Abstract
Aim
Advanced age is associated with vascular endothelial dysfunction, characterized by reductions in endothelium-dependent vasodilation of conduit and resistance arteries, in part from decreased nitric oxide (NO) bioavailability. Although vascular smooth muscle function (SMF), assessed by responsiveness to an exogenous NO donor, is typically reported to be intact, many of these studies are limited by small sample size. Therefore, the purpose of this meta-analysis is to systematically review and determine whether vascular SMF is different between older versus young healthy subjects.
Design
We conducted a systematic search of MEDLINE, Cochrane and Scopus, since their inceptions until January 2014 for articles evaluating SMF in the brachial artery and/or resistance arteries (BASMF and RASMF, respectively), as assessed by the endothelium-independent vasodilator response to exogenous NO donors in older (≥60 years) and young (<30 years) groups of healthy subjects. Meta-analyses were performed to compare the mean difference (MD) in BASMF and the standardized mean difference (SMD) in RASMF between older and young groups. Subgroup analyses were performed to identify sources of heterogeneity.
Results
Fifteen studies assessing BASMF and 20 studies assessing RASMF were included, comprising 550 older and 516 young healthy subjects. After data pooling, BASMF and RASMF were lower in older compared with young groups (MD=−1.89 %, P=0.04; SMD=−0.46, P=0.0008, respectively). Significant heterogeneity was observed in the BASMF (I2=74 %; P<0.00001) and RASMF (I2=57 %; P=0.0008) meta-analyses. Subgroup analyses revealed that studies with (predominantly) males showed similar SMF responses between older and young groups.
Conclusions
Based on current published studies, vascular SMF is reduced in conduit and resistance arteries of otherwise healthy older subjects, particularly in women.
Keywords: advanced age, vascular smooth muscle function, conduit artery, resistance arteries
INTRODUCTION
Older age is a primary risk factor for the development of cardiovascular diseases [1, 2]. The influence of advanced age on increased cardiovascular risk is, at least partly, mediated through its effects on the arterial vasculature [1]. In this regard, ageing is associated with vascular endothelial dysfunction in humans, characterized by reduced endothelium-dependent vasodilator function in conduit [3–13] and resistance [6, 9, 14–24] arteries. Alterations in the nitric oxide (NO) signaling pathway and/or decreased NO bioavailability have been proposed to contribute to the age-related decrease in endothelium-dependent vasodilator function [25, 26].
The ability of vessels to dilate is dependent on the function of the endothelium, but is also subordinated to vascular smooth muscle (vasodilator) function (SMF). Smooth muscle responsiveness represents the final, and frequently underappreciated, step of endothelium-dependent vasodilation. However, relatively little is known about the impact of advanced age on SMF. Some reports from animal studies indicate that advanced age is associated with reduced vascular SMF [27–29]; nonetheless, this finding is not universal [25]. In humans, studies assessing vascular SMF in healthy older and young subjects have reported variable results, possibly as a result of small sample sizes [3–6, 8–10, 12, 14–24, 30–43].
Therefore, the primary aim of this study was to perform a systematic review and meta-analyses of available studies comparing vascular SMF in older and young healthy subjects. We hypothesized that SMF would be reduced in conduit and resistance arteries of older compared with young healthy adults. We selected studies that assessed SMF, determined by the endothelium-independent vasodilator response to exogenous NO donors, in the brachial artery (BASMF) and in resistance arteries (RASMF) of healthy young and healthy older subjects.
METHODS
The review is reported according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) Group guidelines [44].
Data sources and searches
Our systematic search included MEDLINE, Cochrane and Scopus, since their inceptions until January 2014. We used combinations of the subject headings “older”, “vascular smooth muscle”, “endothelium independent”, “nitroglycerin”, “sodium nitroprusside”, “vascular function”, “vasodilation” and “vascular reactivity”; the search strategy for MEDLINE is shown in Supplemental Figure S1. We also performed hand searching in reference citations of articles included in meta-analysis and related citations in MEDLINE.
Study selection
To be included in the analysis, an observational report had to assess BASMF and/or RASMF in an older group (mean age ≥60 years) and a young group (mean age <30 years) of healthy subjects. In the event of multiple publications pertaining to the same research, the first published or most comprehensive study was included. Inclusion of studies was not limited by publication status or language.
Data extraction and quality assessment
The following variables were extracted into a pre-formatted spreadsheet: authors, year of publication, characteristics of study participants (n, gender, age, height, weight, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), physical activity, maximal oxygen consumption (VO2max), morbidities, risk factors to health, medication) and vascular variables (vascular region, vasodilation parameter, baseline vascular tone, NO donor, dosage, time of analysis after NO donor administration, wall-tracking system, BASMF, RASMF, endothelial function). A systematic appraisal of quality for observational research (SAQOR) [45] previously applied in meta-analysis of observational studies evaluating arterial function [46] was performed to provide assessment of study quality. The SAQOR was adjusted to assess 1) the older sample group, 2) the young sample group, 3) quality of vascular SMF measurement, 4) confounding variables and 5) data. Overall, the SAQOR was scored out of 16, quality deemed better with a greater score (Table S1 and S2).
Data synthesis and analysis
The meta-analyses and subgroup analyses were performed using Review Manager software (RevMan 5.2, Cochrane Collaboration, Oxford, UK). The primary outcomes were the mean difference (MD) in BASMF and the standardized mean difference (SMD) in RASMF between older and young groups. SMD summary statistic allowed us to standardize RASMF values expressed in different units into a uniform scale to complete this meta-analysis. Each MD and SMD was weighted according to the inverse variance method [47] and they were pooled with a random-effects model [48]. SMD of 0.2, 0.5, and 0.8 represents small, medium, and large effect sizes, respectively [49]. Heterogeneity between studies was assessed using the chi-squared test for heterogeneity and I2 statistics. Potential moderating factors were evaluated by subgroup analysis comparing studies grouped by dichotomous or continuous variables potentially influencing vascular SMF. Median values of continuous variables were used as cut-off values for grouping studies. Publication bias was evaluated by estimating the asymmetry of the Begg and Mazumdar’s funnel plot [50]. A P value of less than 0.05 was considered statistically significant.
RESULTS
Study selection and characteristics
The flow diagram of the process of study selection is shown in Figure 1, which resulted in the inclusion of 33 articles. Thirteen of these articles assessed BASMF, 19 assessed RASMF and 1 assessed both BASMF and RASMF. One of the articles assessing BASMF presented two groups of older subjects, each of which had been independently compared with a location-matched young group [43]. Therefore, these data were evaluated as two individual studies. Table 1 shows the main clinical characteristics of the 15 BASMF studies and 20 RASMF studies, comprising a total of 550 subjects in the older group and 516 subjects in the young group. Older and young groups were gender-matched in all studies (omitting 1 study in which gender-related data was not available [22]). All subjects were free from co-morbidities and risk factors according to cut-off values, non-smokers (except for 1 study allowing < 5 cigarettes per day [23]) and not taking medications (other than oral contraceptives reported in 1 study [31]). The quality of the studies was moderate-to-high according to a previously validated scale [45, 51]. The mean score was 13.9±1.2 for studies assessing BASMF and 11.8±1.2 for studies assessing RVSMF, out of a possible 16 points (Table S1 and S2). As for the evaluation of potential bias, the Begg and Mazumdar’s funnel plot for the MD in BASMF was moderately asymmetric, suggesting the presence of publication bias and/or other biases (Figure S2). The Begg and Mazumdar’s funnel plot for the SMD in RVSMF was relatively symmetrical (Figure S3).
FIGURE 1.
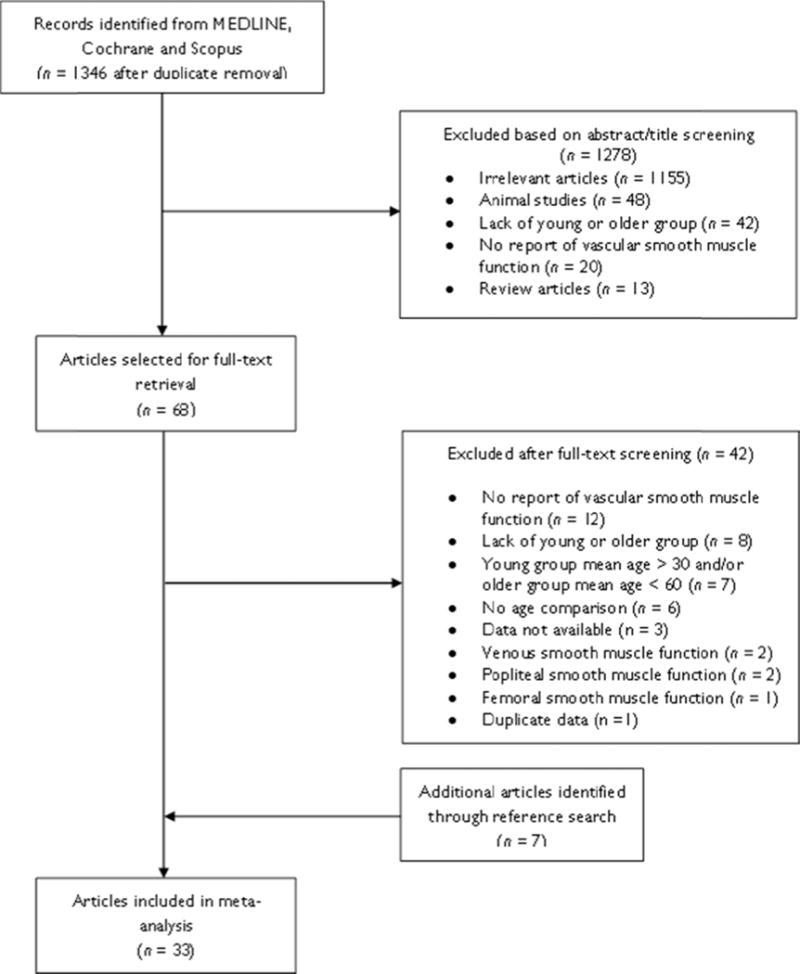
Flow diagram of the process of study selection
TABLE 1.
Main clinical characteristics of studies included in the meta-analyses
| n | Females (%) | Age (years) | BMI (m2/kg) | SBP (mm Hg) | DBP (mm Hg) | Morbidities | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study, year of publication | YNG | OLD | YNG | OLD | YNG | OLD | YNG | OLD | YNG | OLD | YNG | OLD | |
| Studies assessing brachial artery SMF | |||||||||||||
| DeVan et al [4], 2013 | 21 | 25 | 12 | 17 | 24±5 | 62±6* | 23±5 | 26±6* | 111±11 | 121±12* | 60±5 | 75±6* | none |
| Pierce et al [42], 2011 | 16 | 18 | 0 | 0 | 25±4 | 63±5* | 26±4 | 27±3 | 118±13 | 124±11 | 69±9 | 78±5* | none |
| Black et al [3], 2009 | 9 | 8 | 100 | 100 | 26±3 | 60±6* | 23±3 | 31±6* | 101±12 | 122±14* | 56±9 | 67±8* | none |
| Donato et al [6], 2009 | 27 | 23 | 0 | 0 | 22±5 | 62±5* | 25±5 | 26±5* | 117±11 | 124±10* | 63±11 | 78±10* | none |
| Walker et al [12], 2009 | 26 | 15 | 0 | 0 | 23±10 | 66±4* | 24±5 | 26±4 | 116±10 | 124±12* | 61±5 | 74±8* | none |
| Donato et al [5], 2007 | 51 | 44 | 0 | 0 | 23±7 | 63±7* | 24±7 | 26±7* | 114±7 | 121±13* | 62±7 | 75±7* | none |
| Gates et al [39], 2007 | 10 | 12 | 50 | 50 | 21±3 | 60±7* | 22±3 | 25±3* | 105±6 | 114±17 | 60±6 | 70±10* | none |
| Parker et al [41], 2006 | 8 | 8 | 100 | 100 | 22±4 | 70±7* | 22±3 | 25±3* | 109±15 | 131±11* | 68±7 | 78±11* | none |
| Eskurza et al [36], 2006 | 9 | 9 | 11 | 11 | 26±3 | 64±6* | 24±4 | 26±2 | 110±6 | 108±9 | 60±9 | 71±9* | none |
| Eskurza et al [38], 2005 | 10 | 9 | 0 | 0 | 22±3 | 62±6* | 23±3 | 26±3 | 110±9 | 111±12 | 58±6 | 69±3* | none |
| Heiss et al [40], 2005 | 20 | 20 | 50 | 65 | 25±4 | 61±9* | 22±4 | 24±4 | 117±9 | 122±13 | 77±9 | 81±9 | none |
| Eskurza et al [37], 2004 | 11 | 9 | 0 | 0 | 25±3 | 64±6* | 24±3 | 26±3 | 113±7 | 116±12 | 63±7 | 73±12* | none |
| McCrohon et al [10], 2000 | 10 | 10 | 100 | 100 | 28±5 | 61±3* | 23±2 | 24±2 | 106±13 | 124±9* | 75±10 | 79±12 | none |
| Woo et al A [43], 1997 | 19 | 19 | 68 | 68 | 29±5 | 63±5* | 24±8 | 23±4 | 115±11 | 129±14* | 76±10 | 83±8 | none |
| Woo et al B [43], 1997 | 19 | 19 | 68 | 68 | 28±6 | 63±4* | 23±3 | 25±3 | 108±9 | 127±13* | 75±7 | 79±11 | none |
| Mean | 18 | 17 | 37 | 39 | 25 | 63 | 23 | 26 | 111 | 121 | 66 | 75 | – |
| Studies assessing resistance artery SMF | |||||||||||||
| Millet et al [30], 2012 | 20 | 42 | 85 | 86 | 24±3 | 69±7* | 22±3 | 23±4 | 116±8 | 128±10* | 67±7 | 71±7* | none |
| Donato et al [8], 2011 | 16 | 22 | 25 | 32 | 25±4 | 64±5* | 24±3 | 28±5* | 111±12 | 126±14* | 71±8 | 78±9* | none |
| Westby et al [24], 2011 | 14 | 14 | 0 | 0 | 25±4 | 61±7* | 24±2 | 26±2* | 114±11 | 125±11* | 66±7 | 78±7* | none |
| Kirby et al [16], 2010 | 13 | 13 | 38 | 38 | 21±4 | 66±11* | 22±2 | 24±3* | N/A | N/A | N/A | N/A | none |
| Tew et al [19], 2010 | 15 | 14 | 20 | 29 | 27±2 | 65±6* | 25 | 27 | 119±12 | 124±12 | 75±9 | 79±7 | none |
| Donato et al [6], 2009 | 15 | 18 | 0 | 0 | 21±4 | 62±4* | 25±4 | 26±4* | 117±8 | 124±8* | 63±8 | 78±8* | none |
| Nicholson et al [31], 2009 | 10 | 10 | 40 | 40 | 29±9 | 68±4* | 24±3 | 25±2 | 116±16 | 122±13 | 62±8 | 69±12 | none |
| Kirby et al [17], 2009 | 14 | 14 | 36 | 36 | 22±4 | 65±7* | 25±3 | 24±3 | N/A | N/A | N/A | N/A | none |
| Donato et al [32], 2008 | 11 | 14 | 18 | 29 | 23±7 | 64±4* | 24±2 | 26±3 | 110±10 | 123±11* | 71±7 | 78±7* | none |
| Galetta et al [9], 2006 | 16 | 16 | 50 | 31 | 27±2 | 65±4* | 23±2 | 24±2 | 118±7 | 122±6 | 75±3 | 80±2* | none |
| Al-Shaer et al [21], 2006 | 15 | 17 | 47 | 24 | 22±4 | 62±12* | 25±4 | 28±4* | 116±12 | 126±12* | 67±12 | 78±8* | none |
| Newcomer et al [33], 2005 | 8 | 8 | 0 | 0 | 24±6 | 67±6* | 25±3 | 26±3 | 113±11 | 129±8* | 74±11 | 81±8 | none |
| Weverling-Rijnsburger et al [34], 2004 | 7 | 8 | 0 | 0 | 22 | 80* | N/A | N/A | N/A | N/A | N/A | N/A | none |
| Ahlers et al [14], 2004 | 14 | 10 | 0 | 0 | 23±3 | 62±7* | N/A | N/A | 118±11 | 120±19 | 74±11 | 72±13 | none |
| Smith et al [35], 2003 | 10 | 20 | 0 | 0 | 28±3 | 62±4* | 25±5 | 29±3 | 115±9 | 124±9 | 64±9 | 81±9* | none |
| Minson et al [18], 2002 | 10 | 10 | 50 | 50 | 22±6 | 77±16* | 24±3 | 24±3 | 120±13 | 137±19* | 71±9 | 79±13 | none |
| DeSouza et al [15], 2002 | 22 | 41 | 0 | 0 | 28±5 | 61±6* | 23±6 | 27±4* | 114±14 | 122±13* | 65±9 | 76±13* | none |
| Wang et al [20], 2002 | 12 | 10 | 0 | 0 | 24±3 | 67±3* | 22±5 | 23±4 | 127±17 | 139±22 | 77±10 | 73±16 | none |
| Taddei et al [23], 2000 | 12 | 12 | 33 | 33 | 27±2 | 63±6* | 23±4 | 24±4 | 119±5 | 119±6 | 77±3 | 78±3 | none |
| Gerhard et al [22], 1996 | 11 | 7 | N/A | N/A | 20–29 | 60–69* | N/A | N/A | N/A | N/A | N/A | N/A | none |
| Mean | 13 | 16 | 23 | 23 | 24 | 66 | 24 | 26 | 116 | 126 | 70 | 77 | – |
Data are n, % of females, mean or mean ± SD. One study presented two groups of older subjects, each of which had been independently compared with a young group [43], thus they were evaluated as individual studies (distinguished by A and B).
significantly different from young group at P<0.05
BMI, body mass index; DBP, diastolic blood pressure; OLD, older group; SBP, systolic blood pressure; SMF, smooth muscle function; YNG, young group
Brachial artery smooth muscle function (BASMF)
All studies assessing BASMF evaluated the vasodilator response to 0.4 mg of nitroglycerin by means of high-resolution ultrasound (Table 2). Resting brachial diameter ranged from 3.0 to 4.4 mm, with older groups commonly presenting a larger resting brachial diameter than young groups. After data pooling, the meta-analysis revealed that BASMF was lower in older compared with young groups (15 studies, MD=−1.89%; P=0.04) (Figure 2). Significant heterogeneity was detected (I2=74%; P<0.00001). In subgroup analyses, studies above the median in presence of females in the study group showed lower BASMF in older compared with young groups (8 studies, MD=−3.38%; P=0.01). In contrast, studies below the median in presence of females had similar BASMF in older and young groups (7 studies, MD=0.19%; P=0.80). Both sex subgroups were significantly different when compared with each other (P=0.02) (Table S3). In addition, lower brachial artery endothelial function in older compared with young groups was related to lower BASMF (P=0.03, Table S3). No other potential moderating factor (n, age, height, weight, BMI, SBP, DBP, VO2max, vascular assessment, methodological quality, year of publication) significantly influenced the MD in BASMF between older and young groups in subgroup analyses (Table S3).
TABLE 2.
Brachial artery smooth muscle function (SMF) assessment of studies included in the meta-analysis
| Study, year of publication | Artery | Wall-tracking system | Resting diameter (mm) | SMF | EF | |||
|---|---|---|---|---|---|---|---|---|
| YNG | OLD | Stimulus | Dose (mg) | OLD vs. YNG | OLD vs. YNG | |||
| DeVan et al [4], 2013 | brachial | computerized | 4.0±0.4 | 4.0±0.5 | NTG sublingual | 0.4 | ↔ | ↓ |
| Pierce et al [42], 2011 | brachial | computerized | 4.0±0.4 | 4.1±0.5 | NTG sublingual | 0.4 | ↔ | ↓ |
| Black et al [3], 2009 | brachial | computerized | 3.3±0.1 | 3.6±0.6 | NTG sublingual | 0.4 | ↔ | ↓ |
| Donato et al [6], 2009 | brachial | computerized | N/A | N/A | NTG sublingual | 0.4 | ↔ | ↓ |
| Walker et al [12], 2009 | brachial | computerized | 4.1±0.4 | 4.4±0.5* | NTG sublingual | 0.4 | ↔ | ↓ |
| Donato et al [5], 2007 | brachial | computerized | N/A | N/A | NTG sublingual | 0.4 | ↔ | ↓ |
| Gates et al [39], 2007 | brachial | computerized | 3.7±0.4 | 4.0±0.8 | NTG sublingual | 0.4 | ↔ | ↓ |
| Parker et al [41], 2006 | brachial | computerized | 3.0±0.5 | 3.1±0.4 | NTG sublingual | 0.4 | ↓ | ↓ |
| Eskurza et al [36], 2006 | brachial | computerized | 4.0±0.6 | 4.3±0.7 | NTG sublingual | 0.4 | ↔ | ↓ |
| Eskurza et al [38], 2005 | brachial | computerized | 4.1±0.4 | 4.4±0.5 | NTG sublingual | 0.4 | ↔ | ↓ |
| Heiss et al [40], 2005 | brachial | computerized | 3.7±0.9 | 4.2±0.5* | NTG sublingual | 0.4 | ↔ | ↓ |
| Eskurza et al [37], 2004 | brachial | manual | 4.1±0.3 | 4.3±0.3 | NTG sublingual | 0.4 | ↔ | ↓ |
| McCrohon et al [10], 2000 | brachial | manual | 3.1±0.3 | 3.6±0.4* | NTG sublingual | 0.4 | ↓ | ↓ |
| Woo et al A [43], 1997 | brachial | manual | 3.5±0.5 | 3.6±0.6 | NTG sublingual | 0.4 | ↔ | ↔ |
| Woo et al B [43], 1997 | brachial | manual | 3.4±0.6 | 3.7±0.5 | NTG sublingual | 0.4 | ↔ | ↓ |
Data are dose or mean ± SD. One study presented two groups of older subjects, each of which had been independently compared with a location-matched young group [43], thus they were evaluated as individual studies (distinguished by A and B).
significantly different from young group at P<0.05
EF, endothelial function; N/A, data not available; NMD, nitrate-mediated dilation; NTG, nitroglycerin; OLD, older group; SMF, smooth muscle function; YNG, young group; ↔, no significant difference between older and young groups; ↓, significant decrease in older compared with young groups
FIGURE 2.
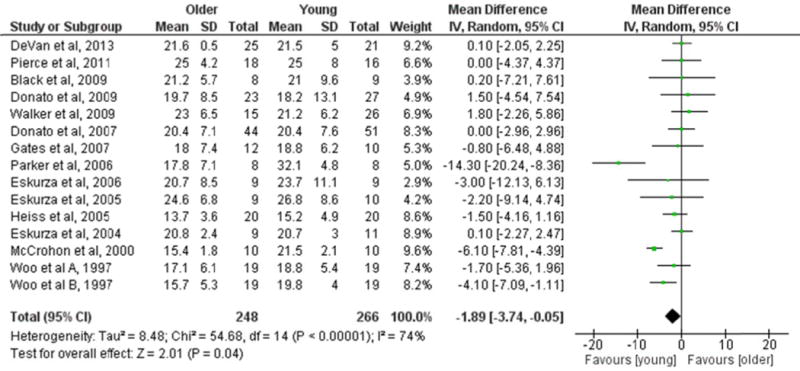
Forest plot of the mean difference (MD) in brachial artery smooth muscle function (BASMF) between older and young groups. BASMF was expressed as the percentage increase in brachial artery diameter from baseline to maximal vasodilation. Squares represent the MD in BASMF for each study. The diamond represents the pooled MD in BASMF across studies. CI, confidence interval; IV, inverse variance; SD, standard deviation
Resistance artery smooth muscle function (RASMF)
RASMF was determined by evaluating the absolute peak [6, 14, 15, 18, 23, 24, 30–33, 35], percent increase from baseline [8, 9, 16, 17, 19–21, 34] or slope [22] in vasodilation in response to sodium nitroprusside (Table 3). After data pooling, the RASMF was lower in older compared with young groups (20 studies, SMD=−0.46; P=0.0008) (Figure 3). Significant heterogeneity was detected (I2=57%; P=0.0008). In subgroup analyses, studies above the median in presence of females showed lower RASMF in older compared with young groups (10 studies, SMD=−0.77; P=0.001). In contrast, studies below the median in presence of females presented similar RASMF in older and young groups (9 studies, SMD=−0.17; P=0.20). Both sex subgroups were significantly different when compared with each other (P=0.02) (Table S3). No other potential moderating factor (n, age, height, weight, BMI, SBP, DBP, VO2max, vascular assessment, methodological quality, year of publication) significantly influenced the SMD in RASMF between older and young groups (Table S3).
TABLE 3.
Resistance artery smooth muscle function (SMF) assessment in studies included in the meta-analysis
| Study, year of publication | Vascular region | Technique | SMF | EF | ||
|---|---|---|---|---|---|---|
| Stimulus | OLD vs. YNG | Stimulus | OLD vs. YNG | |||
| Millet et al [30], 2012 | supramedial malleolar (skin) | laser Doppler | SNP iontophoresis | ↔ | local heating | ↔ |
| Donato et al [8], 2011 | forearm | plethysmography | SNP infusion | ↔ | ACh infusion | ↓ |
| Westby et al [24], 2011 | forearm | plethysmography | SNP infusion | ↔ | ACh infusion | ↓ |
| Kirby et al [16], 2010 | forearm | plethysmography | SNP infusion | ↔ | ACh infusion | ↓ |
| Tew et al [19], 2010 | forearm (skin) | laser Doppler | SNP iontophoresis | ↓ | ACh iontophoresis | ↓ |
| Donato et al [6], 2009 | forearm | plethysmography | SNP infusion | ↔ | ACh infusion | ↓ |
| Nicholson et al [31], 2009 | forearm | plethysmography | SNP infusion | ↔ | N/A | N/A |
| Kirby et al [17], 2009 | forearm | plethysmography | SNP infusion | ↓ | ACh infusion | ↓ |
| Donato et al [32], 2008 | forearm | plethysmography | SNP infusion | ↔ | ACh infusion | ↓ |
| Galetta et al [9], 2006 | forearm | plethysmography | SNP infusion | ↔ | ACh infusion | ↓ |
| Al-Shaer et al [21], 2006 | forearm | plethysmography | SNP infusion | ↓ | ACh infusion | ↓ |
| Newcomer et al [33], 2005 | lega | ultrasound | SNP infusion | ↔ | ACh infusion | ↓ |
| Weverling-Rijnsburger et al [34], 2004 | forearm | plethysmography | SNP infusion | ↔ | ACh infusion | ↔ |
| Ahlers et al [14], 2004 | forearm | plethysmography | SNP infusion | ↔ | ACh infusion | ↓ |
| Smith et al [35], 2003 | forearm | plethysmography | SNP infusion | ↔ | Bk infusion | ↔ |
| Minson et al [18], 2002 | forearm (skin) | laser Doppler | SNP microdialysis | ↓ | local heating | ↓ |
| DeSouza et al [15], 2002 | forearm | plethysmography | SNP infusion | ↔ | ACh infusion | ↓ |
| Wang et al [20], 2002 | lower leg (skin) | laser Doppler | SNP iontophoresis | ↔ | ACh iontophoresis | ↓ |
| Taddei et al [23], 2000 | forearm | plethysmography | SNP infusion | ↓ | ACh infusion | ↓ |
| Gerhard et al [22], 1996 | forearm | plethysmography | SNP infusion | ↔ | ACh infusion | ↓ |
Leg and forearm resistance artery SMF were reported. Leg resistance artery SMF was used in meta-analysis because of lower (as compared with forearm) coefficient of variation
ACh, acetylcholine; Bk, bradykinin; EF, endothelial function; N/A, data not available; OLD, older group; SMF, smooth muscle function; SNP, sodium nitroprusside; YNG, young group; ↔, no significant difference between older and young groups; ↓, significant decrease in older compared with young groups
Figure 3.
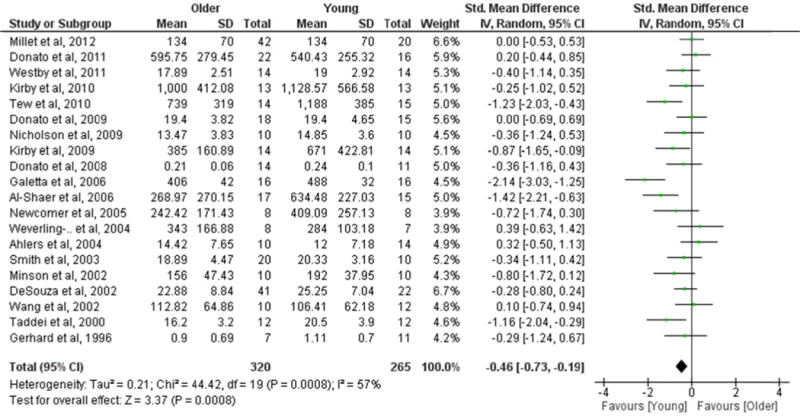
Forest plot of the standardized mean difference (SMD) in resistance artery smooth muscle function (RASMF) between older and young groups. Squares represent the SMD in RASMF for each study. The diamond represents the pooled SMD in RASMF across studies. CI, confidence interval; IV, inverse variance; SD, standard deviation
DISCUSSION
In this systematic review and meta-analysis, we pooled and analyzed data from 33 articles comparing vascular SMF, determined by the vasodilator response to exogenous NO donors, in 550 older and 516 young healthy subjects. The main finding of this meta-analysis is that vascular SMF is significantly lower in older compared to young individuals, a finding that is similarly present in conduit and resistance arteries (Figure 2 and 3). In subgroup analyses, we found that sex altered the impact of age on vascular SMF. More specifically, studies including females only or a higher ratio of females to males displayed a significant and marked impact of age on conduit and resistance artery SMF, whilst studies involving males only or a lower ratio of females to males reported no effect of age on SMF (Table S3).
The impact of age on vascular SMF had not been explored in detail in most previous individual studies (Table 2 and 3), possibly because of low statistical power and presence of confounding factors inherent in cross-sectional comparisons. Results from this meta-analysis indicate that, in addition to endothelial dysfunction, older age is associated with smooth muscle dysfunction in conduit and resistance arteries, as represented by reduced smooth muscle sensitivity to a NO donor. Consistent with this finding, studies performed in older rats demonstrated reduced vasodilator response to NO [27–29] and decreased vascular smooth muscle expression of soluble guanylyl cyclase (sGC) [27, 52] (i.e., the principal intracellular receptor of NO and mediates vasodilation via formation of cyclic guanosine monophosphate (cGMP) [53]). In humans, decreased expression and activity of sGC in brain tissue have been related to advanced age and Alzheimer’s disease, respectively, although no specific vascular measures were provided [54, 55]. Whether an age-related reduction in expression and activity of sGC in vascular smooth muscle contributes to a blunted vascular responsiveness to NO needs further investigation.
A consistent finding from previous studies is that brachial artery flow-mediated dilation, as well as resistance artery responses to acetylcholine, are impaired in older healthy humans. As most of these previous studies found no difference in the endothelium-independent vasodilation to exogenous NO donors, the general consensus was that the lower flow-mediated dilation and resistance artery responses to acetylcholine supported the presence of a dysfunctional endothelium. Our results, however, suggest the presence of a small-to-moderate but significant age-related impairment in SMF that is present across the vascular tree. Moreover, our finding also suggests that a portion of the impaired NO-dependent responses in conduit and resistance arteries in older healthy subjects may be in part due to reduced smooth muscle sensitivity to NO. This important observation should be taken into consideration when interpreting the lower NO-dependent responses in healthy older humans.
Another important finding of this meta-analysis was the influence of sex on the difference in vascular SMF between young and older humans. Studies that involved (predominantly) males, which is a common observation in studies in the field of cardiovascular physiology, found similar BASMF and RASMF between older and young groups. However, studies with females only or studies with a relatively high ratio of females to males reported a significantly lower BASMF and RASMF in the older group compared with the young group. One potential explanation for this finding may be related to menopausal status in women. Indeed, reduced vasodilation to exogenous NO donors has been reported in conduit and resistance arteries of healthy older women but not in those on hormonal replacement therapy. This suggests that hormone replacement therapy in postmenopausal women may preserve the sensitivity of vascular smooth muscle to NO [56–58]. In this regard, it is important to emphasize that none of the studies included in our meta-analyses involved women on hormonal therapy. Consequently, based on the assumption that the majority of older women were post-menopausal, the lower vascular SMF in older groups could be a result of the loss of vascular protection from circulating estrogens associated with post-menopause. Nevertheless, removal of estrogen with ovariectomy in rodents does not appear to alter vasodilator responses to sodium nitroprusside [59, 60], suggesting that loss of estrogen after menopause may not be the sole factor contributing to reduced vascular SMF in older women. Estrogen-independent alterations in the NO signaling pathway could underlie the altered vascular SMF in older women in view of the decreased NO-stimulated vascular cGMP formation in middle-aged and older women (with no report of menopause) compared with men [61]. Whilst speculative, the sex-related difference in vascular sensitivity to NO might partly explain why women remain more symptomatic and present with higher resting heart rate and lower exercise capacity compared with men after long-term nitrate therapy for heart failure [62].
A potential explanation for a lower NO-mediated dilation of the brachial artery in older subjects may relate to the larger baseline diameter. As demonstrated in several previous studies, the brachial artery dilator response to ischemia or glyceryl trinitrate is inversely related to the baseline diameter [63–67]. Nonetheless, in the present study, the difference in baseline brachial diameter between older and young groups did not modify the MD in BASMF according to subgroup analysis (Table S3). Moreover, the magnitude of the difference in baseline brachial diameter between older men and young men (4 studies) was not different than the difference between older women and young women (3 studies) (P=0.24). Furthermore, the impact of sex on BASMF is not explained by artery diameter [68]. Taken together, we believe differences in brachial artery diameter between older and young groups do not entirely explain the age-related decline in BASMF observed in the present meta-analysis.
As for the prognostic value of vascular SMF, adverse cardiovascular events are associated with reduced smooth muscle sensitivity to NO in high-risk populations when assessed in conduit [69] and resistance arteries [70] by means of ultrasound and plethysmography, respectively. It should be noted that blood flow responses in the microcirculation assessed via plethysmography and laser Doppler techniques—such as those used in the studies included in the RASMF meta-analysis—may be, to a certain degree, dependent on microvascular structure, which in turn is strongly associated with cardiovascular events [71]. Conversely, NO-dependent responses determined by micromyography did not predict cardiovascular events in high-risk patients, suggesting that resistance artery function per se has low clinical relevance [72]. Similarly, it cannot be discarded that the age-related reduction in RASMF is an observation dependent on differences in microvascular structure. Further research is warranted to determine the extent to which vascular structural changes with ageing influence the non-invasive estimates of smooth muscle responsiveness to NO and their clinical significance.
There are some limitations in this meta-analysis. First, cross-sectional comparisons may be misleading when addressing the question of the impact of advanced age [73]. Second, we were unable to accurately determine the level of physical activity or fitness in the included studies. Since physical activity or exercise training has well-established effects on endothelial function [74], we were unable to identify the potential impact of this factor on our results. Likewise, body composition, fat distribution and blood lipids could be suggested as potential moderating factors, albeit not investigated in this meta-analysis. Third, although single-dose (0.4 mg of nitroglycerin) is the common procedure to evaluate BASMF (Table 2), there are physiological and methodological variables that may affect the reproducibility of BASMF [75, 76]. In addition, the administration of 0.4 mg of nitroglycerin is considered to induce a maximal NO-mediated vasodilator response [75], which could be dissociated from vascular smooth muscle submaximal responses to lower or step-wise doses of NO. In this respect, dose-response studies might have higher sensitivity to detect small differences in BASMF between older and young groups [75].
In conclusion, the current meta-analysis provides evidence that advanced age is associated with a relatively small, but significant impairment in conduit and resistance artery SMF in healthy humans. The magnitude of vascular smooth muscle dysfunction seems more prevalent in women than in men, potentially related to the loss of circulating estrogens in women after menopause. A potential implication of our finding is that the impairment in conduit and resistance artery endothelial function reported with advanced age may, at least in part, be attributed to changes in vascular SMF in healthy adults. Further studies are needed to determine sex-related mechanisms that may influence the ageing-associated alterations in vascular SMF in humans.
Supplementary Material
Acknowledgments
None.
Source of Funding: DHJT is financially supported by the Netherlands Heart Foundation (2009T064).
GLP is supported by the National Institutes of Health grant AG043722-01 and the American Heart Association grant 13SDG143400012. JP is supported by the American Heart Association grant 14SDG20320006.
Footnotes
Conflicts of Interest
The authors report no conflicts of interest.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart disease and stroke statistics–2014 update: a report from the american heart association. Circulation. 2014;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7(1):29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- 3.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. 2009;297(3):24. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeVan AE, Eskurza I, Pierce GL, Walker AE, Jablonski KL, Kaplon RE, et al. Regular aerobic exercise protects against impaired fasting plasma glucose-associated vascular endothelial dysfunction with aging. Clin Sci. 2013;124(5):325–31. doi: 10.1042/CS20120291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100(11):1659–66. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 6.Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K, et al. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297(1):H425–32. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauer T, Heiss C, Balzer J, Kehmeier E, Mangold S, Leyendecker T, et al. Age-dependent endothelial dysfunction is associated with failure to increase plasma nitrite in response to exercise. Basic Research in Cardiology. 2008;103(3):291–7. doi: 10.1007/s00395-008-0714-3. [DOI] [PubMed] [Google Scholar]
- 8.Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA, Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. Journal of Physiology. 2011;589(18):4545–54. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galetta F, Franzoni F, Virdis A, Ghiadoni L, Taddei S, Salvetti A, et al. Endothelium-dependent vasodilation and carotid artery wall remodeling in athletes and sedentary subjects. Atherosclerosis. 2006;186(1):184–92. doi: 10.1016/j.atherosclerosis.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 10.McCrohon JA, Woo KS, Celermajer DS. A comparison of endothelial function in Caucasian and Chinese women before and after the menopause. Maturitas. 2000;35(1):31. doi: 10.1016/s0378-5122(00)00102-x. [DOI] [PubMed] [Google Scholar]
- 11.Haykowsky MJ, Herrington DM, Brubaker PH, Morgan TM, Hundley WG, Kitzman DW. Relationship of flow-mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. Journals of Gerontology – Series A Biological Sciences and Medical Sciences. 2013;68(2):161–7. doi: 10.1093/gerona/gls099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker AE, Eskurza I, Pierce GL, Gates PE, Seals DR. Modulation of vascular endothelial function by low-density lipoprotein cholesterol with aging: Influence of habitual exercise. American Journal of Hypertension. 2009;22(3):250–6. doi: 10.1038/ajh.2008.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boegli Y, Gremion G, Golay S, Kubli S, Liaudet L, Leyvraz PF, et al. Endurance Training Enhances Vasodilation Induced by Nitric Oxide in Human Skin. Journal of Investigative Dermatology. 2003;121(5):1197–204. doi: 10.1046/j.1523-1747.2003.12518.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahlers BA, Parnell MM, Chin-Dusting JP, Kaye DM. An age-related decline in endothelial function is not associated with alterations in L-arginine transport in humans. J Hypertens. 2004;22(2):321–7. doi: 10.1097/00004872-200402000-00016. [DOI] [PubMed] [Google Scholar]
- 15.DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, et al. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. Journal of Physiology. 2002;542(1):255–62. doi: 10.1113/jphysiol.2002.019166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Vasodilatory responsiveness to adenosine triphosphate in ageing humans. Journal of Physiology. 2010;588(20):4017–27. doi: 10.1113/jphysiol.2010.197814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: Impact of acute ascorbic acid administration. Journal of Physiology. 2009;587(9):1989–2003. doi: 10.1113/jphysiol.2008.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol. 2002;93(5):1644–9. doi: 10.1152/japplphysiol.00229.2002. [DOI] [PubMed] [Google Scholar]
- 19.Tew GA, Klonizakis M, Saxton JM. Effects of ageing and fitness on skin-microvessel vasodilator function in humans. European Journal of Applied Physiology. 2010;109(2):173–81. doi: 10.1007/s00421-009-1342-9. [DOI] [PubMed] [Google Scholar]
- 20.Wang JS, Lan C, Chen SY, Wong MK. Tai Chi Chuan training is associated with enhanced endothelium-dependent dilation in skin vasculature of healthy older men. Journal of the American Geriatrics Society. 2002;50(6):1024–30. doi: 10.1046/j.1532-5415.2002.50256.x. [DOI] [PubMed] [Google Scholar]
- 21.Al-Shaer MH, Choueiri NE, Correia ML, Sinkey CA, Barenz TA, Haynes WG. Effects of aging and atherosclerosis on endothelial and vascular smooth muscle function in humans. Int J Cardiol. 2006;109(2):201–6. doi: 10.1016/j.ijcard.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelium-dependent vasodilation in forearm resistance vessels of humans. Hypertension. 1996;27(4):849–53. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 23.Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, et al. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101(25):2896–901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- 24.Westby CM, Weil BR, Greiner JJ, Stauffer BL, DeSouza CA. Endothelin-1 vasoconstriction and the age-related decline in endothelium-dependent vasodilatation in men. Clin Sci (Lond) 2011;120(11):485–91. doi: 10.1042/CS20100475. [DOI] [PubMed] [Google Scholar]
- 25.Delp MD, Behnke BJ, Spier SA, Wu G, Muller-Delp JM. Ageing diminishes endothelium-dependent vasodilatation and tetrahydrobiopterin content in rat skeletal muscle arterioles. J Physiol. 2008;586(4):1161–8. doi: 10.1113/jphysiol.2007.147686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107(3):490–7. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 27.Kloss S, Bouloumie A, Mulsch A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension. 2000;35(1 Pt 1):43–7. [PubMed] [Google Scholar]
- 28.Moritoki H, Tanioka A, Maeshiba Y, Iwamoto T, Ishida Y, Araki H. Age-associated decrease in histamine-induced vasodilation may be due to reduction of cyclic GMP formation. Br J Pharmacol. 1988;95(4):1015–22. doi: 10.1111/j.1476-5381.1988.tb11734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shirasaki Y, Su C, Lee TJ, Kolm P, Cline WH, Jr, Nickols GA. Endothelial modulation of vascular relaxation to nitrovasodilators in aging and hypertension. J Pharmacol Exp Ther. 1986;239(3):861–6. [PubMed] [Google Scholar]
- 30.Millet C, Roustit M, Blaise S, Cracowski JL. Aging is associated with a diminished axon reflex response to local heating on the gaiter skin area. Microvascular Research. 2012;84(3):356–61. doi: 10.1016/j.mvr.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Nicholson WT, Vaa B, Hesse C, Eisenach JH, Joyner MJ. Aging Is Associated with reduced prostacyclin-mediated dilation in the human forearm. Hypertension. 2009;53(6):973–8. doi: 10.1161/HYPERTENSIONAHA.108.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Donato AJ, Eskurza I, Jablonski KL, Gano LB, Pierce GL, Seals DR. Cytochrome P-450 2C9 signaling does not contribute to age-associated vascular endothelial dysfunction in humans. J Appl Physiol. 2008;105(4):1359–63. doi: 10.1152/japplphysiol.90629.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newcomer SC, Leuenberger UA, Hogeman CS, Proctor DN. Heterogeneous vasodilator responses of human limbs: Influence of age and habitual endurance training. American Journal of Physiology – Heart and Circulatory Physiology. 2005;289(158–1):H308–H15. doi: 10.1152/ajpheart.01151.2004. [DOI] [PubMed] [Google Scholar]
- 34.Weverling-Rijnsburger AWE, Blauw GJ, Meinders AE. Effect of atorvastatin on impaired vascular function in healthy old men. Journal of Clinical Pharmacy and Therapeutics. 2004;29(2):157–64. doi: 10.1111/j.1365-2710.2004.00548.x. [DOI] [PubMed] [Google Scholar]
- 35.Smith DT, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA. Effects of ageing and regular aerobic exercise on endothelial fibrinolytic capacity in humans. J Physiol. 2003;546(Pt 1):289–98. doi: 10.1113/jphysiol.2002.027870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eskurza I, Kahn ZD, Seals DR. Xanthine oxidase does not contribute to impaired peripheral conduit artery endothelium-dependent dilatation with ageing. Journal of Physiology. 2006;571(3):661–8. doi: 10.1113/jphysiol.2005.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. Journal of Physiology. 2004;556(1):315–24. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin augments endothelium-dependent dilatation in sedentary but not in habitually exercising older adults. Journal of Physiology. 2005;568(3):1057–65. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gates PE, Boucher ML, Silver AE, Monahan KD, Seals DR. Impaired flow-mediated dilation with age is not explained by L-arginine bioavailability or endothelial asymmetric dimethylarginine protein expression. Journal of Applied Physiology. 2007;102(1):63–71. doi: 10.1152/japplphysiol.00660.2006. [DOI] [PubMed] [Google Scholar]
- 40.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. Journal of the American College of Cardiology. 2005;45(9):1441–8. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 41.Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: A comparison of dilatory responsiveness in the brachial and popliteal arteries. American Journal of Physiology – Heart and Circulatory Physiology. 2006;291(6):H3043–H9. doi: 10.1152/ajpheart.00190.2006. [DOI] [PubMed] [Google Scholar]
- 42.Pierce GL, Donato AJ, LaRocca TJ, Eskurza I, Silver AE, Seals DR. Habitually exercising older men do not demonstrate age-associated vascular endothelial oxidative stress. Aging Cell. 2011;10(6):1032–7. doi: 10.1111/j.1474-9726.2011.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woo KS, McCrohon JA, Chook P, Adams MR, Robinson JT, McCredie RJ, et al. Chinese adults are less susceptible than whites to age-related endothelial dysfunction. J Am Coll Cardiol. 1997;30(1):113–8. doi: 10.1016/s0735-1097(97)00111-3. [DOI] [PubMed] [Google Scholar]
- 44.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 45.Ross LE, Grigoriadis S, Mamisashvili L, Koren G, Steiner M, Dennis CL, et al. Quality assessment of observational studies in psychiatry: an example from perinatal psychiatric research. Int J Methods Psychiatr Res. 2011;20(4):224–34. doi: 10.1002/mpr.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sprung VS, Atkinson G, Cuthbertson DJ, Pugh CJ, Aziz N, Green DJ, et al. Endothelial function measured using flow-mediated dilation in polycystic ovary syndrome: a meta-analysis of the observational studies. Clin Endocrinol. 2013;78(3):438–46. doi: 10.1111/j.1365-2265.2012.04490.x. [DOI] [PubMed] [Google Scholar]
- 47.Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration. 2011 Available from www.cochrane-handbook.org. Accessed 25 August 2013.
- 48.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 49.Cohen J. In: Statistical Power Analysis for the Behavioral Sciences. 2nd. Hillsdale N, editor. Lawrence Erlbaum Associates Publishers; 1988. [Google Scholar]
- 50.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sprung VS, Atkinson G, Cuthbertson DJ, Pugh CJ, Aziz N, Green DJ, et al. Endothelial function measured using flow-mediated dilation in polycystic ovary syndrome: a meta-analysis of the observational studies. Clin Endocrinol (Oxf) 2013;78(3):438–46. doi: 10.1111/j.1365-2265.2012.04490.x. [DOI] [PubMed] [Google Scholar]
- 52.Chen L, Daum G, Fischer JW, Hawkins S, Bochaton-Piallat ML, Gabbiani G, et al. Loss of expression of the beta subunit of soluble guanylyl cyclase prevents nitric oxide-mediated inhibition of DNA synthesis in smooth muscle cells of old rats. Circ Res. 2000;86(5):520–5. doi: 10.1161/01.res.86.5.520. [DOI] [PubMed] [Google Scholar]
- 53.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43(2):109–42. [PubMed] [Google Scholar]
- 54.Ibarra C, Nedvetsky PI, Gerlach M, Riederer P, Schmidt HH. Regional and age-dependent expression of the nitric oxide receptor, soluble guanylyl cyclase, in the human brain. Brain Res. 2001;907(1–2):54–60. doi: 10.1016/s0006-8993(01)02588-4. [DOI] [PubMed] [Google Scholar]
- 55.Bonkale WL, Winblad B, Ravid R, Cowburn RF. Reduced nitric oxide responsive soluble guanylyl cyclase activity in the superior temporal cortex of patients with Alzheimer’s disease. Neurosci Lett. 1995;187(1):5–8. doi: 10.1016/0304-3940(95)11323-o. [DOI] [PubMed] [Google Scholar]
- 56.Lim SC, Caballero AE, Arora S, Smakowski P, Bashoff EM, Brown FM, et al. The effect of hormonal replacement therapy on the vascular reactivity and endothelial function of healthy individuals and individuals with type 2 diabetes. J Clin Endocrinol Metab. 1999;84(11):4159–64. doi: 10.1210/jcem.84.11.6160. [DOI] [PubMed] [Google Scholar]
- 57.Arora S, Veves A, Caballaro AE, Smakowski P, LoGerfo FW. Estrogen improves endothelial function. J Vasc Surg. 1998;27(6):1141–6. doi: 10.1016/s0741-5214(98)70016-3. discussion 7. [DOI] [PubMed] [Google Scholar]
- 58.Parker BA, Smithmyer SL, Proctor DN. Hormone therapy is associated with preserved smooth muscle structure and dilation in the arterial vasculature of the leg in older women. Maturitas. 2008;59(1):46–54. doi: 10.1016/j.maturitas.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riveiro A, Mosquera A, Calvo C, Alonso M, Macia M, Cores M. Long-term effect of bilateral ovariectomy on endothelial function in aortic rings of spontaneously hypertensive rats: role of nitric oxide. Gynecol Endocrinol. 2001;15(2):158–64. [PubMed] [Google Scholar]
- 60.Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, et al. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc Res. 2000;45(2):454–62. doi: 10.1016/s0008-6363(99)00359-4. [DOI] [PubMed] [Google Scholar]
- 61.Witte K, Hachenberger J, Castell MF, Vahl CF, Haller C. Nitric oxide-sensitive soluble guanylyl cyclase activity is preserved in internal mammary artery of type 2 diabetic patients. Diabetes. 2004;53(10):2640–4. doi: 10.2337/diabetes.53.10.2640. [DOI] [PubMed] [Google Scholar]
- 62.Levine TB, Levine AB, Kaminski P, Stomel RJ. Reversal of heart failure remodeling in women. J Womens Health Gend Based Med. 2000;9(5):513–9. doi: 10.1089/15246090050073594. [DOI] [PubMed] [Google Scholar]
- 63.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 64.Silber HA, Bluemke DA, Ouyang P, Du YP, Post WS, Lima JA. The relationship between vascular wall shear stress and flow-mediated dilation: endothelial function assessed by phase-contrast magnetic resonance angiography. J Am Coll Cardiol. 2001;38(7):1859–65. doi: 10.1016/s0735-1097(01)01649-7. [DOI] [PubMed] [Google Scholar]
- 65.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol. 2008;295(5):H1927–34. doi: 10.1152/ajpheart.00405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thijssen DH, Willems L, van den Munckhof I, Scholten R, Hopman MT, Dawson EA, et al. Impact of wall thickness on conduit artery function in humans: is there a “Folkow” effect? Atherosclerosis. 2011;217(2):415–9. doi: 10.1016/j.atherosclerosis.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Atkinson G, Batterham AM, Thijssen DH, Green DJ. A new approach to improve the specificity of flow-mediated dilation for indicating endothelial function in cardiovascular research. J Hypertens. 2013;31(2):287–91. doi: 10.1097/HJH.0b013e32835b8164. [DOI] [PubMed] [Google Scholar]
- 68.Dengel DR, Jacobs DR, Steinberger J, Moran AM, Sinaiko AR. Gender differences in vascular function and insulin sensitivity in young adults. Clin Sci (Lond) 2011;120(4):153–60. doi: 10.1042/CS20100223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Akamatsu D, Sato A, Goto H, Watanabe T, Hashimoto M, Shimizu T, et al. Nitroglycerin-mediated vasodilatation of the brachial artery may predict long-term cardiovascular events irrespective of the presence of atherosclerotic disease. J Atheroscler Thromb. 2010;17(12):1266–74. doi: 10.5551/jat.5181. [DOI] [PubMed] [Google Scholar]
- 70.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–8. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 71.Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, et al. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108(18):2230–5. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- 72.Rizzoni D, Porteri E, De Ciuceis C, Boari GE, Zani F, Miclini M, et al. Lack of prognostic role of endothelial dysfunction in subcutaneous small resistance arteries of hypertensive patients. J Hypertens. 2006;24(5):867–73. doi: 10.1097/01.hjh.0000222756.76982.53. [DOI] [PubMed] [Google Scholar]
- 73.DiPietro NA. Methods in epidemiology: observational study designs. Pharmacotherapy. 2010;30(10):973–84. doi: 10.1592/phco.30.10.973. [DOI] [PubMed] [Google Scholar]
- 74.Thijssen DH, Maiorana AJ, O’Driscoll G, Cable NT, Hopman MT, Green DJ. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol. 2010;108(5):845–75. doi: 10.1007/s00421-009-1260-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ayer JG, Harmer JA, David C, K SS, Seale JP, Celermajer DS. Severe obesity is associated with impaired arterial smooth muscle function in young adults. Obesity (Silver Spring) 2011;19(1):54–60. doi: 10.1038/oby.2010.114. [DOI] [PubMed] [Google Scholar]
- 76.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


