Abstract
Introduction
Skeletal muscle oxidative capacity decreases and fatigability increases after spinal cord injury. Transcription factor peroxisome proliferator-activated receptor δ (PPARδ) promotes a more oxidative phenotype.
Methods
We asked whether PPARδ overexpression could ameliorate these deficits in the medial gastrocnemius (MG) of spinal cord transected (ST) adult mice.
Results
Time-to-peak tension and half-relaxation times were longer in PPARδ-Con and PPARδ-ST compared to littermate wild-type (WT) controls. Fatigue index was 50% higher in PPARδ-Con than WT-Con and 70% higher in the PPARδ-ST than WT-ST. There was an overall higher percent of darkly stained fibers for succinate dehydrogenase in both PPARδ groups.
Discussion
The results indicate a conversion towards slower, more oxidative, and less fatigable muscle properties with overexpression of PPARδ. Importantly, the elevated fatigue resistance was maintained after ST, suggesting that enhanced PPARδ expression, and possibly small molecule agonists, could ameliorate the increased fatigability routinely observed in chronically paralyzed muscles.
Keywords: oxidative metabolism, fatigability, spinal cord injury, isometric contractile properties, myosin heavy chain
INTRODUCTION
Most skeletal muscles are comprised of a mixture of fibers categorized as slow (type I) or fast (type II) based on their morphologic, metabolic, and mechanical properties. Slow muscle fibers are predominantly dependent on oxidative phosphorylation for energy, have a relatively high capillary density, are highly resistant to fatigue, contain type I myosin heavy chain (MHC), are small in size, have relatively slow isometric twitch properties, and exhibit a relatively slow shortening velocity1,2. In contrast, fast fibers generally exhibit opposite characteristics, i.e., have higher dependence on the glycolytic pathways for energy, are less resistant to fatigue, have relatively fast isometric twitch properties, and exhibit a faster shortening velocity1,2. In general, the muscle speed properties reflect its fiber type composition3.
It is well documented that after a spinal cord injury there is marked atrophy and a pronounced shift in the MHC profile from type I to type II in the muscles that are innervated below the level of injury4–8. A reduction in the oxidative potential and an increase in the fatigability of hindlimb muscles comprised predominantly of fast fibers also have been reported in in situ preparations9,10. Similarly, paralyzed human subjects show increased neuromuscular fatigability in muscles below the site of spinal cord injury in both acute and chronic11,12 stages after injury. Therefore, reversing or reducing this fatigability most likely would result in significant functional benefits for spinal cord injured subjects.
Nuclear hormone receptors have been shown to alter muscle fiber phenotype, oxidative capacity, and substrate usage13,14. For example, peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors expressed in a variety of tissues and play key roles in lipid metabolism15. One PPAR subtype, PPARδ plays a critical role in transcriptional regulation of skeletal muscle metabolism by switching fuel usage from glucose and fatty acids to predominantly fatty acids, thereby increasing the oxidative capacity of the muscle16,17. Overexpression of constitutively active PPARδ in skeletal muscles of transgenic mice increased the number of oxidative muscle fibers and enhanced running endurance by ~100% compared to wild-type littermates14. Similarly, in vivo transfection of active PPARδ (VP16-PPARδ) in pre-existing fibers of the extensor digitorum longus (EDL) muscle of adult rats resulted in a transformation of fast fibers towards a slower, more oxidative phenotype18. For example, the number of I/IIa hybrids tripled, IIa fibers increased from 14% to 25%, and IIb fibers decreased from 55% to 45% in PPARδ-transfected EDL muscles after 14 days. These findings suggest that PPARδ modulation could be used to prevent the increased fatigability and shift towards faster mechanical and metabolic properties that occur after muscle paralysis.
The purpose of this study was to determine and compare the effects of PPARδ overexpression on the phenotype of the medial gastrocnemius (MG, a predominantly fast plantar flexor) muscle in intact and complete spinal cord transected (ST) adult wild-type and PPARδ transgenic mice. We hypothesized that overexpression of PPARδ, which results in a higher percentage of slow (high oxidative and fatigue resistant) fibers than observed in wild-type mice18,14, would attenuate the decrease in fatigue resistance normally observed in predominantly fast muscles after complete ST19,9,10. The MG was studied because it is a mixed fast muscle and has a fiber type composition and distribution that is representative of most muscles in the hindlimb. We find that overexpression of PPARδ enhances fatigue resistance of the MG, and this enhancement is maintained after ST, suggesting that activation of its target gene network could have potential therapeutic benefits.
MATERIALS AND METHODS
Experimental groups
Age- and gender-matched mice used in the study were derived from the strain originally described by Wang, et.al.14 Adult PPARδ transgenic and age-matched wild-type (WT) littermates (n=6–8/group) were assigned randomly to a WT-Control (Con), WT-ST, PPARδ-Con, and PPARδ-ST group. All mice in the ST groups underwent ST surgery. All procedures were approved by the UCLA Office of Animal Research Oversight and followed the American Physiological Society Animal Care Guidelines.
Surgical procedures and animal care
All surgical procedures were performed under aseptic conditions as described previously20. All ST mice were anesthetized deeply using isoflurane gas via facemask to effect. A longitudinal midline skin incision was made dorsal to the spinal column from the T6–T10 levels. The muscles overlying the spinal column were separated from the spinal column, and a partial laminectomy was performed to expose the spinal cord at the T7–T8 levels. The spinal cord, including the dura, then was transected completely with micro-dissection scissors. To verify the completeness of the transection, 1) a probe was passed between the cut ends of the spinal cord, and 2) the cut ends of the spinal cord were lifted gently using fine forceps. Gelfoam was packed between the cut ends of the spinal cord. The fascia surrounding the spinal column was sutured (5.0 Dexon) over the transection site, and the skin incision closed using 5.0 Ethilon. The mice were placed in an incubator until fully awake and transferred to individual plastic cages amply lined with CareFresh. Baytril, a general antibiotic, and Buprenex, an analgesic, were administered (5 mg/kg and 0.05 mg/kg subcutaneously twice daily, respectively) during the first 2 days of recovery. Post-surgical care included manual expression of the bladder three times daily for the first week and twice daily thereafter as previously described21,20. Once daily the hindlimbs of each mouse were stretched gently through a full range of motion at each joint to help sustain joint mobility.
In situ mechanical properties
Four weeks after ST, the MG from all mice were tested for their in situ mechanical properties as described by Roy et al.22. The mice were anesthetized with a mixture of ketamine (133 mg/kg body weight) and xylazine (9 mg/kg body weight) and given supplemental doses of ~20% the initial dose as needed. The MG nerve was isolated carefully with a glass probe, cut as far proximal as possible, and placed on a bipolar silver electrode for stimulation. The MG tendon was freed at its distal end and secured to a stiff wire near the musculotendinous junction that was attached to a force transducer-lever system (Cambridge Instruments) to measure the muscle isometric properties (see below). The mice were placed in a prone position on a heating pad maintained at 36 ±1°C. Using a combination of bars, pins, and clamps, the hip, knee, and ankle joints were stabilized at ~90° with the MG in a horizontal position. The skin in the lower leg was freed from connective tissue and used to form a warm mineral oil pool that was maintained at 35 ±1°C using radiant heat. The temperature of the muscle was allowed to equilibrate with the mineral oil for at least 30 min prior to physiological testing, and the temperature was monitored with a digital thermocouple inserted in an adjacent muscle with feedback capability.
The average time-to-peak-tension (TPT), half-relaxation time (HRT), and maximum twitch tension (Pt) were determined from 10 consecutive isometric twitch responses at the optimum muscle length at 2 times threshold stimulation intensity. Peak isometric tension development was measured at frequencies of 5, 10, 20, 30, 40, 50, 75, 100, 200, and 250 Hz for a duration (between 100 ms and 1 s) that resulted in a plateau in the tension output. The highest tension achieved regardless of stimulation frequency was considered the maximum isometric tetanic tension (Po). The isometric fatigue properties were determined by 330-ms trains of 40 Hz delivered once per sec for 2 min, and the fatigue index was calculated as a ratio of the tension produced at 2 min divided by the maximum tension measured during the fatigue test. Specific tension was calculated as Po/muscle weight (g/mg).
Tissue harvesting
The MG muscle was removed bilaterally, trimmed of fat and connective tissue, and weighed (wet weight). The MG muscles used for the physiology experiments were pinned on cork at near physiological length, quick-frozen in isopentane cooled with liquid nitrogen, and stored at −80°C until they underwent histochemical and immunohistochemical analyses. The muscles not used for the physiological experiments were quick frozen in liquid nitrogen and stored at −80°C until they underwent RNA analyses.
Quantitative RT-PCR
Assays were performed as described previously23. Briefly, for each biological sample, quantitative PCR reactions were performed in triplicate, and expression of PPARδ was normalized to Gapdh expression. The averaged values of PPARδ in the MG were expressed relative to the WT values.
Quantitative RT-PCR
To determine if expression of PPARδ is altered after ST, we analyzed PPARδ expression by quantitative RT-PCR (QPCR) in VP16-PPARδ transgenic mice and WT controls as described previously23. Briefly, for each biological sample, quantitative PCR reactions were performed in triplicate, and expression of PPARδ was normalized to Gapdh expression. The results confirmed that transgenic mice had an 18- to 20-fold increase in expression of PPARδ compared to WT-Con (data not shown).
Immunohistochemical analyses
Serial cross sections (10 µm thick) from the mid-belly of each MG were cut in a cryostat maintained at −20°C and mounted on gelatin-coated slides. The MHC profiles of ~100 adjacent fibers from a representative area from deep (the region of the muscle near the tibia) and superficial (the region of the muscle away from the tibia) regions were determined. A series of monoclonal antibodies specific to mouse MHC isoforms were used for the immunohistochemical analyses as described by Talmadge et al.8. Anti-slow and anti-fast antibodies (Vector Laboratories, Burlingame, CA) were used to identify slow and fast MHC isoforms, respectively. The avidin-biotin immunohistochemical procedure was used to localize and amplify the antigen-antibody binding complex (Vectastain ABC kits, Vector Laboratories). Additional tissue sections were stained without primary antibody incubation to control for nonspecific binding. Fibers were considered to contain a specific MHC if there was a visually detectable reaction in the fiber to the appropriate monocolonal antibody. Fibers were classified as type I (positive for the slow antibody), type I+II (positive for both the slow and fast antibodies), and type II (positive for the fast antibody).
Succinate dehydrogenase (SDH) analysis
Histochemical staining for succinate dehydrogenase (SDH) was carried out as described by Luquet et al.24. Briefly, fresh-frozen sections from the mid-belly of the MG were cut at 10 µm in a cryostat and placed on gelatin-coated slides. Slides then were incubated in 0.5 mM phosphate buffer (pH 7.6) containing sodium succinate and nitro-blue tetrazolium for 20 min at 37°C. Slides were transferred to 0.9% NaCl briefly, dehydrated through a serial dilution of ethanol, cleared with Citri-solv (Fisher Scientific, Pittsburgh, PA), and mounted with Permount. Individual fibers were assessed qualitatively as staining either darkly or lightly for SDH. To assess the level of SDH staining across the entire muscle cross section, the area containing a relatively high percentage of fibers staining darkly for SDH was measured using Axiovision (Zeiss), divided by the total area of the muscle cross section, and reported as a percent.
Statistical analyses
All data are presented as mean ± SEM. Overall mean differences were determined using a 2 × 2 analysis of variance (ANOVA) model followed by the Neuman-Kuels post hoc test for determining pairwise mean differences. The Prism software package (GraphPad) was used for all analyses. Significant differences were determined at P< 0.05.
RESULTS
Body and MG weights
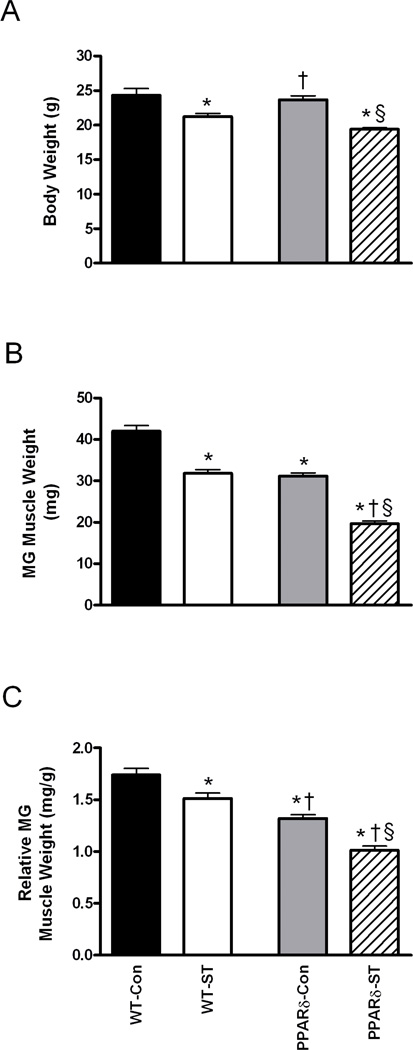
The initial mean body weights were similar across groups: WT-Con, WT-ST, PPARδ-Con, and PPARδ-ST were 23.3±0.8, 23.9±0.7, 22.9±0.5, and 23.5±0.3 g, respectively. At the 4-week time point, however, the mean body weights for the WT-ST and PPARδ-ST groups were 13% and 18% lower than their respective Con groups (Fig. 1A), consistent with previous reports25,26.
Figure 1.
Body weight (A) for wild-type Control (WT-Con, n=8), WT-spinal cord transected (WT-ST, n=7), PPARδ-Con (n=6), and PPARδ-ST (n=8) groups. Absolute (B) and relative to body weight (C) medial gastrocnemius (MG) weights for each group. Values are means ± SEM. *, †, and § denote a significant difference from WT-Con, WT-ST, and PPARδ-Con groups, respectively, at P < 0.05.
At the 4-week time point, the mean absolute MG weight was 26% smaller in the PPARδ-Con compared to the WT-Con group (Fig. 1B). The absolute MG weights were 24% and 37% smaller in the WT-ST and PPARδ-ST mice compared to their respective Con groups. In addition, the absolute MG weight was 38% smaller in the PPARδ-ST compared to the WT-ST group. Relative (to body weight) MG weight was 35% smaller in the PPARδ-Con than in the WT-Con group (Fig. 1C). The relative weights of the WT-ST and PPARδ-ST groups were 14% and 16% smaller than their respective Con groups, with the relative weight of the PPARδ-ST group being 26% smaller than the WT-ST group. These results indicate that the effects of ST on all measures of muscle weight were greater in the PPARδ than in the WT mice.
MG mechanical properties
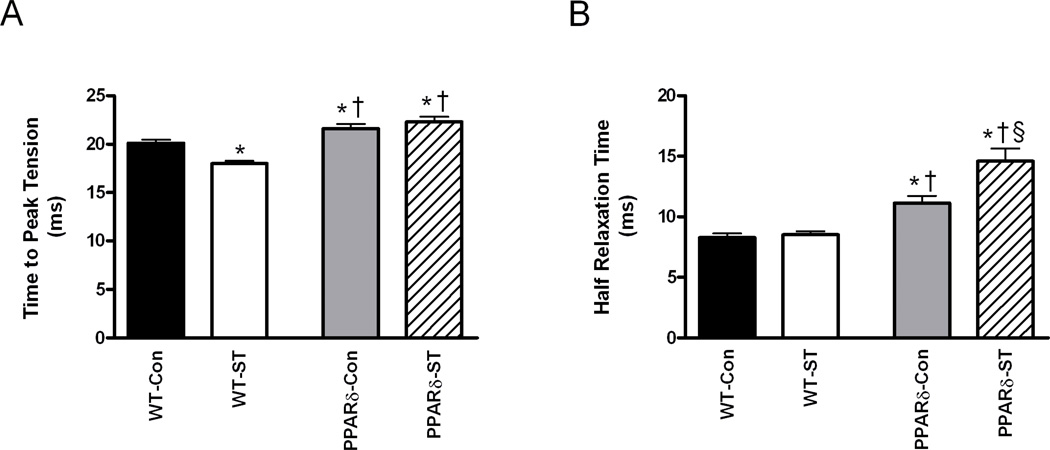
Mean twitch TPT was longer in the PPARδ-Con than in the WT-Con group and longer in the PPARδ-ST than in the WT-ST group (Fig. 2A). ST resulted in a shorter TPT in the WT-ST than WT-Con group, but there was no effect in the PPARδ mice. Mean HRT was longer in both PPARδ groups compared to their respective control groups (Fig. 2B). Unexpectedly, mean HRT was longer in the PPARδ-ST than the PPARδ-Con group. Overall these results are consistent with a lengthening of the twitch duration of the MG with PPARδ overexpression.
Figure 2.
Time-to-peak-tension (A) and half-relaxation times (B) of the MG from WT-Con, WT-ST, PPARδ-Con, and PPARδ-ST groups at the end of the 4-week experimental period. Values are means ± SEM. *, †, and § denote a significant difference from WT-Con, WT-ST, and PPARδ-Con groups, respectively, at P < 0.05.
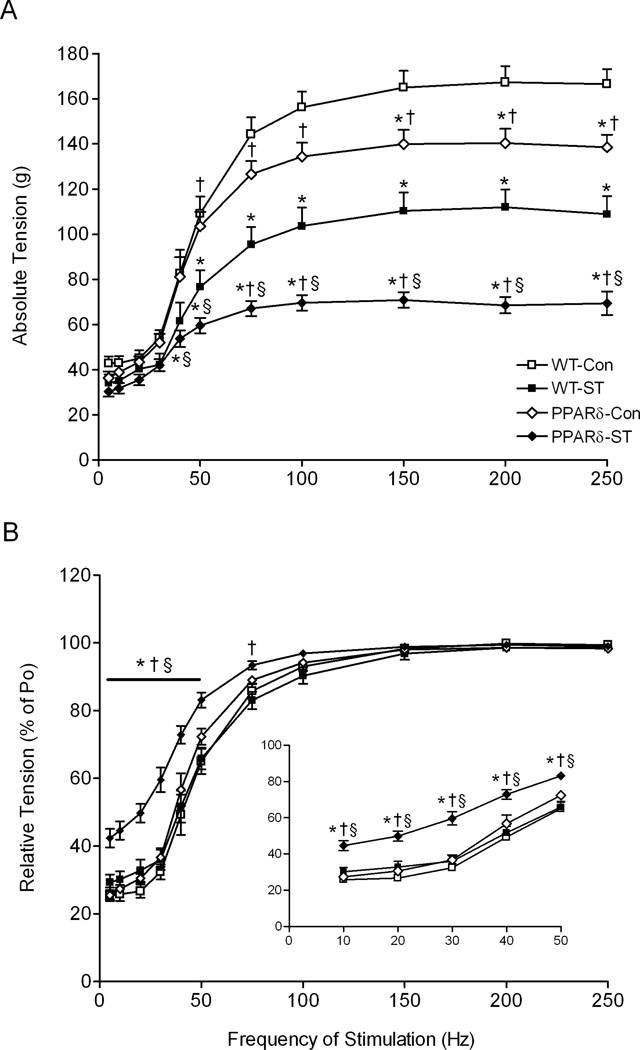
The mean absolute tension produced in the PPARδ-Con was lower than in the WT-Con group at all stimulation frequencies above 150 Hz (Fig. 3A). These values were lower than both Con groups at all frequencies above 50 Hz and above 40 Hz for the WT-ST and PPARδ-ST groups, respectively. In addition, the mean absolute tension was lower in the PPARδ-ST than the WT-ST at all frequencies above 40 Hz. Interestingly, the mean relative tension (expressed as a percent of Po) was similar for the PPARδ-Con, WT-Con, and WT-ST groups at all stimulation frequencies (Fig. 3B). In contrast, the tensions were higher (curve shifted to the left) in the PPARδ-ST than all other groups at all frequencies up to 50 Hz, and higher than WT-ST up to 75 Hz. This leftward shift in the curve in the PPARδ-ST group suggests a shift toward slower muscle properties.
Figure 3.
Absolute (A) and relative [% of maximum tetanic tension (B)] frequency of stimulation-tension responses for the MG muscles for the WT-Con, WT-ST, PPARδ-Con, and PPARδ-ST groups at the end of the 4-week experimental period. Inset in (B) is the expanded relative frequency of stimulation-tension response from 10 to 50 Hz. Values are shown as means ± SEM. Po, maximum tetanic tension. *, †, and § denote a significant difference from WT-Con, WT-ST, and PPARδ-Con groups, respectively, at P < 0.05.
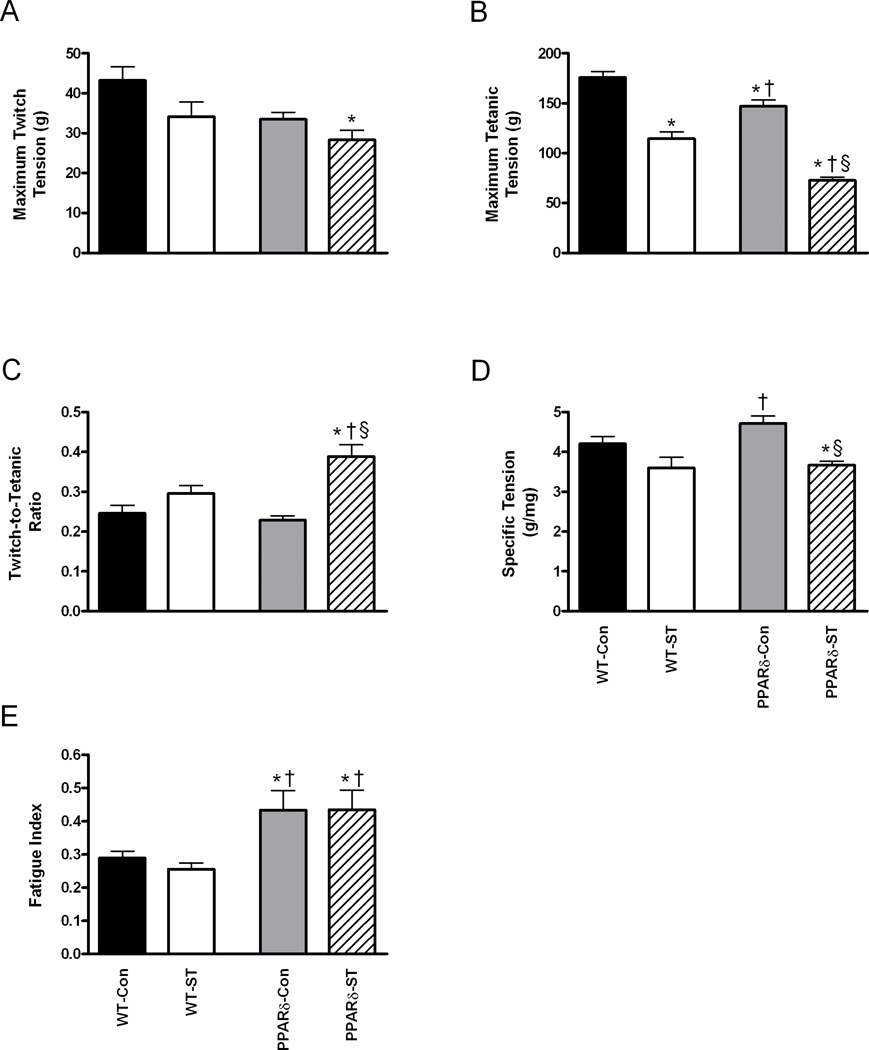
The mean Pt was quite variable and was not different across groups (Fig. 4A). The mean Po, however, was 16% lower in the PPARδ-Con than in the WT-Con group (Fig. 4B), and ST resulted in a mean Po that was 35% lower in the WT-ST than the WT-Con and 51% lower in the PPARδ-ST than the PPARδ-Con groups (Fig. 4B). Significantly, the mean Po was 51% lower in the PPARδ-ST than the WT-ST group. Mean Pt/Po in the PPARδ-ST was 70% and 32% larger than in the PPARδ-Con and WT-ST groups, respectively (Fig. 4C). Specific tension was 22% lower in the PPARδ-ST than in the PPARδ-Con group (Fig. 4D). Finally, the fatigue index was 50% higher in the PPARδ-Con than the WT-Con group and unexpectedly 70% higher in the PPARδ-ST than in the WT-ST group, indicating a marked improvement in fatigue resistance with PPARδ overexpression (Fig. 4E).
Figure 4.
Maximum twitch tension (A), maximum tetanic tension (B), twitch-to-tetanic tension ratio (C), specific tension (D), and fatigue index (E) of the MG from WT-Con, WT-ST, PPARδ-Con, PPARδ-ST groups at the end of the 4-week experimental period. Specific tension is expressed as maximum tetanic tension (g) per muscle weight (mg). Values are means ± SEM. *, †, and § denote a significant difference from WT-Con, WT-ST, and PPARδ-Con groups, respectively, at P < 0.05.
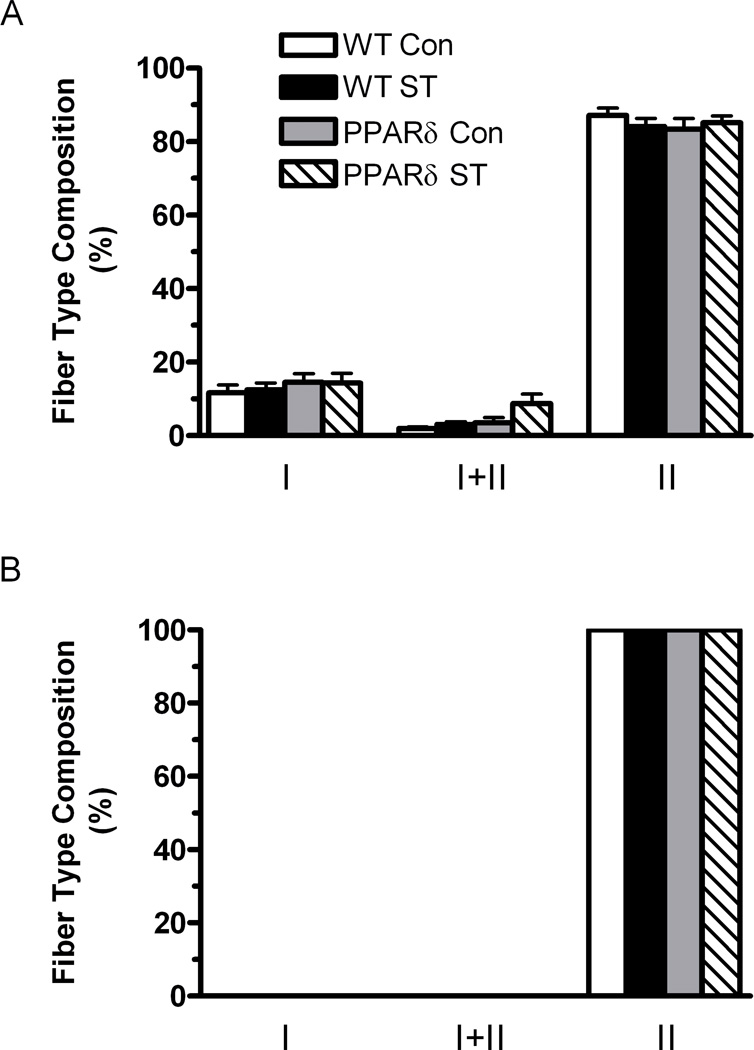
Fiber type composition is not affected in the MG of VP16-PPARδ mice
The deep region of the MG of WT-Con mice was composed of 12, 1, and 87% types I, I+II, and II fibers, respectively (Fig. 5A). The MG superficial region in all groups was composed of fibers containing only the type II isoform (Fig. 5B). There were no significant differences in the fiber type composition in either the deep or superficial regions of the MG among groups.
Figure 5.
Fiber type composition determined by MHC immunohistochemistry. The data are expressed as a percentage of the total number of fibers sampled in the deep (A) (close to the bone) and superficial (B) (away from the bone) regions of the MG from WT-Con, WT-ST, PPARδ-Con, and PPARδ-ST groups. Values are means ± SEM.
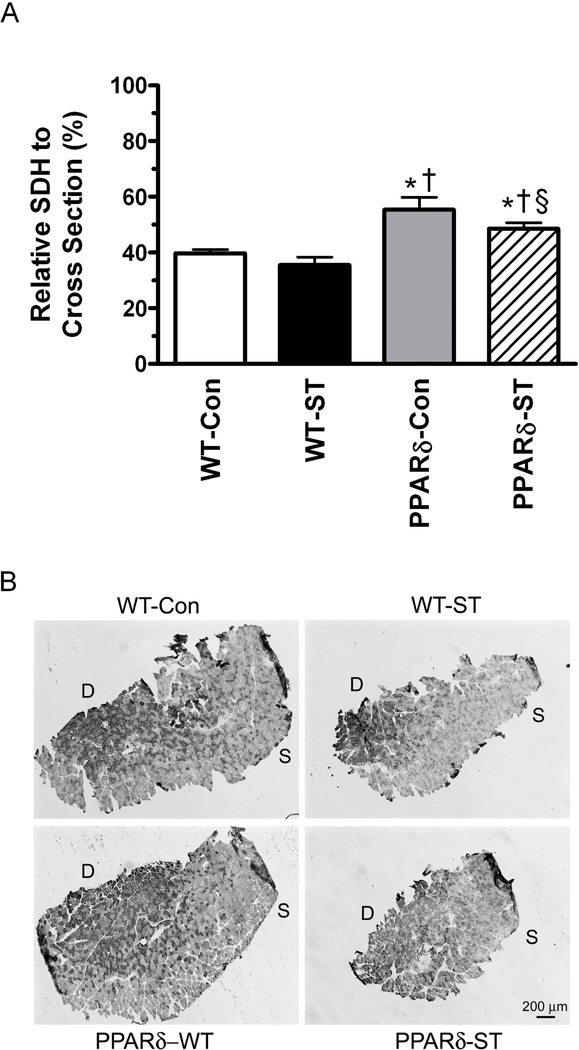
Oxidative profile of the MG
The area of the MG cross-section with a high proportion of oxidative fibers was ~40% greater in both PPARδ groups compared to their respective control group (Fig. 6). In addition, this percent was lower in the PPARδ-ST than the PPARδ-Con group.
Figure 6.
Relative SDH levels per whole muscle cross section (A) and representative cross-sections from the mid-belly of the MG (B) from WT-Con, WT-ST, PPARδ-Con, and PPARδ-ST groups. Values are means ± SEM. D, deep region of the muscle close to the bone; S. superficial region of the muscle away from the bone. *, †, and § denote a significant difference from WT-Con, WT-ST, and PPARδ-Con groups, respectively, at P < 0.05.
DISCUSSION
There is growing evidence that PPARδ has a strong influence in the maintenance of and transformation to the slow and oxidative skeletal muscle phenotype. For example, a recent study of biopsies from the vastus lateralis muscles of elite athletes, spinal cord injured individuals, and normally active individuals showed a strong correlation between the amount of type I fibers and PPARδ expression27. Elite cyclists had the highest proportion of type I fibers and the highest expression of PPARδ mRNA, whereas the spinal cord injured subjects had the lowest proportion of type I fibers (large increase in type IIb) and lowest PPARδ expression levels. Accordingly, we explored whether enhanced PPARδ expression in skeletal muscle could mitigate the shift towards faster mechanical properties, lower oxidative capacity, and increased fatigability associated with ST. Using the MG, an ankle plantar flexor comprised predominantly of fast fibers, our data clearly demonstrate that activation of the PPARδ signaling pathway results in a shift towards slower isometric twitch and frequency-tension properties and reduced fatigability compared to WT-Con and that these properties are maintained for at least 4 weeks after a complete mid-thoracic ST.
Effects of PPARδ overexpression on muscle weight
The effects of PPARδ overexpression on muscle properties appear to be dependent on the type of muscle, i.e., slow vs. fast, and the mechanism by which the gene is expressed, i.e., transgenic vs. viral. For example, our studies show that in the soleus, a predominantly slow ankle plantar flexor, the relative muscle weight (compared to body weight) was 58% higher (data not shown), whereas in the MG it was 26% smaller in the PPARδ transgenic compared to the WT littermates (Fig. 1). Similarly, although Lunde and colleagues18 did not report muscle weight data, consistent with this decrease in muscle mass of a fast ankle extensor is the report of an overall 14% decrease in fiber cross-sectional area in adult rat extensor digitorum longus muscles (a fast flexor) 5 days after they were transfected with a VP16-PPARδ fusion construct. In contrast, in homozygous transgenic PPARδ mice there were no significant changes in the absolute muscle weight in the tibialis anterior, soleus, and plantaris muscles compared to control littermates24. The observed differences between the studies above could stem from developmental differences in PPARδ transgenic expression, i.e., whether the PPARδ is overexpressed using a CRE-recombinase system24 or through generation of a VP16-PPARδ construct14, or due to differences in the promoters, such as VP16 used here. Moreover, the lack of change in body weight despite the marked decrease in muscle weight may reflect increases in bone density as previously observed in ovariectomized mice given the PPARβ/δ-specific agonist GW50151628.
Effects of PPARδ overexpression on skeletal muscle isometric mechanical properties
The atrophy and associated decrease in maximum force generation in the MG of both WT and PPARδ mice in response to ST is consistent with that observed in a number of species and spinal cord injury models22,10. The shorter TPT in WT-ST compared with WT-Con also is consistent with faster isometric twitch speed properties after ST as reported previously29,9,10. In contrast, there was no difference in TPT in the PPARδ-Con and PPARδ-ST and a longer HRT after ST. This prolonged HRT is consistent with the shift of the frequency-tension curve to the left in the PPARδ transgenic mice, indicating that the MG has slower isometric mechanical properties after ST. These results may reflect an adaptation in calcium kinetics during excitation-contraction coupling, particularly calcium re-uptake during relaxation30,31 and are consistent with the reported increase in slow and decrease in fast forms of troponin I in PPARδ transgenic mice14. In addition, changes in the function and concentration of Ca2+-ATPase have been suggested to explain, at least in part, the slowing of relaxation time in the quadriceps muscles of spinal cord injured subjects 24 weeks post-injury32.
Effects of PPARδ overexpression on skeletal muscle MHC composition
The MHC composition between the deep and superficial regions was not different between the WT and PPARδ groups. This is in contrast to what was observed in normal adult rat EDL muscles transfected with a constitutively active PPARδ construct for 14 days in which there was a shift in the MHC fiber type composition towards the slower phenotype, i.e., increased type I+IIa and IIa fibers with concomitant decreases in MHC IIb18. The number of “hybrid” I+II fibers was not different in the PPARδ-Con compared to the WT-Con group in our study. Even 4-weeks after ST the number of hybrid fibers in both the deep and superficial regions did not change. Thus in our hands it appears that the appearance of hybrid fibers often associated with changes in muscle activity did not occur as reported previously in the soleus muscle after 360 days of ST in adult rats33. The mice used in our study are born with excess PPARδ, and any changes associated with increased PPARδ may have occurred during development. The conversion of type II to type I fibers has been observed in mice that overexpress calcium/calmodulin-dependent protein kinase IV or PGC-1α34,35, but overexpression of PPARδ does not promote the appearance of type I fibers in TA and plantaris muscles24. This is consistent with our observations that show no changes in MHC composition of type I fibers across groups. We did not distinguish between the different fast MHC isoforms, i.e., IIa, IIx, and IIb, thus we cannot determine if there were any changes in the expression of these isoforms among groups.
Effects of PPARδ overexpression on skeletal muscle metabolic and fatigue properties
Lunde et al.18 reported that the overall fiber SDH activity level in the EDL based on quantitative histochemistry was 43% higher in the PPARδ-transfected than control rats. SDH activity was elevated in all fast fiber types. Type IIb fibers, that normally have the lowest levels of SDH activity, had levels similar to that normally found in IIx fibers, and type IIx and IIa fibers had 82% and 20% increases in SDH activity, respectively. Luquet et al.24 also reported an increase in the number of fibers staining darkly for SDH in the tibialis anterior and plantaris muscles and in the level of citrate synthase activity in the tibialis anterior of double transgenic PPARδ mice. In our study we observed an increase in the percent of the muscle cross section containing a high proportion of fibers staining darkly for SDH, a finding consistent with the overall increase in oxidative capacity reported with PPARδ overexpression previously18,24,36,14. Wang et al.14 found an approximately 2-fold increase in type I fibers (using a metachromic dye-ATPase method) in the gastrocnemius of PPARδ transgenic mice and reported 67% and 92% increases in running time and distance, respectively, when the mice were run to exhaustion on an oxygen-infused enclosed treadmill. In the present study, the fatigue resistance of the MG was significantly higher in the PPARδ-Con than in the WT-Con group, consistent with the reported higher oxidative capacity and the higher number of fatigue-resistant fibers in PPARδ transgenic mice18,14.
PPARδ maintains skeletal muscle fatigue properties after ST
The MG in PPARδ transgenic mice was more fatigue resistant than in WT mice, and this difference (70%) was maintained after ST. This is important because a decrease in fatigue resistance is one of the primary deficits observed after spinal cord injury in animal models and in humans37. Most studies of spinal cord injury report a shift in the MHC composition from slow type I and fast type IIa towards fast type IIx and IIb that may contribute to greater fatigability due to the lesser efficiency of fast vs. slow myosin38. This shift in fiber type composition also is associated with a decrease in the oxidative potential of the muscle that directly and negatively impacts its fatigue resistance properties. Our results demonstrate that PPARδ overexpression can change the isometric mechanical properties of a predominantly fast, fatigable muscle towards a more oxidative, fatigue resistant muscle. Moreover, these properties are maintained even 4 weeks after complete ST. These results are particularly important when considering the advances that have been made in rehabilitating motor function in spinal cord injured subjects using a number of interventions such as motor training, spinal cord epidural stimulation, and/or administration of pharmacological agents39–41. The maintenance of fatigue resistance in the muscles affected by spinal cord injury certainly would be beneficial for the level of recovery. Moreover, attenuating this response could have important therapeutic implications given the deleterious effects that increased fatigability has on muscle function and subsequent locomotor performance after ST.
Limitations
How the changes in the contractile properties are linked to changes in the MHC profile (composition) and other muscle-related properties, i.e., SERCA, troponin, etc remain unknown and should be considered to further elucidate the mechanisms (pathways) by which PPARδ may be targeting muscle function after a ST. Although the myosin isoform is linked closely to contractile speed3, it is clear that it is not the only controller. This is readily obvious when considering the large range in maximum shortening velocity for a population of single muscle fibers expressing the same MHC isoform42. Our data also show that the metabolic changes as reflected by SDH staining intensity do not correlate with changes in MHC composition, suggesting that the oxidative potential of fibers in a muscle are not strongly dependent on muscle fiber phenotype. Since we were unable to separate the type II MHC isoforms, however, a more detailed analysis of the MHC composition may show that there is an association between the metabolic changes and the subtypes of fast MHC isoforms in the MG, an extensor muscle comprised predominantly of fast fibers. Whether the adaptations induced by PPARδ administration would be similar in a predominantly slow muscle such as the soleus is unknown.
Therapeutic implications
Muscle phenotype changes are likely to be influenced by a cascade of highly coordinated events including, but not limited to, those controlling myosin protein composition, metabolism, mitochondrial function, and vascularization. Factors such as diet, gene overexpresssion, and pharmacological interventions can trigger these events, but these factors most likely will simultaneously impact other physiological such as cardiovascular, respiratory, and other support systems. Overexpression of the nuclear hormone receptor estrogen receptor-related receptor γ (ERRγ) enhances the type I muscle phenotype in the normally fast gastrocnemius muscle and increases its vascularization and mitochondria content resulting in an increased resistance to fatigue13. Furthermore mice with ERRγ-remodeled muscle have an increase in oxygen consumption and lower respiratory exchange ratio and a subsequent increase in endurance and resistance to weight gain. Similarly, administration of the PPARδ agonist GW501516 results in a robust activation of genes involved in lipid metabolism such as uncoupling protein 3 (Ucp3), muscle carnitine palmitoyl transferase I (mCPT I), and pyruvate dehydrogenase kinase 4 (Pdk4)36,43. PPARδ agonism however, does not activate mitochondrial gene expression and function in vitro and in vivo nor does it induce a fiber type switch from glycolytic to oxidative43. In fact the effects of PPARδ agonism increases mitochondrial gene expression only when combined with exercise36, demonstrating that pharmacological intervention alone is not sufficient to have a beneficial effect on factors such as running time and distance, elements that are critical when developing a potential rehabilitative paradigm. Furthermore, PPARδ agonism induced by GW610742X administration appears to have a positive therapeutic potential for type II diabetic patients. The potential has limited or negative long-term implications, as PPARδ agonism can impair muscle function by inhibiting carbohydrate oxidation during exercise at intensities where carbohydrates are the mainstay of energy delivery44. Thus, the significant differences in metabolic and biochemical properties in skeletal muscles of PPARδ agonism vs. PPARδ overexpression suggest the complex nature of this gene and its potential as a therapeutic intervention, and warrants further investigation.
Acknowledgments
The authors thank Dr. Jeffrey Gornbein from the Department of Biomathematics, and Sharon Zdunowski, and Parag Gad for their help with the statistical analyses. The authors also thank Maynor Herrera and a large number of undergraduate students for excellent care of the animals.
Funding: This work was supported by the Christopher and Dana Reeve Foundation award #VEC-2010 (V.R.E.) and the National Institutes of Health Grant DK057978, HL105278, The Ellison Medical Foundation, The Leona M. and Harry B. Helmsley Charitable Trust #2012-PG-MED002, the Glenn Foundation for Medical Research and the Howard Hughes Medical Institute (R.M.E.).
ABBREVIATIONS
- Con
control
- CSA
cross-sectional area
- EDL
extensor digitorum longus
- ERRγ
estrogen receptor-related receptor γ
- HRT
half-relaxation time
- MG
medial gastrocnemius
- MHC
myosin heavy chain
- PDK4
pyruvate dehydrogenase kinase 4
- PPARδ
peroxisome proliferator-activated receptor δ
- Pt
maximum twitch tension
- Po
maximum tetanic tension
- SDH
succinate dehydrogenase
- ST
spinal cord transection
- TPT
time-to-peak tension
- WT
wild-type
Footnotes
Competing interests: There are no competing interests.
REFERENCES
- 1.Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Reviews of physiology, biochemistry and pharmacology. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- 2.Pette D, Staron RS. Transitions of muscle fiber phenotypic profiles. Histochemistry and cell biology. 2001;115(5):359–372. doi: 10.1007/s004180100268. [DOI] [PubMed] [Google Scholar]
- 3.Caiozzo VJ. Plasticity of skeletal muscle phenotype: mechanical consequences. Muscle & nerve. 2002;26(6):740–768. doi: 10.1002/mus.10271. [DOI] [PubMed] [Google Scholar]
- 4.Andersen JL, Mohr T, Biering-Sorensen F, Galbo H, Kjaer M. Myosin heavy chain isoform transformation in single fibres from m. vastus lateralis in spinal cord injured individuals: effects of long-term functional electrical stimulation (FES) Pflugers Arch. 1996;431(4):513–518. doi: 10.1007/BF02191897. [DOI] [PubMed] [Google Scholar]
- 5.Burnham R, Martin T, Stein R, Bell G, MacLean I, Steadward R. Skeletal muscle fibre type transformation following spinal cord injury. Spinal Cord. 1997;35(2):86–91. doi: 10.1038/sj.sc.3100364. [DOI] [PubMed] [Google Scholar]
- 6.Graham SC, Roy RR, Navarro C, Jiang B, Pierotti D, Bodine-Fowler S, Edgerton VR. Enzyme and size profiles in chronically inactive cat soleus muscle fibers. Muscle & nerve. 1992;15(1):27–36. doi: 10.1002/mus.880150106. [DOI] [PubMed] [Google Scholar]
- 7.Roy RR, Kim JA, Grossman EJ, Bekmezian A, Talmadge RJ, Zhong H, Edgerton VR. Persistence of myosin heavy chain-based fiber types in innervated but silenced rat fast muscle. Muscle & nerve. 2000;23(5):735–747. doi: 10.1002/(sici)1097-4598(200005)23:5<735::aid-mus11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Talmadge RJ, Roy RR, Edgerton VR. Prominence of myosin heavy chain hybrid fibers in soleus muscle of spinal cord-transected rats. Journal of applied physiology. 1995;78(4):1256–1265. doi: 10.1152/jappl.1995.78.4.1256. [DOI] [PubMed] [Google Scholar]
- 9.Roy RR, Talmadge RJ, Hodgson JA, Oishi Y, Baldwin KM, Edgerton VR. Differential response of fast hindlimb extensor and flexor muscles to exercise in adult spinalized cats. Muscle & nerve. 1999;22(2):230–241. doi: 10.1002/(sici)1097-4598(199902)22:2<230::aid-mus11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 10.Talmadge RJ, Roy RR, Caiozzo VJ, Edgerton VR. Mechanical properties of rat soleus after long-term spinal cord transection. J Appl Physiol. 2002;93(4):1487–1497. doi: 10.1152/japplphysiol.00053.2002. [DOI] [PubMed] [Google Scholar]
- 11.Castro MJ, Apple DF, Jr, Staron RS, Campos GE, Dudley GA. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999;86(1):350–358. doi: 10.1152/jappl.1999.86.1.350. [DOI] [PubMed] [Google Scholar]
- 12.Gerrits HL, De Haan A, Hopman MT, van Der Woude LH, Jones DA, Sargeant AJ. Contractile properties of the quadriceps muscle in individuals with spinal cord injury. Muscle & nerve. 1999;22(9):1249–1256. doi: 10.1002/(sici)1097-4598(199909)22:9<1249::aid-mus13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 13.Narkar VA, Fan W, Downes M, Yu RT, Jonker JW, Alaynick WA, Banayo E, Karunasiri MS, Lorca S, Evans RM. Exercise and PGC-1alpha-independent synchronization of type I muscle metabolism and vasculature by ERRgamma. Cell metabolism. 2011;13(3):283–293. doi: 10.1016/j.cmet.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS biology. 2004;2(10):e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muoio DM, Koves TR. Skeletal muscle adaptation to fatty acid depends on coordinated actions of the PPARs and PGC1 alpha: implications for metabolic disease. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2007;32(5):874–883. doi: 10.1139/H07-083. [DOI] [PubMed] [Google Scholar]
- 16.Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Molecular endocrinology (Baltimore, Md. 2003;17(12):2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- 17.Schuler M, Ali F, Chambon C, Duteil D, Bornert JM, Tardivel A, Desvergne B, Wahli W, Chambon P, Metzger D. PGC1alpha expression is controlled in skeletal muscles by PPARbeta, whose ablation results in fiber-type switching, obesity, and type 2 diabetes. Cell metabolism. 2006;4(5):407–414. doi: 10.1016/j.cmet.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Lunde IG, Ekmark M, Rana ZA, Buonanno A, Gundersen K. PPARdelta expression is influenced by muscle activity and induces slow muscle properties in adult rat muscles after somatic gene transfer. The Journal of physiology. 2007;582(Pt 3):1277–1287. doi: 10.1113/jphysiol.2007.133025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy RR, Sacks RD, Baldwin KM, Short M, Edgerton VR. Interrelationships of contraction time, Vmax, and myosin ATPase after spinal transection. J Appl Physiol. 1984;56(6):1594–1601. doi: 10.1152/jappl.1984.56.6.1594. [DOI] [PubMed] [Google Scholar]
- 20.Fong AJ, Cai LL, Otoshi CK, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci. 2005;25(50):11738–11747. doi: 10.1523/JNEUROSCI.1523-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai LL, Fong AJ, Otoshi CK, Liang Y, Burdick JW, Roy RR, Edgerton VR. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J Neurosci. 2006;26(41):10564–10568. doi: 10.1523/JNEUROSCI.2266-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy RR, Zhong H, Monti RJ, Vallance KA, Edgerton VR. Mechanical properties of the electrically silent adult rat soleus muscle. Muscle & nerve. 2002;26(3):404–412. doi: 10.1002/mus.10219. [DOI] [PubMed] [Google Scholar]
- 23.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell metabolism. 2007;6(1):13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Luquet S, Lopez-Soriano J, Holst D, Fredenrich A, Melki J, Rassoulzadegan M, Grimaldi PA. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. Faseb J. 2003;17(15):2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- 25.Landry E, Frenette J, Guertin PA. Body weight, limb size, and muscular properties of early paraplegic mice. Journal of neurotrauma. 2004;21(8):1008–1016. doi: 10.1089/0897715041651060. [DOI] [PubMed] [Google Scholar]
- 26.Ung RV, Lapointe NP, Guertin PA. Early adaptive changes in chronic paraplegic mice: a model to study rapid health degradation after spinal cord injury. Spinal Cord. 2008;46(3):176–180. doi: 10.1038/sj.sc.3102119. [DOI] [PubMed] [Google Scholar]
- 27.Kramer DK, Ahlsen M, Norrbom J, Jansson E, Hjeltnes N, Gustafsson T, Krook A. Human skeletal muscle fibre type variations correlate with PPAR alpha, PPAR delta and PGC-1 alpha mRNA. Acta physiologica (Oxford, England) 2006;188(3–4):207–216. doi: 10.1111/j.1748-1716.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 28.Scholtysek C, Katzenbeisser J, Fu H, Uderhardt S, Ipseiz N, Stoll C, Zaiss MM, Stock M, Donhauser L, Böhm C. PPAR [beta]/[delta] governs Wnt signaling and bone turnover. Nature medicine. 2013;19(5):608–613. doi: 10.1038/nm.3146. [DOI] [PubMed] [Google Scholar]
- 29.Lieber RL, Johansson CB, Vahlsing HL, Hargens AR, Feringa ER. Long-term effects of spinal cord transection on fast and slow rat skeletal muscle. I. Contractile properties. Experimental neurology. 1986;91(3):423–434. doi: 10.1016/0014-4886(86)90041-5. [DOI] [PubMed] [Google Scholar]
- 30.Briggs FN, Poland JL, Solaro RJ. Relative capabilities of sarcoplasmic reticulum in fast and slow mammalian skeletal muscles. The Journal of physiology. 1977;266(3):587–594. doi: 10.1113/jphysiol.1977.sp011783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brody IA. Regulation of isometric contraction in skeletal muscle. Experimental neurology. 1976;50(3):673–683. doi: 10.1016/0014-4886(76)90036-4. [DOI] [PubMed] [Google Scholar]
- 32.Castro MJ, Apple DF, Jr, Rogers S, Dudley GA. Influence of complete spinal cord injury on skeletal muscle mechanics within the first 6 months of injury. European journal of applied physiology. 2000;81(1–2):128–131. doi: 10.1007/PL00013785. [DOI] [PubMed] [Google Scholar]
- 33.Talmadge RJ, Roy RR, Edgerton VR. Persistence of hybrid fibers in rat soleus after spinal cord transection. Anat Rec. 1999;255:188–201. doi: 10.1002/(SICI)1097-0185(19990601)255:2<188::AID-AR9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Wu H, Tarr PT, Zhang C-Y, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 35.Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296(5566):349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- 36.Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134(3):405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pelletier CA, Hicks AL. Muscle characteristics and fatigue properties after spinal cord injury. Critical reviews in biomedical engineering. 2009;37(1–2):139–164. doi: 10.1615/critrevbiomedeng.v37.i1-2.40. [DOI] [PubMed] [Google Scholar]
- 38.MacIntosh B, Gardiner P, McComas A. Human Kinetics. 2nd ed. Champaign, IL: 2006. Skeletal muscle: From and function. [Google Scholar]
- 39.Courtine G, Micera S, DiGiovanna J, Millan Jdel R. Brain-machine interface: closer to therapeutic reality? Lancet. 2013;381(9866):515–517. doi: 10.1016/S0140-6736(12)62164-3. [DOI] [PubMed] [Google Scholar]
- 40.Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annual review of neuroscience. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- 41.Fong AJ, Roy RR, Ichiyama RM, Lavrov I, Courtine G, Gerasimenko Y, Tai YC, Burdick J, Edgerton VR. Recovery of control of posture and locomotion after a spinal cord injury: solutions staring us in the face. Progress in brain research. 2009;175:393–418. doi: 10.1016/S0079-6123(09)17526-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardetto P, Schluter J, Fitts R. Contractile function of single muscle fibers after hindlimb suspension. Journal of applied physiology. 1989;66(6):2739–2749. doi: 10.1152/jappl.1989.66.6.2739. [DOI] [PubMed] [Google Scholar]
- 43.Kleiner S, Nguyen-Tran V, Bare O, Huang X, Spiegelman B, Wu Z. PPAR{delta} agonism activates fatty acid oxidation via PGC-1{alpha} but does not increase mitochondrial gene expression and function. The Journal of biological chemistry. 2009;284(28):18624–18633. doi: 10.1074/jbc.M109.008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Constantin-Teodosiu D, Baker DJ, Constantin D, Greenhaff PL. PPARdelta agonism inhibits skeletal muscle PDC activity, mitochondrial ATP production and force generation during prolonged contraction. The Journal of physiology. 2009;587(Pt 1):231–239. doi: 10.1113/jphysiol.2008.164210. [DOI] [PMC free article] [PubMed] [Google Scholar]