Abstract
Endometrial carcinoma is the most common gynecologic malignancy is the United States, and accounts for 6% of all cancers in women. The disease is classified as Type I or Type II based on clinicopathologic and molecular features. It is a multifactorial disease with a number of risk factors, including environmental exposures. How environmental exposures, such as flame retardants, may affect the incidence of endometrial cancer is a topic of current and ongoing interest. Tetrabromobisphenol A (TBBPA) is a widely used brominated flame retardant found in a variety of household products. A recent two-year National Toxicology Program carcinogenicity study found that exposure to TBBPA was associated with a marked increase in the development of uterine tumors, specifically uterine carcinomas, in Wistar Han rats. Molecularly, TBBPA-induced uterine carcinomas in Wistar Han rats were characterized by a marked increase in Tp53 mutation compared to spontaneous uterine carcinomas, as well as overexpression of Her2. Similar to spontaneous carcinomas, tumors in TBBPA-exposed rats were ERα positive and PR negative by immunohistochemistry. The morphologic and molecular features of uterine carcinomas in TBBPA-exposed rats resemble those of high-grade Type I tumors in women, and these data suggest that exposure to TBBPA may pose an increased cancer risk.
Keywords: Tetrabromobisphenol A, endometrial carcinoma, Tp53, Ctnnb1, Kras, Ccnd1, Her2, Cdh1, Wistar Han rats
Introduction
Endometrial cancer is one of the leading causes of cancer morbidity and mortality in women in the United States, with an estimated 49,560 new cases and 8,190 deaths in 2013, and even higher estimates for 2014 (ACS 2013, 2014). Endometrial carcinoma (EC) in women is classified into two subtypes, Type I and Type II, based on clinicopathologic and molecular features. These two subtypes of EC have unique dualistic phenotypic and molecular features that correlate relatively well with the biologic behavior of these neoplasms (Horn et al., 2007; Lax, 2004; Liu, 2007; Llobet et al., 2009; Matias-Guiu et al., 2001; Ryan et al., 2005). Type I ECs, account for 80–90% of cases and, generally have a more favorable prognosis, while Type II ECs, which represent approximately 10–20% of cases, are associated with a poor prognosis. While the features of the two EC subtypes may overlap to some extent, subtyping is of particular utility when determining clinical outcome, prognosis, or therapeutic options for these tumors.
Based on clinicopathologic features, Type I tumors occur predominately in postmenopausal women, although they are also less frequently reported in premenopausal and perimenopausal women. Type I tumors are more often associated with nulliparity, are estrogen receptor-alpha (ERα) and progesterone receptor (PR) positive, and are slowly progressive with a lower risk of myometrial invasion (Mills and Longacre, 2011). Alternatively, Type II ECs occur in postmenopausal women, are associated with multiparity, and are high-grade with an aggressive clinical course. Morphologically, Type I tumors have endometrioid differentiation, characterized by irregularly shaped, variably sized glandular structures forming a merged, cribiform pattern or papillary proliferations, and associated with a desmoplastic stroma (Horn et al., 2007). Type I ECs are typically low-grade and arise within a background of atypical hyperplasia, but high-grade Type I tumors do occur that behave more aggressively. Type II ECs (nonendometrioid) are much more poorly differentiated, characterized by tubulocystic, papillary, or solid forms composed of small, cuboidal to “hobnail” shaped cells with pleomorphic nuclei, frequent mitoses, and often bizarre nuclear forms. Necrosis may be common. Type II ECs are subdivided into a “clear cell” subtype based on cytoplasmic glycogen, or a “serous” subtype based on eosinophilic cytoplasm with apically located nuclei and focal cytoplasmic clearing. Features of both phenotypes may be present in the same tumor. Type II ECs arise within an estrogen-deficient, atrophic uterine epithelium and are associated with a precursor lesion of endometrial intraepithelial carcinoma. They are highly aggressive and infiltrative tumors, often spreading along the peritoneal cavity, and harbor a poor prognosis.
Aside from clinical and morphologic features, ECs in women can also be subclassified based on molecular phenotype (Horn et al., 2007; Lax, 2004; Liu, 2007; Llobet et al., 2009; Matias-Guiu et al., 2001; Ryan et al., 2005). Type I EC is associated with a higher incidence of PTEN inactivation, microsatellite instability, and mutation of the KRAS, CTNNB1, and PIK3CA genes. Conversely, Type II EC is associated with a very low incidence of the above molecular alterations, but rather has a very high incidence of TP53 mutation, as well as overexpression of Human Epidermal Growth Factor Receptor 2 (HER2), Cyclin D1 (CCND1) and Cyclins D1/E1 (CCND/E1), inactivation of P16, and alterations in E-cadherin (CDH1), expression.
While all Type II ECs are considered high-grade, an accepted and widely used histologic grading system has been developed for Type I ECs to distinguish more aggressive, high-grade Type I tumors from low-grade Type I tumors. High-grade Type I ECs have overlapping morphologic and molecular features of Type I and Type II tumors, including increased local invasion, loss of ERα and PR expression, increased KI67 protein expression, high rates of TP53 mutation, and a poor prognosis, relative to low-grade Type I ECs (Liu, 2007; McConechy et al. 2012; Zannoni et al., 2010). Table 1 lists some of the clinicopathologic and molecular features of the subtypes of ECs (Horn et al., 2007; Liu, 2007; McConechy et al., 2012; 2001; Ryan et al., 2005; Zannoni et al., 2010).
Table 1.
Clinicopathologic and molecular features of human endometrial carcinoma (EC) subtypes.
| L ow grade Type I ECa | High grade Type I ECa | Type II ECa | |
|---|---|---|---|
| Incidence | 80–90% | ~3% | 10–20% |
| Age | Pre/peri/post-menopausal | Pre/peri/post-menopausal | Post-menopausal |
| Parity | Nulliparous | Nulliparous | Multiparous |
| Morphology | Endometrioid | Overlapping Type I & Type II features | Clear/Serous cell |
| Background | Hyperplastic | Hyperplastic | Atrophic |
| Invasion | Less invasive | Local invasion | Infiltrative/peritoneal seeding |
| Behavior | Stable | Agressive | Aggressive |
| Prognosis | Favorable | Poor (relative to Low grade Type I) | Poor |
| ER-α expression | Present | Absent | Absent |
| PR expression | Present | Absent | Absent |
| TP53 mutation | 10–20% | Higher than Low grade | 80–90% |
A major risk factor for EC in women is hormone dysregulation. Many of the contributing factors to the development of EC influence estrogen levels, and increased estrogen is associated with EC development. Hormone (estrogen) therapy in menopausal women is associated with increased risk (Beral et al., 2005), whereas the use of oral contraceptives and multiparity are protective (Modan et al., 2001). Early menarche and late menopause are risk factors, and nulliparity is a risk factors, since pregnancy shifts the balance of hormones from estrogen to progesterone (Emons et al., 2000). Other risk factors include family history, diabetes, obesity, tamoxifen therapy, and ovarian pathologies such as granulosa cell tumors and polycystic ovarian syndrome, which are associated with unabated estrogen secretion (Emons et al., 2000). Aside from family history, the majority, if not all of these risk factors, are associated with hormone imbalance. It is becoming evident that an increasing number of compounds that people are exposed to in their environment, whether they are environmental pollutants or occupational compounds, have the ability to influence hormone production and function.
Tetrabromobisphenol A (TBBPA), a compound structurally related to Bisphenol A and formed by bromination of Bisphenol A, is a widely used flame retardant in electrical equipment, plastics, paper, furniture, and carpeting. It is a high volume production chemical in the United States, and the most widely used brominated flame retardant in the world (Schauer et al., 2006). It has been identified in fish, shellfish, and drinking water. Like Bisphenol A, TBBPA has been shown to have endocrine disrupting activity in vitro and in vivo, and in the uterotropic assay in ovariectomized mice, exposure to 500mg/kg TBBPA resulted in increased uterus:body weight ratios (Kitamura et al., 2005). It is known that estrogens, estrogenic chemicals, and estrogen agonists can act as tumor promoters as well as carcinogens (Vollmer, 2003). Previous studies have shown that TBBPA is possibly a very weak estrogenic agonist in vitro, but results have been equivocal (Hamers et al, 2006). It seems to stabilize and prevent turnover of estrogen levels through inhibition of estrogen sulfotransferase, a hormone metabolizing enzyme (Gosavi et al, 2013). However, there is little known about the long-term toxicologic and carcinogenic effects of TBBPA in humans. In a recent National Toxicology Program (NTP) two-year carcinogenicity study, exposure of Wistar Han rats to TBBPA by corn-oil gavage was associated with a treatment-related increase in uterine carcinomas (NTP TR 587, 2013). Given the relevance of this compound to human exposures and the potential for endocrine disruption, investigation of the molecular mechanisms of uterine tumorigenesis due to TBBPA exposure in Wistar Han rats was warranted. The objective of this study was to characterize uterine carcinomas in Wistar Han rats exposed to TBBPA based on histopathologic and molecular criteria relevant to the human disease, to better understand the similarities and differences between human and rat EC, and to better understand the molecular events which occur in uterine carcinomas from TBBPA-treated Wistar Han rats.
Materials and Methods
Animals, histology, and immunohistochemistry
TBBPA-induced uterine carcinomas were obtained from Wistar Han [Crl:WI(Han)] rats in the two-year NTP bioassay. The care of animals on this study was according to National Institutes of Health (NIH) procedures as described in the “ The U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals”, available from the Office of Laboratory Animal Welfare, National Institutes of Health, Department of Health and Human Services, RKLI, Suite 360, MSC 7982, 6705 Rockledge Drive, Bethesda, MD 20892–7982 or online at http://grants.nih.gov/grants/olaw/olaw.htm#pol. The use of animals was approved by the NIEHS animal care and use committee. Forty-one formalin-fixed, paraffin-embedded (FFPE) uterine carcinomas from TBBPA-exposed animals were used in this study (10 low-dose, 15 mid-dose, 16 high-dose). A total of ten FFPE spontaneous carcinomas were available from control animals from various NTP studies. Due to the low incidence of spontaneously occurring uterine carcinomas in the current TBBPA carcinogenicity bioassay (two spontaneous tumors in corn-oil vehicle gavage control group), additional FFPE spontaneous tumors were obtained from other available Wistar Han rat carcinogenicity bioassay studies: one from polybrominated diethyl ether mixture (DE71) (corn-oil vehicle gavage), two from green tea extract (GTE-NTP TR 585, 2014) (deionized water vehicle), two from metal working fluids (MWF-NTP TR 586, 2014) (whole body inhalation), and three from antimony trioxide (AT) (whole body inhalation). Five FFPE normal uteri from corn-oil vehicle gavage control females in the concurrent TBBPA study were used as normal uterus controls. Four board-certified anatomic veterinary pathologists reviewed the histology of all uterine carcinomas using the standardized system of nomenclature and diagnostic criteria (SSNDC) guide for female reproductive proliferative lesions in rats to morphologically characterize the endometrial adenocarcinomas as well differentiated or poorly differentiated tumors (Table 2). If a tumor had areas that were well differentiated and poorly differentiated, then the tumor was classified as poorly differentiated. Tumors available for molecular biology included those > 0.5cm at the time of necropsy. These tumors were sectioned in half, and one half was flash frozen in liquid nitrogen for molecular analysis, and the other half fixed in 10% neutral buffered formalin (NBF) for histology and immunohistochemistry (IHC). Frozen tissue was available from six uterine carcinomas from TBBPA-exposed animals, and nine vehicle control animals from the above studies (one from TBBPA, two from GTE, three from AT, two from DE71, and one from MWF). The above frozen tissues and paired FFPE tissues were used for qPCR and IHC analysis, respectively. For IHC, 5um paraffin-embedded sections were obtained and immunolabeled using the Vector ABC system with the following antibodies using routine methods: ERα (1:50, Beckman Coulter Inc., Fullerton CA), PR (1:50, Beckman Coulter Inc., Fullerton CA), TP53 (1:500, Vector Laboratories, Burlingame CA), and KI67 (1:100, Dakocytomation Corporation, Carpinteria CA). The expression of TP53 protein in uterine carcinomas was graded by a board-certified pathologist based on a severity grade of 0–3 (0 = no immunoreactivity, 1+ = minimal, 2+ = moderate, 3+ = strong immunoreactivity). KI67 was graded based on a scale of 0 = no immunoreactivity, 1+ = <25%, 2+ = 25–50%, and 3+ = >50% of tumor cell immunoreactivity.
Table 2.
Standardized system of nomenclature and diagnostic criteria (SSNDC) classification of endometrial adenocarcinomas (Dixon et al 1999; 2014).
| Well-Differentiated Endometrial Adenocarcinomas |
| Usually cuboidal or columnar neoplastic epithelial cells arranged in well-formed solid nests, cords, papillary or acinar structures within or supported by stroma |
| Poorly Differentiated Endometrial Adenocarcinomas |
| Neoplastic epithelia exhibit cellular and nuclear atypia and pleomorphism. Multiple cell layers thick and may pile upon or crowd one another |
DNA Isolation, PCR Amplification, and Autosequencing
DNA was isolated and extracted from 22 FFPE TBBPA-induced uterine carcinomas and ten FFPE spontaneous uterine carcinomas from rat control animals with DNeasy Tissue Kit (Qiagen, Valencia, CA). Amplification reactions were carried out by semi-nested polymerase chain reaction (PCR) using the designed primer sets (Table 3) for Kras exons 1 and 2 (codons 12, 13, 61), Ctnnb1 exon 2 (corresponds to exon 3 in human), Tp53 exons 5–8, and Pten exons 1–9 for 6 cDNA treated samples. Controls lacking DNA were run with all sets of reactions. PCR products were purified using a QIAquick Gel Extraction Kit (Qiagen, Valencia, CA). The purified PCR products were cycled with Terminal Ready Reaction Mix-Big Dye (Perkin Elmer, Foster City, CA), and the extension products were purified using the DyeEx 2.0 Spin Kit (Qiagen, Valencia, CA). The lyophilized PCR products were sequenced with an automatic sequencer (Perkin-Elmer ABI Model 3100). An electropherogram was used for comparison between normal uterus from vehicle controls and uterine carcinomas from vehicle controls and TBBPA-exposed animals.
Table 3.
Primers used to amplify hotspot regions of rat Kras Ctnnb1 Tp53 and Pten.
| Gene | Exon | Codon | Primer | Strand | Sequence |
|---|---|---|---|---|---|
| Kras | 1 | 12–13 | KrasF25849 | Sense | 5’-AAAGTACTTGATAATTCTTGTGTGG-3’ |
| KrasR25489 | Antisense | 5’-AGAAGTGGGGTTCCTTGCACCG-3’ | |||
| KrasR25497 | Antisense | 5’-GGTTCCTTGCACCGATGGTTCCC-3’ | |||
| 2 | 61 | KrasF14399 | Sense | 5’-GCTCTGCCTATTTGGACTGGG-3’ | |
| KrasF14444 | Sense | 5’-CGAGTGCTTGCTGACCACTTG-3’ | |||
| KrasR13986 | Antisense | 5’-CAGGAATTCTACATACTTGACAC-3’ | |||
| Ctnnb1 | 2 | 5–80 | CatF272 | Sense | 5’-ACATAATCAACAAGCCACCC-3’ |
| CatF431 | Sense | 5’-ACTCAGGCAGCATTCTCAGTGCAT-3’ | |||
| CatR7 25 | Antisense | 5’-GGAAGGTAACACAGAGAGTTGCTT-3’ | |||
| CatR799 | Antisense | 5’-ATGTGAGACTCCGTTGCC-3’ | |||
| Tp53 | 5 | 124–184 | p53Ex5OF1366 | Sense | 5’-CCTAGTTGGCTTGTCCG-3’ |
| p53Ex5OR1671 | Antisense | 5’-AGCAAGAATAAGTCAGAGGC-3’ | |||
| p53Ex5IF1382 | Sense | 5’-CGCTGACCTTTGATTCTTTCTCC-3’ | |||
| p53Ex5IR1639 | Antisense | 5’-GACAACCAGTTCTAAACCCCACAG-3’ | |||
| 6 | 185–259 | p53Ex6OF1620 | Sense | 5’-TGGGGTTAGAACTGGTTG-3’ | |
| p53Ex6OR1963 | Antisense | 5’-GAACAAAAACAGGCCGAG-3’ | |||
| p53Ex6IF1645 | Sense | 5’-TCTCCCGGCCTCTGACTTATTC-3’ | |||
| p53Ex6IR1927 | Antisense | 5’-CAGCCCAACCTGGCACAC-3’ | |||
| 7 | 260–304 | p53Ex7OF2101 | Sense | 5’-AGCTCCAGATAGGACAAG-3’ | |
| p53Ex7OR2434 | Antisense | 5’-TGGGCAGTGCTATGGAAG-3’ | |||
| p53Ex7IF2166 | Sense | 5’-AGCTTTCTTACTGCCTTGTG-3’ | |||
| p53Ex7IR2402 | Antisense | 5’-TGACTTTGGGGTGAAGCTG-3’ | |||
| 8 | 305–329 | p53Ex8OF2333 | Sense | 5’-GGAGTGCAAAGAGAGGTG −3’ | |
| p53Ex8R2602 | Antisense | 5’-TGCGCTCTGACGATAATG-3’ | |||
| p53Ex8IF2386 | Sense | 5’-GCTTCACCCCAAAGTCAC-3’ | |||
| p53Ex8IR2549 | Antisense | 5’- GCGTTTTGTGTCCTAGACTTAG-3’ | |||
| Pten | 1–7 | 1–260 | RPtenex1F | Sense | 5’-TGACAGCCATCATCAAAGAG-3’ |
| RPtenex7R | Antisense | 5’-CTTTGAGCATCTTGTTCTGTT-3’ | |||
| 6–9 | 173–404 | RPtenex6F | Sense | 5’-GGAGTAACTATTCCCAGTCAGAG-3’ | |
| RPtenex9R | Antisense | 5’-TCAGACTTTTGTAATTTGTGAAT-3’ | |||
qPCR
RNA was extracted and purified from frozen samples using the Invitrogen TRIzol Kit (Invitrogen, Carlsbad, CA). Fold increases and decreases in gene expression were determined by quantification of cDNA from target samples relative to a calibrator sample of controls. The 18s RNA gene was used as the endogenous control for normalization of initial RNA levels, and the expression of genes relevant for human EC (P16, Her2, Ccnd1, Cdh1, Msh2, Msh3, Mlh1, Irf1, Pten) was assessed.
Statistical Analysis
Incidences of neoplasms and non-neoplastic lesions were tested using one-sided poly-3 tests (Bailer and Portier, 1988; Portier and Bailer, 1989; Piegorsch and Bailer, 1997). This method modifies the Cochran-Armitage linear trend test and Fisher’s exact test to take length of survival into account, by more closely approximating the total number of animal-years at risk. The poly-3 trend test assesses the significance of the linear trend of incidences with dose, while the pairwise poly-3 tests assesses the significance of pairwise differences in incidences of each dose group compared to the control group. Differences are considered statistically significant if the one-sided p-value is less than 0.05.
Results
Exposure to TBBPA was associated with a treatment-related increase in uterine carcinomas in Wistar Han rats
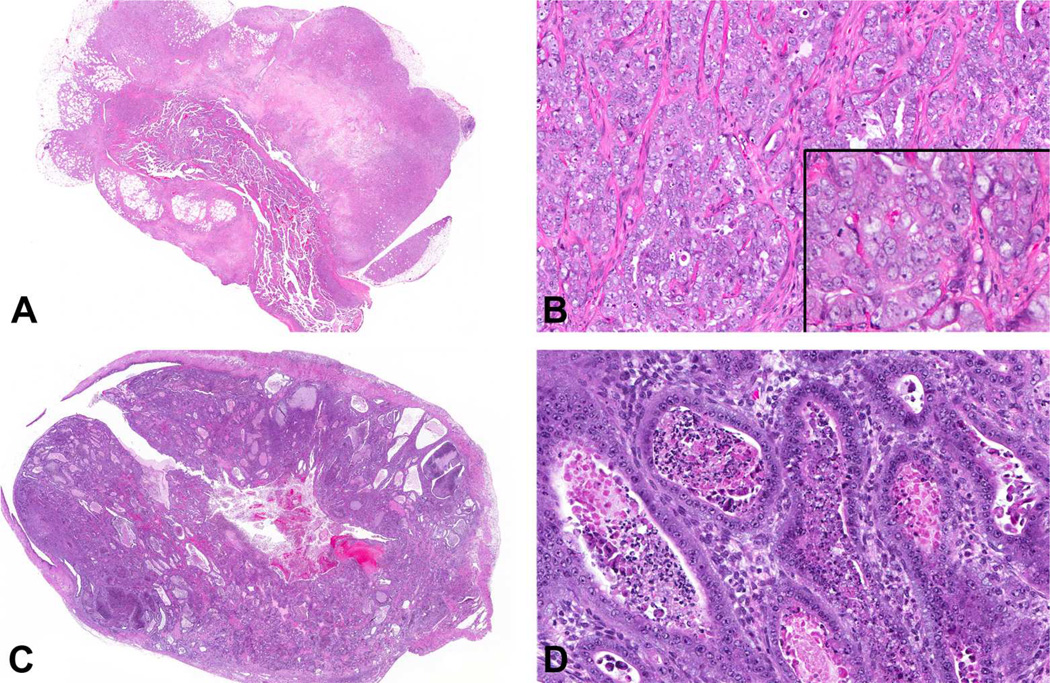
The results of the two-year NTP chronic bioassay indicated that TBBPA exposure resulted in a statistically significant (p < 0.05) increase in the incidence of uterine carcinomas in the top 2 dose levels of female Wistar Han rats (Table 4). Based on histomorphologic criteria (Table 2), both TBBPA-induced (28 of 41, 68%) and spontaneous (7 of 10, 70%) uterine carcinomas in Wistar Han rats were classified as poorly differentiated, high-grade carcinomas, characterized by deeply infiltrative lobules, cords, and nests of poorly differentiated epithelial cells with marked cellular pleomorphism, atypia, high mitotic rate, and large foci of necrosis (Figure 1A–B and Table 5). The remaining carcinomas were considered fairly well-differentiated, as endometrial stromal invasion was limited to the superficial and mid-endometrium rather than replacing large areas of the deep stroma. Additionally, neoplastic glands exhibited milder nuclear atypia and pleomorphism as well as less necrosis (Figure 1C–D).
Table 4.
Incidence of epithelial uterine neoplasms in TBBPA-exposed Wistar Han rats in the two-year NTP bioassay.
| UTERUSa | 0mg/kg | 250mg/kg | 500mg/kg | 1000mg/kg |
|---|---|---|---|---|
| Adenoma (includes multiples) | 3 (6%) | 2 (4%) | 4 (8%) | 6 (12%) |
| Poly-3 testb | P=0.103 | P=0.489N | P=0.437 | P=0.242 |
| Adenocarcinoma (includes multiples) | 4 (8%) | 10 (20%) | 15 (30%) | 16 (32%) |
| Poly-3 test | P=0.002^ | P=0.089 | P=0.002* | P=0.002* |
n = 50 animals examined per group
The p-value in the control column corresponds to the trend test; p-values in the dose columns correspond to pairwise comparisons with the control group.
N indicates a tumor-rate decrease compared to controls.
Statistically significant (p < 0.05) based on poly-3 test for linear trend.
Statistically significant (p < 0.05) based on poly-3 test for pairwise comparisons to the controls.
Figure 1.
Uterine carcinomas from TBBPA-exposed Wistar Han rats. (A) Poorly differentiated uterine carcinoma characterized by marked invasion through the uterine wall into the peri-uterine tissues, accompanied by large zones of necrosis. (B) Poorly differentiated uterine carcinomas were characterized by solid clusters, cords and nests of poorly differentiated epithelial cells with marked pleomorphism and cellular atypia, including large vesicular nuclei, multiple nucleoli, marked anisocytosis and anisokaryosis (inset), and mitotic figures. (C) Well-differentiated carcinomas were fairly well circumscribed with stromal invasion relatively limited to the superficial and mid-endometrium rather than replacing large areas of the deep stroma, and a glandular phenotype. (D) Glands were generally lined by one or more layers of fairly well-differentiated cuboidal to columnar epithelial cells with minimal to mild pleomorphism and few mitotic figures.
Table 5.
Morphologic and immunohistochemical features of uterine carcinomas from control and TBBPA-exposed WH rats.
| TBBPA-treated | Control | |
|---|---|---|
| Morphology | ||
| Poorly-differentiated | 21/22 (95%) | 8/10 (80%) |
| Well-differentiated | 1/22 (5%) | 2/10 (20%) |
| Estrogen receptor-α expression | 6/6 (100%) | 9/9 (100%) |
| Progesterone receptor expression | 2/6 (33%) | 6/9 (67%) |
| TP53 expression | 6/6 [2.3]a | 5/9 [1.0] a |
| KI67 expression | 6/6 [2.8] a | 9/9 [1.7] a |
Average intensity scores are an average of the severity grade (0 = no immunoreactivity, 1+ = minimal, 2+ = moderate, 3+ = strong immunoreactivity) for each animal divided by the total number of animals in each cohort (n=6 for TBBPA-treated rats; n=9 for control rats).
Uterine carcinomas in TBBPA-exposed Wistar Han rats show a tendency towards loss of PR expression and increased TP53 protein expression compared to spontaneous uterine carcinomas
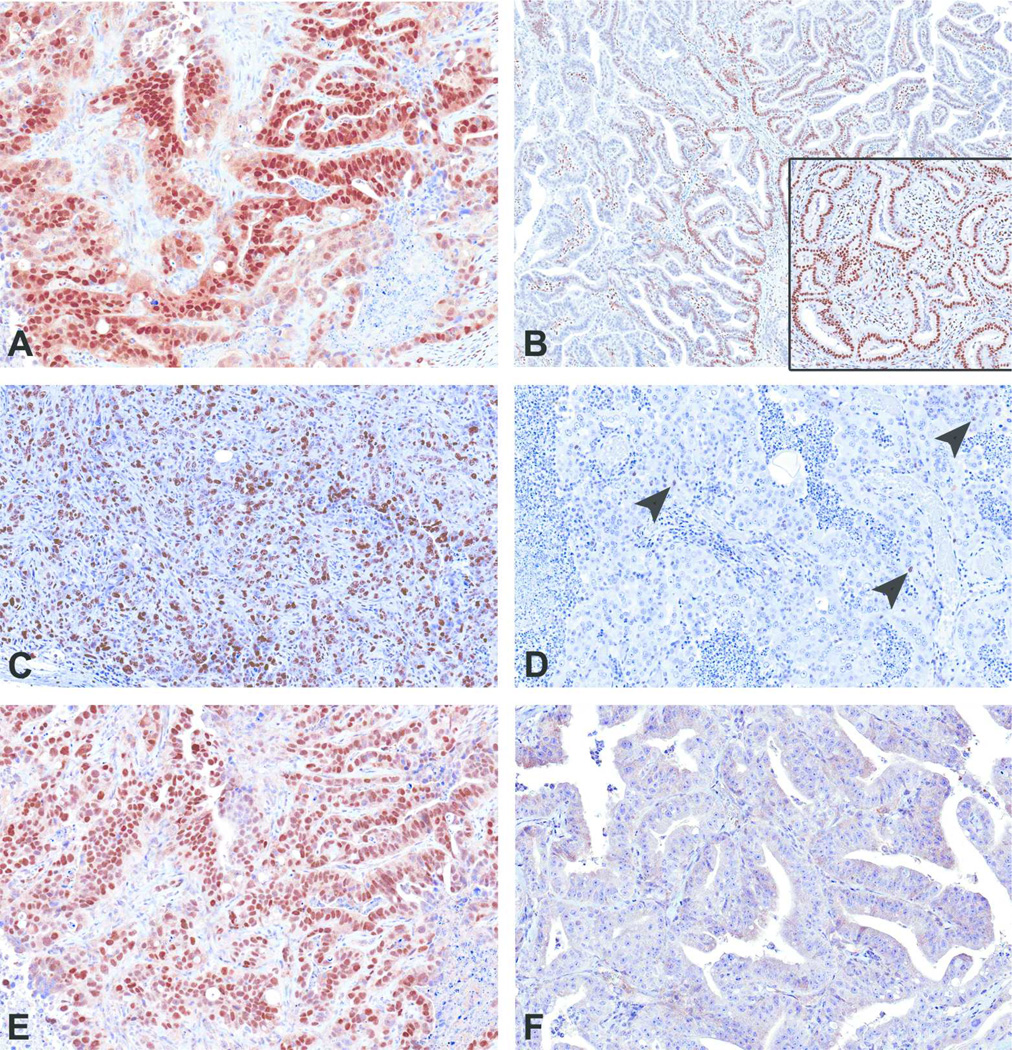
Since criteria for classifying human Type I and II endometrial carcinoma relies partly on hormone (ERα/PR) expression, proliferation, and Tp53 mutations, uterine carcinomas from TBBPA-exposed animals and spontaneous uterine carcinomas from vehicle controls and controls were examined for expression of ERα, PR, KI67, and Tp53 by immunohistochemistry. All TBBPA-exposed (6 of 6, 100%) and spontaneous (9 of 9) uterine carcinomas in Wistar Han rats examined had diffuse positive nuclear immunoreactivity to antibodies against ERα. All spontaneous and TBBPA-exposed uterine carcinomas in animals had nuclear immunoreactivity in the endometrial stroma and myometrium, similar to the staining pattern seen in normal uteri. The neoplastic epithelial component in 2 of 6 uterine carcinomas in TBBPA-exposed animals had focal nuclear immunoreactivity for PR (Figure 2B), whereas in control animals, there was diffuse nuclear immunoreactivity in 6 of 9 tumors (Figure 2B inset). Poorly differentiated tumors from control and TBBPA exposed animals had increased nuclear immunoreactivity to KI67 (Figure 2C), whereas well-differentiated tumors had little to no KI67 expression (Figure 2D). Lastly, all examined uterine carcinomas (6 of 6) from TBBPA-exposed animals displayed diffuse and moderate to strong nuclear immunoreactivity to antibodies against TP53 (Figure 2E and Table 5). Five of nine spontaneous uterine carcinomas displayed nuclear immunoreactivity, with an average intensity score of 1.0; two displayed strong intensity (3+) and were associated with poor differentiation. Three spontaneous tumors had weak immunoreactivity (1+), and four were negative (Figure 2F). The two well-differentiated spontaneous uterine carcinomas did not express TP53.
Figure 2.
Immunohistochemical features of uterine carcinomas. (A) All spontaneous and TBBPA-induced uterine adenocarcinomas had diffuse nuclear immunoreactivity to ER α. (B) Two uterine carcinomas from TBBPA-exposed animals had focal nuclear immunoreactivity to PR antibody, and one spontaneous tumor showed diffuse nuclear immunoreactivity (inset). (C) Poorly differentiated uterine carcinomas showed diffuse KI67 expression, whereas well-differentiated tumors (D) had single cell (arrowheads) to no expression of KI67. (E) Uterine carcinomas from all TBBPA-exposed animals showed strong nuclear immunoreactivity to antibodies to Tp53, whereas 4/9 spontaneous tumors were negative (F).
Uterine carcinomas in TBBPA-exposed Wistar Han rats had increased incidence of Tp53 mutations compared to spontaneous uterine carcinomas
Since mutations of several cancer genes are associated with clinical outcomes in humans, we screened spontaneous and TBBPA-associated uterine carcinomas for mutations relevant for EC in women. FFPE uterine carcinomas from TBBPA-exposed animals (n = 22) and spontaneous uterine carcinomas from vehicle controls (n = 10) were examined for mutations in Kras, Ctnnb1, and Tp53 (Table 6). In TBBPA-exposed animals, 15 of 22 (68%) tumors harbored mutations in Tp53, often with multiple mutations per tumor (Table 7), whereas in spontaneous uterine carcinomas, 2 of 10 (20%) tumors had a mutation in this gene (Table 6). Of 22 uterine carcinomas from TBBPA-exposed animals that were assessed for mutations in Ctnnb1 and Kras, 3 of 22 harbored mutations in Ctnnb1, and 1 of 22 animals had a Kras mutation (Table 6). Although, neither Ctnnb1 nor Kras mutations were observed in spontaneous uterine carcinomas in this study (0 of 10) (Table 6) the incidence in uterine carcinomas from TBBPA-exposed rats was very small, and the relative differences in the number examined (10 spontaneous vs. 22 TBBPA-exposed) relatively large; therefore, this difference is likely not statistically significant, and may reflect variations in normal biology of these tumors in this strain.
Table 6.
Mutation frequency of Tp53, Ctnnb1, and Kras in uterine carcinomas from control and TBBPA-exposed WH rats.
| TBBPA-treated | Control | |
|---|---|---|
| Tp53 mutation | 15/22 (68%) | 2/10 (20%) |
| Ctnnb1 mutation | 3/22 (14%) | 0/10 (0%) |
| Kras mutation | 1/22 (4.5%) | 0/10 (0%) |
Table 7.
Morphologic and molecular features in uterine carcinomas from humans and TBBPA-exposed WH rats.
| Morphologic |
|||||
|---|---|---|---|---|---|
| Human Type I ECa | Human Type II ECa | TBBPA-exposed WH ratb | |||
| Relative Incidence | 80–90% | 10–20% | ~27% (41/150) | ||
| Age | Pre/peri/post-menopausal | Post-menopausal | Late onset | ||
| Histology | Endometrioid | Serous/clear cell | Endometrioid | ||
| ERa expression | Present | Absent | Present | ||
| Background | Hyperplastic | Atrophic | |||
| Differentiation | Low grade | High grade | High > Low grade | ||
| Invasion | Minimal | Deep | Deep > Minimal | ||
| Behavior | Stable | Progressive | |||
| Prognosis | Favorable | Poor | |||
|
Molecular |
|||||
| Human Type I ECa | Human Type II ECa | TBBPA-exposed WH ratb | |||
| PTEN inactivation | 35–80% | 10% | 0% | ||
| Microsatellite instability | 20–45% | 0–5% | ND | ||
| KRAS mutation | 10–30% | 0–5% | 4.5% | ||
| CTNNB1 mutation | 20–40% | 0–5% | 14% | ||
| PIK3CA mutation | 30% | 5% | ND | ||
| TP53 mutation | 10–20% | 80–90% | 68% | ||
| HER2 overexpression | 10–30% | 40–80% | 100% (30+ fold) | ||
| P16 inactivation | 10% | 40% | 0% | ||
| CDH1 downregulation | 10–20% | 60–90% | 0% | ||
| CCND1 overexpression | 2–5% | 26–42% | 100% (6+ fold) | ||
Horn et al., 2007; Llobet et al., 2009; Lax 2004; Matias-Guiu et al., 2001; Ryan et al., 2005; Liu 2006.
Current study
ND = not determined
Uterine carcinomas from TBBPA-exposed rats had increased HER2 expression compared to spontaneous uterine carcinomas
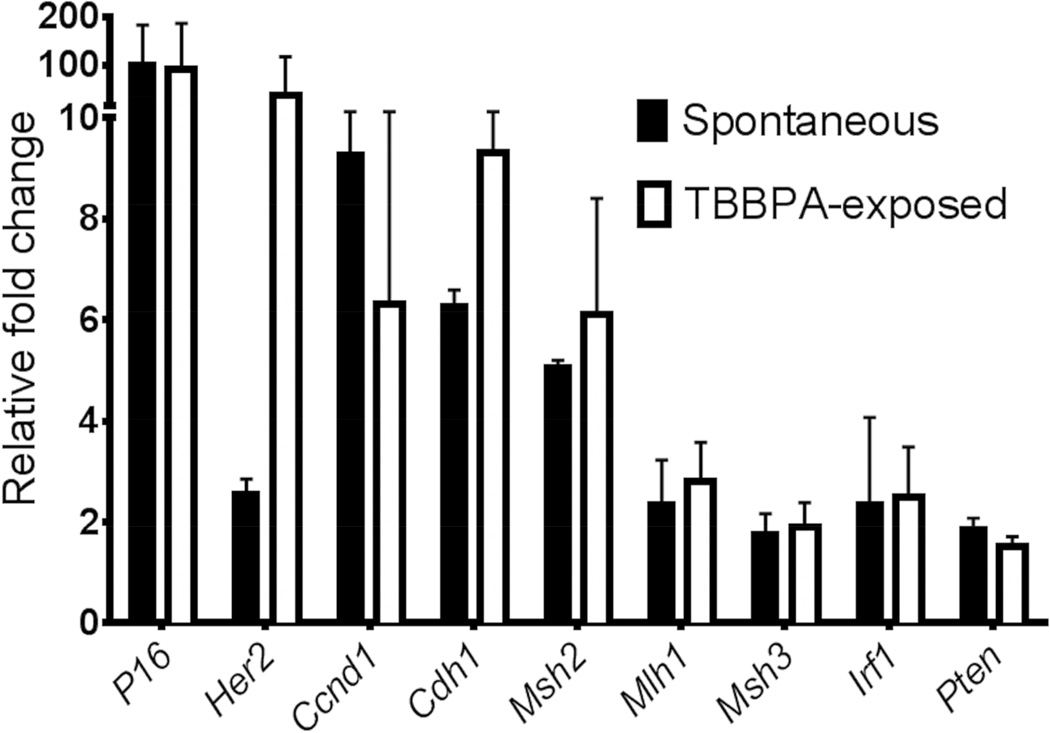
Since alterations in gene expression of a variety of growth factors, oncogenes, and tumor suppressor genes are also present in ECs in women, including HER2, CCND1, and CDH1, we evaluated uterine carcinomas for alterations in the expression of these genes (Figure 3 and Table 7). All uterine carcinomas in TBBPA-exposed animals and vehicle control animals overexpressed Her2; expression in uterine carcinomas from TBBPA-exposed animals averaged a 30-fold increase compared to a 2-fold increase in spontaneous carcinomas in vehicle control rats. Approximately 40–80% of Type II tumors in humans overexpress HER2, as opposed to 10–30% of Type I EC tumors. All uterine carcinomas examined overexpressed Ccnd1 and Cdh1, and the fold increase in expression was not significantly different between spontaneous uterine carcinomas and uterine carcinomas from TBBPA-exposed rats. In contrast to ECs in women, loss of Pten and P16 tumor suppressor gene expression was not observed in spontaneous or TBBPA-associated carcinomas. In addition, both spontaneous and TBBPA-associated uterine carcinomas had an increased expression of microsatellite markers (Msh2, Mlh1, Msh3) instead of the characteristic decreased expression associated with microsatellite instability in ECs in women. Additionally, there was no difference in expression of Irf1, a tumor suppressor gene that has been shown to decrease with the grade of endometrial adenocarcinomas in women (Kuroboshi et al., 2003) between TBBPA-induced and spontaneous tumors.
Figure 3.
Real time quantitative PCR gene expression of mediators involved in human endometrial carcinogenesis. Uterine carcinomas in TBBPA-exposed animals averaged a 30 fold overexpression of Her2 compared to a 2-fold increase in spontaneous uterine carcinomas. Uterine carcinomas from control and TBBPA-exposed animals overexpressed Ccnd1 and Cdh1, although the fold increase in expression was not significantly different between tumors.
Discussion
Long-term exposure of Wistar Han rats to TBBPA resulted in treatment-related increased frequencies in uterine carcinomas. The majority of uterine carcinomas in both TBBPA-exposed animals and spontaneous tumors were considered poorly differentiated. Uterine carcinomas in this study varied from a relatively more well-differentiated glandular and papillary form to poorly differentiated, infiltrative clusters, ribbons, or solid sheets of anaplastic glandular epithelial cells. A majority of poorly differentiated carcinomas also expressed high levels of KI67, in contrast to well-differentiated tumors. In women, KI67 expression is a prognostic factor correlated with higher grade, more aggressive ECs and a poor prognosis (Lax, 2004; Oreskovic et al., 2004; Salvesen et al., 1999; Zannoni et al., 2010). In addition to similar morphologic features, for the most part alterations in gene expression and mutation spectra relevant for human EC were similar in Wistar Han rat uterine carcinomas regardless of TBBPA exposure. Uterine carcinomas in both groups had nuclear expression of ERα, increased expression of Her2, Pten, P16, Irf1, Ccnd1 and Cdh1, and microsatellite markers. Loss of Pten and P16 tumor suppressor gene expression are common events in Type I and Type II EC in women, respectively, and increased expression of Ccnd1 is a common feature of Type II tumors. While EC in women is generally associated with microsatellite instability (loss of Msh2, Mlh1, Msh3) and loss of Cdh1, P16, and Pten function, both spontaneous and TBBPA-associated uterine carcinomas had increased expression of these mediators.
The primary molecular differences observed in uterine carcinomas from TBBPA-exposed animals compared to spontaneous tumors included a marked increase in Tp53 mutation and increased Her2 gene expression, and possibly a reduction in PR expression. While all uterine carcinomas, irrespective of differentiation or TBBPA exposure, showed nuclear expression of ERα, PR expression was reduced or lost in a majority of uterine carcinomas from TBBPA-exposed animals and fewer spontaneous tumors. Expression of ERα and loss of PR expression in TBBPA-exposed Wistar Han rats may suggest that these tumors remain estrogen responsive, but have lost receptor functionality for progesterone. Interestingly, research has shown that PR status may be a more reliable prognostic factor than ERα status in EC in women, and corresponds with a more aggressive, high-grade tumor (Oreskovic et al., 2004; Zannoni et al., 2010).
Spontaneous uterine carcinomas and carcinomas from TBBPA-exposed rats both shared morphologic and molecular features with Type I and II EC in women. Features common to both spontaneous and TBBPA-exposed tumors that were shared with Type I EC tumors in women included late onset, endometrioid differentiation, and ERα hormone receptor expression. Features shared between rat uterine carcinomas and human Type II EC include poor differentiation and deep invasion, high mitotic rate and KI67 index, increased cellular pleomorphism and atypia, Her2 expression and Tp53 mutation. While both spontaneous uterine carcinomas and those from TBBPA-exposed rats share features of Type I and Type II human EC, carcinomas from TBBPA-exposed rats have significant differences from spontaneous tumors. In addition to the above mentioned features, uterine carcinomas from TBBPA-exposed rats had significantly increased Tp53 mutation and Her2 expression compared to spontaneous tumors, features more often observed in Type II EC in women. Taken together, the morphologic and molecular features of uterine carcinomas in TBBPA-exposed rats overlap those of Type I and II EC in women, most consistent with high-grade Type I EC (Table 7), suggesting that uterine carcinomas in TBBPA-exposed rats may closely resemble this type of EC. However, many human EC tumors also share features of both subtypes, and a clear distinction based on histomorphologic and molecular features is sometimes difficult.
By far, the most striking differences between uterine carcinomas in TBBPA-exposed animals and controls were the marked increase in Her2 gene expression and Tp53 mutation, two of the major genetic alterations in Type II EC in women (Liu, 2007). Human epidermal growth factor receptor 2 (Her2 or Her2/neu) is a receptor tyrosine kinase that is an upstream regulator of a number of important cell proliferation and anti-apoptosis pathways. It is highly upregulated in Type II EC, and has been linked to adverse prognostic outcome and decreased overall survival (Doll et al., 2008). The TP53 tumor suppressor gene is responsible for cell cycle checkpoint maintenance, apoptosis, and genomic stability (Blagosklonny, 2000). Loss of TP53 tumor suppressor function is important in a wide variety of human cancers, including the development of aggressive endometrial cancers. TP53 mutation is associated with high-grade tumors, advanced disease, and a poor prognosis (Liu, 2007). TP53 mutations are extremely common in Type II EC (80–90%), and found in a subset (10–20%) of high-grade Type I EC in women (Liu, 2007; Llobet et al., 2009; Oreskovic et al., 2004; Zannoni et al., 2010); therefore, it is thought to possibly occur as a late event in the development of aggressive endometrial cancer. In this study, the high rate of Tp53 mutation in uterine carcinomas from TBBPA-exposed rats compared to spontaneous tumors suggests that the increased incidence of uterine carcinomas in treated animals may be driven through a Tp53-dependent mechanism. While some degree of TP53 protein expression was observed in both spontaneous and TBBPA-associated uterine carcinomas, those tumors in TBBPA-exposed animals showed a much higher degree of immunoreactivity, consistent with the increase in mutation rate. The presence of nuclear immunoreactivity did not always correspond with the presence of a mutation in Tp53 in this series of samples. However, these types of discordant results have been reported previously, and may be a result of gain of function of the TP53 gene, accumulation of wildtype protein due to DNA damage (Blagosklonny, 2000), or a reflection of a low percentage of mutation-positive tumor cells (Bian et al., 2001; George et al., 2007; Kropveld et al., 1996). This latter explanation could also account for nuclear expression of TP53 in spontaneous samples, which was much lower than that of the TBBPA samples, in which there was a high rate of mutation. Alternatively, gene mutations may have occurred in unsequenced exons.
Other features of Type I and II EC in women include Ctnnb1 and Kras mutation. Beta-catenin is the protein product of Ctnnb1 and is an essential part of the WNT signaling pathway, regulating cellular processes including proliferation, motility, and survival (Wu et al., 2007). CTNNB1 mutation in EC in women occurs in approximately 20–40% of Type I tumors compared to 0–5% in Type II tumors (Llobet et al., 2009). KRAS mutation, seen in 10–30% of Type I EC in women, (Llobet et al., 2009), is associated with constitutive activation, enhanced cellular proliferation, transformation, and cell survival, and has been associated with adverse clinical outcomes in women (Matias-Guiu et al., 2001). Ctnnb1 and Kras mutations were not observed to play a significant role in either spontaneous uterine carcinomas or those from TBBPA-exposed animals in this study; the general lack of these mutations suggests that these are not critical pathways for the generation of these tumors in the Wistar Han rat model.
The results of this study suggest that uterine carcinomas in TBBPA-exposed Wistar Han rats most closely resemble high-grade Type I EC in women on the molecular level, which are typified in humans by conservation of ERα expression, a high rate of TP53 mutation, more aggressive clinical course and a poor prognosis compared to other typical low-grade Type I tumors (Liu, 2007; McConechy et al., 2012; Zannoni et al., 2010). Interestingly, the BDII Han rat model develops a high rate of spontaneous uterine carcinomas (~90%) which, based on molecular features, have also been classified as similar to high-grade Type I ECs in women (Adamovic et al., 2008; Behboudi et al., 2001; Nordlander et al., 2005; Samuelson et al., 2009). They have an early onset, endometrioid differentiation, and are estrogen responsive, but have high rates of Tp53 mutation, similar to the uterine carcinomas observed in TBBPA-exposed Wistar Han rats in this study. However, carcinomas in TBBPA-exposed rats have additional features of Type II ECs, including overexpression of Her2.
In summary, uterine carcinomas were observed with a higher frequency in Wistar Han rats exposed to TBBPA, have higher rates of proliferation, an increased rate of Tp53 mutation, a tendency toward decreased PR expression, and increased HER2 expression compared to spontaneous uterine carcinomas in Wistar Han rats. These features overlap with those of Type I and II EC in women, and are most concordant with high-grade Type I tumors, which are clinically more aggressive and harbor a poorer prognosis compared to other Type I tumors. Furthermore, the presence of such a high rate of Tp53 mutations in TBBPA-exposed animals compared to controls suggests that uterine carcinogenesis in Wistar Han rats exposed to TBBPA is partially Tp53-dependent. Since alteration of TP53 is relevant for aggressive endometrial cancer in women, and plays a predominant role in the pathogenesis of a number of other cancers in humans, it is reasonable to hypothesize that TBBPA exposures may pose a cancer risk to humans through similar mechanisms. However, inherent species differences in the metabolism of TBBPA or sensitivity to its biologic effects on uterine pathophysiology may exist, and further functional studies on the carcinogenic effects of TBBPA on the female reproductive tract are warranted.
Acknowledgements
The authors would like to thank the CMPB Necropsy Core Laboratory personnel, Histology, Immunohistochemistry, and Sequencing Core Laboratories for their technical expertise; and Ms. Beth Mahler and Ms. Eli Ney (NIEHS) for images and figures.
This work was supported by the National Institutes of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), and The Division of the National Toxicology Program (DNTP). This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions or conclusions contained therein do not necessarily represent the statements, opinions or conclusions of the NIEHS, NIH, or the United States government.
Abbreviations
- TBBPA
Tetrabromobisphenol A
- EC
Endometrial carcinoma
- ERα
Estrogen receptor-alpha
- PR
Progesterone receptor
- HER2
Human Epidermal Growth Factor Receptor 2
- CCND/E1
Cyclins D1/E1
- PTEN
Phosphatase and tensin homolog
- P16
Cyclin-dependent kinase inhibitor 2A, multiple tumor suppressor 1
- CDH1
E-cadherin
- Tp53
Tumor protein 53
- Ctnnb1
Catenin (cadherin-associated protein), beta 1
- Kras
Kirsten rat sarcoma viral oncogene homolog
- PIK3CA
Phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha
- NTP
National Toxicology Program
- FFPE
Formalin-fixed, paraffin-embedded
- DE71
Polybrominated diethyl ether mixture
- AT
Antimony trioxide
- SSNDC
Standardized system of nomenclature and diagnostic criteria
- Msh2/Msh3
MutS protein homolog 2/3
- Mlh1
MutL homolog 1
- Irf1
Interferon regulatory factor 1
References
- American Cancer Society: Cancer Facts and Figures. Atlanta, Ga: American Cancer Society; 2013. [Google Scholar]
- American Cancer Society: Cancer Facts and Figures. Atlanta, Ga: American Cancer Society; 2014. http://www.cancer.org/cancer/endometrialcancer/detailedguide/endometrial-uterine-cancer-key-statistics. [Google Scholar]
- Adamovic T, Hamta A, Roshani L, Lu X, Rohme D, Helou K, Klinga-Levan K, Levan G. Rearrangement and allelic imbalance on chromosome 5 leads to homozygous deletions in the CDKN2A/2B tumor suppressor gene region in rat endometrial cancer. Cancer genetics and cytogenetics. 2008;184:9–21. doi: 10.1016/j.cancergencyto.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Bailer AJ, Portier CJ. Effects of treatment-induced mortality and tumor-induced mortality on tests for carcinogenicity in small samples. Biometrics. 1988;44:417–431. [PubMed] [Google Scholar]
- Behboudi A, Levan G, Hedrich HJ, Klinga-Levan K. High-density marker loss of heterozygosity analysis of rat chromosome 10 in endometrial adenocarcinoma. Genes Chromosomes Cancer. 2001;32:330–341. doi: 10.1002/gcc.1198. [DOI] [PubMed] [Google Scholar]
- Beral V, Bull D, Reeves G. Endometrial cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2005;365:1543–1551. doi: 10.1016/S0140-6736(05)66455-0. [DOI] [PubMed] [Google Scholar]
- Bian Y-S, Osterheld M-C, Bosman FT, Benhattar J, Fontolliet C. p53 Gene Mutation and Protein Accumulation during Neoplastic Progression in Barrett's Esophagus. Mod Pathol. 2001;14:397–403. doi: 10.1038/modpathol.3880324. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. p53 from complexity to simplicity: mutant p53 stabilization, gain-of-function, and dominant-negative effect. Faseb J. 2000;14:1901–1907. doi: 10.1096/fj.99-1078rev. [DOI] [PubMed] [Google Scholar]
- Dixon D, Leininger JR, Valerio MG, Johnson AN, Stabinski LG, Firth CH. Proliferative lesions in the ovary, uterus, vagina, cervix, and oviduct in rats. Washington, DC: Guides for Toxicologic Pathology, STP/ARP/AFIP; 1999. pp. 1–32. [Google Scholar]
- Dixon D, Alison R, Bach U, Colman K, Foley GL, Harleman J, Haworth R, Herbert Heuser A, Long G, Mirsky M, Regan K, van Esch E, Westwood FR, Vidal J, Yoshida M. INHAND: Nonproliferative and Proliferative Lesions of the Rat and Mouse Female Reproductive Tract. J Toxicol. Pathol. 2014 doi: 10.1293/tox.27.1S. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll A, Abal M, Rigau M, Monge M, Gonzalez M, Demajo S, Colas E, Llaurado M, Alazzouzi H, Planaguma J, Lohmann MA, Garcia J, Castellvi S, Ramon y Cajal J, Gil-Moreno A, Xercavins J, Alameda F, Reventos J. Novel molecular profiles of endometrial cancer-new light through old windows. J Steroid Biochem Mol Biol. 2008;108:221–229. doi: 10.1016/j.jsbmb.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Emons G, Fleckenstein G, Hinney B, Huschmand A, Heyl W. Hormonal interactions in endometrial cancer. Endocr Relat Cancer. 2000;7:227–242. doi: 10.1677/erc.0.0070227. [DOI] [PubMed] [Google Scholar]
- George B, Datar RH, Wu L, Cai J, Patten N, Beil SJ, Groshen S, Stein J, Skinner D, Jones PA, Cote RJ. p53 gene and protein status: the role of p53 alterations in predicting outcome in patients with bladder cancer. J Clin Oncol. 2007;25:5352–5358. doi: 10.1200/JCO.2006.10.4125. [DOI] [PubMed] [Google Scholar]
- Gosavi RA, Knudsen GA, Birnbaum LS, Pedersen LC. Mimicking of estradiol binding by flame retardants and their metabolites: a crystallographic analysis. Environ Health Perspect. 2013;121:1194–1199. doi: 10.1289/ehp.1306902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Kester MH, Andersson PL, Legler J, Brouwer A. In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol Sci. 2006;92:157–173. doi: 10.1093/toxsci/kfj187. [DOI] [PubMed] [Google Scholar]
- Horn LC, Meinel A, Handzel R, Einenkel J. Histopathology of endometrial hyperplasia and endometrial carcinoma: an update. Ann Diagn Pathol. 2007;11:297–311. doi: 10.1016/j.anndiagpath.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S. Comparative study of the endocrine-disrupting activity of bisphenol A and 19 related compounds. Toxicol Sci. 2005;84:249–259. doi: 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- Kuroboshi H, Okubo T, Kitaya K, Nakayama T, Daikoku N, Fushiki S, Honjo H. Interferon regulatory factor-1 expression in human uterine endometrial carcinoma. Gynecologic Oncology. 2003;91:354–358. doi: 10.1016/s0090-8258(03)00515-8. [DOI] [PubMed] [Google Scholar]
- Kropveld A, van Mansfeld ADM, Nabben N, Hordijk GJ, Slootweg PJ. Discordance of p53 status in matched primary tumours and metastases in head and neck squamous cell carcinoma patients. European Journal of Cancer. Part B: Oral Oncology. 1996;32:388–393. doi: 10.1016/s0964-1955(96)00030-9. [DOI] [PubMed] [Google Scholar]
- Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004;444:213–223. doi: 10.1007/s00428-003-0947-3. [DOI] [PubMed] [Google Scholar]
- Liu FS. Molecular carcinogenesis of endometrial cancer. Taiwan J Obstet Gynecol. 2007;46:26–32. doi: 10.1016/S1028-4559(08)60102-3. [DOI] [PubMed] [Google Scholar]
- Llobet D, Pallares J, Yeramian A, Santacana M, Eritja N, Velasco A, Dolcet X, Matias-Guiu X. Molecular pathology of endometrial carcinoma: practical aspects from the diagnostic and therapeutic viewpoints. Journal of clinical pathology. 2009;62:777–785. doi: 10.1136/jcp.2008.056101. [DOI] [PubMed] [Google Scholar]
- Matias-Guiu X, Catasus L, Bussaglia E, Lagarda H, Garcia A, Pons C, Munoz J, Arguelles R, Machin P, Prat J. Molecular pathology of endometrial hyperplasia and carcinoma. Hum Pathol. 2001;32:569–577. doi: 10.1053/hupa.2001.25929. [DOI] [PubMed] [Google Scholar]
- McConechy MK, Ding J, Cheang MC, Wiegand KC, Senz J, Tone AA, Yang W, Prentice LM, Tse K, Zeng T, McDonald H, Schmidt AP, Mutch DG, McAlpine JN, Hirst M, Shah SP, Lee CH, Goodfellow PJ, Gilks CB, Huntsman DG. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228:20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AM, Longacre TA. Atypical endometrial hyperplasia and well differentiated endometrioid adenocarcinoma of the uterine corpus. Surg Pathol. 2011;4:149–198. doi: 10.1016/j.path.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Mittal KR, Barwick KW. Diffusely infiltrating adenocarcinoma of the endometrium. A subtype with poor prognosis. Am J Surg Pathol. 1988;12:754–758. doi: 10.1097/00000478-198810000-00003. [DOI] [PubMed] [Google Scholar]
- Modan B, Hartge P, Hirsh-Yechezkel G, Chetrit A, Lubin F, Beller U, Ben-Baruch G, Fishman A, Menczer J, Struewing JP, Tucker MA, Wacholder S. Parity, oral contraceptives, and the risk of ovarian cancer among carriers and noncarriers of a BRCA1 or BRCA2 mutation. N Engl J Med. 2001;345:235–240. doi: 10.1056/NEJM200107263450401. [DOI] [PubMed] [Google Scholar]
- Nordlander C, Behboudi A, Levan G, Levan KK. Allelic imbalance on chromosome 10 in rat endometrial adenocarcinomas. Cancer genetics and cytogenetics. 2005;156:158–166. doi: 10.1016/j.cancergencyto.2004.05.001. [DOI] [PubMed] [Google Scholar]
- NTP. Toxicology studies of tetrabromobisphenol a (CAS No. 79–94-7) in f344/ntac rats and b6c3f1/n mice and toxicology and carcinogenesis studies of tetrabromobisphenol a in wistar han [crl:wi(han)] rats and b6c3f1/n mice (gavage studies) 2013 NTP Technical Report Series: http://ntp.niehs.nih.gov/ntp/about_ntp/trpanel/2013/october/draft_tr-587.pdf.
- NTP. Toxicology studies of green tea extract in f344/ntac rats and b6c3f1/n mice and toxicology and carcinogenesis studies of green tea extract in wistar han[crl:wi(han)] rats and b6c3f1/n mice (gavage studies) 2014 NTP Technical Report Series: http://ntp.niehs.nih.gov/?objectid=BD499CD4-123F-7908-7B1F22BDE9A5D1D7.
- NTP. Toxicology studies of antimony trioxide 10676-v (CASRN: 1309–64-4) in wistar han [crl:wi(han)] rats and b6c3f1/n mice and toxicology and carcinogenesis studies of antimony trioxide 10676-v in wistar han[crl:wi(han)] rats and b6c3f1/n mice (inhalation studies) 2014 NTP Technical Report Series: http://ntp.niehs.nih.gov/?objectid=BCD52E6A-123F-7908-7BD5298A5D912AF9.
- NTP. Toxicology studies of cimstar 3800 in f344/ntac rats and b6c3f1/n mice and toxicology and carcinogenesis studies of cimstar 3800 in wistar han [crl:wi (han)] rats and b6c3f1/n mice (inhalation studies). NTP Technical Report Series: http://ntp.niehs.nih.gov/ntp/about_ntp/trpanel/2014/may/draft_tr586_508.pdf#search=cimstar%2 03800. 2014 doi: 10.22427/NTP-TR-586. [DOI] [PMC free article] [PubMed]
- NTP. Toxicology studies of pentabromodiphenyl oxide (technical) (DE 71) - M20287 (CASRN: 32534–81-9) in f344/n rats and b6c3f1/n mice and toxicology and carcinogenesis studies of pentabromodiphenyl oxide (technical) (DE 71) - M20287 in wistar han [crl:wi(han)] rats and b6c3f1/n mice (gavage studies) 2014 NTP Technical Report Series: http://ntp.niehs.nih.gov/?objectid=BD73BA18-123F-7908-7BD4AEF6AB4318BF.
- Oreskovic S, Babic D, Kalafatic D, Barisic D, Beketic-Oreskovic L. A significance of immunohistochemical determination of steroid receptors, cell proliferation factor Ki-67 and protein p53 in endometrial carcinoma. Gynecol Oncol. 2004;93:34–40. doi: 10.1016/j.ygyno.2003.12.038. [DOI] [PubMed] [Google Scholar]
- Piegorsch WW, Bailer AJ. Statistics for Environmental Biology and Toxicology, Section 6.3.2. London: Chapman and Hall; 1997. [Google Scholar]
- Portier CJ, Bailer AJ. Testing for increased carcinogenicity using a survival-adjusted quantal response test. Fundamental and Applied Toxicology. 1989;12:731–737. doi: 10.1016/0272-0590(89)90004-3. [DOI] [PubMed] [Google Scholar]
- Ryan AJ, Susil B, Jobling TW, Oehler MK. Endometrial cancer. Cell and tissue research. 2005;322:53–61. doi: 10.1007/s00441-005-1109-5. [DOI] [PubMed] [Google Scholar]
- Salvesen HB, Iversen OE, Akslen LA. Prognostic significance of angiogenesis and Ki-67, p53, and p21 expression: a population-based endometrial carcinoma study. J Clin Oncol. 1999;17:1382–1390. doi: 10.1200/JCO.1999.17.5.1382. [DOI] [PubMed] [Google Scholar]
- Samuelson E, Hedberg C, Nilsson S, Behboudi A. Molecular classification of spontaneous endometrial adenocarcinomas in BDII rats. Endocr Relat Cancer. 2009;16:99–111. doi: 10.1677/ERC-08-0185. [DOI] [PubMed] [Google Scholar]
- Schauer UM, Volkel W, Dekant W. Toxicokinetics of tetrabromobisphenol a in humans and rats after oral administration. Toxicol Sci. 2006;91:49–58. doi: 10.1093/toxsci/kfj132. [DOI] [PubMed] [Google Scholar]
- Vollmer G. Endometrial cancer:experimental models useful for studies on molecular aspects of endometrial cancer and carcinogenesis. Endocr Relat Cancer. 2003;10:23–42. doi: 10.1677/erc.0.0100023. [DOI] [PubMed] [Google Scholar]
- Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, Hanash S, Misek DE, Katabuchi H, Williams BO, Fearon ER, Cho KR. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer cell. 2007;11:321–333. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Zannoni GF, Vellone VG, Arena V, Prisco MG, Scambia G, Carbone A, Gallo D. Does high-grade endometrioid carcinoma (grade 3 FIGO) belong to type I or type II endometrial cancer? A clinical-pathological and immunohistochemical study. Virchows Arch. 2010;457:27–34. doi: 10.1007/s00428-010-0939-z. [DOI] [PubMed] [Google Scholar]