Summary
Background
Patients with locally advanced rectal cancer who achieve a pathological complete response to neoadjuvant chemoradiation have an improved prognosis. The need for surgery in these patients has been questioned, but the proportion of patients achieving a pathological complete response is small. We aimed to assess whether adding cycles of mFOLFOX6 between chemoradiation and surgery increased the proportion of patients achieving a pathological complete response.
Methods
We did a phase 2, non-randomised trial consisting of four sequential study groups of patients with stage II–III locally advanced rectal cancer at 17 institutions in the USA and Canada. All patients received chemoradiation (fluorouracil 225 mg/m2 per day by continuous infusion throughout radiotherapy, and 45.0 Gy in 25 fractions, 5 days per week for 5 weeks, followed by a minimum boost of 5.4 Gy). Patients in group 1 had total mesorectal excision 6–8 weeks after chemoradiation. Patients in groups 2–4 received two, four, or six cycles of mFOLFOX6, respectively, between chemoradiation and total mesorectal excision. Each cycle of mFOLFOX6 consisted of racemic leucovorin 200 mg/m2 or 400 mg/m2, according to the discretion of the treating investigator, oxaliplatin 85 mg/m2 in a 2-h infusion, bolus fluorouracil 400 mg/m2 on day 1, and a 46-h infusion of fluorouracil 2400 mg/m2. The primary endpoint was the proportion of patients who achieved a pathological complete response, analysed by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT00335816.
Findings
Between March 24, 2004, and Nov 16, 2012, 292 patients were registered, 259 of whom (60 in group 1, 67 in group 2, 67 in group 3, and 65 in group 4) met criteria for analysis. 11 (18%, 95% CI 10–30) of 60 patients in group 1, 17 (25%, 16–37) of 67 in group 2, 20 (30%, 19–42) of 67 in group 3, and 25 (38%, 27–51) of 65 in group 4 achieved a pathological complete response (p=0.0036). Study group was independently associated with pathological complete response (group 4 compared with group 1 odds ratio 3.49, 95% CI 1.39–8.75; p=0.011). In group 2, two (3%) of 67 patients had grade 3 adverse events associated with the neoadjuvant administration of mFOLFOX6 and one (1%) had a grade 4 adverse event; in group 3, 12 (18%) of 67 patients had grade 3 adverse events; in group 4, 18 (28%) of 65 patients had grade 3 adverse events and five (8%) had grade 4 adverse events. The most common grade 3 or higher adverse events associated with the neoadjuvant administration of mFOLFOX6 across groups 2-4 were neutropenia (five in group 3 and six in group 4) and lymphopenia (three in group 3 and four in group 4). Across all study groups, 25 grade 3 or worse surgery-related complications occurred (ten in group 1, five in group 2, three in group 3, and seven in group 4); the most common were pelvic abscesses (seven patients) and anastomotic leaks (seven patients).
Interpretation
Delivery of mFOLFOX6 after chemoradiation and before total mesorectal excision has the potential to increase the proportion of patients eligible for less invasive treatment strategies; this strategy is being tested in phase 3 clinical trials.
Funding
National Institutes of Health/National Cancer Institute R01 CA090559; and Core Grant P30 CA008748
Introduction
Patients with locally advanced rectal cancer are treated with neoadjuvant chemoradiation before total mesorectal excision to induce tumour regression, increase the probability of achieving resection with negative margins, and reduce the risk of local recurrence. Postoperative adjuvant chemotherapy is also recommended in these patients to reduce the risk of distant metastasis. This multimodality treatment achieves high levels of local tumour control and good long-term survival.1 However, total mesorectal excision is associated with some mortality, morbidity, and long-term sequelae that have a substantial negative effect on quality of life.2
Rectal cancer response to chemoradiation is variable, and patients with tumours who achieve a pathological complete response have a better prognosis than do non-responders.3 Local relapse is uncommon and survival is excellent in patients with locally advanced rectal cancer who have a pathological complete response, questioning the added value of total mesorectal excision in these patients. Several institutional case series have reported the feasibility of a watch-and-wait approach in patients with locally advanced rectal cancer who have a complete response to chemoradiation.4,5 However, the proportion of patients achieving a complete response and potentially benefiting from expectant management is small. Therefore, strategies to improve tumour response can alter the present treatment algorithm by maximising the proportion of patients eligible for less invasive surgical approaches.
A common strategy to improve tumour regression in patients with locally advanced rectal cancer is to intensify neoadjuvant treatment by adding systemic chemotherapy before chemoradiation. Findings from several phase 2 trials6–11 have shown enhanced tumour response in patients with a large primary tumour approaching the mesorectal fascia by delivering some of the systemic adjuvant chemotherapy before chemoradiation. In these trials, a slight improvement was noted in the proportion of patients achieving a pathological complete response. However, evidence suggests that tumour response to chemoradiation is time dependent, and complete tumour regression might take months.12–15 Surgeons have historically been reluctant to delay surgery beyond 8 weeks because of the concern that radiation-induced pelvic fibrosis could increase the technical difficulty of the operation and the risk of surgical complications. There are also concerns about disease progression if surgery is not done promptly after chemoradiation. Still, delivering systemic chemotherapy after chemoradiation rather than before might have a greater effect on tumour response by allowing the tumour more time to regress while also providing some treatment to reduce the risk of developing systemic disease. Therefore, we aimed to investigate the effect of delivering two, four, or six cycles of mFOLFOX6 between chemoradiation and surgery on the proportion of patients achieving a pathological complete response and on surgical complications.
Methods
Study design and participants
We did a phase 2, non-randomised, open-label trial at 17 institutions in the USA and Canada. Patients aged at least 18 years, with clinical stage II (T3–4, N0) or III (any T, N1–2) invasive rectal adenocarcinoma who had a distal tumour border within 12 cm of the anal verge by proctoscopy were eligible for inclusion. Local staging was done by endorectal ultrasound or phased-array MRI. Before treatment, patients underwent a full colonoscopy, CT scan of the abdomen and pelvis, and chest radiograph or CT. Patients were required to have an Eastern Collaborative Oncology Group performance status score of 0 or 1 or a comparable Karnofsky score. Patients with a history of pelvic radiation, polyposis syndromes, inflammatory bowel disease, recurrent rectal cancer, metastatic disease, or other primary tumours within the previous 5 years were ineligible. Patients with substantial cardiac disease; seizure disorders; neurological disease; psychiatric disorders; or renal, hepatic, or bone marrow dysfunction were also ineligible.
A central institutional review board and the institutional review boards at each participating institution approved the study protocol. Patients provided written informed consent before enrolment in the study and the start of all study-specific procedures. The central institutional review board assessed and approved the ethical considerations associated with the trial protocol.
Procedures
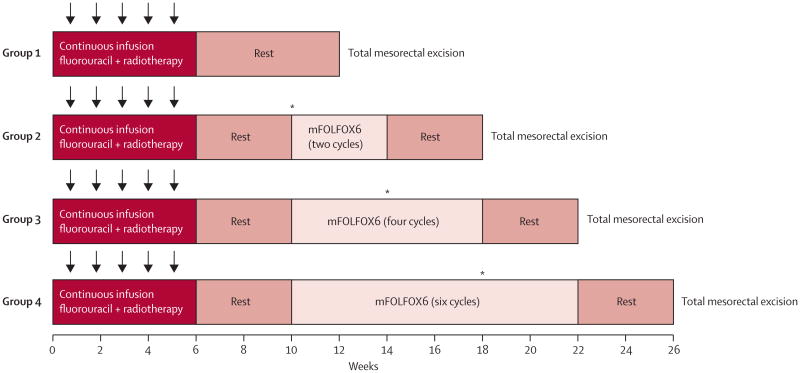
The trial consisted of a series of four sequential phase 2 study groups. In group 1, patients were treated with fluorouracil-based chemoradiation and total mesorectal excision to establish the proportion of patients achieving a pathological complete response at baseline. In groups 2–4, patients received two, four, or six cycles of mFOLFOX6 between chemoradiation and total mesorectal excision (figure 1). Patients in all study groups were treated with fluorouracil 225 mg/m2 per day by continuous infusion, 7 days per week throughout radiation. Fluorouracil infusion was given for 5-6 weeks, depending on the number of radiation boosts given. Radiation treatment was given once a day at 1.8 Gy per day, 5 days per week for 5 weeks, for a total of 45 Gy in 25 fractions, followed by a minimum boost of 5.4 Gy. In patients in whom the entire small bowel could be excluded from the final cone down, a second boost of 3.6 Gy (54 Gy total cumulative dose) was given. A linear accelerator using a minimum 6 MV energy in three to four fields was delivered. Intensity-modulated radiation treatment was permitted if approved by the supervising radiation oncologist.
Figure 1. Trial protocol.
Radiotherapy was given 5 days per week for 5 weeks (arrows) for a total of 45 Gy with a minimum boost of 5.4 Gy. Fluorouracil was given as a 225 mg/m2 per day continuous infusion for 7 days per week during radiation therapy for 5-6 weeks, depending on the number of radiation boosts given. mFOLFOX6 was given in 2-week cycles of leucovorin 200 mg/m2 or 400 mg/m2 and oxaliplatin 85 mg/m2 in a 2-h infusion, bolus fluorouracil 400 mg/m2 on day 1, and a 46-h infusion of fluorouracil 2400 mg/m2. *Interim assessments were done by proctoscopic examination; total mesorectal excision was done if the patient had stable or progressive disease.
Patients in group 1 had surgery 6–8 weeks after chemoradiation. Patients in groups 2–4 received cycles of mFOLFOX6 4-5 weeks after the completion of chemoradiation: patients in group 2 received two cycles, those in group 3 four cycles, and those in group 4 six cycles of mFOLFOX6. Each cycle consisted of racemic leucovorin 200 mg/m2 or 400 mg/m2, according to the discretion of the treating investigator, oxaliplatin 85 mg/m2 in a 2-h infusion, bolus fluorouracil 400 mg/m2 on day 1, and a 46-h infusion of fluorouracil 2400 mg/m2. Patients had surgery 3–5 weeks after the last cycle of mFOLFOX6. To ensure patients in groups 2–4 were not placed at risk of disease progression during the lengthened chemoradiation-to-surgery interval, tumour response was assessed by Response Evaluation Criteria in Solid Tumors guidelines16 during the neoadjuvant treatment course. Patients with progressive or stable disease at interim assessment did not receive additional mFOLFOX6 and had total mesorectal excision without delay.
Surgery was done according to the principles of sharp mesorectal excision. Specimens were assessed according to the recommendations of the Association of Directors of Anatomic and Surgical Pathology.17 Postoperative chemotherapy to complete a total of eight cycles of mFOLFOX6 was recommended, but not dictated by the trial, and was delivered at the discretion of the treating physician.
Outcomes
The primary endpoint of the study was the proportion of patients achieving a pathological complete response (defined as the absence of tumour cells in the surgical specimen, both at the primary tumour site and at regional lymph nodes) in each study group. We also collected information on the proportion of patients who achieved pathological partial response (defined as having at least a 30% decrease in tumour width, or length in circumferential tumours), stable disease (between a 30% decrease and a 20% increase), and progressive disease (at least a 20% increase). The secondary endpoints were the frequency, severity, and attribution of adverse events associated with neoadjuvant treatment, technical difficulty of surgery using both subjective (eg, perceived technical difficulty of the operation by the surgeon on an arbitrary scale) and objective criteria (ie, estimated blood loss, time of operation, and intraoperative complications), and the frequency, grade, and attribution of surgical complications. Primary and secondary endpoints were measured through the 30-day postoperative period across all four study groups.
Conflicting results for response were resolved by consensus. Surgical complications were graded according to the Clavien-Dindo classification.18 Long-term follow-up to measure oncological outcomes to 5 years after surgery remains ongoing for patients who provided further consent to be assessed beyond the trial's original design.
Adverse events during chemoradiation and mFOLFOX6 treatment were measured using the Common Terminology Criteria for Adverse Events Version 3.0. Perioperative and operative details were collected prospectively. Data on pelvic fibrosis and surgical difficulty were collected at the time of surgery using an arbitrary scale. The study pathologist reviewed pathology reports and slides from each diagnostic biopsy and surgical specimen to confirm the diagnosis, whereas pathological staging was done at the treating institutions.
Statistical analysis
The design within each study group was a Simon's two-stage minimax in which the threshold for deciding patient accrual in groups 2–4 was dependent on the empirical proportion of patients achieving a pathological complete response noted in the immediately preceding study group. We set the expected proportion of patients achieving a pathological complete response in group 1 at 15% on the basis of the best responses in the published work at the time. The sample size for each subsequent study group was based on an estimated 5% increase in the proportion of patients achieving a pathological complete response, type I error of 5%, power of 90%, and estimated attrition of 15%.
Analyses and comparisons between study groups were made on an intention-to-treat basis. Responses across treatment groups were compared using two-sided Fisher's exact tests for categorical variables and the Jonckheere-Terpstra test for ordinal variables. Unless otherwise specified, all other p values are based on ANOVA tests for proportions and continuous endpoints.
We used multivariable logistic regression to model the probability of a pathological complete response using clinically relevant variables. Three models were developed to specifically assess the efficacy of treatment using an intention-to-treat approach (assessment by study group) and on the basis of treatment received (cycles of mFOLFOX6, and chemoradiation-to-surgery interval).
This trial is registered with ClinicalTrials.gov, number NCT00335816.
Role of the funding source
The funder participated in study design, but had no role in data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had the final responsibility for the decision to submit for publication.
Results
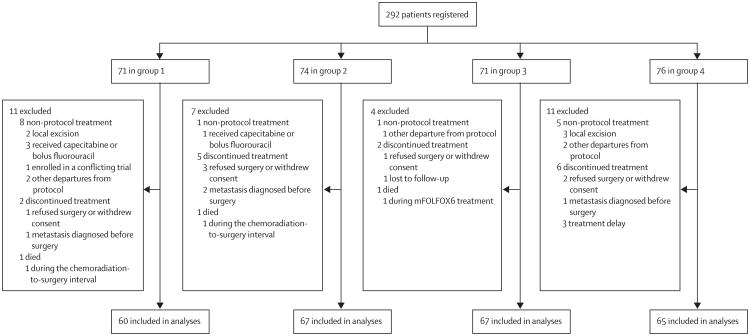
Between March 24, 2004, and Nov 16, 2012, 292 patients (71 in group 1, 74 in group 2, 71 in group 3, and 76 in group 4) were registered to the trial (figure 2; appendix p 1). Of these, 33 patients were excluded from the final analysis for non-protocol treatment, discontinuation of treatment, or death on study (figure 2). Table 1 lists patient demographics and tumour characteristics. Age, sex, tumour size, clinical stage, and distance from anal verge were similar between groups.
Figure 2. Trial profile.
Table 1. Baseline patient and tumour characteristics.
| Group 1 (n=60) | Group 2 (n=67) | Group 3 (n=67) | Group 4 (n=65) | p value | |
|---|---|---|---|---|---|
| Age (years) | 57 (34–87) | 56 (32–84) | 56 (21–76) | 58 (33–72) | 0.15 |
|
| |||||
| Sex | |||||
| Female | 23 (38%) | 30 (45%) | 30 (45%) | 24 (37%) | 0.70 |
| Male | 37 (62%) | 37 (55%) | 37 (55%) | 41 (63%) | ·· |
|
| |||||
| ECOG performance status | |||||
| 0 | 55 (92%) | 60 (90%) | 56 (84%) | 51 (78%) | 0.14 |
| 1 | 5 (8%) | 7 (10%) | 11 (16%) | 14 (22%) | ·· |
|
| |||||
| T stage | |||||
| T2 | 2 (3%) | 6 (9%) | 7 (10%) | 5 (8%) | 0.86 |
| T3 | 57 (95%) | 60 (90%) | 57 (85%) | 55 (85%) | ·· |
| T4 | 1 (2%) | 1 (1%) | 3 (4%) | 3 (5%) | ·· |
| Tx | 0 | 0 | 0 | 2 (3%) | ·· |
|
| |||||
| N stage | |||||
| N0 | 19 (32%) | 12 (18%) | 15 (22%) | 18 (28%) | 0.076 |
| N1 | 39 (65%) | 52 (78%) | 43 (64%) | 36 (55%) | ·· |
| N2 | 1 (2%) | 3 (4%) | 9 (13%) | 11 (17%) | ·· |
| Nx | 1 (2%) | 0 | 0 | 0 | ·· |
|
| |||||
| Staged by | |||||
| Endorectal ultrasound only | 53 (88%) | 52 (78%) | 51 (76%) | 39 (60%) | 0.004 |
| MRI only | 3 (5%) | 12 (18%) | 7 (10%) | 18 (28%) | ·· |
| Both | 4 (7%) | 3 (4%) | 9 (13%) | 8 (12%) | ·· |
|
| |||||
| Clinical stage | |||||
| II | 19 (32%) | 12 (18%) | 15 (22%) | 18 (28%) | 0.29 |
| III | 41 (68%) | 55 (82%) | 52 (78%) | 47 (72%) | ·· |
|
| |||||
| Distance from anal verge (cm) | 6.9 (3.0) | 6.2 (3.1) | 7.1 (2.9) | 6.7 (3.4) | 0.42 |
|
| |||||
| Size (cm) | 4.6 (1.5) | 5.0 (2.0) | 4.6 (1.8) | 5.3 (2.1) | 0.10 |
Data are median (range), number (%), or mean (SD). Some percentages do not add up to 100 because of rounding. p values test the null hypothesis of equal means or proportions across study groups. ECOG=Eastern Cooperative Oncology Group.
Three patients died during the course of neoadjuvant treatment and thus were excluded from assessment for pathological complete response and surgical morbidity (figure 2). One patient in group 1 died of cardiac arrest during the interval between chemoradiation and total mesorectal excision, one patient in group 2 with chronic pulmonary disease transitioned to palliative measures alone, and one patient in group 3 died of cardiac arrest during the first cycle of mFOLFOX6.
Eight patients were diagnosed with metastatic disease during treatment (three [5%] in group 1, two [3%] in group 2, one [1%] in group 3, and two [3%] in group 4). Of these, four were found to have metastatic disease before surgery and therefore discontinued protocol treatment (one in group 1, two in group 2, and one in group 4) and were excluded from the analyses for pathological complete response and surgical complications (figure 2). The other four patients (two in group 1, one in group 3, and one in group 4) completed protocol treatment and metastatic disease was identified during surgery.
Patients in all study groups received chemoradiation at the outset of neoadjuvant treatment. The cumulative dose of continuous infusion fluorouracil did not differ between groups (p=0.21), nor did the cumulative dose of radiation received (p=0.17), or the number of unscheduled interruptions during chemoradiation (p=0.73).
The mean number of mFOLFOX6 cycles received preoperatively per patient increased across study groups according to the intended trial design, ranging from no cycles in group 1, to 1.7 cycles (SD 0.7) in group 2, 3.5 cycles (1.1) in group 3, and 5.0 cycles (2.1) in group 4 (p<0.0001; appendix p 2). The total dose of oxaliplatin received preoperatively also increased according to study group (p<0.0001; appendix p 2). The mean interval from completion of chemoradiation to total mesorectal excision also increased, from 8.5 weeks (SD 4.2) in group 1, to 11.1 weeks (2.9) in group 2, 15.4 weeks (2.6) in group 3, and 19.3 weeks (4.2) in group 4 (p=0.0001; table 2). As expected from the study design, we found an association between the number of cycles of mFOLFOX6 received and the time interval from completion of chemoradiation to surgery (Pearson correlation 0.806; p<0.0001). The proportion of patients completing all planned cycles of mFOLFOX6 in groups 2–4 did not differ: 55 of 67 (82%) in group 2, 54 of 67 (81%) in group 3, and 50 of 65 (77%) in group 4 (p=0.50).
Table 2. Surgical results.
| Group 1 (n=60) | Group 2 (n=67) | Group 3 (n=67) | Group 4 (n=65) | p value | |
|---|---|---|---|---|---|
| Time from start of chemoradiation to surgery (weeks) | 14.2 (4.3) | 17.1 (2.9) | 21.0 (27) | 25.2 (4.0) | 0.0001 |
|
| |||||
| Time from end of chemoradiation to surgery (weeks) | 8.5 (4.2) | 11.1 (2.9) | 15.4 (2.6) | 19.3 (4.2) | 0.0001 |
|
| |||||
| Sphincter-saving surgery | 46 (77%) | 50 (75%) | 50 (75%) | 44 (68%) | 0.68 |
| Ileostomy | 38/46 (83%) | 43/50 (86%) | 47/50 (94%) | 38/43 (88%)* | 0.33 |
|
| |||||
| Resection with negative margins | 59 (98%) | 67 (100%) | 64 (96%) | 64 (100%)† | 0.089 |
|
| |||||
| Number of nodes examined | 12 (2–31) | 14 (2–30) | 13 (2–30) | 11 (1–47) | 020 |
|
| |||||
| Pelvic fibrosis‡ | 24 (17) | 3.9 (2.6) | 4.4 (2.4) | 3.9 (2.4) | 0.0001 |
|
| |||||
| Technical diffi culty§ | 4.6 (27) | 4.9 (2.8) | 5.1 (2.5) | 4.8 (2.4) | 0.80 |
|
| |||||
| Estimated blood loss (mL) | 200 (50–1200) | 225 (25–1500) | 200 (50–1000) | 150 (0–1000) | 0.62 |
Data are mean (SD), number (%), n/N (%), or median (range). p values test the null hypothesis of equal means or proportions across study groups.
Information on whether an ileostomy was created or not was not available for one patient.
Data missing for one patient.
Scale ranges from 1 (none) to 10 (maximum).
Scale ranges from 1 (easy) to 10 (difficult).
The proportion of patients achieving a pathological complete response among the 259 assessable patients increased with each study group: 11 (18%, 95% CI 10–30) of 60 in group 1, 17 (25%, 16–37) of 67 in group 2, 20 (30%, 19–42) of 67 in group 3, and 25 (38%, 27–51) of 65 in group 4 (p=0.0036; table 3). Across all four study groups, no patients experienced progression of the primary tumour during treatment. In group 1, five (8%) of 60 patients had stable disease on pathological assessment. One patient in group 3 and one in group 4 had stable disease, but neither received any cycles of chemotherapy because of chemoradiation-related adverse events. Thus, every patient who received some chemotherapy after chemoradiation achieved either a partial or complete response of the primary tumour.
Table 3. Pathological tumour response.
| Group 1 (n=60) | Group 2 (n=67) | Group 3 (n=67) | Group 4 (n=65) | p value | |
|---|---|---|---|---|---|
| Pathological complete response | 11 (18%) | 17 (25%) | 20 (30%) | 25 (38%) | 0.0036 |
| Partial response | 44 (73%) | 50 (75%) | 46 (69%) | 39 (60%) | ·· |
| Stable disease | 5 (8%) | 0 | 1 (1%) | 1 (2%) | ·· |
Data are number (%). p value tests the null hypothesis of equal proportions across study groups.
68 (26%) of 259 patients experienced grade 3 adverse events from chemoradiation, with the most common adverse events being diarrhoea (15 patients; 6%) or lymphopenia (15 patients; 6%). Six patients (2%) had grade 4 complications from chemoradiation. No deaths occurred during chemoradiation. The proportion of patients who experienced grade 3 or 4 complications during chemoradiation did not differ between study groups (data not shown).
The proportion of patients experiencing adverse events during mFOLFOX6 treatment increased from group 2 to group 4. In group 2, two (3%) of 67 patients had grade 3 adverse events and one (1%) had a grade 4 adverse event; in group 3, 12 (18%) of 67 patients had grade 3 adverse events; in group 4, 18 (28%) of 65 patients had grade 3 adverse events and five (8%) had grade 4 adverse events. The most common grade 3 or higher adverse events from mFOLFOX6 across study groups 2-4 were neutropenia in 11 patients (6%; five in group 3 and six in group 4) and lymphopenia in seven patients (4%; three in group 3 and four in group 4). 18 (9%) of patients experienced neuropathy during mFOLFOX6 treatment, all of which were either grade 1 or 2. One patient had grade 1 neuropathy in group 2, six had grade 1 and one had grade 2 neuropathy in group 3, and nine had grade 1 and one had grade 2 neuropathy in group 4.
Table 2 summarises surgical results. The proportion of patients who received a sphincter-saving surgery and resection with negative margins was not significantly different between study groups (p=0.68 and p=0.089, respectively). The number of nodes examined and estimated blood loss were similar across all study groups (p=0.20 and p=0.62, respectively). Pelvic fibrosis, as measured by surgeon scoring from 1 (none) to 10 (maximum), increased in groups 2–4 (p=0.0001). However, the technical difficulty of the operation, as scored by the surgeon, was not significantly different across study groups (p=0.80).
No patient died during or after surgery in any study group. There was no significant difference in the number of grade 3 or worse complications across study groups (all p>0.1; table 4). Grade 3 or worse complications were noted for nine (15%) patients in group 1, four (6%) patients in group 2, three (4%) patients in group 3, and six (9%) patients in group 4, when counting the maximum Clavien-Dindo grade complication for each patient. Of the 25 grade 3 or worse complications reported across all study groups, the most common were pelvic abscesses (seven patients: three in group 1, two in group 3, and two in group 4) and anastomotic leaks (seven patients: three in group 1, one in group 2, one in group 3, and two in group 4).
Table 4. Summary of surgical complications by Clavien-Dindo grading.
| Group 1 (n=60) | Group 2 (n=67) | Group 3 (n=67) | Group 4 (n=65) | p value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Number of patients* | Number of events | Number of patients* | Number of events | Number of patients* | Number of events | Number of patients* | Number of events | ||
| None | 36 (60%) | NA | 41 (61%) | NA | 44 (66%) | NA | 37 (57%) | NA | ·· |
| Grade 1 | 11 (18%) | 16 | 12 (18%) | 18 | 10 (15%) | 16 | 11 (17%) | 14 | 0.88 |
| Grade 2 | 4 (7%) | 6 | 10 (15%) | 12 | 10 (15%) | 13 | 11 (17%) | 16 | 0.04 |
| Grade 3a | 2 (3%) | 2 | 1 (1%) | 2 | 1 (1%) | 1 | 4 (6%) | 5 | 0.27 |
| Grade 3b | 5 (8%) | 6 | 2 (3%) | 2 | 2 (3%) | 2 | 2 (3%) | 2 | 0.11 |
| Grade 4a | 2 (3%) | 2 | 1 (1%) | 1 | 0 | 0 | 0 | 0 | 018 |
Some patients had more than one complication. Some percentages do not add up to 100 because of rounding. p values test the null hypothesis of equal proportions of number of events across study groups for each grade. NA=not applicable.
The maximum grade complication is counted for each patient.
Using univariable logistic regression, we assessed known clinically relevant variables and study groups and their association with pathological complete response. The comparison of group 4 (the most intense regimen) with group 1 (the standard neoadjuvant regimen) showed a significant association with pathological complete response (p=0.028; table 5). We then used multivariable logistic regression to model the probability of pathological complete response, examining whether a treatment effect on pathological complete response was present after adjusting for other known clinically relevant variables. In an intention-to-treat analysis that included study group, radiation dose, tumour stage, size, and distance to anal verge as variables, we found study group to be the only significant predictor of pathological complete response (p=0.048; table 5). Patients in group 4 were significantly more likely to achieve a pathological complete response than were patients in group 1 (odds ratio 3.49, 95% CI 1.39–8.75; p=0.011). In preplanned analyses, we tested two additional models in which we substituted study group for the treatment delivered as measured by cycles of mFOLFOX6 or the chemoradiation-to-surgery interval; both were significant predictors of pathological complete response (p=0.028 and p=0.018, respectively).
Table 5. Univariable and multivariable regression for pathological complete response.
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Radiation dose | 1.00 (0.99–1.00) | 0.40 | 1.00 (0.99–1.00) | 0.13 |
|
| ||||
| Clinical stage | ||||
| II | 1.22 (0.66-2.25) | 0.53 | 1.26 (0.63-2.51) | 0.52 |
| III | 1.00 | ·· | 1.00 | ·· |
|
| ||||
| Tumour size | 0.93 (0.80-1.09) | 0.38 | 0.90 (0.76-1.07) | 0.24 |
|
| ||||
| Distance from anal verge | 1.02 (0.93-1.12) | 0.65 | 0.98 (0.89-1.09) | 0.73 |
|
| ||||
| Study group | ||||
| 1 | 1.00 | 0.092* | 1.00 | 0.048* |
| 2 | 1.52 (0.64-3.56) | 0.67 | 1.58 (0.59-4.23) | 0.63 |
| 3 | 1.90 (0.82-4.38) | 0.61 | 1.95 (0.75-5.07) | 0.79 |
| 4 | 2.78 (1.22-6.34) | 0.028 | 3.49 (1.39-8.75) | 0.011 |
p values are based on the Wald χ2 test and show whether a variable or a specific comparison is associated with pathological complete response. OR=odds ratio.
Used for categorical variables, the type 3 Wald χ2 test shows whether the study group as a whole is associated with pathological complete response.
Discussion
In this multi-institutional trial with sequential study groups, we show that adding mFOLFOX6 after chemoradiation and lengthening the chemoradiation-to-surgery interval increases the proportion of patients with locally advanced rectal cancer who achieve a pathological complete response. 25 (38%) of 65 patients in group 4, who were assigned to receive six cycles of mFOLFOX6 after chemoradiation, achieved a pathological complete response, which is one of the highest proportions reported so far for stage II-III rectal cancer. Of equal importance, we show that this approach seems to be safe from both an oncological and surgical standpoint; it did not increase the risk of tumour progression, technical difficulty, or surgical complications. If these findings can be reliably reproduced, a far greater number of patients with locally advanced rectal cancer will be eligible for organ preservation, which would improve functional outcomes and quality of life.
The rationale for this study was based on the finding that the response to radiation in rectal cancer is time dependent. In the Lyon R90-01 study, Francois and colleagues13 revealed that 14% of patients who had surgery 8 weeks after radiation achieved pathological complete response compared with 7% when surgery was done 4 weeks after radiation. Findings from several retrospective case series have confirmed that a longer chemoradiation-to-surgery interval is associated with higher proportions of patients achieving a pathological complete response.14,19–24 We chose pathological complete response as our primary endpoint to assess how many patients are potentially eligible for a non-operative approach and to establish whether the proportion of patients achieving a pathological complete response could be increased by lengthening the duration between chemoradiation and surgery. However, at the time that this trial was designed, lengthening the interval between chemoradiation and surgery was thought to be unsafe because it would delay the administration of adjuvant systemic chemotherapy, potentially increasing the risk of metastasis. There were also concerns about the safety of delaying surgery beyond 8 weeks after chemoradiation because of the potential development of pelvic fibrosis that could increase technical difficulty and postoperative complications. To address these concerns, we designed our trial to have a sequence of study groups in which increasing cycles of mFOLFOX6 were delivered after chemoradiation. Having established the proportion of patients achieving a pathological complete response and who experienced complications at baseline in group 1, in which patients were treated according to standard practice, the opening of each subsequent group was decided only after proving the safety of the previous one. Therefore, the study design linked the number of cycles of mFOLFOX6 with the time interval between chemoradiation and surgery, so their relative contributions to the increase in pathological complete response cannot be ascertained from this trial.
The proportion of patients who achieved pathological complete response in group 1 of our trial (18%) is within the range reported in large phase 3 trials25–28 that included patients with equivalent stage rectal cancers treated with similar chemoradiation regimens and chemoradiation-to-surgery intervals. The proportion of patients who achieved pathological complete response increased in each subsequent study group when analysed in an intention-to-treat fashion, and modelling on the basis of the number of mFOLFOX6 cycles received or the time interval between chemoradiation and surgery was independently associated with pathological complete response.
The alternative approach of sequencing chemotherapy with a fluoropyrimidine and oxaliplatin before chemoradiation in the neoadjuvant setting has shown slight increases in the proportion of patients achieving a pathological complete response and possibly improved long-term oncological outcome in several studies.6–11 In the Grupo Cáncer de Recto 3 phase 2 study,29 the proportion of patients achieving a pathological complete response did not differ when the combination of capecitabine and oxaliplatin was provided before chemoradiation and surgery compared with the postoperative setting. Only findings from the AVACROSS study30 showed a proportion of patients achieving a pathological complete response equivalent to that in our study. In the AVACROSS study,30 the addition of bevacizumab to capecitabine plus oxaliplatin before chemoradiation increased the proportion of patients who achieved a pathological complete response to 36%, but at the cost of more serious surgery-associated complications; two (4%) patients died while receiving neoadjuvant treatment, and 11 (24%) needed additional surgical intervention, mostly because of anastomotic failures.30 Although our trial design did not provide a direct comparison with these induction chemotherapy approaches, our approach of delivering systemic mFOLFOX6 after chemoradiation seemed to result in a higher proportion of patients who achieved pathological complete response compared with equivalent doses of systemic chemotherapy before chemoradiation. We believe this might signify that the time from chemoradiation to surgery has a greater contribution to pathological complete response than the administration of mFOLFOX6, but this remains speculative. This discussion could be further informed by findings from the RAPIDO trial,31 which is comparing 3-year disease-free survival in patients randomly assigned to pre operative short-course radiation followed by six cycles of capecitabine and oxaliplatin and total mesorectal excision versus traditional preoperative long-course chemoradiation and total mesorectal excision.
Lengthening the neoadjuvant treatment time by delivering mFOLFOX6 before total mesorectal excision did not increase the risk of disease progression. We did not note progression of the primary tumour on pathological assessment in any study group, and the two patients with stable disease in groups 2–4 actually never received systemic chemotherapy. Thus, every patient who received chemotherapy after chemoradiation achieved at least a partial response. The proportion of patients diagnosed with distant metastatic disease during neoadjuvant treatment and at the time of surgery did not differ between study groups. The amount of metastasis diagnosed during the treatment period was equivalent to that reported in clinical trials of similar patients treated with conventional neoadjuvant treatment protocols.26,27 The increased number of adverse events reported in relation to the administration of mFOLFOX6 in groups 2–4 is consistent with that noted with similar regimens in the adjuvant setting.27
Findings from this trial show that delivering systemic chemotherapy after chemoradiation and delaying total mesorectal excision to up to 20 weeks after completion of chemoradiation does not increase the surgical technical difficulty or the risk of surgical complications—a concern that, to the best of our knowledge, has not been addressed in a prospective manner until this point.32,33 Although there was an increase in pelvis fibrosis from group 1 to groups 2–4, technical difficulty did not increase. However, these results should be interpreted with caution because surgeons were not masked to study group. A prolonged chemoradiation-to-surgery interval did not decrease the proportion of patients who had a resection with negative margins or a sphincter-saving procedure. Finally, overall complications and the proportion of specific complications, such as pelvic abscesses and anastomotic leaks, did not differ between study groups.
Several limitations of our trial deserve mention. Because the study was a non-randomised, phase 2 trial, unrecognised factors might have contributed to the differences reported between study groups. Although our findings lend support to the hypothesis that mFOLFOX6 after chemoradiation increases the proportion of patients achieving a pathological complete response, they should still be regarded as exploratory and in need of confirmation in a randomised trial. There was a change in the preferred staging method during the study period, with numerically decreased staging by endorectal ultrasound and increased use of MRI instead from group 1 to group 4. Although this change could have introduced a staging bias, tumour size was not significantly different between study groups, which lessens this concern. A small number of patients with excellent clinical response to neoadjuvant treatment ultimately refused surgery or opted for local excision and were therefore excluded from analyses. Thus, the proportion of patients achieving a pathological complete response might have been underestimated. This phase 2 trial was powered to assess the proportion of patients achieving a pathological complete response, rather than surgical complications or long-term oncological outcomes. However, our findings do not support concerns that lengthening the interval between chemoradiation and surgery will increase the risk of tumour progression during treatment or surgical complications. The measurement of pelvic fibrosis and surgical technical difficulty is limited because they represent two parameters that have no validated instruments or standards for measure. Still, we felt collection of the surgeons' assessment of these parameters was important since they perceived that an increased chemoradiation-to-surgery interval would result in increased fibrosis and surgical difficulty. Finally, our study used pathological complete response as the primary endpoint, and although pathological complete response is associated with improved recurrence-free survival,3,34 the long-term follow-up and survival outcomes for patients in this trial could further inform the design of future randomised trials.
In the years since this trial was designed and run, additional trials and findings have led to an increasing interest in watch-and-wait approaches, which are beginning to be investigated in clinical trials. Although our study protocol did not assess the clinical or radiological response of the cancer immediately before surgery, these measurements will be essential to learn how reliably we can identify complete responders. This unanswered question has contributed to the design of a trial in which patients are being randomly assigned to either chemoradiation followed by chemotherapy— similar to the regimen in the present study—or chemotherapy followed by chemoradiation. After the completion of these regimens, patients will be restaged by clinical examination, endoscopy, and MRI, and the amount of response will be used to decide between total mesorectal excision or watch-and-wait approaches (NCT02008656). This trial will also directly investigate the quality-of-life differences between a watch-and-wait approach and total mesorectal excision,35 for which there are no prospective data at this time.
Supplementary Material
Research in context.
Evidence before this study
Some locally advanced rectal cancers respond completely to neoadjuvant chemoradiation. Patients who have a complete response could be eligible for less invasive surgeries or even a watch-and-wait approach, but the proportion of patients who achieve a pathological complete response remains low. Response to radiation is time dependent and longer intervals from radiation to surgery are associated with an increase in the proportion of patients who achieve a pathological complete response. However, deferral of surgery has encountered resistance because of the hypothetical risk of primary tumour progression in non-responders, the increase in technical difficulty of the surgery, and the delay in adjuvant chemotherapy. Delivering systemic chemotherapy before chemoradiation has been associated with a slight increase in tumour response. We postulated that delivering systemic chemotherapy after chemoradiation would be more effective by expanding the neoadjuvant treatment and lengthening the interval from radiation to surgery.
Added value of this study
In this multicentre trial, a correspondingly greater proportion of patients with locally advanced rectal cancer given increasing cycles of mFOLFOX6 after chemoradiation but before surgery achieved a pathological complete response. We show that the improvement in response is not associated with tumour progression, an increase in technical difficulty, or surgical complications.
Implications of all the available evidence
These findings support efforts to shift systemic treatments into the neoadjuvant setting and suggest that delivering chemotherapy after chemoradiation could be more effective at increasing the proportion of patients achieving a pathological complete response than before chemoradiation. If high proportions of complete responses can be replicated, a greater amount of patients with locally advanced rectal cancer could be eligible for less invasive surgical or watch-and-wait approaches.
Acknowledgments
This study was supported by the National Institutes of Health National Cancer Institute R01 Grant CA090559 (to JG-A).
This study was funded in part by the Cancer Center core grant P30 CA008748.
Footnotes
See Online for appendix
Contributors: JG-A, DDS, JEM, PAC, MGV, ASK, SO, TC, SRH, MJS, CAT, DOH, AF, BNP, DWD, and KA designed the study. JG-A, OSC, DDS, JEM, PAC, MGV, ASK, SO, TC, SRH, MJS, CAT, DOH, AF, BNP, DWD, and KA collected data. DDS and SP did the statistical analysis. JG-A and OSC wrote the manuscript and prepared the figures. All authors contributed towards data analysis, data interpretation, and manuscript revisions.
Declaration of interests: JEM reports grants from MSK. MJS reports grants, personal fees, and stock ownership in Novadaq; grants and personal fees from Gore; personal fees from Ethicon, Olympus, NiTi, Edwards Life Sciences, and CR Bard; and author royalties from Elsevier. KA reports grants from National Institutes of Health. JG-A, OSC, DDS, PAC, MGV, ASK, SO, TC, SRH, CAT, DOH, AF, BNP, DWD, and SP declare no competing interests.
References
- 1.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 2.Williams NS, Johnston D. The quality of life after rectal excision for low rectal cancer. Br J Surg. 1983;70:460–62. doi: 10.1002/bjs.1800700805. [DOI] [PubMed] [Google Scholar]
- 3.Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835–44. doi: 10.1016/S1470-2045(10)70172-8. [DOI] [PubMed] [Google Scholar]
- 4.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240:711–17. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith JD, Ruby JA, Goodman KA, et al. Nonoperative management of rectal cancer with complete clinical response after neoadjuvant therapy. Ann Surg. 2012;256:965–72. doi: 10.1097/SLA.0b013e3182759f1c. [DOI] [PubMed] [Google Scholar]
- 6.Calvo FA, Serrano FJ, Diaz-González JA, et al. Improved incidence of pT0 downstaged surgical specimens in locally advanced rectal cancer (LARC) treated with induction oxaliplatin plus 5-fluorouracil and preoperative chemoradiation. Ann Oncol. 2006;17:1103–10. doi: 10.1093/annonc/mdl085. [DOI] [PubMed] [Google Scholar]
- 7.Schou JV, Larsen FO, Rasch L, et al. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23:2627–33. doi: 10.1093/annonc/mds056. [DOI] [PubMed] [Google Scholar]
- 8.Chua YJ, Barbachano Y, Cunningham D, et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241–48. doi: 10.1016/S1470-2045(09)70381-X. [DOI] [PubMed] [Google Scholar]
- 9.Maréchal R, Vos B, Polus M, et al. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23:1525–30. doi: 10.1093/annonc/mdr473. [DOI] [PubMed] [Google Scholar]
- 10.Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30:1620–27. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 11.Cercek A, Goodman KA, Hajj C, et al. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12:513–19. doi: 10.6004/jnccn.2014.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brierley JD, Cummings BJ, Wong CS, et al. Adenocarcinoma of the rectum treated by radical external radiation therapy. Int J Radiat Oncol Biol Phys. 1995;31:255–59. doi: 10.1016/0360-3016(94)e0102-p. [DOI] [PubMed] [Google Scholar]
- 13.Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol. 1999;17:2396. doi: 10.1200/JCO.1999.17.8.2396. [DOI] [PubMed] [Google Scholar]
- 14.Habr-Gama A, Perez RO, Proscurshim I, et al. Interval between surgery and neoadjuvant chemoradiation therapy for distal rectal cancer: does delayed surgery have an impact on outcome? Int J Radiat Oncol Biol Phys. 2008;71:1181–88. doi: 10.1016/j.ijrobp.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 15.Kerr SF, Norton S, Glynne-Jones R. Delaying surgery after neoadjuvant chemoradiotherapy for rectal cancer may reduce postoperative morbidity without compromising prognosis. Br J Surg. 2008;95:1534–40. doi: 10.1002/bjs.6377. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Jass JR, O'Brien MJ, Riddell RH, Snover DC. Recommendations for the reporting of surgically resected specimens of colorectal carcinoma. Hum Pathol. 2007;38:537–45. doi: 10.1016/j.humpath.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 19.Moore HG, Gittleman AE, Minsky BD, et al. Rate of pathologic complete response with increased interval between preoperative combined modality therapy and rectal cancer resection. Dis Colon Rectum. 2004;47:279–86. doi: 10.1007/s10350-003-0062-1. [DOI] [PubMed] [Google Scholar]
- 20.Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 weeks between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15:2661–67. doi: 10.1245/s10434-008-9892-3. [DOI] [PubMed] [Google Scholar]
- 21.Kalady MF, de Campos-Lobato LF, Stocchi L, et al. Predictive factors of pathologic complete response after neoadjuvant chemoradiation for rectal cancer. Ann Surg. 2009;250:582–89. doi: 10.1097/SLA.0b013e3181b91e63. [DOI] [PubMed] [Google Scholar]
- 22.Wolthuis AM, Penninckx F, Haustermans K, et al. Impact of interval between neoadjuvant chemoradiotherapy and TME for locally advanced rectal cancer on pathologic response and oncologic outcome. Ann Surg Oncol. 2012;19:2833–41. doi: 10.1245/s10434-012-2327-1. [DOI] [PubMed] [Google Scholar]
- 23.Zeng WG, Zhou ZX, Liang JW, et al. Impact of interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer on surgical and oncologic outcome. J Surg Oncol. 2014;110:463–67. doi: 10.1002/jso.23665. [DOI] [PubMed] [Google Scholar]
- 24.Calvo FA, Morillo V, Santos M, et al. Interval between neoadjuvant treatment and definitive surgery in locally advanced rectal cancer: impact on response and oncologic outcomes. J Cancer Res Clin Oncol. 2014;140:1651–60. doi: 10.1007/s00432-014-1718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773–80. doi: 10.1200/JCO.2010.34.4911. [DOI] [PubMed] [Google Scholar]
- 26.Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol. 2012;30:4558–65. doi: 10.1200/JCO.2012.42.8771. [DOI] [PubMed] [Google Scholar]
- 27.Rödel C, Liersch T, Becker H, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13:679–87. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 28.O'Connell MJ, Colangelo LH, Beart RW, et al. Capecitabine and oxaliplatin in the preoperative multimodality treatment of rectal cancer: surgical end points from National Surgical Adjuvant Breast and Bowel Project trial R-04. J Clin Oncol. 2014;32:1927–34. doi: 10.1200/JCO.2013.53.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernández-Martos C, Pericay C, Aparicio J, et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo Cáncer de Recto 3 study. J Clin Oncol. 2010;28:859–65. doi: 10.1200/JCO.2009.25.8541. [DOI] [PubMed] [Google Scholar]
- 30.Nogué M, Salud A, Vicente P, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist. 2011;16:614–20. doi: 10.1634/theoncologist.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nilsson PJ, van Etten B, Hospers GAP, et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer—the RAPIDO trial. BMC Cancer. 2013;13:279. doi: 10.1186/1471-2407-13-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Supiot S, Bennouna J, Rio E, et al. Negative influence of delayed surgery on survival after preoperative radiotherapy in rectal cancer. Colorectal Dis. 2006;8:430–35. doi: 10.1111/j.1463-1318.2006.00990.x. [DOI] [PubMed] [Google Scholar]
- 33.Van den Broek CBM, Vermeer TA, Bastiaannet E, et al. Impact of the interval between short-course radiotherapy and surgery on outcomes of rectal cancer patients. Eur J Cancer. 2013;49:3131–39. doi: 10.1016/j.ejca.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 34.De Campos-Lobato LF, Stocchi L, da Luz Moreira A, et al. Pathologic complete response after neoadjuvant treatment for rectal cancer decreases distant recurrence and could eradicate local recurrence. Ann Surg Oncol. 2011;18:1590–98. doi: 10.1245/s10434-010-1506-1. [DOI] [PubMed] [Google Scholar]
- 35.Temple LK, Bacik J, Savatta SG, et al. The development of a validated instrument to evaluate bowel function after sphincter-preserving surgery for rectal cancer. Dis Colon Rectum. 2005;48:1353–65. doi: 10.1007/s10350-004-0942-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.