Abstract
Context:
The prognosis of combined septoplasty and endoscopic dacryocystorhinostomy (En-DCR) for moderate nasal septum deviation (NSD) has not yet been fully investigated.
Purpose:
To evaluate whether septoplasty improves the prognosis of En-DCR for moderate NSD.
Settings and Design:
A retrospective cohort study in a real-world clinical setting.
Methods:
The postoperative FICI DCR ostium grading scores and functional and anatomical information at 1, 2, 3, and 6 months were determined for consecutive patients with chronic dacryocystitis (CD) and moderate NSD who underwent En-DCR.
Statistical Analysis Used:
Univariate and generalized estimating equation multivariate analyses were used to compare the outcomes of the septoplasty and non-septoplasty groups.
Results:
En-DCR and septoplasty were concurrently performed for 32 (20.1%, 32/158) cases. The total FICI DCR ostial scores for the septoplasty and non-septoplasty groups were highest at the first (4.97 ± 0.177 vs. 4.97 ± 0.176, P > 0.05) and lowest at the sixth (4.41 ± 1.341 vs. 4.50 ± 1.355, P > 0.05) postoperative months. At the end of follow-up, the two groups showed comparable proportions of patients requiring definitive intervention for the ostium (6.3% vs. 7.1%, P > 0.05), comparabe functional success rates (87.5% vs. 90.5%, P > 0.05) and anatomical success rates (93.8% vs. 92.9%, P > 0.05). Only the non-septoplasty group experienced nasal mucosal adhesions (3.2%, 4/126).
Conclusions:
In patients with CD and moderate NSD, nasal septoplasty did not impact En-DCR prognosis, but reduced the complications. Skilled surgeons should reconsider septoplasty in the absence of otolaryngological indications.
Keywords: Chronic dacryocystitis, endoscopic dacryocystorhinostomy, nasal septum deviation, nasolacrimal duct obstruction, septoplasty
Chronic dacryocystitis (CD) is associated with nasolacrimal duct obstruction (NLDO) and stenosis.[1,2] Nasal endoscopic dacryocystorhinostomy (En-DCR) is safe and successful in addressing obstruction and restoring tear flow.[3,4,5] Unlike traditional external DCR (gold standard),[6] En-DCR does not require facial incisions and prevents damage to the pump mechanism. In addition, it allows the repair of intranasal disorders, such as enlarged middle turbinate or nasal septal deviation (NSD), during surgery.[7]
En-DCR failure is linked to NSD.[8] Patients with NLDO and ipsilateral NSD often lack a sufficiently wide En-DCR surgical corridor. Thus, a simultaneous endoscopic septoplasty can provide a clearer and more accessible surgical approach to the lacrimal sac.[9] However, recent studies have reported that performing septoplasty concurrently with En-DCR yields surgical success rates similar to those of En-DCR alone.[10] For patients with asymptomatic NSD, additional septoplasty does not offer long-term relief from nasal obstruction symptoms.[11] Hence, the severity of NSD should be considered when deciding on concomitant septoplasty. It is most beneficial for patients with severe and symptomatic NSD to prevent En-DCR surgical failure, potentially alleviating nasal obstruction.
In moderate NSD associated with CD, assessing the necessity of septoplasty is complex owing to asymptomatic or tolerable nasal obstruction. In addition, determining whether additional septoplasty improves En-DCR prognosis is challenging. Comparative studies on combined septoplasty and En-DCR versus En-DCR alone for this condition are lacking. Therefore, this study aimed to determine whether septoplasty improves the prognosis of En-DCR for moderate NSD.
Methods
Participants and study design
This retrospective cohort study was conducted in the Ophthalmology Department of Henan Provincial People’s Hospital. We evaluated consecutive patients with CD and moderate NSD who underwent En-DCR performed by the lacrimal and orbit teams from June 2018 to March 2022. The inclusion criteria were as follows: (a) moderate NSD located near the middle turbinate axilla that partially obstructed the endoscopic view for DCR; (b) any unilateral septoplasty performed on the same side as En-DCR; and (c) records of comprehensive clinical examination with consistent follow-up. Patients with a history of external DCR surgery, dacryocyst or paranasal sinus cancer, neurogenic lacrimal hypersecretory disorders, or severe nasal bony deformities were excluded. After chart review, 158 individuals were included and further classified into two groups: En-DCR with septoplasty group (32 patients) and En-DCR alone or “non-septoplasty” group (126 patients). The conceptual framework and design of this study are illustrated in Fig. 1.
Figure 1.
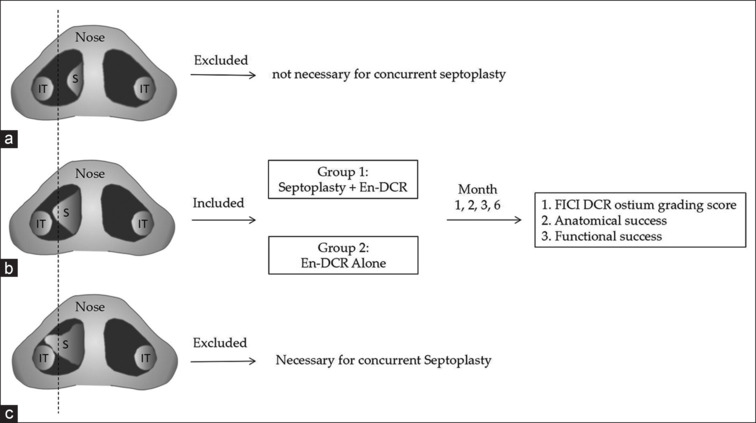
The study concept ion and design diagram. (a) Mild NSD: deviated septum affecting < 50% of the nasal passage. (b) Moderate NSD: deviated septum affecting >50% of the nasal passage. (c) Severe NSD: deviated septum touching the lateral nasal wall. IT = inferior turbinate, NSD = nasal septal deviation, S = septal deviation
All the participants provided informed consent. They were provided explanations about En-DCR, as well as the benefits and potential risks associated with additional septoplasty. Subsequently, everyone was allowed to decide whether to undergo concurrent septoplasty. This study adhered to the principles of the Declaration of Helsinki and was approved by the Henan Eye Hospital Scientific Research Commission.
Detection of moderate NSD
Anterior rhinoscopy and 0° rigid nasal endoscopic examination (STORZ, Tuttlingen, Germany) were performed to locate and assess NSD. The Nasal NSD Evaluation System,[12] which uses a simplified 0–3 scale, was used to determine the severity of NSD. A score of 2, indicative of moderate NSD, was characterized by a septum affecting more than 50% of the nasal passage without contacting the lateral nasal wall [Fig. 2a].
Figure 2.
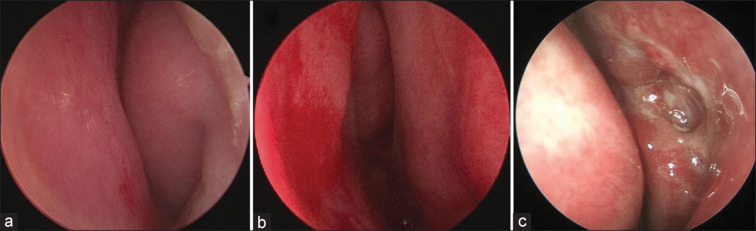
Endoscopic views of the nasal cavity and En-DCR ostium. (a) Preoperative view of moderate NSD to the left. (b) Septoplasty finished: preoperative view of moderate NSD to the left. The nasal cavity is enlarged. (c) En-DCR without septoplasty: the surgical anastomosis healed well, and no nasal mucosal adhesion occurred at the end of visit. En-DCR = endoscopic dacryocystorhinostomy, NSD = nasal septal deviation
Clinical examination and outcomes
Routine nasal endoscopic examination and lacrimal duct computed tomography (CT) angiography were performed for all patients. Standard nasolacrimal lavage was used to identify the patent nasolacrimal passages. Functional rhinostomy was performed using a fluorescein endoscopic dye test (FEDT). A positive FEDT result was indicated by a spontaneous drop of fluorescein across the conjunctival fornix from the rhinostomy side. Rigid nasoendoscopy was used to examine DCR ostium characteristics such as membranes or granulomas over the internal common opening (ICO).
The primary outcome was surgical success, as assessed using DCR ostium score (0–5) and grade (poor, good, and excellent). According to the FICI DCR ostium grading score system,[13] DCR ostium score is the total score for the four ostial parameters that influence the anatomical and functional outcomes of DCR surgery. The first parameter is the FEDT score (range 0–2); the scores were 2 for spontaneous FEDT, 1 for non-spontaneous but positive with irrigation, and 0 for negative and non-patency with irrigation. The other three parameters were ICO dynamicity, cicatricial ostium closure, and ICO threat. A score of 1 was assigned when ICO dynamicity was favorable, there was no cicatricial ostium closure, and no ICO threat was present. Otherwise, a score of 0 was assigned. Thus, the total FICI DCR ostium scores ranged from 0 to 5. The FICI grades were classified as excellent (score of 5, no intervention needed), good (score of 3–4, minimal intervention needed), and poor (score of 0–1, definitive intervention and corrective measures needed).[13]
The secondary outcomes were functional and anatomical success at the 3- and 6-month follow-up visits. Given that the silicone tubes were removed 2–3 months postoperatively, their potential influence on outcomes was eliminated. Functional success was determined by the absence of epiphora and graded as none (score 0), moderate (score 1), or high (score 2). Anatomical success was professionally assessed by a senior ophthalmologist using lacrimal irrigation and ostium patency via nasal endoscopy. These assessment criteria were aligned with the recommendations of Olver.[14]
Surgical technique
Senior lacrimal and sinus surgeons with more than 5 years of surgical experience endoscopically performed the procedures under general anesthesia. Nasal packs soaked in 1% lidocaine and 1:100,000 epinephrine hydrochloride diluted with saline were applied to the nasal mucosa to facilitate vasoconstriction. Local anesthetic infiltration was performed on the lateral nasal wall mucosa, where the dacryoscyst projects, using 20 mg/ml lidocaine and 0.0125 mg/ml epinephrine hydrochloride. An incision of approximately 8 mm was made on the mucosa in front of the middle turbinate attachment, and two perpendicular incisions were created at the upper and lower edges. A mucosal flap was formed with a Freer elevator. The flap was extended posteriorly, and the uncinate process was lifted to expose the lacrimomaxillary suture, which served as a reliable marker for locating the underlying lacrimal sac. Based on this mark, a power system was used to coarsely grind the frontal process of the maxilla to thin it out and improve transillumination. The bone overlying the lacrimal sac was removed, if necessary, using a powered osteotomy to completely expose the sac fundus. A suitable bone hole (approximately 1.0 × 1.2 cm) was created according to the size of the lacrimal sac. A Bowman’s probe was inserted into the lacrimal sac from the superior punctum. The entire lacrimal sac was excised to reach the blockade site. The nasal mucosa and lacrimal sac flaps were repaired. The silicone tubes were implanted into the upper and lower puncta. For hemostasis, absorbable gelatin sponge pieces were placed around the anastomotic site.
Concurrent endoscopic septoplasty may be required before En-DCR under local anesthesia. An L-shaped incision is typically made in the mucosa opposite to the nasolacrimal duct. To restore the nasal septum, a nasal endoscope was used to separate the mucoperiosteal flaps on both sides, and any deformed bone or cartilage was removed. Subsequently, ipsilateral en-DCR was performed. Finally, the nasal mucosal incision was sutured, and the nasal cavities on both sides were filled with gauze [Fig. 2b].
Review of postoperative visit records
Following surgery, all patients were routinely administered intravenous antibiotics, eyewash, and budesonide nasal spray. After 72 h, the gauze strips were removed from the nasal cavity and appropriate postoperative care was provided. The patients underwent nasal endoscopy-guided visits at intervals of 2 weeks and 1, 2, 3, and 6 months. During these sessions, any blood scabs, secretions, granulomas, membranes, or synechiae were removed as needed. Silicone tubes were removed between the second and third months, depending on the preoperative condition of the lacrimal canalicular stenosis and the postoperative healing status of the epithelial perianastomotic stroma. The minimum follow-up duration was 6 months.
All patient data from these visits were stored in the Hospital Information System (HIS), from which we retrospectively extracted relevant information through chart reviews.
Statistical analysis
Data analysis was performed using IBM Statistical Package for the Social Sciences (SPSS) 19.0. (SPSS, Inc., Chicago, IL, USA). Statistical figures were generated and compiled using the Origin 2022 software. The qualitative and quantitative variables are expressed as n (%) or median (range). Chi-squared or Fisher’s exact test was used to compare groups based on anatomical or functional success, ICO dynamicity, cicatricial ostium closure, and ICO threats at each follow-up interval. Mann–Whitney U tests were used to compare FEDT scores, DCR ostium grades, and DCR ostium scores of the groups. We also used the generalized estimating equation (GEE) model to examine the group differences in DCR ostium grade and functional and anatomical success, adjusting for factors such as participant age, sex, visits, and the affected eye. Statistical significance was set at P < 0.05.
Results
All patients underwent unilateral En-DCR. Of the 32 participants in the septoplasty group, six (18.8%) were male and 21 (65.6%) had left eye involvement. In the non-septoplasty group, 23 (18.3%) were male and 70 (55.6%) had left eye involvement. The average ages of the septoplasty and non-septoplasty groups were 53.9 ± 12.4 and 50.5 ± 13.5 years, respectively.
Fig. 3 shows the proportions of the scores for the four ostial parameters for the participants in the septoplasty and non-septoplasty groups at each visit. Both groups consistently showed high proportions of participants with spontaneous FEDT scores (2 scores, >81%), dynamic ICO scores (1 score, >78%), absence of cicatricial ostium closure (1 score, >93%), and absence of ICO threats (1 score, >93%) across all visits, without significant group differences (P > 0.05).
Figure 3.
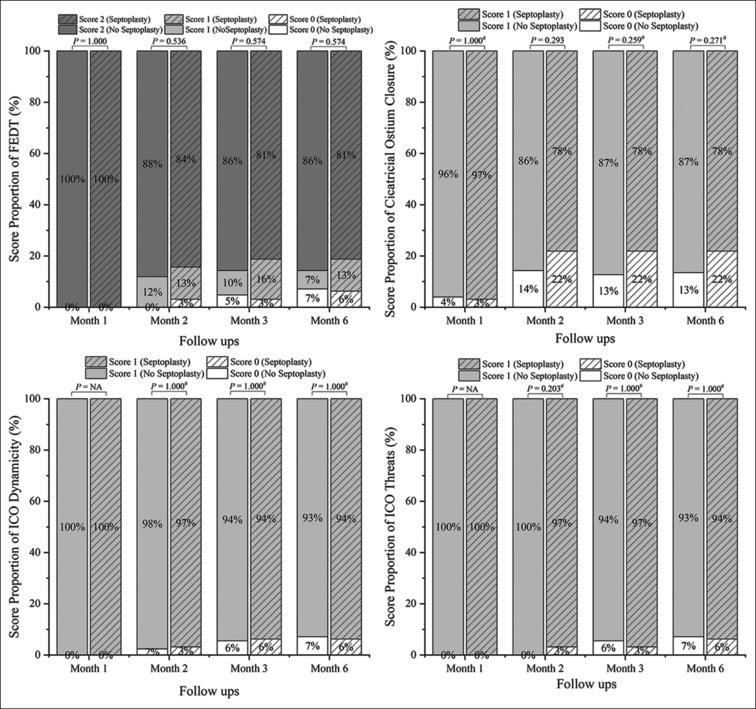
The four FICI ostial parameters’ score proportions in septoplasty and non-septoplasty groups at each follow-up point
After tube removal, total FICI DCR ostial scores for the septoplasty and non-septoplasty groups were 4.47% and 4.58%, respectively (P = 0.335). By the end of the last visit, these scores had decreased to 4.41 and 4.50, respectively (P = 0.357). The proportion of patients with “poor” FICI DCR ostial grades, indicating a need for definitive intervention and corrective measures, increased from 5.6% to 7.1% for the non-septoplasty group. However, this was comparable to that of the septoplasty group (P > 0.05) [Table 1].
Table 1.
The primary and secondary outcomes of two groups after tube removal and at the visit end
| Outcomes | Septoplasty (n=32) | No septoplasty (n=126) | Z/χ2 | P | ||||
|---|---|---|---|---|---|---|---|---|
| DCR ostium score after TB | 4.47±1.164, 5 (0–5) | 4.58±1.162, 5 (0–5) | 0.965 | 0.335 | ||||
| DCR ostium score at the last visit | 4.41±1.341, 5 (0–5) | 4.50±1.355, 5 (0–5) | 0.920 | 0.357 | ||||
| Poor DCR ostium after TB | 2 (6.3) | 7 (5.6) | 0.023 | 0.880 | ||||
| Poor DCR ostium at the last visit | 2 (6.3) | 9 (7.1) | 0.025 | 0.874 | ||||
| Anatomical success after TB | 30 (93.8) | 119 (94.4) | 0.000 | 1.000 | ||||
| Anatomical success at the last visit | 30 (93.8) | 117 (92.9) | 0.000 | 1.000 | ||||
| Functional success after TB | 29 (90.6) | 116 (92.1) | - | 0.728a | ||||
| Functional success at the last visit | 28 (87.5) | 114 (90.5) | 0.029 | 0.865 |
DCR=Dacryocystorhinostomy, TB=Tube removal. Data are shown as mean±SD, median (range), or (n, %). aStatistical analysis was calculated using Fisher’s exact tests
Both groups demonstrated high and similar functional and anatomical success rates after tube removal, with rates exceeding 90% (P > 0.05). These success rates showed a slight decrease (ranging between 0% and 3.1%) at the last visit, but remained notably high at over 87.5% (P > 0.05) [Table 1].
The findings of GEE multivariate analysis were consistent with those of the univariate analysis. After adjustments for follow-up duration, affected eye, sex, and age, there were no significant differences in the FICI DCR ostium grade, functional success, or anatomical success between the septoplasty and non-septoplasty groups (P > 0.05) [Table 2].
Table 2.
GEE multivariate analysis of the primary and secondary outcomes in septoplasty versus non-septoplasty groups
| Outcomes | β | SD | Wald χ2 | P | ||||
|---|---|---|---|---|---|---|---|---|
| FICI DCR ostium grade | -0.513 | 0.505 | 1.032 | 0.310 | ||||
| Anatomical success | 0.048 | 0.818 | 0.003 | 0.954 | ||||
| Functional success | 0.288 | 0.633 | 0.207 | 0.649 |
DCR=Dacryocystorhinostomy, GEE=Generalized estimating equation, SD=Standard deviation
The main complication was postoperative nasal mucosal adhesions in the non-septoplasty group (3.2%, 4/126) [Fig. 2c]. DCR ostium closure was observed using nasoendoscopy. One patient in the septoplasty group had blood seeping from the ipsilateral nostril, which was stopped by the addition of another gauze. Potential complications related to rhinostomy, such as scar and granuloma, were reported as part of the FICI DCR ostium parameters. No other complications, including septoplasty-related signs, were observed.
Discussion
As a common nasal abnormality, NSD often requires intervention for a wide surgical field using the endonasal approach.[15] Septoplasty is typically performed in patients with symptomatic nasal airway obstruction.[16] For patients with CD and NSD, additional endoscopic septoplasty may be performed regardless of the primary indication to facilitate En-DCR, particularly for moderate NSD.[17] In the present study, we retrospectively compared the prognosis of En-DCR combined with septoplasty with that of En-DCR alone for patients with CD. To the best of our knowledge, this is the first comprehensive comparison of the outcomes of a large series of patients with moderate NSD. Across all visits, both groups demonstrated favorable surgical, functional, and anatomical outcomes with no significant differences.
A recent evidence-based review provided low-level evidence on the efficacy of concurrent septoplasty.[18] Our study provides additional insights to guide ophthalmologists in clinical practice. High success rates (87%) and a minimal need for postoperative intervention (93%) highlight the benefits of concurrently addressing nasal anatomical disorders during En-DCR.[17,19] Moreover, simultaneous septoplasty could reduce the risk of mucosal tissue adhesion observed in 3.2% of patients who undergo En-DCR alone because of the proximity of healing mucosal tissues to a moderately deviated septum.[10] However, concurrent septoplasty had similar prognostic benefits. This implies that although additional septoplasty facilitated the surgical procedure, it did not influence the outcomes of En-DCR. In asymptomatic patients with moderate NSD, the indications for septoplasty may not have been met. Patients may opt for En-DCR alone, especially if extra septoplasty, meant to aid En-DCR, does not offer further prognostic advantages. Such scenarios require surgeons with greater expertise and skill.
The success rates for En-DCR alone ranged from 84% to 98%,[3,7,10,20,21,22,23,24,25] while those for En-DCR combined with septoplasty ranged from 67% to 96%,[10,20,26] as reported in recent publications. The anatomical and functional success rates in our study were close to the upper limits of the existing data. This could be attributed to advanced endoscopic techniques and extensive experience in rhinology. Greater surgeon expertise facilitates successful En-DCR without concurrent septoplasty for moderate NSD. In addition, multidisciplinary collaboration cannot be overemphasized. Mutual support of surgeons with ophthalmic and otolaryngological backgrounds is highly valued. As reported by Nogueira et al.,[27] the epiphora resolution rate for En-DCR conducted by an otolaryngologist increased from 75% to 92% with the involvement of an ophthalmologist. The ophthalmologist was more professional in inserting the probes into the lacrimal canaliculi during surgery.
Most studies have focused on the size of DCR ostium when assessing it.[21,28,29,30] In our study, the FICI DCR ostium score was used as the primary outcome. This simple and physician-friendly tool uses four parameters optimized from the 10 parameters for earlier DCR ostium (DOS) scoring.[13,31] The final score determined the ostium grade, which served as a guide for intervention. Notably, only 6.3% of patients in the septoplasty group required definitive postoperative intervention, a proportion comparable to that of the En-DCR alone group. This study, for the first time, demonstrated that septoplasty for moderate NSD did not affect the FICI DCR ostium score.
Previous studies with the same design conducted by Cikrikci et al.[20] and Koval et al.[10] demonstrated similar results. However, none highlighted the severity of NSD. Moreover, no studies have assessed the health of DCR ostium, which is closely associated with DCR failure.[31] In contrast, our study comprehensively investigated the ostia in patients with moderate NSD for the following considerations. Severe NSD, defined as a deviated septum touching the lateral nasal wall,[12] significantly affects the nasal passage, leading to obvious nasal obstruction symptoms. Concurrent septoplasty is necessary to satisfy patient needs and ensure the success of En-DCR. For mild NSD, where the nasal passage is affected less than 50%, it remains wide enough for En-DCR alone. Cikrikci et al.[20] additionally evaluated patient symptoms using the Lund–Mackay symptom score and the Lund–Kennedy system. No association between symptoms and surgical success was found.
A significant strength of our study is that all the participants had moderate NDS and CD. Comparative analysis of the septoplasty and non-septoplasty groups was performed using the GEE model. After adjusting for age, sex, visit intervals, and affected eyes and incorporating several novel outcomes, our conclusions became more robust. However, our study has some limitations. First, this is the largest moderate NDS series to date; however, the sample sizes for the two cohorts were unbalanced (1:4), which may have reduced the statistical power. However, we included participants from real-world scenarios, and 20.1% underwent concurrent septoplasty, which is in line with published data.[10] Second, to eliminate potential bias, we included only participants with ipsilateral, moderate NDS and unilateral CD. This selection may have undermined the authenticity of the findings. Third, silicone stents are routinely used for primary En-DCR. However, the rationality of this stent intubation procedure in DCR remains controversial. Finally, our evaluation focused solely on En-DCR–related ocular outcomes. The symptoms of nasal pathology were excluded. Future research should incorporate outcomes such as the Lund–Mackay score and the Lund–Kenedy score.
In conclusion, concomitant septoplasty and En-DCR yielded a high functional and anatomical success rate and satisfactory FICI ostium grade with fewer complications than En-DCR alone. However, in patients with moderate NSD, the addition of septoplasty did not significantly improve the outcomes of En-DCR. When En-DCR is performed by experienced surgeons and the otolaryngological indications for septoplasty are ambiguous, the necessity of septoplasty should be reconsidered even for cases of moderate NDS.
Institutional review board statement
The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the ethics committees of Henan Eye Hospital.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Data availability statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical reasons.
Financial support and sponsorship
This research was supported by the project “Medical Science and Technology Tackling Plan of Henan Province (LHGJ20210078).”
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wang Y, Liu F, Cao M, Xie L, Tan S, Liu L, et al. Efficacy and safety of modified seamless endoscopic dacryocystorhinostomy in patients with chronic dacryocystitis. J Ophthalmol. 2022;2022:3061859. doi: 10.1155/2022/3061859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luo B, Li M, Xiang N, Hu W, Liu R, Yan X, et al. The microbiologic spectrum of dacryocystitis. BMC Ophthalmol. 2021;21:29. doi: 10.1186/s12886-020-01792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jawad A, Kausar A, Iftikhar S, Akhtar N, Rabbani Z. Results of endoscopic endonasal dacryocystorhinostomy: A prospective cohort study. J Pak Med Assoc. 2021;71:1420–3. doi: 10.47391/JPMA.187. [DOI] [PubMed] [Google Scholar]
- 4.Yu B, Tu YH, Zhou GM, Shi JL, Wu ED, Wu WC, et al. Management of chronic dacryocystitis cases after failed external dacryocystorhinostomy using endoscopic technique with a novel lacrimal ostium stent. Int J Ophthalmol. 2022;15:413–9. doi: 10.18240/ijo.2022.03.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kumar S, Mishra AK, Sethi A, Mallick A, Maggon N, Sharma H, et al. Comparing outcomes of the standard technique of endoscopic DCR with its modifications: A retrospective analysis. Otolaryngol Head Neck Surg. 2019;160:347–54. doi: 10.1177/0194599818813123. [DOI] [PubMed] [Google Scholar]
- 6.Sobel RK, Aakalu VK, Wladis EJ, Bilyk JR, Yen MT, Mawn LA, et al. A comparison of endonasal dacryocystorhinostomy and external dacryocystorhinostomy: A report by the American Academy of Ophthalmology. Ophthalmology. 2019;126:1580–5. doi: 10.1016/j.ophtha.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Marcet MM, Kuk AK, Phelps PO. Evidence-based review of surgical practices in endoscopic endonasal dacryocystorhinostomy for primary acquired nasolacrimal duct obstruction and other new indications. Curr Opin Ophthalmol. 2014;25:443–8. doi: 10.1097/ICU.0000000000000084. [DOI] [PubMed] [Google Scholar]
- 8.Lin GC, Brook CD, Hatton MP, Metson R. Causes of dacryocystorhinostomy failure: External versus endoscopic approach. Am J Rhinol Allergy. 2017;31:181–5. doi: 10.2500/ajra.2017.31.4425. [DOI] [PubMed] [Google Scholar]
- 9.Karpishchenko SA, Vereshchagina OE, Karpov AA. Endoscopic septoplasty as a stage of endonasal dacryocystorhinostomy. Vestn Otorinolaringol. 2020;85:56–9. doi: 10.17116/otorino20208506156. [DOI] [PubMed] [Google Scholar]
- 10.Koval T, Zloto O, Yakirevitch A, Ben Simon GJ, Ben-Shoshan J, Ben Artsi E, et al. No impact of nasal septoplasty on the outcome of endoscopic dacryocystorhinostomy. Eye (Lond) 2020;34:1454–8. doi: 10.1038/s41433-019-0696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyake MM, Gregorio LL, Freitag SK, Lefebvre DR, Gray ST, Holbrook EH, et al. Impact of endoscopic dacryocystorhinostomy on sinonasal quality of life. Am J Rhinol Allergy. 2016;30:189–91. doi: 10.2500/ajra.2016.30.4332. [DOI] [PubMed] [Google Scholar]
- 12.Şahin B, Çomoğlu Ş, Aydemir L, Pamuk S, Keleş TM. The simplified nasal septal deviation evaluation system and nasal obstruction symptom evaluation scale correlation. J Craniofac Surg. 2020;31:1782–4. doi: 10.1097/SCS.0000000000006590. [DOI] [PubMed] [Google Scholar]
- 13.Ali MJ, Gupta A, Lakshmi CS, Ali MH. The FICI grading for a dacryocystorhinostomy ostium. Eur J Ophthalmol. 2022;32:129–33. doi: 10.1177/1120672121994747. [DOI] [PubMed] [Google Scholar]
- 14.Olver JM. The success rates for endonasal dacryocystorhinostomy. Br J Ophthalmol. 2003;87:1431. doi: 10.1136/bjo.87.11.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali MJ, Psaltis AJ, Wormald PJ. The frequency of concomitant adjunctive nasal procedures in powered endoscopic dacryocystorhinostomy[J] Orbit. 2015;34:142–5. doi: 10.3109/01676830.2015.1014509. [DOI] [PubMed] [Google Scholar]
- 16.Watters C, Brar S, Yapa S. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. Septoplasty. [Updated 2022 Nov 8] Available from: https://www.ncbi.nlm.nih.gov/books/NBK567718/ [Last accessed on 2023 Apr 12] [PubMed] [Google Scholar]
- 17.Goel R, Nagpal S, Kumar S, Kamal S, Dangda S, Bodh SA. Our experience with transcanalicular laser-assisted endoscopic dacryocystorhinostomy (TCLADCR) in patients of chronic dacryocystitis with deviated nasal septum. Int Ophthalmol. 2015;35:811–7. doi: 10.1007/s10792-015-0052-z. [DOI] [PubMed] [Google Scholar]
- 18.Yim M, Wormald PJ, Doucet M, Gill A, Kingdom T, Orlandi R, et al. Adjunctive techniques to dacryocystorhinostomy: An evidence-based review with recommendations. Int Forum Allergy Rhinol. 2021;11:885–93. doi: 10.1002/alr.22699. [DOI] [PubMed] [Google Scholar]
- 19.Knisely A, Harvey R, Sacks R. Long-term outcomes in endoscopic dacryocystorhinostomy. Curr Opin Otolaryngol Head Neck Surg. 2015;23:53–8. doi: 10.1097/MOO.0000000000000121. [DOI] [PubMed] [Google Scholar]
- 20.Cikrikci S, Erkan E, Agdas F. Association between septoplasty, Lund-Mackay score and Lund-Kennedy score with endoscopic dacryocystorhinostomy results. Orbit. 2021;40:274–80. doi: 10.1080/01676830.2020.1782441. [DOI] [PubMed] [Google Scholar]
- 21.Tadke K, Lahane V, Lokhande P. Ostium characteristics and its relevance in successful outcome following endoscopic dacryocystorhinostomy. Indian J Otolaryngol Head Neck Surg. 2022;74:900–10. doi: 10.1007/s12070-020-01970-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miragall V, Oeken J, Güldner C. Results and clinical aspects of primary endonasal endoscopic dacryocystorhinostomy with silicone tube. Eur Arch Otorhinolaryngol. 2022;279:2409–15. doi: 10.1007/s00405-021-07004-z. [DOI] [PubMed] [Google Scholar]
- 23.Sun S, Song D, Liu W, Zhao Y, Liu Y. Effect of endonasal endoscopic dacryocystorhinostomy combined with lacrimal duct drainage tube implantation in treating lacrimal duct obstruction. Chin J Clin Otolaryngol Head Neck Surg. 2022;36:845–8. doi: 10.13201/j.issn.2096-7993.2022.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García CF, Juantegui AM, Balaguer GR. Factors involved in the success and failure of endoscopic dacryocystorhinostomy from our experience. Acta Otorrinolaringol Esp (Engl Ed) 2022;73:11–8. doi: 10.1016/j.otoeng.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Sung JY, Lee YH, Kim KN, Kang TS, Lee SB. Surgical outcomes of endoscopic dacryocystorhinostomy: Analysis of age effect. Sci Rep. 2019;9:19861. doi: 10.1038/s41598-019-56491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raposo A, Piqueras F, García-Purriños F, Martínez-Martinez ML, Lajara J. Influence of septal deviation on the prognosis of transcanalicular diode laser-assisted dacryocystorhinostomy. J Ophthalmol. 2016;2016:9573760. doi: 10.1155/2016/9573760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nogueira A, Zaragoza P, Toledano N, Genol I, Plaza G. [Endoscopic dacryocystorhinostomy: Role of the ophthalmologist] Arch Soc Esp Oftalmol. 2014;89:157–60. doi: 10.1016/j.oftal.2012.09.027. [DOI] [PubMed] [Google Scholar]
- 28.Torun MT, Yılmaz E. The role of the rhinostomy ostium size on functional success in dacryocystorhinostomy. Braz J Otorhinolaryngol. 2022;88(Suppl 1):S57–62. doi: 10.1016/j.bjorl.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al HR, Al-Faky YH. Late endoscopic evaluation of the ostium size after external dacryocystorhinostomy. Eur J Ophthalmol. 2021;31:3425–9. doi: 10.1177/1120672120976044. [DOI] [PubMed] [Google Scholar]
- 30.Bertaux PJ, Gan G, Hirtz G, Mouret P, El-Hachem F, Lhuillier L, et al. Evaluation of ostium size following endoscopic dacryocystorhinostomy as a predictive factor of outcome: A prospective study. J Fr Ophtalmol. 2021;44:397–403. doi: 10.1016/j.jfo.2020.05.024. [DOI] [PubMed] [Google Scholar]
- 31.Ali MJ, Psaltis AJ, Wormald PJ. Dacryocystorhinostomy ostium: Parameters to evaluate and DCR ostium scoring. Clin Ophthalmol. 2014;8:2491–9. doi: 10.2147/OPTH.S73998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical reasons.


