Abstract
Objectives
The aim of the current study was to evaluate the value of preoperative 18F-FDG (FDG) PET/CT in predicting cervical lymph node (LN) metastasis in patients with papillary thyroid carcinoma (PTC).
Methods
One hundred and ninety-three newly diagnosed PTC patients (M: F = 25:168, age = 46.8 ± 12.2) who had undergone pretreatment FDG PET/CT and had neck node dissection were included in this study. The FDG avidity of the primary tumor and the SUVmax of the primary tumor (pSUVmax) were analyzed for prediction of LN metastasis. Detectability by ultrasonography (US) and FDG PET/CT for cervical LN metastasis were also assessed and compared with the pSUVmax.
Results
The FDG avidity of the primary tumor was identified in 118 patients (FDG avid group: 61.0%, M: F = 16:102, age 47.0 ± 12.7 years) and pSUVmax ranged from 1.3 to 35.6 (median 4.6) in the FDG avid group. The tumor size in the FDG avid group was bigger and there was a higher incidence of LN metastasis compared to the FDG non-avid group (0.93 vs. 0.59 cm, p <0.001 and 49.2 vs. 33.3%, p <0.05). In the FDG avid group, patients with LN metastasis had higher pSUVmax than patients without LN metastasis (8.7 ± 8.3 vs. 5.7 ± 5.1, p <0.001). The incidence of central LN metastasis in patients with a pSUVmax >4.6 was 54%; however, the detectability of central LN metastasis by US and FDG PET/CT were 10.3% and 3.6%, respectively.
Conclusion
A high FDG avidity of the primary tumor was related to LN metastasis in PTC patients. Therefore, patients with a high pSUVmax should be cautiously assessed for LN metastasis and might need a more comprehensive surgical approach.
Introduction
Papillary thyroid carcinoma (PTC) is an endocrine neoplasm with a high incidence of lymphatic metastasis. The incidence of cervical lymph node (LN) metastasis in patients with well-differentiated thyroid carcinoma has been reported to be 20–90% [1]. The metastatic spread of PTC to cervical LN increases the risk of local or regional recurrence of the tumor and the need for further surgery [2, 3]. In addition, some large studies even reported reduced survival because of cervical LN metastases in PTC patients [4, 5]. Since determining the presence of cervical LN metastasis may suggest a different operation method, it is clinically important. Current guidelines recommend removal of all clinically involved metastatic LNs in the central and lateral compartments, which are visualized by preoperative radiological investigations using, for example, ultrasonography (US), enhanced computed tomography (CT), and magnetic resonance imaging (MRI) [6]. However, preoperative CT, MRI, cervical US and 18F-fluoro-2-deoxy-D-glucose-positron emission tomography–computerized tomography (FDG PET/CT) has a low sensitivity (30–40%) for detecting cervical LN metastasis [7].
FDG PET/CT is widely used in the staging of various malignancies and is useful in identifying occult LN or distant metastasis [8–14]. Although FDG PET/CT has been used primarily for recurrence work-up in differentiated thyroid carcinoma patients with increased serum thyroglobulin level but negative radioiodine whole body scan, [15, 16] a substantial portion of PTC patients have preoperative FDG PET/CT by incidental first recognition of the PTC on the FDG PET/CT which was performed for other purposes, such as the staging of other malignancies and work-up for fever of unknown origin [17]. However, so far, only a few studies on the value of FDG PET/CT in the preoperative regional LN staging of differentiated thyroid carcinoma have been done. Its role has not yet been fully evaluated [18]. In previous studies, the value of FDG PET/CT for detecting metastatic LN was assessed and the results of the studies precluded the use of FDG PET/CT for detecting metastatic LNs because of a limited ability to detect the metastatic lymph nodes [19, 20]. The limited ability of FDG PET/CT for detecting metastatic LNs warrants other FDG PET/CT approaches to the achievement of better accuracy for predicting LN metastasis. Metabolic parameters of primary tumor on FDG PET/CT has been used to predict occult LN metastasis in various cancers including lung cancer [21], cervical cancer [22], and breast cancer [23]. Even though Byun et al. reported that the highest SUV (SUVmax) are valuable for predicting LN metastasis from papillary thyroid carcinoma, this previous study evaluated only small sized papillary thyroid carcinoma less than 1cm [24]. 45% of enrolled patients had FDG non-avid primary tumor and they assessed the value of FDG PET/CT in the patients with FDG avid primary tumor. Furthermore, they didn’t statistically compared the diagnostic accuracy of SUVmax criterion to that of US. The authors in the current study therefore investigated the value of preoperative FDG PET/CT in predicting cervical LN metastasis in patients with PTC, regardless of tumor size and FDG avidity. Ultimately, we compared the diagnostic accuracy of SUVmax criterion to that of US.
Materials and Methods
Patients
In this retrospective study, we analyzed the clinical data of 236 patients with PTC who had preoperative neck US and FDG PET/CT from January 2012 to December 2012. The patients who underwent the surgery in other institutions were excluded because they had no any information and pathologic reports (n = 33). And the patients didn’t received LN dissection were also excluded because the regional LN cannot be assessed (n = 10). Finally, 193 patients (mean age: 46.8 ± 12.2) were enrolled in this study. A hemithyroidectomy with central LN dissection (n = 66) was performed in patients with preoperative cytological diagnoses of PTC but with no evidence of extrathyroid extension or LN metastasis and no suspicious lesions in the contralateral lobes on preoperative US. Otherwise, a total thyroidectomy with LN dissection was performed (n = 127).
Ethics Statement
The survey was approved by the institutional review board (IRB) of Kyungpook National University School of Medicine/Hospital, Korea. The patient records and information was anonymized and de-identified prior to analysis.
FDG PET/CT
Patients fasted for at least 6 h and blood glucose levels were checked before the administration of FDG. Patients with elevated blood glucose levels had their examinations rescheduled. None of the patients had blood glucose levels exceeding 150 mg/dL. Approximately 8.1 megabecquerel (MBq) of FDG per kilogram of body weight was injected intravenously and the scans were performed 1 h after FDG injection. FDG PET/CT scans were performed using a Reveal RT-HiREZ 6-slice CT apparatus (CTI Molecular Imaging) and a 16-slice CT Discovery standard test equipment (STE) apparatus (GE Healthcare). For attenuation correction before the PET scan, a low-dose CT scan was obtained without contrast enhancement from the skull base to the upper thigh, with the patient supine and breathing quietly. PET scans with a maximum spatial resolution of 6.5 mm (Reveal PET/CT) and 5.5 mm (Discovery PET/CT) were also obtained from the skull base to the upper thigh at 3 min per bed position. PET images obtained by the Reveal PET/CT and Discovery PET/CT scanners were reconstructed with a 128 × 128 matrix, an ordered-subset expectation maximum iterative reconstruction algorithm (4 iterations, 8 subsets), a Gaussian filter of 5.0 mm, and a slice thickness of either 3.0 mm (Reveal PET/CT) or 3.27 mm (Discovery PET/CT).
US
Thyroid US was performed with a 5- to 12-MHz linear transducer (HDI 5000; Philips Healthcare, Bothell, WA). The cervical LNs were divided into levels in accordance with the system of the American Joint committee on Cancer [25]. LNs showing any of the following findings on US were considered suggestive of metastatic nodes: a short- to long-axis ratio of greater than 0.5, hypoechogenicity, cystic changes, loss of an echogenic fatty hilum, microcalcification, and abnormal blood flow on Doppler US.
Image analysis
The FDG PET/CT images and FDG avidity were interpreted by 2 experienced nuclear medicine physicians and a final consensus was reached for all patients. If the primary tumor had focal uptake with underlying diffuse uptake, it was also considered as FDG avidity. Regions of interest were manually placed over the area of maximal activity on slices of the primary tumor lesions in attenuation-corrected images and the pSUVmax within the region of interest over primary lesion was obtained (pSUVmax). The pSUVmax was measured only in the FDG avid group because the exact location of the primary tumor on the PET/CT in the FDG non-avid group, due to the pSUVmax of the FDG non-avid group, was equal to or lower than that of the surrounding normal thyroid tissue.
The pSUVmax was calculated using the following formula:
Statistical analysis
The positive and negative predictive values of US and FDG PET/CT for detecting cervical LN metastasis were calculated, as well as the sensitivity, specificity, and diagnostic accuracy for predicting the presence of the metastasis on a level-by-level analysis. Surgical pathologic results were used as reference standards. A chi-squared (χ2) test and comparison of proportions were used to compare the sensitivity and specificity of US and FDG PET/CT for predicting LN metastasis. An independent t test was applied to compare the differences in the degrees of FDG uptake (pSUVmax) in accordance with pathologic parameters such as tumor size, multiplicity, extrathyroid extension, and LN metastasis. Fisher’s exact test was applied to determined risk factors of LN metastasis. Receiver-operating characteristic (ROC) curves for risk factors were then analyzed to predict LN metastasis. To assess the predictive role of pSUVmax for LN metastasis, Fisher’s exact test and ROC analyses were performed. To minimize the effect of tumor size, we made several subgroups according to primary tumor size and then McNemar test was performed. Finally, authors determined the optimal criterion for the prediction of central LN metastasis and made a comparison with the sensitivity of US, using comparison of proportions. Medcalc version 15.4 (Medcalc Software) was used for all analyses. All P values were two sided and statistical significance was accepted for P values of <0.05.
Results
Patients’ characteristics
Table 1 shows the characteristics of 193 subjects. 61.1% (n = 118) of the patients showed FDG avidity of primary tumor on preoperative FDG PET/CT and the SUVmax of the tumor with FDG avidity was 7.2 ± 7.0. Pathologic tumor size was 0.8 ± 0.5 cm (range: 0.2–3.9cm), microcarcinoma was seen in 78.2% (n = 151) of the patients and 43.0% (n = 83) had LN metastasis.
Table 1. Characteristics of 193 subjects enrolled in this study.
| Characteristics | Value | |
|---|---|---|
| Mean age (year) | 46.8 ± 12.2 | |
| Gender | ||
| Female | 168 (87.0%) | |
| Male | 25 (13.0%) | |
| Pathologic characteristics | ||
| Operation type | ||
| Total thyroidectomy | 127 (65.8%) | |
| Hemithyroidectomy | 66 (34.2%) | |
| Primary tumor size (cm) | 0.8 ± 0.5 | |
| Microcarcinoma | 151 (78.2%) | |
| TNM stage* | ||
| T stage | T1 | 87 (45.1%) |
| T2 | 0 | |
| T3 | 84 (43.5%) | |
| T4 | 22 (11.4%) | |
| N stage | N0 | 110 (57.0%) |
| N1 | 83 (43.0%) | |
| Metabolic parameters | ||
| FDG uptake at primary tumor | ||
| FDG avidity | 118 (61.1%) | |
| FDG non-avidity | 75 (38.9%) | |
| pSUVmax | 7.2 ± 7.0 | |
pSUVmax = SUVmax of primary tumor
*Based on TNM (tumor, node, metastasis) staging system defined by the American Joint Committee on Cancer [25]
Clinical variables associated with central LN metastasis
Table 2 shows the prevalence of LN metastasis according to the presence of FDG avidity at the primary tumor. There was a significant difference in the prevalence of LN metastasis between the FDG avid and FDG non-avid groups. (Figs 1 and 2) shows that the pSUVmax was associated with both LN metastasis (p < 0.001) and N stage (p < 0.001) in the FDG avidity group (n = 118).
Table 2. Characteristics of patients according to FDG avidity.
| Characteristics | FDG non-avid group | FDG avid group | p value |
|---|---|---|---|
| Mean age (year) | 46.5 ± 11.3 | 47.0 ± 12.7 | 0.76 |
| Gender | |||
| Female | 66 | 102 | 0.93 |
| Male | 9 | 16 | |
| Pathologic characteristics | |||
| Primary tumor size (cm) | 0.59 ± 0.51 | 0.93 ± 0.50 | <0.001 |
| T stage* | |||
| T1/T2 | 49 | 38 | <0.001 |
| T3/T4 | 26 | 80 | |
| N stage* | |||
| N0 | 50 | 60 | <0.05 |
| N1 | 25 | 58 |
* Based on TNM (tumor, node, metastasis) staging system defined by the American Joint Committee on Cancer [25].
Fig 1. Relationship between SUVmax of primary tumor (pSUVmax) and lymph node metastasis.
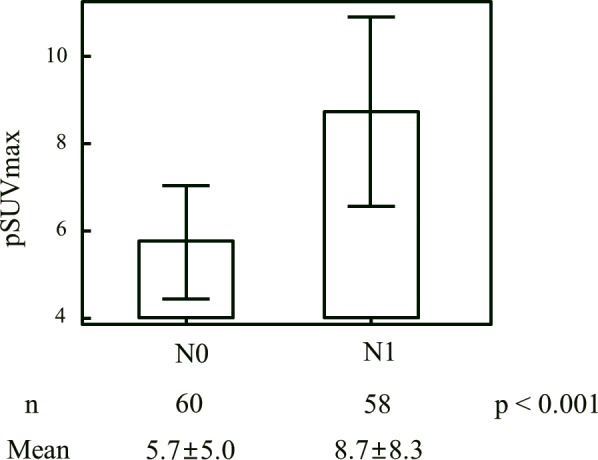
Fig 1 shows that the pSUVmax was associated with both LN metastasis (p < 0.001) in the FDG avidity group (n = 118).
Fig 2. Relationship between pSUVmax and N stage*.
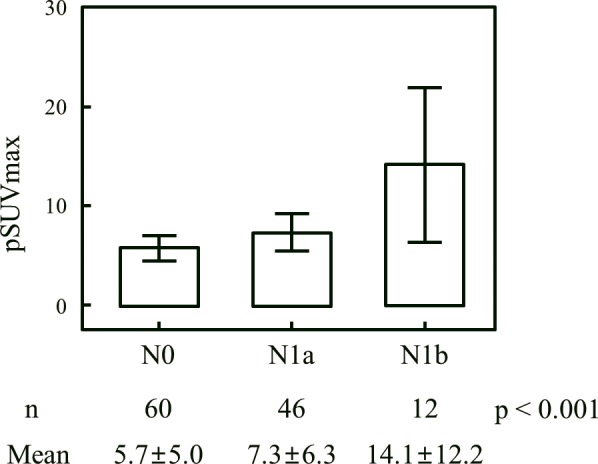
Fig 2 shows that the pSUVmax was associated with N stage (p < 0.001) in the FDG avidity group (n = 118).* Based on TNM (tumor, node, metastasis) staging system defined by the American Joint Committee on Cancer.
Clinicopathologic variables according to FDG avidity
Table 2 shows the characteristics of patients according to FDG avidity at the primary tumor. The tumor sizes of the FDG non-avidity group were significantly smaller than those of the FDG avid group (0.59 vs. 0.93 cm, p <0.001). There was also a significant difference in the prevalence of LN metastasis between the FDG avid and FDG non-avid groups (49.2 vs. 33.3%, p < 0.05).
US and FDG PET for detecting LN metastasis
Among 83 patients with LN metastasis, 68 had the metastasis only at the central compartment, 4 had the metastasis only at the lateral compartment, and 11 had the metastasis at both compartments. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of FDG PET/CT for LN metastasis were 3.6, 94.6, 33.3, and 56.5%, respectively. Those for US were 25.3, 92.7, 72.4, and 62.2%, respectively. The sensitivity, specificity, PPV, and NPV for FDG PET/CT for LN metastasis of the central compartment were 2.9, 100, 100, and 62.5%, respectively. Those for US were 10.3, 96.4, 63.6, and 63.5%, respectively.
Prediction of central LN metastasis
The optimal cutoff of pSUVmax for predicting central LN metastasis was 4.6 by ROC analysis and the sensitivity, specificity, PPV, and NPV of the pSUVmax cutoff for predicting central LN metastasis were 58.7, 61.7, 53.6, and 66.4%, respectively. Patients with a pSUVmax >4.6 revealed a higher incidence of central LN metastasis compared to patients with a pSUVmax ≤4.6 (54% vs. 32%, p < 0.05). Compared to US, the pSUVmax criterion had a significantly higher sensitivity (p <0.001) for predicting central LN metastasis, but a lower specificity (p < 0.05) (Fig 3).
Fig 3. Predictability of central lymph node metastasis by SUVmax of primary tumor (pSUVmax) and ultrasonography (US).
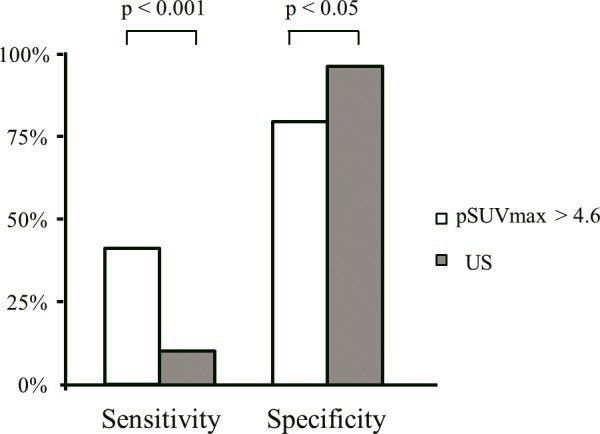
Compared to US, the pSUVmax criterion had a significantly higher sensitivity (p <0.001) for predicting central LN metastasis, but a lower specificity (p < 0.05).
Subgroup analysis for size correction
Of these patients, we made several subgroups according to primary tumor size; group A (tumor size≤1.0, n = 151), group B (tumor size≤2.0, n = 185) and group C (tumor size>2.0, n = 8). In these group A and B, there were significant differences in the prevalence of LN metastasis between the FDG avid and FDG non-avid groups (p < 0.005 and p = 0.0001, respectively), and between the patients with a pSUVmax >4.6 and patients with a pSUVmax ≤4.6 (p < 0.005 and p < 0.005, respectively). While, there was no significant difference in the prevalence of LN metastasis between two groups separated by FDG avid and pSUVmax in group C (p = 1.0 and p = 1.0).
Discussion
LN metastasis is quite common in patients with PTC and the incidence of cervical LN metastasis has been reported as up to 90% at initial diagnosis [1, 26]. Several studies reported that cervical LN metastasis has prognostic importance for recurrence and survival of PTC [2–5]. In addition to excision of the primary tumor with surrounding normal thyroid tissue, a complete removal of LN metastasis following accurate diagnosis is essential for the cure of PTC. The complete removal of primary and metastatic lesions reduces the recurrence rate. The general agreement on guidelines from various associations about central node dissection (CND) is to dissect central nodes only in cases of confirmed LN metastasis preoperatively or in T3/T4 tumors. However, the role of prophylactic CND remains controversial [27, 28]. Accurate assessment of the cervical LN status is essential for risk stratification and establishment of a treatment plan.
US, the most common imaging modality for preoperative LN staging in patients with thyroid cancer, revealed a relatively high specificity (approaching 95%); however, its sensitivity was quite low (as low as approximately 25%) [20, 29]. It is well known that the detection of metastasis on US is heavily dependent on careful searching by radiologists. In the current study, the sensitivity of US for detecting cervical LN metastasis was lower than in previous reports. This might be partially related to the characteristics of the patients in the current study, given that 78% of the participants had a microcarcinoma. The early thyroid cancer might produce a small metastatic lesion, which is undetectable and incapable of changing the anatomical features of the cervical LN; therefore, the metastatic LN might not be easily detected on US. In addition, the low sensitivity may be related to the surgical protocol of our institution which includes a routine central neck dissection. This subsequently decreases motivation for paying close attention to the central neck node.
FDG PET/CT has been reported to be efficient in staging, restaging, and prognosticating various malignancies, including lung, breast, head, and neck cancers [11, 30]. Although there are published reports [19, 31] about the effectiveness of preoperative FDG PET/CT evaluation for thyroid cancer, it has not been widely used for preoperative staging in patients with differentiated thyroid cancer. This is presumably because of the excellent prognosis of the disease and the low diagnostic sensitivity of FDG PET/CT for the disease. Because of the high incidence of dormant thyroid cancer [32–35], it is one of the common incidentally-found malignancies in the FDG PET/CT study, which was performed for the staging of other malignancies or cancer screening. Therefore, some of the thyroid cancer patients have preoperative FDG PET/CT. The current study aimed to assess the clinical value of preoperative FDG PET/CT in patients with PTC.
Prediction of LN metastasis by pSUVmax had been reported in other cancers [22, 23]. In the patients with gastric cancer, the pSUVmax was an independent indicator of LN metastasis [36]. Using the pSUVmax cutoff value of 3.2, the sensitivity and specificity were 74.6% and 74.0% respectively. Kim et al. reported that a high pSUVmax showed an association with an increased risk of LN metastasis in clinical N0 squamous cell lung carcinoma patients [21]. Using the pSUVmax cutoff value of 8.8, the sensitivity and specificity were 91.7% and 52.9% respectively.
The results of the current study demonstrate that the size of the primary tumor was significantly larger in the FDG avid group than in the FDG non-avid group and that the FDG avidity was significantly associated with LN metastasis. The prevalence of central LN metastasis was higher in PTC patients with pSUVmax >4.6 than in PTC patients with pSUVmax ≤4.6. In addition, a high pSUVmax was associated with the presence of central LN metastasis in patients with microcarcinoma (p < 0.005), even though it was reported that the patients with microcarcinoma had less frequent central LN metastasis [24]. After tumor size correction, group A and B still showed significant correlation between pSUVmax and LN metastasis. In group C, FDG avidity and pSUVmax was not significantly associated with LN metastasis, it might derived from the small number of patients (n = 8), The results of the current study reveal that the pSUVmax might be a better predictor than US for predicting central LN metastasis in patients with PTC. Therefore, central LN dissection might be undertaken in patients with high pSUVmax in order to reduce the chance of residual LN metastasis in the central compartment of the neck.
The study has limitations. Partial volume correction of the SUVmax was not performed in the current study. SUVmax can be underestimated by the partial volume effect, especially in small tumors [37]. Hoetjes et al. reported that correction of the effect may improve the accuracy of the diagnostic performance of SUVmax [38]. Although pSUVmax predicted central LN metastasis successfully in the current study, application of the partial volume correction may improve predictability of pSUVmax for the LN metastasis. Predictability of pSUVmax for lateral LN metastasis, which is more valuable in the clinical field, was not performed in the current study due to inborn referral bias, i.e., lateral neck dissection was performed solely in patients who demonstrated evidence of lateral neck LN metastasis on preoperative radiological evaluation or fine needle aspiration cytology. Lastly, there was relatively wide overlap between patients with LN metastasis and without, therefore, pSUVmax can be used more usefully with other clinical risk factors.
Conclusion
FDG avidity of primary tumor on preoperative FDG PET/CT can be used to predict LN metastasis with clinical risk factors in patients with PTC. Patients with a high pSUVmax should be cautiously assessed for LN metastasis and might need a more comprehensive surgical approach.
Acknowledgments
This research was supported by National Nuclear R&D Program through the National Research Foundation of Korea(NRF) funded by the Ministry of Education, Science and Technology (No.2012M2A2A7014020) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C0001).
These results were previously presented in poster form as Ji-hoon Jung, Seung Hyun Son, Choon-Young Kim, Do-Hoon Kim, Chae Moon Hong, Shin Young Jeong, Sang-Woo Lee, Jaetae Lee, and Byeong-Cheol Ahn: Preoperative prediction of lymph node metastasis by 18F-FDG PET/CT in patients with papillary thyroid carcinoma J Nucl Med May 2014 55:1953 General Clinical Specialties—MTA II: Endocrinology/Neuroendocrine Tumors Posters http://jnm.snmjournals.org/content/55/supplement_1/1953.short
Data Availability
Due to ethical restrictions imposed by the Institutional Review Board of the Kyungpook National University School of Medicine and Hospital, Daegu, Republic of Korea, data are available upon request. Request for data may be submitted to the Institutional Review Board of the Kyungpook National University School of Medicine and Hospital. Address: 135 DONGDEOK-RO, JUNG-GU, DAEGU, KOREA 700-412, Tel: 82-53-420-5430, Fax: 82-53-426-7465, E-mail: knuhmrc@knu.ac.kr.
Funding Statement
This research was supported by National Nuclear R&D Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2012M2A2A7014020) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C0001).
References
- 1. Wang TS, Dubner S, Sznyter LA, Heller KS. Incidence of metastatic well-differentiated thyroid cancer in cervical lymph nodes. Arch Otolaryngol Head Neck Surg. 2004;130(1):110–3. Epub 2004/01/21. 10.1001/archotol.130.1.110 . [DOI] [PubMed] [Google Scholar]
- 2. Mazzaferri EL, Kloos RT. Clinical review 128: Current approaches to primary therapy for papillary and follicular thyroid cancer. J Clin Endocrinol Metab. 2001;86(4):1447–63. Epub 2001/04/12. 10.1210/jcem.86.4.7407 . [DOI] [PubMed] [Google Scholar]
- 3. Lee BJ, Wang SG, Lee JC, Son SM, Kim IJ, Kim YK. Level IIb lymph node metastasis in neck dissection for papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg. 2007;133(10):1028–30. Epub 2007/10/17. 10.1001/archotol.133.10.1028 . [DOI] [PubMed] [Google Scholar]
- 4. Lundgren CI, Hall P, Dickman PW, Zedenius J. Clinically significant prognostic factors for differentiated thyroid carcinoma: a population-based, nested case-control study. Cancer. 2006;106(3):524–31. Epub 2005/12/22. 10.1002/cncr.21653 . [DOI] [PubMed] [Google Scholar]
- 5. Hughes DT, White ML, Miller BS, Gauger PG, Burney RE, Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010;148(6):1100–6; discussion 006–7. Epub 2010/12/08. 10.1016/j.surg.2010.09.019 . [DOI] [PubMed] [Google Scholar]
- 6. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16(2):109–42. Epub 2006/01/20. 10.1089/thy.2006.16.ft-1 . [DOI] [PubMed] [Google Scholar]
- 7. Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011;121(3):487–91. Epub 2011/02/24. 10.1002/lary.21227 . [DOI] [PubMed] [Google Scholar]
- 8. Kim DH, Jung JH, Son SH, Kim CY, Hong CM, Jeong SY, et al. Difference of clinical and radiological characteristics according to radioiodine avidity in pulmonary metastases of differentiated thyroid cancer. Nuclear medicine and molecular imaging. 2014;48(1):55–62. Epub 2014/06/06. 10.1007/s13139-013-0239-z ; PubMed Central PMCID: PMCPmc4035156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim S, Chung JK, Min HS, Kang JH, Park do J, Jeong JM, et al. Expression patterns of glucose transporter-1 gene and thyroid specific genes in human papillary thyroid carcinoma. Nuclear medicine and molecular imaging. 2014;48(2):91–7. Epub 2014/06/06. 10.1007/s13139-013-0249-x ; PubMed Central PMCID: PMCPmc4028475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee JW, Min HS, Lee SM, Kwon HW, Chung JK. Relations Between Pathological Markers and Radioiodine Scan and (18)F-FDG PET/CT Findings in Papillary Thyroid Cancer Patients With Recurrent Cervical Nodal Metastases. Nuclear medicine and molecular imaging. 2015;49(2):127–34. Epub 2015/06/19. 10.1007/s13139-015-0324-6 ; PubMed Central PMCID: PMCPmc4463869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kresnik E, Mikosch P, Gallowitsch HJ, Kogler D, Wiesser S, Heinisch M, et al. Evaluation of head and neck cancer with 18F-FDG PET: a comparison with conventional methods. Eur J Nucl Med. 2001;28(7):816–21. Epub 2001/08/16. . [PubMed] [Google Scholar]
- 12. Pieterman RM, van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koeter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343(4):254–61. Epub 2000/07/27. 10.1056/nejm200007273430404 . [DOI] [PubMed] [Google Scholar]
- 13. Bohuslavizki KH, Klutmann S, Kroger S, Sonnemann U, Buchert R, Werner JA, et al. FDG PET detection of unknown primary tumors. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2000;41(5):816–22. Epub 2000/05/16. . [PubMed] [Google Scholar]
- 14. Park HL, Yoo Ie R, Lee N, Yoon H, Choi EK, Choi HS, et al. The Value of F-18 FDG PET for Planning Treatment and Detecting Recurrence in Malignant Salivary Gland Tumors: Comparison with Conventional Imaging Studies. Nuclear medicine and molecular imaging. 2013;47(4):242–8. Epub 2014/06/06. 10.1007/s13139-013-0222-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang W, Macapinlac H, Larson SM, Yeh SD, Akhurst T, Finn RD, et al. [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography localizes residual thyroid cancer in patients with negative diagnostic (131)I whole body scans and elevated serum thyroglobulin levels. J Clin Endocrinol Metab. 1999;84(7):2291–302. Epub 1999/07/15. 10.1210/jcem.84.7.5827 . [DOI] [PubMed] [Google Scholar]
- 16. Alnafisi NS, Driedger AA, Coates G, Moote DJ, Raphael SJ. FDG PET of recurrent or metastatic 131I-negative papillary thyroid carcinoma. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2000;41(6):1010–5. Epub 2000/06/16. . [PubMed] [Google Scholar]
- 17. Blokhuis GJ, Bleeker-Rovers CP, Diender MG, Oyen WJ, Draaisma JM, de Geus-Oei LF. Diagnostic value of FDG-PET/(CT) in children with fever of unknown origin and unexplained fever during immune suppression. European journal of nuclear medicine and molecular imaging. 2014;41(10):1916–23. Epub 2014/05/30. 10.1007/s00259-014-2801-z . [DOI] [PubMed] [Google Scholar]
- 18. Jeong HS, Baek CH, Son YI, Choi JY, Kim HJ, Ko YH, et al. Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: comparison with ultrasound and contrast-enhanced CT. Clin Endocrinol (Oxf). 2006;65(3):402–7. Epub 2006/08/22. 10.1111/j.1365-2265.2006.02612.x . [DOI] [PubMed] [Google Scholar]
- 19. Morita S, Mizoguchi K, Suzuki M, Iizuka K. The accuracy of (18)[F]-fluoro-2-deoxy-D-glucose-positron emission tomography/computed tomography, ultrasonography, and enhanced computed tomography alone in the preoperative diagnosis of cervical lymph node metastasis in patients with papillary thyroid carcinoma. World journal of surgery. 2010;34(11):2564–9. Epub 2010/07/21. 10.1007/s00268-010-0733-8 . [DOI] [PubMed] [Google Scholar]
- 20. Choi WH, Chung YA, Han EJ, Sohn HS, Lee SH. Clinical value of integrated [18F]fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in the preoperative assessment of papillary thyroid carcinoma: comparison with sonography. J Ultrasound Med. 2011;30(9):1267–73. Epub 2011/08/31. . [DOI] [PubMed] [Google Scholar]
- 21. Kim DH, Song BI, Hong CM, Jeong SY, Lee SW, Lee J, et al. Metabolic parameters using F-FDG PET/CT correlate with occult lymph node metastasis in squamous cell lung carcinoma . European journal of nuclear medicine and molecular imaging. 2014. Epub 2014/07/06. 10.1007/s00259-014-2831-6 . [DOI] [PubMed] [Google Scholar]
- 22. Crivellaro C, Signorelli M, Guerra L, De Ponti E, Buda A, Dolci C, et al. 18F-FDG PET/CT can predict nodal metastases but not recurrence in early stage uterine cervical cancer. Gynecologic oncology. 2012;127(1):131–5. Epub 2012/07/10. 10.1016/j.ygyno.2012.06.041 . [DOI] [PubMed] [Google Scholar]
- 23. Keam B, Im SA, Koh Y, Han SW, Oh DY, Cho N, et al. Predictive value of FDG PET/CT for pathologic axillary node involvement after neoadjuvant chemotherapy. Breast cancer (Tokyo, Japan). 2013;20(2):167–73. Epub 2012/02/09. 10.1007/s12282-011-0323-0 . [DOI] [PubMed] [Google Scholar]
- 24. Byun BH, Jeong UG, Hong SP, Min JJ, Chong A, Song HC, et al. Prediction of central lymph node metastasis from papillary thyroid microcarcinoma by 18F-fluorodeoxyglucose PET/CT and ultrasonography. Ann Nucl Med. 2012;26(6):471–7. Epub 2012/04/03. 10.1007/s12149-012-0594-3 . [DOI] [PubMed] [Google Scholar]
- 25. Edge SB BD, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual (7th ed). New York, NY: Springer; 2010. [Google Scholar]
- 26. Machens A, Hinze R, Thomusch O, Dralle H. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World journal of surgery. 2002;26(1):22–8. Epub 2002/03/19. 10.1007/s00268-001-0176-3 . [DOI] [PubMed] [Google Scholar]
- 27. White ML, Gauger PG, Doherty GM. Central lymph node dissection in differentiated thyroid cancer. World journal of surgery. 2007;31(5):895–904. Epub 2007/03/10. 10.1007/s00268-006-0907-6 . [DOI] [PubMed] [Google Scholar]
- 28. Ito Y, Tomoda C, Uruno T, Takamura Y, Miya A, Kobayashi K, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World journal of surgery. 2006;30(1):91–9. Epub 2005/12/22. 10.1007/s00268-005-0113-y . [DOI] [PubMed] [Google Scholar]
- 29. Kim E, Park JS, Son KR, Kim JH, Jeon SJ, Na DG. Preoperative diagnosis of cervical metastatic lymph nodes in papillary thyroid carcinoma: comparison of ultrasound, computed tomography, and combined ultrasound with computed tomography. Thyroid. 2008;18(4):411–8. Epub 2008/03/25. 10.1089/thy.2007.0269 . [DOI] [PubMed] [Google Scholar]
- 30. Kao J, Vu HL, Genden EM, Mocherla B, Park EE, Packer S, et al. The diagnostic and prognostic utility of positron emission tomography/computed tomography-based follow-up after radiotherapy for head and neck cancer. Cancer. 2009;115(19):4586–94. Epub 2009/06/23. 10.1002/cncr.24493 . [DOI] [PubMed] [Google Scholar]
- 31. Palmedo H, Bucerius J, Joe A, Strunk H, Hortling N, Meyka S, et al. Integrated PET/CT in differentiated thyroid cancer: diagnostic accuracy and impact on patient management. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2006;47(4):616–24. Epub 2006/04/06. . [PubMed] [Google Scholar]
- 32. Choi JY, Lee KS, Kim HJ, Shim YM, Kwon OJ, Park K, et al. Focal thyroid lesions incidentally identified by integrated 18F-FDG PET/CT: clinical significance and improved characterization. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2006;47(4):609–15. Epub 2006/04/06. . [PubMed] [Google Scholar]
- 33. Lee S, Park T, Park S, Pahk K, Rhee S, Cho J, et al. The Clinical Role of Dual-Time-Point (18)F-FDG PET/CT in Differential Diagnosis of the Thyroid Incidentaloma. Nuclear medicine and molecular imaging. 2014;48(2):121–9. Epub 2014/06/06. 10.1007/s13139-013-0247-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Are C, Hsu JF, Schoder H, Shah JP, Larson SM, Shaha AR. FDG-PET detected thyroid incidentalomas: need for further investigation? Annals of surgical oncology. 2007;14(1):239–47. Epub 2006/10/07. 10.1245/s10434-006-9181-y . [DOI] [PubMed] [Google Scholar]
- 35. Nilsson IL, Arnberg F, Zedenius J, Sundin A. Thyroid incidentaloma detected by fluorodeoxyglucose positron emission tomography/computed tomography: practical management algorithm. World journal of surgery. 2011;35(12):2691–7. Epub 2011/10/13. 10.1007/s00268-011-1291-4 . [DOI] [PubMed] [Google Scholar]
- 36. Oh HH, Lee SE, Choi IS, Choi WJ, Yoon DS, Min HS, et al. The peak-standardized uptake value (P-SUV) by preoperative positron emission tomography-computed tomography (PET-CT) is a useful indicator of lymph node metastasis in gastric cancer. J Surg Oncol. 2011;104(5):530–3. Epub 2011/05/28. 10.1002/jso.21985 . [DOI] [PubMed] [Google Scholar]
- 37. Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1998;39(5):904–11. Epub 1998/05/20. . [PubMed] [Google Scholar]
- 38. Hoetjes NJ, van Velden FH, Hoekstra OS, Hoekstra CJ, Krak NC, Lammertsma AA, et al. Partial volume correction strategies for quantitative FDG PET in oncology. European journal of nuclear medicine and molecular imaging. 2010;37(9):1679–87. Epub 2010/04/28. 10.1007/s00259-010-1472-7 ; PubMed Central PMCID: PMCPmc2918791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to ethical restrictions imposed by the Institutional Review Board of the Kyungpook National University School of Medicine and Hospital, Daegu, Republic of Korea, data are available upon request. Request for data may be submitted to the Institutional Review Board of the Kyungpook National University School of Medicine and Hospital. Address: 135 DONGDEOK-RO, JUNG-GU, DAEGU, KOREA 700-412, Tel: 82-53-420-5430, Fax: 82-53-426-7465, E-mail: knuhmrc@knu.ac.kr.


