institute.
Footnotes
Competing interest statement: The authors declare that they have no conflict of interest.
References
- 1.US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2012 Incidence and Mortality Web-based Report. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; Atlanta: 2015. Available at: www.cdc.gov/uscs. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse S, et al., editors. [Last date accessed 5 July 2015];SEER Cancer Statistics Review, 1975–2010. 2013 Available at: http://seer.cancer.gov/csr/1975_2010/
- 3.Maitra A, Hruban RH. Pancreatic cancer. Ann Rev Pathol: Mechan Dis. 3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cullen JJ, Weydert C, Hinkhouse MM, Ritchie J, Domann FE, Spitz D, et al. The role of manganese superoxide dismutase in the growth of pancreatic adenocarcinoma. Cancer research. 2003;63(6):1297–303. [PubMed] [Google Scholar]
- 5.Holley AK, Dhar SK, Xu Y, St Clair DK. Manganese superoxide dismutase: beyond life and death. Amino acids. 2012;42(1):139–58. doi: 10.1007/s00726-010-0600-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ough M, Lewis A, Zhang Y, Hinkhouse MM, Ritchie JM, Oberley LW, et al. Inhibition of Cell Growth by Overexpression of Manganese Superoxide Dismutase (MnSOD) in Human Pancreatic Carcinoma. Free Radical Research. 2004;38(11):1223–1233. doi: 10.1080/10715760400017376. [DOI] [PubMed] [Google Scholar]
- 7.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, et al. Mitogenic signaling mediated by oxidants in Ras-transformed fibroblasts. Science. 1997;275(5306):1649–52. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 8.Meng X, Wu J, Pan C, Wang H, Ying X, Zhou Y, et al. Genetic and epigenetic down-regulation of microRNA-212 promotes colorectal tumor metastasis via dysregulation of MnSOD. Gastroenterology. 2013;145(2):426–36. e1–6. doi: 10.1053/j.gastro.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Fang F, Zhang J, Josson S, St Clair WH, St Clair DK. miR-17* suppresses tumorigenicity of prostate cancer by inhibiting mitochondrial antioxidant enzymes. PloS one. 2010;5(12):e14356. doi: 10.1371/journal.pone.0014356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji G, Lv K, Chen H, Wang T, Wang Y, Zhao D, et al. MiR-146a regulates SOD2 expression in H2O2 stimulated PC12 cells. PloS one. 2013;8(7):e69351. doi: 10.1371/journal.pone.0069351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nature reviews Genetics. 2004;5(7):522–31. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 12.Bushati N, Cohen SM. microRNA functions. Annual review of cell and developmental biology. 2007;23:175–205. doi: 10.1146/annurev.cellbio.23.090506.123406. [DOI] [PubMed] [Google Scholar]
- 13.Liu N, Olson EN. MicroRNA regulatory networks in cardiovascular development. Developmental cell. 2010;18(4):510–25. doi: 10.1016/j.devcel.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Developmental cell. 2006;11(4):441–50. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Qin S, Zhang C. MicroRNAs in vascular disease. Journal of cardiovascular pharmacology. 2011;57(1):8–12. doi: 10.1097/FJC.0b013e318203759b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lynam-Lennon N, Maher SG, Reynolds JV. The roles of microRNA in cancer and apoptosis. Biological reviews of the Cambridge Philosophical Society. 2009;84(1):55–71. doi: 10.1111/j.1469-185X.2008.00061.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Jamaluddin MS, Weakley SM, Yao Q, Chen C. Roles and mechanisms of microRNAs in pancreatic cancer. World journal of surgery. 2011;35(8):1725–31. doi: 10.1007/s00268-010-0952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Liu Y, Wang L, Chen R, Ge W, Lin Z, et al. Down-regulation of miR-301a suppresses pro-inflammatory cytokines in TLR-triggered macrophages. Immunology. 2013 doi: 10.1111/imm.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma F, Zhang J, Zhong L, Wang L, Liu Y, Wang Y, et al. Upregulated microRNA-301a in breast cancer promotes tumor metastasis by targeting PTEN and activating Wnt/beta-catenin signaling. Gene. 2014;535(2):191–7. doi: 10.1016/j.gene.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Li C, Yu B, Su L, Li J, Ju J, et al. Overexpressed miR-301a promotes cell proliferation and invasion by targeting RUNX3 in gastric cancer. Journal of gastroenterology. 2013 doi: 10.1007/s00535-012-0733-6. [DOI] [PubMed] [Google Scholar]
- 21.Xu XD, He XJ, Tao HQ, Zhang W, Wang YY, Ye ZY, et al. Abnormal expression of miR-301a in gastric cancer associated with progression and poor prognosis. Journal of surgical oncology. 2013;108(3):197–202. doi: 10.1002/jso.23374. [DOI] [PubMed] [Google Scholar]
- 22.Zhou P, Jiang W, Wu L, Chang R, Wu K, Wang Z. miR-301a is a candidate oncogene that targets the homeobox gene Gax in human hepatocellular carcinoma. Digestive diseases and sciences. 2012;57(5):1171–80. doi: 10.1007/s10620-012-2099-2. [DOI] [PubMed] [Google Scholar]
- 23.Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, et al. Expression profiling identifies microRNA signature in pancreatic cancer. International journal of cancer Journal international du cancer. 2007;120(5):1046–54. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Chen LY, Dai HY, Wang P, Gao S, Wang K. miR-301a promotes pancreatic cancer cell proliferation by directly inhibiting Bim expression. Journal of cellular biochemistry. 2012;113(10):3229–35. doi: 10.1002/jcb.24200. [DOI] [PubMed] [Google Scholar]
- 25.Lu Z, Li Y, Takwi A, Li B, Zhang J, Conklin DJ, et al. miR-301a as an NF-kappaB activator in pancreatic cancer cells. The EMBO journal. 2011;30(1):57–67. doi: 10.1038/emboj.2010.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Li C, Yu B, Su L, Li J, Ju J, et al. Overexpressed miR-301a promotes cell proliferation and invasion by targeting RUNX3 in gastric cancer. Journal of gastroenterology. 2013;48(9):1023–1033. doi: 10.1007/s00535-012-0733-6. [DOI] [PubMed] [Google Scholar]
- 27.Huang L, Liu Y, Wang L, Chen R, Ge W, Lin Z, et al. Down-regulation of miR-301a suppresses pro-inflammatory cytokines in Toll-like receptor-triggered macrophages. Immunology. 2013;140(3):314–322. doi: 10.1111/imm.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure S1 (Vector construction map, and miR-301a antisense sequence)