Abstract
Arrestins are intracellular scaffolding proteins known to regulate a range of biochemical processes including G-protein coupled receptor (GPCR) desensitization, signal attenuation, receptor turnover and downstream signaling cascades. Their roles in regulation of signaling network have lately been extended to receptors outside of the GPCR family, demonstrating their roles as important scaffolding proteins in various physiological processes including proliferation, differentiation, and apoptosis. Recent studies have demonstrated a critical role for arrestins in immunological processes including key functions in inflammatory signaling pathways. In this review, we provide a comprehensive analysis of the different functions of the arrestin family of proteins especially related to immunity and inflammatory diseases.
INTRODUCTION
Arrestins are scaffolding proteins that include multiple members divided into subfamilies based on sequence homology and tissue distribution: (i) visual rod arrestin (S antigen), and cone arrestin (C- or X- arrestin) and (ii) β–arrestins (β-arrestin1 and β-arrestin2). More recently, another set of arrestin-domain containing proteins (called α-arrestins) have been included into this family. While the visual rod and cone arrestins are restricted to rods and cones respectively in the visual system, β-arrestins (1 and 2) are ubiquitously expressed. Arrestins are highly conserved across the animal kingdom, with 39–50% sequence homology observed between vertebrates and invertebrates; and 44–84% homology within the vertebrate animals1. Even though originally discovered for their role in GPCR functions, arrestins, especially β-arrestins are now recognized to be critical players in a number of physiological and pathophysiological processes, with various functions indentified to be independent of GPCRs. In this review, we provide only a brief outline of the canonical roles of β-arrestins in GPCR signaling. For in depth information regarding β-arrestins' role in canonical GPCR signaling, readers are referred to some excellent reviews 2. We focus this review mainly on the non-canonical roles of β-arrestins. In particular, we will address the new and emerging roles of β-arrestins in inflammation and inflammatory disease processes.
CANONICAL ROLES OF β-ARRESTINS
β-arrestin was first identified as a protein involved in β-adrenergic receptor desensitization3 along with G protein coupled regulatory kinases (GRKs)4. Both arrestins and GRKs are now established as critical regulators of the canonical G-protein coupled receptor (GPCR) desensitization4–7. Classical GPCR activation is initiated by ligand-binding induced conformational change in the receptor, allowing it to interact with and activate associated heterotrimeric G protein. G protein activation involves exchange of bound GDP for GTP and its dissociation into its functional subunits, Gα and Gβγ. This induces signaling cascade through second messengers such as cAMP, calcium and diacylglycerol (DAG). In addition to initiating downstream signaling, agonist binding also leads to receptor phosphorylation by GRKs and recruitment of arrestin to this phosphorylated GPCR. A receptor thus bound to arrestin is sterically unable to interact with G proteins, thereby terminating further signaling (desensitization). In many cases, this is followed by receptor internalization through clathrin-coated pits followed by either receptor recycling or degradation (Fig 1). To allow for a check on uninhibited GPCR activation that could be detrimental to the host, signal termination and receptor desensitization is thus brought about by concerted actions of GRKs and arrestins. Over the course of time, this simplified view has considerably expanded to include contribution of β-arrestins to other aspects of GPCR signaling including scaffolds for signal transduction, transcriptional control and apoptosis regulation to name a few.
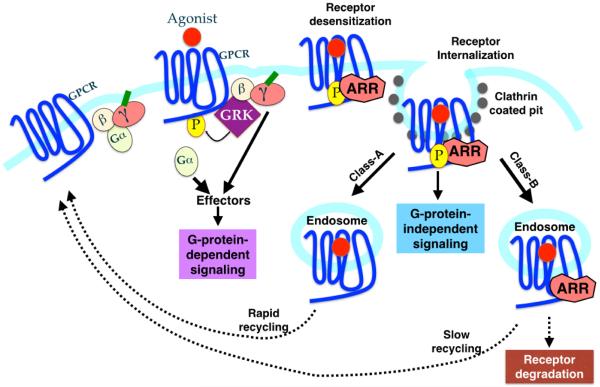
Fig 1. Classical and New Roles of Arrestins in GPCR lifecycle.
Activation of GPCR occurs by binding of the agonist, which stimulates heterotrimeric G-proteins. This G-protein-dependent signaling results in the production of second messengers. Simultaneously, GRKs phoshorylate the receptor leading to the arrestin binding and inhibition of G-protein-dependent signaling (desensitization). In addition to desensitizing the receptor by uncoupling the receptor from G-proteins, arrestins also recruit clathrin and adaptin molecules to target the desensitized receptor to clathrin coated pit. Thus arrestins play a crucial role in receptor internalization. Arrestins bound to the phosphorylated GPCRs also serve as a scaffolding platform for the MAPK modules (MAPKKK-MAPKK-MAPK). This results in the prevention of nuclear translocation of the MAPK, leading to an increase in phosphorylation of the cytosolic targets. This causes the “second-wave” of signaling, that is G-protein-independent. Affinity and duration of arrestin interaction with endocytosed GPCRs has also led to a classification of receptors into class A and B. See text for details.
Association of β-arrestins with GPCRs divides the latter into two distinct categories on the basis of receptor trafficking. Class A receptors including, adrenergic (β2, α1B), μ opioid, endothelin A and dopamine D1A receptors have a higher affinity for β-arrestin2 compared to β-arrestin1 and interact transiently with β-arrestins. Class B receptors (such as angtiotensin II, vasopressin V2, neurotensin 1 and neurokinin NK1) on the other hand have equal affinities for both β-arrestins and their interaction is more stable. While class A receptors recycle rapidly, class B receptors recycle slowly (Fig 1).
The two β-arrestins have 78% identical amino acid sequence with most differences observed in the C-terminus2. Studies over the past couple of decades have clearly demonstrated that their role in GPCR signaling is non-redundant with both distinct and overlapping functions. While both β-arrestins have a nuclear localization signal located at the amino-terminus (1–185)8; β-arrestin2 additionally has a nuclear export signal located at C-terminus (384–409) that leads to its cytoplasmic shuttling8, leading to distinct subcellular localization patterns for the two proteins. β-arrestins further have the ability to form homo- and hetero-dimers, through a inositol hexakisphosphate (IP6) binding site. This oligomerization further affects the subcellular localization of β-arrestin1, such that monomers are nuclear while oligomers exhibit cytoplasmic localization9. This differential regulation of subcellular localization has implications in signaling modules employed by the two β-arrestins as discussed in following sections.
Even though individual knockouts develop normally, double knockouts are embryonically lethal10. β-arrestin1 (53 kda, chromosome 7) knockout mice are fertile, produce normal litter size and do not exhibit any overt histopathological evidence of disease in organs. They also display normal blood chemistry in terms of hemoglobin, hematocrit, WBC and RBC count and splenic lymphocyte reconstitution11. β-arrestin2 (46 KDa, chromosome 11) knockout mice too are viable with no gross abnormalities and no differences in basal body temperature12. The β-arrestin2 knockout mice were reported to have slightly lower body weight and fat proportion once they reach the age of 12 weeks13; this may be mouse colony dependent since in our mouse colony we have observed that young knockout mice have reduced body weight that recovers as the mice get older (unpublished observations).
Role of β-arrestins in receptor endocytosis
The canonical role of β-arrestins involves receptor desensitization and internalization wherein they act as scaffolds for proteins involved in the life-cycle of the GPCR. Receptor internalization is an important feature of GPCR life-cycle occurring through clathrin-mediated or caveolae-mediated endocytic vesicles. Early studies found an important role for β-arrestin recruitment in receptor internalization and formation and trafficking of clathrin coated pits. β-arrestin (1 and 2) through C terminus was also shown to interact stoichiometrically with clathrin protein heavy chain motif (LIEL/P) following β-adrenergic agonist stimulation14,15, 16. The two β-arrestins however, differ in their mode of interaction with internalization machinery and consequent signaling. β-arrestin1 dephosphorylation at Ser-412 residue in response to agonist stimulation is required for its interaction with clathrin heavy chain, receptor internalization17 and signaling through Src and ERK1/218. On the other hand, β-arrestin2 dephosphorylation at Thr-383 residue in response to agonist stimulation is irrelevant to its interaction with clathrin molecules and downstream signaling19, 20. β-arrestins also interact with other components of clathrin endocytic machinery including clathrin adapter protein AP-221,22 and NSF (N-ethylmaleimide sensitive fusion) protein, an ATPase essential for intracellular transport23. Through their interacting partners, β-arrestins initiate the process of receptor endocytosis, and internalization and therefore are now established as critical regulators of these physiological processes.
NON-CANONICAL SIGNALING ROLES OF β-ARRESTINS
In addition to their role in GPCR life cycle, β-arrestins also act as “signaling scaffolds” for a number of signaling pathways both GPCR-dependent and -independent. These non-canonical β-arrestin roles differ in context based on cell type and receptors engaged; therefore only studies with implications in inflammatory responses (and pertaining to GPCRs) are discussed below.
Interaction of β-arrestins with MAPK family members
c-Jun N-terminal kinases
c-Jun N-terminal kinases (JNK) are activated following a stress response. The family includes three genes (JNK1, JNK2, JNK3). The cascade includes sequential phosphorylation by MAP3K (eg. ASK1, MEKK, MLK) of MAP2K (eg. MKK4, MKK7) that consequently phosphorylates and activates JNK. Activated JNK can phosphorylate various cytosolic and nuclear targets8, 24, 25. β-arrestin2 constitutively colocalizes with JNK3 in COS-7 cells even under basal conditions and is specifically required for ASK-1 mediated JNK3 activation. This interaction was specific for β-arrestin2 and JNK3 since neither β-arrestin1 nor JNK1 co-immunoprecipitated with a corresponding partner. β-arrestin2 interacts directly with ASK-1 and JNK3 through amino- and carboxy- termini respectively. Although its interaction with upstream MKK4 is indirect, the signalosome forms a complete MAPK module through β-arrestin2 mediated interaction26. The interaction site for JNK3 interaction on β-arrestin2 was shown to require residues 186–410 and further pinned to be at residues 196–19827. Further, nuclear export signal (NES) of β-arrestin2 is critical for sub-cellular localization of JNK3 with it's loss leading to nuclear sequesteration of both β-arrestin2 and JNK328. In vitro studies demonstrate β-arrestin2 (arrestin-3) to be a true scaffold for MKK4-JNK3α2 signaling module29. Further studies using peptide array technology have uncovered binding sites on both β-arrestins and JNK. Interestingly using this approach, molecular determinants of binding of JNK signaling module (including ASK, MKK4 and JNK) to β-arrestin1 and 2 were identified30, 31. In the context of GPCR signaling, angiotensin-induced JNK3 activation and endosomal translocation are both β-arrestin2-mediated, demonstrating the ability of β-arrestin2 to regulate activation and subcellular localization of JNK MAPK module26. Arrestins interact with and cause nuclear export of JNK3 even under basal state, suggesting the existence of a complex poised to respond to the stimuli8, 24, 25. This regulation of JNK3 pathway by β-arrestins can have major implications in physiological processes like stress survival, apoptosis, mitogenesis and differentiation regulated by JNK. However, role of this scaffolding function in immune cell function and in inflammatory diseases is not well known. There is some recent evidence that N-methyl-D-aspartate (NMDA) activation in the liver during liver injury downregulates inflammasomes in part via β-arrestin-2-JNK pathway32.
Extracellular signal-related kinase 1/2
Extracellular signal-related kinase (ERK) activation is associated with proliferation, survival and differentiation and involves sequential activation of various kinases. Following activation, ERK translocates to the nucleus and induces transcription of target genes involved in mitogenesis and proliferation. The ability of β-arrestins to affect ERK1/2 activation was first shown in β2 adrenergic signaling, wherein its interaction with c-src and ability to induce receptor internalization were required for optimal ERK activation18. Since then several studies using different experimental models have demonstrated a role for β-arrestins in regulating the ERK signaling cascade (reviewed in2, 33). In response to protease activated receptor2 (PAR2) agonist, β-arrestin forms a multi-molecular complex in the cytosol with the receptor (PAR2), Raf-1 and activated ERK1/2. Since p-ERK1/2 is sequestered in cytosol, its role in transcription activation and mitogenic potential is rendered ineffective in the presence of β-arrestin34. Similar β-arrestin scaffold (angiotensin type 1a receptor, β-arrestin, Raf-1, and ERK complex)-mediated sequestration of activated ERK1/2 in cytosol led to decreased Elk-driven transcription in response to angiotensin-II35 36. Substance P mediated ERK1/2 signaling, however, occurs through a complex between NK1R, β-arrestin and Src, followed by nuclear translocation of pERK1/2 and downstream effects of proliferation and protection from apoptosis37. Therefore, similar to its regulation of JNK signaling, β-arrestins can bind to ERK and its upstream signaling module (Src, Raf-1) and can regulate the activation status and sub-cellular localization of ERK MAPK module. Studies have also suggested that nuclear and cytosolic ERK localization may have different consequences such that nuclear ERK activates Elk driven transcription and cell proliferation; while cytosolic ERK phosphorylates various cellular targets, perhaps affecting crosstalk with other pathways38,39. In accordance with these functional studies wherein, β-arrestin has the potential to act as scaffold for this multimeric complex; a protein-protein docking model was recently shown to validate the feasibility of this complex and identify the docking sites on β-arrestin for each molecule in a receptor/β-arrestin/Src/Raf/MEK/ERK complex40.
p38 MAPK
p38 MAPK is another family of MAPK activated in response to stress, including cytokines, lipopolysaccharide (LPS) and growth factors. p38 activation in response to β2 adrenergic agonist acts in a biphasic manner, with the first peak observed at 10 minutes, followed by another peak at 90 minutes, which is sustained for 6 hours. While, the early activation of p38 was β-arrestin1 dependent, late activation was Gs/cAMP/PKA mediated41. β-arrestin1 knockdown additionally inhibited Rac-1 membrane translocation, which was required for early p38 activation, in addition to NADPH oxidase activity41. Early p38 activation therefore occurs via a β-arrestin1/Rac1/NADPH oxidase pathway while late activation occurs through canonical Gs signaling. While other studies have shown a role for β-arrestins in p38 activation in chemokine receptors42, κ-opioid receptors43 and cytomegalovirus US28 receptor44, direct β-arrestin-mediated interaction of p38 in the context of immune cell signaling in inflammatory disease is currently lacking.
Nuclear Factor κB (NFκB) Pathway
Nuclear Factor κ-B (NFκB) includes a family of transcription factors that in basal state is kept inactivated by interaction with inhibitory family of proteins, Inhibitory κBα (IκBα). Following activation, IκBα is phosphorylated and degraded allowing, release and nuclear translocation of active NFκB. It then binds to and activates transcription from promoters of target genes. β-arrestins can bind directly to IκBα through its N-terminus45 thereby leading to its stabilization in response to TNFα stimulation46. The precise role of β-arrestins in NFκB is specific to the context of receptor engaged. β2 adrenergic receptor-induced NFκB activation is negatively regulated by β-arrestin2, which binds to and stabilizes IκBα46. β-arrestin2 also inhibits UV induced NFκB activation and anti-apoptotic signaling, that is further enhanced by simultaneous β2 adrenergic stimulation47. On the other hand, β-arrestin2 acts as a positive regulator of this pathway in response to lysophosphatidic acid (LPA)48. Similarly, β-arrestin1 also acts as a positive regulator of endothelin induced NFκB activation, perhaps through its nuclear interaction49. It is not yet clear if these differential roles of β-arrestins in NFκB activation are due to specific receptors engaged or the cell type under investigation.
Nuclear roles of β-arrestins: Transcriptional regulation
Epigenetic modulation in the form of histone acetylation mediated by a family of histone acetylases (HAT) and histone deacetylases (HDAC) regulate chromatin structure and promoter accessibility as an important mode of transcriptional control. β-arrestin1 through its nuclear interaction with HAT p300 mediates cAMP response element binding protein (CREB)-dependent promoter acetylation and transcription of proteins p27 and c-fos following δ-opioid receptor signaling50. β-arrestin1 also regulates histone acetylation mediated gene transcription of anti apoptotic gene bcl-2 in CD4+ T cells51. Furthermore, in T cells isolated from primary biliary cirrhosis patients, β-arrestin1 can modulate expression of genes by virtue of affecting histone H4 acetylation52. These examples ascribe a critical scaffolding role to nuclear β-arrestin1 in regulating gene transcription.
Role of β-arrestins in post-translational modifications
Ubiquitination
Mdm2 is an E3 ubiquitin ligase protein that mediates p53 ubiquitination and proteosomal degradation. Its role in GPCR signaling was identified through its interaction with and ubiquitination of β-arrestins and β2 adrenergic receptor. β-arrestin scaffolds this interaction and ubiquitination of β-arrestin and the receptor was essential for receptor internalization and degradation respectively53. β-arrestin2 by virtue of having a Nuclear Export signal (NES) also critically affects subcellular localization of mdm254. In response to agonist binding (dopamine or β2 adrenergic) receptor, β-arrestin and mdm2 form a ternary complex through their amino and carboxy-termini respectively. This led to lower mdm2 self-ubiquitination, reduced p53 ubiquitination and its consequent degradation. β-arrestin2 expression is thus able to affect p53-mediated apoptosis with higher level leading to mdm2 sequestration, consequently higher p53 mediated apoptosis and vice versa54. β-arrestins therefore, provide an important link between GPCR and p53 signaling.
Role of β-arrestins in Apoptosis
The signal for apoptosis regulation by β-arrestins can originate from both GPCR and non-GPCR stimuli utilizing multiple pathways including MAPK and PI3K-AKT pathways. As discussed in previous sections, β-arrestins through regulation of MAPK and other signaling modules can affect survival and apoptotic stimuli. Depending on the stimuli and pathway involved the role of β-arrestins can be characterized as pro or anti-apoptotic as summarized in table 1.
Table 1.
Role of β-arrestins in apoptosis
| Signal Inducer | β-arrestin | Role | Mechanism | Reference |
|---|---|---|---|---|
| IGF-1 | 1 | Protective | PI3K-AKT | 154 |
| IL-8/CXCR2 | 1/2 | Protective | MAPK | 155 |
| GPCR (fpR,CXCR2) | 1/2 | Protective | PI3K, MAPK | 156 |
| H2O2 | 1/2, 2 | Protective | ASK-1, ERK/AKT/BAD | 157,158 |
| H2O2 + Angiotensin | 2 | Protective | ERK/AKT/BAD | 158 |
| Morphine | 2 | Protective | AKT | 159 |
| Morphine + HIVgpl20 | 2 | Protective | 160 | |
| TRAIL | 2 | Protective | Hsp27/Src | 161 |
| Resveratrol | 2 | Protective | AKT/GSK3β | 162 |
| Staurosporine | 1 | Protective | PI3K/AKT | 163 |
| Staurosporine + β2-adrenergic agonist | 1/2 | Protective | Hsp27 | 164 |
| GLP1R | 1 | Protective | ERK/bad | 165 |
| GPCR | 2 | Susceptible | Mdm2/p53 | 54 |
| GABAB | 1/2 | Susceptible | JNK | 166 |
| M-CSF withdrawal | 2 | Susceptible | 58 |
The stimuli is mentioned in bold, secondary signaling that is cytoprotective is underlined while the one that further aggravates apoptosis is in italics.
β-ARRESTINS IN IMMUNE SYSTEM
Immune system development is largely unaffected by lack of either of the β-arrestins. Equivalent proportion of neutrophils, lymphocytes, monocytes and eosinophils are observed in bone marrow55. Further splenic11, 56, 57, thymic57 and blood56 composition of immune cells is unaffected by loss of β-arrestins. Equivalent CSF-1 and F4/80 expression is observed in bone marrow derived macrophages (BMDMs) from WT or β-arrestin2 knockout mice58. Inflammatory response in the absence of β-arrestins is altered by various stimuli via involvement of distinct and multiple pathways as described in following sections.
G-PROTEIN COUPLED RECEPTORS
Chemokine receptors
Chemotaxis leads to migration of immune cells to the site of inflammation and is a critical component of immune response. Since most chemokine receptors are GPCRs, the role of β-arrestins in chemotaxis stems from their ability to regulate GPCR desensitization and signaling. β-arrestins can associate with various chemokine receptors, including CCR2 (mono mac-1 cells)59, CXCR160, CXCR260,61, CXCR462,63,42, CXCR4:CXCR7 heterodimers64 and CCR542. The role of β-arrestins in receptor desensitization and signaling following ligand binding is presented in table 2. Chemokine signaling induced migration and other responses including degranulation are also regulated by β-arrestins and are summarized below.
Table 2.
Role of β-arrestins in chemokine receptor trafficking and signaling.
| Receptor | Receptor trafficking | Signaling | β-arrestin | Reference |
|---|---|---|---|---|
| FPR | Effective recycling but not internalization | - | 1/2 | 167 |
| CXCR1 | Internalization | - | 1/2 | 168 |
| CXCR2 | Internalization | Higher calcium mobilization, GTPase activity and superoxide production | 2 | 65 |
| CXCR4 | Internalization and desensitization | ERK and p38 activation | 2 | 62,42,63 |
| CXCR4/7 (heterodimer) | ERK, p38 and JNK activation | 2 | 64 | |
| CCR5 | Desensitization | ERK and p38 activation; formation of multimeric complex containing Lyn, PI3K, Pyk2 and ERK | 1/2 | 42,169,71 |
| CCR5/C5aR (heterodimer) | Internalization | ? | 1/2 | 170 |
| PAR2 | Internalization (1) | ERK activation | 1/2 | 171,34,55,72 |
Neutrophils
β-arrestins play important and sometimes even distinct roles in chemokine receptor-mediated signaling and migration. For example, even though CXCR2 mediated signaling is dependent on β-arrestins65, neutrophil infiltration in air pouch and cutaneous wound healing models was significantly elevated in β-arrestin2 knockout mice65. Neutrophil infiltration was also significantly elevated in β-arrestin2 KO mice in response to intraperitoneal oyster glycogen injection66. In a model of DNFB induced contact hypersensitivity, neutrophil infiltration to the site of inflammation was significantly increased in mice lacking β-arrestin267. Utilizing bone marrow chimeras this study however, demonstrated an important negative regulatory role for β-arrestin2 in chemokine production from epidermal keratinocytes or other radioresistant cells67. Thus, a cell intrinsic role for β-arrestin2 in neutrophil migration is difficult to assess in vivo in isolation, from the differences in chemokine production that could contribute to the observed phenotype. Another study using leukocytes from bone marrow (60% neutrophils) and thioglycollate-elicited neutrophils demonstrated a drastic reduction in chemotactic response to PAR2 ligand in a transwell migration assay in the absence of β-arrestins (1 and 2)55. β-arrestins (1 and 2) through their interaction with cofilin and chronophin (CIN) were identified as important in process of actin reorganization, pseudopodia polarization and subsequent directional migration55. β-arrestins therefore have the potential to alter directional migration of neutrophils depending on instigating stimuli and cell intrinsic role in actin assembly.
In addition to migration, stimulation of neutrophils with a high concentration of IL-8 can also induce granule release via CXCR1 through Src kinase (c-Hck and Fgr)-mediated process. CXCR1 phosphorylation and association of β-arrestins (1 /2) with phosphorylated Src kinase isoforms was essential for granule release in neutrophils68. β-arrestins therefore play diverse roles in neutrophil migration and degranulation in response to chemokines.
Lymphocytes
Defective chemotaxis was observed in lymphocytes lacking β-arrestin2 in response to CXCL12 (through CXCR4) in both transwell and trans-endothelial assays, even though GTPase activity in response to the chemokine was higher69. Surprisingly, β-arrestin2 deficient T and B cells also have an inconsistent increase in baseline chemokinesis69 even though their numbers in spleen under homeostatic conditions are unaffected. In U87-CD4 cells, migration occurs via the CXCR4:CXCR7 heterodimer activation in response to stromal derived factor-1α (SDF-1α) and is β-arrestin2 dependent64. In allergic asthma model, lung lymphocyte infiltration was largely abrogated in mice lacking β-arrestin2 and this was shown to be dependent on hematopoietic β-arrestin270. As opposed to negative regulation in neutrophils, β-arrestin2 in lymphocytes is perhaps required for directional migration, at least under conditions studied thus far.
Macrophages
In primary human macrophages, knockdown of β-arrestin 1 and 2 drastically decreases chemotactic response to MIP1β (CCR5)71. Other studies investigating macrophages in the context of β-arrestins have mostly focused on their role in inflammatory gene expression and thus regulation of macrophage chemotaxis by β-arrestins is largely unexplored.
Other Cells
In non-immune cells such as HeLa and HEK293 cells, β-arrestin2 positively mediates SDF-1α induced chemotaxis and increases its efficiency42. In HEK293-CCR5 cells, β-arrestin2 overexpression enhances CCR5 mediated chemotaxis in response to RANTES42. PAR2 mediated chemotaxis in breast cancer cell line MDA-MB 231 is impaired in the absence of both β-arrestins (1 and 2)72. β-arrestins are therefore required for chemotaxis of non-immune cells and this regulation can have implications in wound repair and cancer.
Complement receptors
C3aR
C3a receptor is a GPCR that induces chemotaxis and degranulation in mast cells, basophils and eosinophils, thus playing an important role in innate responses and asthma. In basophilic leukemia cell line RBL-2H3, β-arrestin recruitment following agonist binding leads to CCL2 production but inhibits degranulation, suggesting distinct roles for β-arrestin in these processes73. In mast cells, C3aR-induced signaling is differentially regulated by β-arrestins: β-arrestin2 is required for receptor internalization and desensitization but β-arrestin1 is critical for degranulation. Further, while both β-arrestins negatively regulate early ERK activation only β-arrestin2 inhibits C3aR induced NFκB activation and consequent CCL4 (MIP1β) production74. β-arrestins thus have distinct and overlapping roles in C3aR mediated responses.
C5aR
C5a signaling is mediated by two receptors, C5aR expressed at cellular membranes and intracellular C5L2. G protein coupling, however, is exclusively associated with C5aR. In response to agonist binding, C5aR internalizes and colocalizes with C5L2 and β-arrestin75, suggesting an important role for β-arrestin in C5aR internalization. In addition to complement receptor signaling, expression of complement genes can also be modulated by β-arrestins. Expression of C1q genes (a, b and c) is significantly lower in bone marrow macrophages lacking β-arrestin2 (but not β-arrestin1), both basally and in response to LPS stimulation58. Together, these studies demonstrate important role of β-arrestins in regulating the complement receptor system.
Plasminogen activated receptor 2 (PAR2)
PAR2 is activated by protease trypsin leading to calcium mobilization and protein kinase C (PKC) activation. Its desensitization involves irreversible receptor cleavage by trypsin, PKC-mediated termination and endosomal receptor degradation76. Mast cell degranulation activates PAR2 leading to calcium mobilization and redistribution of tight junction proteins ZO-1 and occludin and perijunction protein F-actin. This process leads to increased transepithelial permeability and is affected by β-arrestin mediated ERK activation77. β-arrestins in response to PAR2 form a macromolecular complex with ERK, leading to p-ERK1/2 sequestration in the cytosol; rendering its transcription and mitogenic activity ineffective34. PAR2 regulated increase in transepithelial permeability has huge implications in stress and inflammatory responses and could be modulated by β-arrestins.
Regulation of inflammatory responses by β-arrestins via non-GPCRs
The following section describes the role of β-arrestins in modulating signaling downstream of non-GPCRs either through crosstalk of signaling platforms or direct generation and engagement of GPCR ligands during the course of inflammatory signaling.
Toll Like Receptors (TLRs)
β-arrestins are widely expressed in macrophages and have been shown to affect TLR signaling both in vivo and in vitro in different cell models. The expression of β-arrestin1 itself is decreased in response to TLR2/4 (not TLR3/7) stimulation via a JNK-mediated mechanism involving both reduced transcription and increased degradation of the protein78. Even though expression of β-arrestin2 is unaffected in primary macrophages (thioglycollate elicited) in response to LPS, it is reduced in Raw264.7 macrophage cell line79. Further, upon LPS stimulation, β-arrestin2 stabilizes IκBα, thereby inhibiting NFκB activation and NOSII expression79. This reduction is dependent on TRIF-80 and β2 adrenergic receptor 79. Interestingly, in THP-1 cells, β adrenergic stimulation dampens IL-8 and TNFα production in response to TLR4 stimulation via a β-arrestin2 mediated redistribution of surface TLR4/CD14 receptor81. This inhibition was further shown to involve AMPK pathway in addition to β-arrestin2 82, implicating a β-arrestin2 mediated cross talk between adrenergic, inflammatory and metabolic pathways during inflammation.
The role of β-arrestins in direct TLR4 signaling and interacting partners is complicated with both positive and negative roles ascribed to them in different cell types. In Raw264.7 cells, β-arrestin1 was identified as a binding partner for NFκB1 p105 (p105) and shown to be an inhibitor of downstream TPL2-MEK1/2-induced ERK1/2 activation83. In MEFs, however, both β-arrestin1 and 2 negatively regulated NFκB activation while β-arrestin2 positively mediated ERK1/2 activation. Consequently, IL-6 production was found to be lower following β-arrestin2 knockdown while both β-arrestin1 and 2 were required for IL-8 production84. On the other hand, in studies using BMDMs, β-arrestin2 inhibited LPS-induced cytokine production85,81 or had no effect86. Similarly, β-arrestin2 knockout neutrophils (glycogen oyster elicited) also produced greater extent of IL-6 and TNFα both basally and in response to LPS66. Consistently, thioglycollate elicited neutrophils from β-arrestin2 knockout mice also responded to LPS stimulation with higher production of IL-6 and IL-1056,87.
In addition to TLR4, β-arrestins have been implicated in regulating NFκB activation in response to other TLR ligands (polyIC) as well as IL-1β signaling. β-arrestins bind and inhibit TRAF6 (a E3 ubiquitin ligase protein) mediated NFκB activation. In this regard, the carboxy-terminus of β-arrestins interacts with TRAF6 through its TRAF domain, blocking the polyubiquitination site (K48) required for self-ubiquitination and IκB activation. Consequently, cytokine production was enhanced in β-arrestin2 deficient BMDMs, in response to TLR4, TLR3 and TLR9 ligands85. Recentlly, this interaction of β-arrestin2 with TRAF6 itself has been shown to be regulated by SUMOylation at lysine 295 residue that reduces its affinity for TRAF6 thereby, enhancing signaling through the latter88. Consistent with effects in response to TLR ligands, cytokine production to adenovirus infection has been found to be modulated differentially by β-arrestins as demonstrated in both in vivo and in vitro studies89. Adenovirus-induced cytokine production has been shown to be mediated through TLR, MyD88 and TRIF mediated signaling90, 91. β-arrestin1 and β-arrestin2 act as positive and negative regulators respectively, of adenovirus signaling suggesting that their modulation could alter adenoviral response and influence the use of adenovirus gene therapy.
In addition to cytokine production, β-arrestin2 also protects against serum starvation induced apoptosis aggravated by TLR4 signaling through its ability to stabilize GSK-3β87. β-arrestins can therefore differentially affect multiple facets of TLR signaling in different cell types. Their roles during in vivo models are discussed in the section under inflammatory disease models.
Tumor Necrosis Factor Receptor (TNFR)
In macrophages as well as in HeLa cells, β-arrestin1 directly interacts with IκBα and functions as a negative regulator of TNFα induced NFκB activation45. Although β-arrestin (1/2) knockdown did not affect TNFα induced NFκB activation and IL-6 production in MEFs48. Alternately in 3T3-L1 adipocytes, TNFα induced Src phosphorylation in a β-arrestin1-dependent manner via G-proteins, Gα/q11. Further, downstream ERK and JNK phosphorylation, MMP3 production and lipolysis were found to be dependent on β-arrestin1 but independent of the interacting G protein92. These studies suggest that β-arrestins are important regulators of TNFR with the signaling module and consequent biological response depending on the cell type.
Transforming Growth Factor β Receptor (TGFβR)
β-arrestin2 mediates TGFβRIII internalization, thereby downregulating TGFβ signaling and its anti-proliferative role93. TGFβRIII signaling has been shown to reduce migration via cdc42 and β-arrestin2 dependent actin remodeling94. Further, TGFβ-mediated induction of T-regulator cells (Tregs) was found to be impaired in β-arrestin2 knockout mice in both, in vitro polarization assays and in secondary lymphoid organs during EAE pathogenesis95. Thus, β-arrestin2 can modulate anti-proliferative and T cell differentiation potential downstream of TGFβ signaling, thereby affecting cancer progression, metastasis and T cell-mediated immunopathologies.
Interferon receptors
IFNγ exhibits potent antiviral activity and signals via STAT1 tyrosine phosphorylation followed by its dimerization and nuclear translocation. STAT1 binds to target genes with IFNγ activation site (GAS) and induces transcription. STAT1 function is antagonized by dephosphorylation through nuclear phosphatase TC45. β-arrestin1 was reported to interact with both nuclear STAT1 (active) and TC45 and affect STAT1 dephosphorylation, thus reducing the potency of IFNγ signaling96. MEFs and HeLa cells lacking β-arrestin1, have sustained STAT1 phosphorylation and greater induction of IFNγ inducible genes. Further, in MEFs lacking β-arrestin1 pretreatment with IFNγ leads to lower host cell death and viral loads post VSV infection, demonstrating a negative regulatory role for β-arrestin1 in IFNγ induced antiviral activity96. Viruses can modulate and interfere with STAT1 mediated IFN response97. Interestingly, β-arrestin1 expression was increased for 8 days following Hepatitis-B virus infection before returning to baseline level in the liver (site of infection) but not the spleen96. This expression profile coincided with observed viral loads98, suggesting that β-arrestin1 could be part of the machinery affecting IFN-induced anti-viral response and modulation of its expression could be an effective therapeutic strategy.
Natural Killer inhibitory receptors
NK cells have a repertoire of activating receptors, NKG2D and Natural cytotoxicity receptor (NCR) - NKp46/44/30 that signal to induce cytokine production or cytotoxicity against viral and tumor targets. They also have inhibitory molecules- killer immunoglobulin like receptors (KIRs) and NKG2A/C/E in humans and Ly49 in mice that recruit phosphatases to counter the activation signal. β-arrestin2 was reported as an important player in this KIR mediated cytotoxicity inhibition. It formed a complex with KIR2DL1 and phosphatases, Src homology containing phosphatases-1 and 2 (SHP-1 and SHP-2) and countered signal transduced through activating receptors. Consequently, mouse NK cell cytotoxicity was inversely proportional to β-arrestin2 expression as shown using NK cells from β-arrestin2 transgenic (overexpression) and knockout mice. Further human KIR2DL allelic heterogeneity and inhibitory potential was associated with its ability to recruit β-arrestin2 and SHP-1 to receptor complex99. This ability of β-arrestin2 to affect NK cell mediated cytotoxicity has implications in mouse cytomegalovirus (MCMV) infection model that is largely dependent on NK cell activity. β-arrestin2 transgenic mice, therefore, have higher viral loads, as there is greater inhibition to NK cell cytotoxicity100. T cell Immunoglobulin and ITIM domain receptor (TIGIT) is another inhibitory molecule expressed on NK cells, Tregs, CD8+ and CD4+ T cells. In NK cell line (YTS), TIGIT negatively regulates NFκB activation and IFNγ production in response to its ligand poliovirus receptor (PVR). These inhibitory activities are also dependent on its ability to interact with β-arrestin2101. Thus β-arrestin2 has profound influences on NK cell functions.
T Cell Receptor (TCR)
T cell receptor (TCR) upon binding to its cognate MHC:peptide complex, stimulates cyclic AMP (cAMP) production that activates the inhibitory PKA-Csk1 pathway. This inhibitory effect of cAMP is relieved by CD28-mediated recruitment of phosphodiesterase 4 (PDE4) (that degrades cAMP) and this recruitment of PDE4 was found to be dependent on β-arrestin as well as PI3K pathways. PKB-β-arrestin-PDE4 complex was shown to be sequestered in the membrane fraction following CD3/28 stimulation in a PI3K and Src- dependent manner102. Human primary T cells stimulated with CD3/28 produce lower IL-2 and IFNγ following β-arrestin knockdown, suggesting a positive role for β-arrestins in proximal T cell signaling102. Another study showed altered T cell differentiation in response to TCR stimulation in β-arrestin2 knockout cells. Even though the knockout T cells differentiated into Th1, Th2 and Th17 cells in proportion equivalent to the wild type T cells, their polarization to regulatory T cells (Treg) was impaired. This impaired Treg differentiation has implications in EAE pathogenesis, with the knockouts exhibiting enhanced susceptibility to disease103. Similar β-arrestin2-mediated regulation of Treg differentiation was also observed in our studies using colitis models which led to increased colitic susceptibility by T cells lacking β-arrestin2 (Sharma et al, 2015, Inflammatory Bowel Diseases, Accepted manuscript).
β-arrestin2 expression was found to be higher in T cells isolated from asthma-induced mice as compared to control104, 105. Interestingly, modulating the expression of β-arrestin2 in CD4+ T cells isolated from asthmatic animals affected Th2105 and Th17104 polarization potential and downstream production of IL-4 and IL-17, respectively. On the other hand, T cells lacking β-arrestin1, had equivalent Th1, Th2 and Treg differentiation while Th17 induction was negatively altered106.
In addition to T cell activation, other components of T cell biology such as apoptosis are also affected by β-arrestins during ongoing inflammation. Other studies have shown that TLR2 pretreatment reduces stress-induced loss of splenocytes though a PI3K-Akt pathway that is β-arrestin2 dependent. Stress also alters T cell activation potential, decreasing IL-2 production while increasing IL-4 and this effect was shown to be abrogated by TLR2 agonist via a β-arrestin2 dependent mechanism107. β-arrestin2 knockout mice therefore, exhibit lymphocyte reduction and altered activation pattern that is not rescued by TLR2 agonists107. These processes can have significant consequence in pathogenesis of multiple T cell mediated auto-inflammatory disorders.
Nod-like Receptors (NLRs)
NLR is family of molecules, some of which are capable of forming inflammasomes-multimeric complexes that oligomerize in response to various pathogen and danger associated molecules. NLRs including, NLRP3, NLRC4 and AIM2 are capable of interacting with ASC, forming a complex that recruits casapse-1. This leads to auto-activation of caspase-1 and consequent cleavage of inflammatory cytokines, IL-1β and IL-18 and induction of pyroptotic cell death108. Each inflammasome responds to a specific danger or pathogen-associated signal for example ATP, MSU (monosodium urate crystals) and silica engage NLRP3; while NLRC4 and AIM2 respond to bacterial flagellin and dsDNA respectively. In response to ligand detection, the NLR recruits the adaptor molecule ASC and oligomerize to form the inflammasome complex. This activation is characterized through detection of “ASC-specks” that reflects the cellular localization of the oligomerized complex, secretion of cleaved IL-1β and IL-18 and LDH release that signifies cell death. Given the importance of these cytokines and pyroptosis in pathogen response and inflammatory diseases, inflammasome activation is tightly regulated109. In addition to many known regulators of inflammasome activation, β-arrestin1 was recently shown to specifically be required for optimal NLRP3 and NLRC4 inflammasome activation. ASC aggregation, caspase-1 activation and IL-1β secretion were all reduced in cells lacking β-arrestin1. β-arrestin1 specifically interacted with NLRP3, NLRC4 and NLRP12 through PYD (pyrin) domain but not with AIM2. Further, in vivo peritonitis response to MSU crystals, a NLRP3 ligand as measured by neutrophil infiltration and IL-1β secretion was drastically reduced in mice lacking β-arrestin1. Also, weight loss and IL-1β secretion were lower in β-arrestin1 knockout mice even in response to Salmonella typhimurium infection, a bacteria well known to engage the NLRC4 inflammasome110. These recent data demonstrate yet other important inflammatory pathways that β-arrestins can regulate.
β-ARRESTINS IN INFLAMMATORY DISEASE MODELS
β-arrestins modulate a plethora of cell signaling pathways in response to a variety of ligands in both immune and non-immune cells. Therefore, it is not surprising that β-arrestins play important role in the pathogenesis of inflammatory diseases. In the following section we discuss some of these key findings published in the last few years.
AUTO-INFLAMMATORY DISORDERS
Experimental autoimmune encephalomyelitis (EAE)
Early studies demonstrated a role for β-arrestins in epitope spreading with both autoantibodies111 and T cell proliferative response112 against β-arrestins being significantly higher in MS patients as compared to healthy controls. The T cell response to β-arrestins further correlated positively with the response to myelin binding protein (MBP), a dominant peptide in immunopathology of the disease112. Using a mouse model of EAE113, it was shown that β-arrestin1 knockout mice were protected from disease induction, with lower inflammation and histological damage in spinal cord sections51,113. Complementary to that, β-arrestin1 transgenic mice with β-arrestin1 overexpression had higher clinical scores and greater extent of demyelination in spinal cord sections. Mechanistic studies demonstrated that β-arrestin1 promotes expression of anti-apoptotic gene bcl2 through its nuclear function of histone H4 acetylation, thus reducing apoptosis in both naïve and activated CD4+ T cells. Interestingly, CD4+ T cells from MS patients have higher expression of β-arrestin1 and bcl-2 and importantly, knockdown of β-arrestin1 in these cells increases apoptosis. Therefore, higher survival of CD4+ T cells mediated by β-arrestin1 is posited to be a reason for its role as a positive mediator of the disease pathogenesis51. Another study reported similar upregulation of β-arrestin1 expression in the brains of MS patients and animal model of EAE as compared to respective control along with a concurrent decrease in A1 adenosine receptor expression. Glucocorticoid treatment that alleviates neuroinflammation and associated behavioral deficits was shown to increase adenosine A1AR expression concomitant with a reduction in β-arrestin1 expression, suggesting a reciprocal regulation between the two as an important determinant of MS pathogenesis114.
While β-arrestin1 knockout mice are resistant to EAE pathogenesis, mice lacking β-arrestin2 are highly susceptible to EAE. Mechanistically, the worsened phenotype was found to be associated with lower peripheral Foxp3+ Treg (regulatory T cell) induction103. In fact, T cells lacking β-arrestin2 showed poor conversion to iTregs in vitro, suggesting that lack of regulatory signaling is atleast partially responsible for overt activation of immune response. β-arrestin1 and 2 therefore, modulate EAE inflammation and pathogenesis in a distinct manner115, 116.
Allergic asthma
β-arrestin2 has been shown to be a critical determinant in the pathogenesis of allergic asthma, with both inflammatory and physiological functions ascribed to the protein. In an early study, β-arrestin2 deficient mice displayed drastically reduced physiological and inflammatory responses in OVA sensitized allergy model117. OVA-induced T cell infiltration and Th2 response in the lung were markedly reduced in β-arrestin2 KO mice, while the Th1 response was unaffected. Alternately, T cell chemotaxis to macrophage derived chemokine (MDC) in vitro and its production in vivo was significantly lower in the absence of β-arrestin270. Further studies revealed a divergent role for both hematopoietic and non-hematopoietic β-arrestin2. While hematopoietic β-arrestin2 was required for eosinophil and lymphocyte infiltration, airway hyperresponsiveness was regulated by non-hematopoietic β-arrestin2118. Other lines of evidence supporting a role for β-arrestin2 in regulating inflammation came from studies where PAR2-induced modulation of inflammatory response in asthma was β-arrestin2-dependent119. Expression of β-arrestin2 itself was significantly higher in T cells isolated from asthma-induced mice as compared to control104, 105. Further, modulating the expression of β-arrestin2 in CD4+ T cells isolated from asthmatic animals affected Th2105 and Th17104 polarization potential and downstream production of IL-4 and IL-17, respectively. An important role for β-arrestin2 in affecting physiological process of airway hyerresponsiveness (AHR) came from a study that used an inducible Cre-mediated knockdown of β-arrestin2 once the asthmatic response was established120. Once the inflammatory process and AHR was established, loss of β-arrestin2 had minimal impact on inflammation in terms of cellular infiltration and histological scoring but drastically abrogatesed AHR120. This was a critical study highlighting the diverse roles played by β-arrestin2 in asthma development. While the inflammatory response can be posited to be hematopoietic β-arrestin2 dependent118, AHR itself is perhaps independent of β-arrestin2 driven inflammation120 and instead could be mediated by non-hematopoietic β-arrestin2118. These studies highlight the differential role of β-arrestin2 in cellular compartments during asthma induction or perpetuation104, 105, 119. β-arrestin1, on the other hand had no role to play in OVA-induced inflammation or its PAR2 induced exacerbation119. Thus, β-arrestins can modulate asthma development by regulating various processes and its expression or physiological function could be targeted for therapeutic intervention.
Rheumatoid arthritis
Arthritis is an auto-inflammatory disorder, characterized by chronic inflammation in synovial joint causing cartilage and joint destruction. In a mouse model of collagen antibody induced arthritis (CAIA)121, expression of both β-arrestins was significantly elevated in the joint tissue. Cytokine production by fibroblast-like synoviocytes following hyaluron stimulation was increased in response to β-arrestin1 overexpression but decreased by overexpression of β-arrestin2122. Further, 4-mer hyaluron oligosaccharide, a byproduct of cartilage (hyaluron) breakdown signals through TLR4 for inflammatory cytokine production, a process inhibited by β-arrestin2123. Another study using primary chondrocytes, demonstrated a negative regulatory role for β-arrestin2 in NFκB signaling and downstream cytokine (IL-6, TNFα and IL-17A) production following 4-mer hyaluron stimulation123. This highlights the importance of β-arrestin2 in inflammatory processes that perpetuate cartilage destruction in arthritic joints. These in vitro studies are corroborated by in vivo analysis of disease progression using the collagen antibody induced arthritis (CAIA) model121. In this model of arthritis, consistent with its role as a negative regulator of inflammation, β-arrestin2 knockout mice suffered from more severe arthritis in CAIA model with increased neutrophil and macrophage infiltration observed in the synovial tissue and cavity122. β-arrestin1 expression was higher in PBMCs from patients suffering from RA and in CD4+ T cells in periphery and synovial joints of mice subjected to arthritis106. Consistent with pro-inflammatory role of β-arrestin1 in fibroblasts122, the knockout mice had decreased disease incidence and attenuated joint swelling and destruction in β-arrestin1 knockout mice106. Further, β-arrestin1 knockout arthritic mice had lower IL-17A in synovial joints and β-arrestin1 knockdown in WT mice decreased the production of IL-17 in the joints. The study further demonstrated that β-arrestin1 acts as a scaffold for JAK1-STAT3 mediated IL-6 signaling to positively regulate Th17 polarization106. β-arrestins thus have divergent roles to play in arthritis severity with β-arrestin1 and 2 being pro- and anti-inflammatory respectively.
Inflammatory Bowel Disease (IBD)
IBD is a multifactorial disease perpetuated by a dysregulated immune response. Gut being the site of constant interaction between the immune system and foreign antigens, (dietary or microbial) makes the balance between inflammatory and regulatory responses at this site particularly essential for homeostasis. β-arrestin1 knockout mice were protected from both dextran sodium sulfate (DSS) and trinitrobenzene sulfonic acid (TNBS)124 induced colitis based on clinical signs and histopathological scoring125. These knockout mice also had markedly lower production of IL-6 and higher levels of IL-10 and IL-22 in colons of colitic mice, perhaps restricting inflammation and promoting epithelial cell repair125. On the other hand, β-arrestin2 has distinct functions in pathogenesis of colitis. In a model of DSS induced colitis, β-arrestin2 knockout mice were protected from DSS induced colitis with lower inflammation and higher mucosal apoptosis observed in the colon tissue126. It's own expression is enhanced in colon tissues from colitic human subjects. It was further demonstrated that β-arrestin2 was involved in ER-stress/p-53-upregulated modulator of apoptosis (PUMA) mediated mitochondrial apoptotic signaling and in its absence the ER stress was ameliorated leading to lower epithelial cell apoptosis and alleviated colitis126. On the contrary, we found a role for β-arrestin2 in T cell activation that increased the susceptibility of the β-arrestin2 knockout mice to exhibit higher extent of colitis in DSS mediated colitis that was further independent of microbiota dysbiosis(Sharma et al, 2015, Inflammatory Bowel Diseases, Manuscript Accepted). The difference in the two studies might be due to the differences in the dose of DSS used and degree of inflammation induced making one factor dominant over the other. β-arrestin2 was further shown to inhibit T cell mediated colitis in a RAG T cell transfer model. β-arrestin2 can therefore employ and engage multiple pathways to affect the outcome in colitis. Thus similar to other autoimmune diseases, β-arrestin1 and β-arrestin2 seem to have distinct roles in the pathogenesis of colitis.
Primary biliary cirrhosis
Primary biliary cirrhosis (PBC) is an autoimmune disease associated with extensive humoral and cellular immune response. Autoreactive T cells specific for PDE-C2 antigen mediate destruction of biliary cells127. β-arrestin1 expression was significantly elevated, predominantly in T cells isolated from blood of PBC patients as compared to healthy controls with the increase correlating with Mayo risk score52. Further, altering expression of β-arrestin1 in auto-reactive T cells, by means of over expression and knockdown demonstrated a positive regulatory role for β-arrestin1 in mediating T cell proliferation and IFNγ production. Expression of genes involved in autoimmunity was also shown to be regulated by β-arrestin1 via its role in affecting histone H4 acetylation- while expression of CD40L, LIGHT, IL-17 and IFNγ was upregulated, that of TRAIL, Apo2 and HDAC7A was found to be downregulated128. These studies warrant further investigation into the role of β-arrestins in initiation and development of this T cell mediated autoimmune disease.
Myocardial Infarction
Inflammation during myocardial infarction (MI) is not only important for initiating the healing process but is also responsible for the adverse effects of excessive remodeling. Its control therefore is of prime importance to restore optimum myocardial function. Further, these events of tissue remodeling are perpetuated through aldosterone and adrenergic signaling, with the production and signaling of both being affected by β-arrestins129. β-arrestins could affect the outcome of MI and heart failure in multiple ways130 either through modulating inflammation or by affecting hormonal signaling. In a model of cryoinfarction131, adenoviral mediated adrenal delivery of full length β-arrestin1 and β-arrestin1 C–terminus peptide fragment that act as overexpression and negative inhibitor of β-arrestin1 function respectively were used to delineate the adrenal function of β-arrestin1 in MI and consequent heart failure132. It was found that β-arrestin1 promotes aldosterone production that leads to adverse remodeling and reduced ventricular function. It also correlated with expression of PAI-1 (plasminogen activator inhibitor) and TGFβ, inflammatory mediators of tissue remodeling132. Complementary data for detrimental role of β-arrestin1 was obtained in another study comparing the recovery in response to MI in WT and β-arrestin1 knockout mice133. The β-arrestin1 knockout mice in response to MI had higher survival, lower infarct size, adverse remodeling and reduced circulating levels of catecholamines and aldosterone. Inflammatory cytokines associated with damage and remodeling were significantly lower demonstrating that cardiac response (β-adrenergic), adrenal function and inflammatory response were all dampened in the absence of β-arrestin1 providing a survival benefit133. β-arrestin2 on the other hand had opposing role of mediating protection in MI model134. β-arrestin2 knockout mice had increased early mortality while the cardiac function and hypertrophy, infarct size and fibrotic response were unaffected by loss of β-arrestin2. Inflammation as ascertained by expression of inflammatory mediators and activation of ERK and NFκB pathway was significantly elevated in cardiac macrophages from β-arrestin2 knockout mice. Further, the inflammatory response was modulated by β-arrestin2 in the hematopoietic cellular compartment134. Therefore, β-arrestins through regulation of inflammatory and neurohormonal pathways distinctly regulate reparative response to myocardial infarction, thereby affecting the eventual outcome. Delineating the major players and pathways would be highly informative in designing β-arrestin- biased ligands for therapeutic interventions.
Pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is a fatal disorder of unknown etiology that leads to loss of lung function. It is characterized by inappropriate fibrosis and involves excessive collagen deposition and distortion of lung architecture135. Initiated by an airway injury, TGFβ and MMP are major players in pathophysiology affecting collagen synthesis, fibroblast proliferation, extracellular matrix remodeling and destruction of basement membrane136. In a bleomycin induced mouse model of IPF137, mice lacking either β-arrestins were markedly protected from lung fibrosis and consequent mortality138. Lung architecture distortion and collagen deposition were ameliorated in the absence of β-arrestins even though pulmonary inflammatory infiltration was unaffected. Additionally, TGFβ signaling and chemotaxis to bronchoalveolar fluid (BALF) were similar in primary lung fibroblasts while their invasiveness as assessed by matrigel invasion assay was severely impaired in the absence of β-arrestins (1 or 2). Consistent with that, knockdown of β-arrestin2 in primary fibroblasts isolated from human IPF patients decreased their invasive potential. Genes involved in extracellular matrix degradation and remodeling were thus altered in lung tissue from β-arrestin knockout mice in response to bleomycin induced lung fibrosis138. Therefore, loss of β-arrestins is protective in IPF through regulation of fibroblast invasiveness and their localized inhibition could be a promising potential therapy.
Cystic fibrosis
Cystic fibrosis is a condition caused by loss or mutation of cystic fibrosis transmembrane conductance regulator (CFTR) protein, a cAMP regulated chloride channel 139. Cells from Cystic Fibrosis (CF) patients exhibit higher cAMP signaling as measured by increased activity of cAMP response element binding protein (CREB) and altered cholesterol homeostasis140, 141. The altered cholesterol homeostasis is a critical part of CF pathogenesis such that it can modulate the inflammatory response to microbial challenge and is mediated by CREB activity141. Both these characteristics were affected by β-arrestin2-mediated ERK activation142, 143. β-arrestin2 expression itself was elevated in CF cells in both mouse model and human patients141 and β-arrestin2 overexpression in non-CF cells was sufficient to induce cholesterol accumulation in epithelial cells in a cAMP-dependent manner143. In a CFTR−/− mice loss of β-arrestin2 reduced de novo cholesterol biosynthesis in the liver143 and decreased pERK and pCREB content in nasal epithelium cells to levels comparable to WT mice142. These studies place β-arrestin2 as a critical regulator of cholesterol accumulation observed in pathogenesis of CF and allude to its role in CF associated pathologies.
Cutaneous flushing
Cutaneous flushing is a negative side effect of nicotinic acid treatment used to lower triglycerides, low density lipoproteins (LDL) and raise High density lipoproteins (HDL), lowering the risk of cardiovascular diseases. Nicotinic acid binds to GPR109A144, a 7-transmembrane receptor to induce anti-lipolytic activity and a transient reduction in levels of free fatty acids (FFA)144. This was believed to be the basis of positive effect on levels of serum lipids. Through receptor activation, nicotinic acid also induces production of prostaglandins likely from immune cells and causes the side effect of cutaneous flushing145. The role of β-arrestins in anti-lipolytic and flushing activity was assessed following niacin treatment. Although, serum free fatty acid levels were similarly reduced in WT or β-arrestin (1 or 2) knockout mice in response to nicotinic acid injection, ear perfusion was significantly reduced in β-arrestin1 knockout mice. This was associated with lower cPLA2 (phospholipase) activity observed in response to ex vivo nicotinic acid stimulation in β-arrestin1 knockout macrophages146. This led to the conclusion that β-arrestins mediate the negative side effect of cutaneous flushing without affecting the positive effect of reduction in FFA levels and demonstrates use of GPCR biased ligands as potential therapeutic agents 146. It should however be noted that later studies have contradicted the causal role of GPR109A mediated reduction in FFAs on serum lipid levels147 and thus the physiological role of β-arrestins in GPR109A needs further investigation.
INFLAMMATORY RESPONSE TO PATHOGENS
Endotoxemia
Endotoxemia is the simplest septic shock model involving use of lipopolysaccharide as the instigating pathogenic ligand. It is widely used to study the role of proteins in TLR4 signaling and consequent endotoxic shock148. Given the various role ascribed to β-arrestins in TLR4 signaling and consequent cytokine production, it is perhaps not surprising that the role of β-arrestins in endotoxemia model of sepsis is controversial. In earlier studies, mice lacking β-arrestin2 were shown to be susceptible to D-galactosamine sensitized endotoxemia model with higher level of cytokines observed in plasma85. In contrast, studies from our lab showed lower mortality in response to high dose of LPS that correlated with reduced plasma IFNγ levels in mice lacking either β-arrestins149. Similar to the previous study some cytokines were higher in the β-arrestin2 knockout mice, especially at early time points after LPS injection. Another recent study showed increased mortality in β-arrestin2 in response to LPS injection due to abrogation of anti-inflammatory IL-10 production150. The key differences in these studies with disparate results was use of galactosamine sensitization in Wang et al. and different doses of LPS in the other two; while Porter et al used 20 g/kg that induced 90% mortality in WT mice in 48 hours, Li et al used 10 g/kg LPS dose with less than 30% mortality in WT mice. Thus, role of β-arrestins in endotoxemia model may be dependent on multiple variables including the dose and sensitization of LPS.
Endotoxin supplemented with D-galactosamine and Caerulein sulfate are frequently used as models of acute hepatitis and pancreatitis respectively. A study designed to investigate the role of metabolites, lactate151 and aspartate32 on end organ damage, found that β-arrestin2 mediated the protective effects of these compounds. In primary macrophages, lactate and aspartate signal through GPR81 (G protein receptor 81) and NMDA (N-methyl-D-Aspartate) receptors, respectively to reduce LPS-induced expression of inflammatory genes involved in inflammasome activation- IL-1β, NLRP3 and pro-caspase-1. Further hepatitis and pacreatitis induction and organ injury as measured through ALT and amylase levels were significantly reduced by both metabolites in their specific receptor and β-arrestin2- dependent manner32, 151.
Sepsis
Even though endotoxemia is a simple and convenient model to mimic septic shock, it does not capitulate all features of a human response. A clinically relevant model of sepsis termed Cecal Ligation and Puncture (CLP) that induces a polymicrobial peritonitis is often used as a gold standard for animal models of sepsis152. In this model of sepsis, both β-arrestins negatively regulate polymicrobial sepsis-induced inflammation and consequent mortality57, 86. In both knockout mice, enhanced levels of cytokines are detected in plasma, peritoneal fluid (the site of infection) and lung tissue. Overt activation of NFκB pathway detected in lung tissues of septic mice for both knockouts, suggests inhibition of NFκB by β-arrestins as an important mechanism of controlling sepsis-induced inflammation56, 57. Further, mice heterozygous for both β-arrestins were protected from overt inflammation and enhanced mortality in CLP model of septic peritonitis56, 57 suggesting that one allele is sufficient for inhibiting sepsis-induced inflammation. Bone marrow chimeras generated to understand the cell-compartment-specific role of β-arrestin1 in sepsis-induced inflammation revealed a critical role for non-hematopoietic β-arrestin1 in inhibiting the inflammatory response57. It is however not known whether β-arrestin2 exerts its protective effect in sepsis through either one or both compartments. Since, CLP induces an inflammatory response to necrotic tissue in addition to microbial stimulation153, β-arrestin2 knockout mice were subjected to polymicrobial injection to induce sepsis independent of the incidence of necrotic tissue. In this model as well, mice lacking β-arrestin2 exhibited increased inflammation and mortality, suggesting that the response is higher to microbial stimuli56. Further, in both models of polymicrobial sepsis, β-arrestin2 KO mice have increased neutrophil sequesteration in the lung as ascertained by MPO activity56, 66, 86. This increased neutrophil sequesteration was independent of TLR4 signaling since LPS injection does not have the same effect as microbial stimulation56. This observation highlights the distinct inflammatory response instigated in the absence of β-arrestin2 in the two models of endotoxemia149 and polymicrobial sepsis56, 86 and could be the underlying cause of eventual outcome. Together, these studies underscore the critical role of β-arrestins in sepsis as well as demonstrate important roles for β-arrestins in non-immune cells in modulating sepsis pathogenesis.
Meningitis
Meningitis is an acute inflammation of meninges, the protective membrane in brain and spinal cord induced in response to viral, bacterial or fungal infections. N. meningitides is a gram negative bacterium that causes sepsis and meningitis. Once in the blood stream, it adheres to brain endothelium, multiplies at the cellular surface and crosses blood brain barrier to cause meningitis. Recent studies have shown that the bacterial infection hijacks β2 adenoreceptor/β-arrestin2-biased signaling pathway in the endothelial cells to facilitate bacterial adhesion via src activation. Its penetration into tissue also requires junctional protein delocalization and gap formation that is mediated by β-arrestin115. β2-adrenergic agonists that induce receptor internalization are able to reduce bacterial adhesion underscoring their use as an effective strategy to combat infection. In another study, β-arrestin2 expression was altered in peripheral blood monocyte cells (PBMCs) of patients suffering from meningitis caused by Cryptococcus neoformans, an opportunistic pathogen. Increased β-arrestin2 expression also correlated positively with serum IL-10 and negatively with IFNγ levels. Further β-arrestin2 transfected PBMCs had lower cytotoxic activity while knockdown of β-arrestin2 led to a non-significant increase in cytotoxic activity against C. neoformans116. This suggests a negative role for β-arrestin2 in inducing bacterial killing by perhaps inhibiting IFNγ production. Thus, by regulating bacterial invasion and killing, β-arrestin2 could potentially affect the incidence and perpetuation of meningitis.
Antiviral response
β-arrestin1 negatively impacts antiviral activity of IFNγ signaling as shown by VSV infection mediated cell death and viral loads being lower with β-arrestin1 knockdown in MEFs and HeLa cells96. Further, β-arrestin1 expression was modulated by hepatitis B infection at the site infection (liver), whereas splenic β-arrestin1 expression was unaffected96. Since viruses are capable of affecting JAK-STAT pathway to evade the immune response and potentiate infection97, perhaps regulating β-arrestin1 expression might provide an effective antiviral therapy or potentiate existing IFN therapy. In another study, β-arrestin2 was shown to be an important player in clearance of MCMV infection that is largely dependent on NK cell mediated cytotoxic activity. β-arrestin2 in association with SHP-1 and SHP-2 was able to inhibit cytotoxicity in NK cells such that transgenic mice with higher β-arrestin2 expression have greater viral loads in organs following MCMV infection. Conversely and as expected β-arrestin2 knockout mice had better clearance and lower viral titers100. β-arrestins therefore have distinct ways of regulating anti-viral responses that requires further investigation for potential use in therapeutic development.
Conclusions
In this review we focused mainly on studies dealing with the role of β-arrestins in immunological processes. As noted in the beginning of this review, the “arrestin” family now includes other members such as the arrestin-domain containing proteins called the α-arrestins as well as visual arrestins. While visual arrestins regulation of vision has been well established, studies on the role of α-arrestins especially in GPCR signaling are only beginning to be unraveled and their potential in other signaling pathways deserves further attention. In addition, although β-arrestins appear to be involved in many cell signaling processes, their role in inflammatory disease pathogenesis, appears to be context dependent. While in certain disorders, both β-arrestins have similar functions while in others their roles are contradictory. One characteristic feature of models wherein they have distinct roles is T cell mediated immunopathologies such as EAE, allergic asthma, colitis and arthritis. Further, some studies have attempted to identify the cell type/cellular compartment involved in β-arrestin-mediated inflammatory regulation, in most cases, the involvement of a specific cell type, receptor or ligand remains elusive. Thus future studies focusing on the mechanistic basis of the “inflammatory-context” dependent role of β-arrestins are likely to shed light on the specific role of β-arrestins and identify potential therapeutic targets. Examples of such therapeutics already in existence include biased GPCR ligands that specifically engage β-arrestins to beneficially modulate downstream signaling. Such ligands form an area of emerging interest and provide a promising avenue for future research and development of therapies for inflammatory disorders.
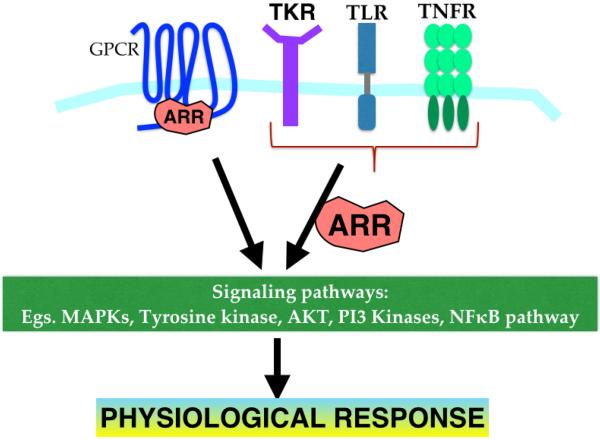
Figure 2. Emerging role of β-arrestins in GPCR and non-GPCR signaling pathways.
As discussed in the text, arrestins modulate various signaling modules in a receptor- and cell-type specific manner. This ability of arrestins to affect important physiological and inflammatory processes has important implications in disease progression.
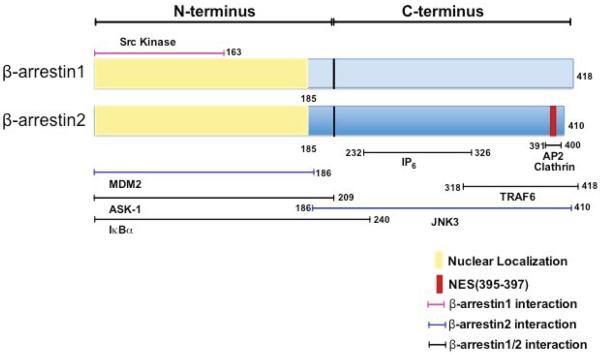
Figure 3. β-arrestin1 and 2 structural domains and interacting proteins.
Structural domains in β-arrestins showing identified binding sites for interacting proteins. Motifs involved in nucleo-cytoplasmic shuttling, the nuclear localization and nuclear export signal (NES) are shown along with segments indispensable for interacting proteins. β-arrestin(1/2) interacting sites are shown in black, those specific for β-arrestin1 or β-arrestin2 are in magenta and blue respectively.
Table 3.
Role of β-arrestins in inflammatory diseases.
| Disease/Model | System | Outcome | Mechanism | Reference |
|---|---|---|---|---|
| MS/EAE | β-arr1−/− | Protected | β-arr1 upregulates bcl-2; survival benefit to activated T cells | 51 |
| β-arr1tg | Susceptible | |||
| β-arr2−/− | Susceptible | Lower Treg induction | 103 | |
| Meningitis/Cytotoxicity to C. neoformans | PBMC-β-arr2 OE | Lower | Negative regulation of IFNγ production | 116 |
| PBMC-β-arr2 KD | Higher | |||
| Asthma/ OVA sensitized model | β-arr2−/− | Protective | Lower immune cell infiltration and airway hyperresponsiveness | 70,118 |
| Endotoxemia/LPS | β-arr1−/− | Protective | Lower systemic response (IFNγ) | 149 |
| β-arr2−/− (20mg/kg) | Protective | Lower systemic response (IFNγ) | ||
| β-arr2−/− (1Omg/kg) | Susceptible | Lower IL-10 production | 150 | |
| β-arr2−/− | Susceptible | Loss of anti-inflammatory regulation | 122 | |
| Endotoxemia/D-galactosamine + LPS | β-arr2−/− | Susceptible | Higher systemic response (TNFα and Il-6) | 85 |
| Sepsis/ cecal ligation and puncture or polymicrobial injection | β-arr1−/− | Susceptible | Higher systemic response (IL-6); nonhematopoietic β-arr1 inhibits inflammation | 57 |
| β-arr1+/− | No effect | Similar systemic response (IL-6) | ||
| β-arr2−/− | Susceptible | Higher systemic response (IL-6) | 86,56 | |
| β-arr2+/− | No effect | Similar systemic response (IL-6) | 56 | |
| Myocardial Infarction | β-arr1−/− | Protective | Tissue remodeling through inflammatory and neuro-hormonal modulation | 134 |
| β-arr1−/− | Protective | |||
| Colitis/ DSS and TNBS Arthritis/ CAIA | β-arr1−/− | Protective | Lower IL-6 and higher IL-22 production Lower Th17 polarization | 125,106 |
| β-arr1−/− | Protective | |||
| Pulmonary Fibrosis/Bleomycin induces lung fibrosis | β-arr1−/− | Protective | Altered expression of genes involved in matrix production and degradation | 138 |
| β-arr2−/− | Protective | |||
| Cystic Fibrosis/CFTR knockout | β-arr2−/− | ? | Lower cholesterol synthesis and CREB activation | 142,143 |
| Cutaneous Flushing/Nicotinic acid injection | β-arr1−/− | Reduced | Lowered prostaglandin D2 production | 146 |
Tg- transgenic, OE- overexpression, KD-knockdown, LPS-lipopolysaccharide, OVA-ovalbumin, CAIA- collages antibody induced arthritis, DSS- dextran sodium sulfate, TNBS- 2,4,6-trinitro benzenesulfonic acid, PBMC- peripheral blood mononuclear cells, Table 1.4(Cont'd)
VSV- vesicular stomatitis virus, MCMV- murine cytomegalovirus, CFTR- cystic fibrosis transmembrane conductor regulator, CREB- cAMP response element-binding protein.
Acknowledgement
Research in Dr. Parameswaran's lab is supported by funding from the National Institutes of Health (grant # HL095637, AR055726, and AI099404).
Footnotes
Authors declare no conflict of interest.
REFERENCES
- 1.Craft CM, Whitmore DH. The arrestin superfamily: cone arrestins are a fourth family. FEBS Lett. 1995;362:247–55. doi: 10.1016/0014-5793(95)00213-s. [DOI] [PubMed] [Google Scholar]
- 2.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 3.Lohse M, Benovic J, Codina J, Caron M, Lefkowitz R. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- 4.Benovic JL, et al. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein) Proc Natl Acad Sci U S A. 1987;84:8879–82. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitcher J, Lohse MJ, Codina J, Caron MG, Lefkowitz RJ. Desensitization of the isolated beta 2-adrenergic receptor by beta-adrenergic receptor kinase, cAMP-dependent protein kinase, and protein kinase C occurs via distinct molecular mechanisms. Biochemistry. 1992;31:3193–7. doi: 10.1021/bi00127a021. [DOI] [PubMed] [Google Scholar]
- 6.Sibley DR, Benovic JL, Caron MG, Lefkowitz RJ. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987;48:913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- 7.Lohse MJ, et al. Receptor-specific desensitization with purified proteins. Kinase dependence and receptor specificity of beta-arrestin and arrestin in the beta 2-adrenergic receptor and rhodopsin systems. Journal of Biological Chemistry. 1992;267:8558–64. [PubMed] [Google Scholar]
- 8.Wang P, Wu Y, Ge X, Ma L, Pei G. Subcellular localization of beta-arrestins is determined by their intact N domain and the nuclear export signal at the C terminus. J Biol Chem. 2003;278:11648–53. doi: 10.1074/jbc.M208109200. [DOI] [PubMed] [Google Scholar]
- 9.Milano SK, Kim YM, Stefano FP, Benovic JL, Brenner C. Nonvisual arrestin oligomerization and cellular localization are regulated by inositol hexakisphosphate binding. J Biol Chem. 2006;281:9812–23. doi: 10.1074/jbc.M512703200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Liu X, Zhang Y, Zhao J. Loss of betaarrestin1 and betaarrestin2 contributes to pulmonary hypoplasia and neonatal lethality in mice. Dev Biol. 2010;339:407–17. doi: 10.1016/j.ydbio.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 11.Conner DA, et al. β-Arrestin1 Knockout Mice Appear Normal but Demonstrate Altered Cardiac Responses to β-Adrenergic Stimulation. Circulation Research. 1997;81:1021–1026. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- 12.Bohn LM, et al. Enhanced Morphine Analgesia in Mice Lacking β-Arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari SL, D.D.P., Glatt V, Goddard DS, Bianchi EN, Lin FT, Manen D, Bouxsein ML. Bone Response to Intermittent Parathyroid Hormone Is Altered in Mice Null for Œ≤-Arrestin2. Endocrinology. 2005;146:1854–1862. doi: 10.1210/en.2004-1282. [DOI] [PubMed] [Google Scholar]
- 14.Goodman OB, Jr, et al. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–50. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 15.Goodman OB, Jr, Krupnick JG, Gurevich VV, Benovic JL, Keen JH. Arrestin/clathrin interaction. Localization of the arrestin binding locus to the clathrin terminal domain. J Biol Chem. 1997;272:15017–22. doi: 10.1074/jbc.272.23.15017. [DOI] [PubMed] [Google Scholar]
- 16.Krupnick JG, Goodman OB, Jr., Keen JH, Benovic JL. Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J Biol Chem. 1997;272:15011–6. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- 17.Lin FT, et al. Clathrin-mediated endocytosis of the beta-adrenergic receptor is regulated by phosphorylation/dephosphorylation of beta-arrestin1. J Biol Chem. 1997;272:31051–7. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- 18.Luttrell L, et al. β-Arrestin-dependent formation of β2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 19.Lin FT, et al. Phosphorylation of beta-arrestin2 regulates its function in internalization of beta(2)-adrenergic receptors. Biochemistry. 2002;41:10692–9. doi: 10.1021/bi025705n. [DOI] [PubMed] [Google Scholar]
- 20.Kim YM, Barak LS, Caron MG, Benovic JL. Regulation of arrestin-3 phosphorylation by casein kinase II. J Biol Chem. 2002;277:16837–46. doi: 10.1074/jbc.M201379200. [DOI] [PubMed] [Google Scholar]
- 21.Laporte SA, et al. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci U S A. 1999;96:3712–7. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. J Biol Chem. 2000;275:23120–6. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- 23.McDonald PH, et al. Identification of NSF as a beta-arrestin1-binding protein. Implications for beta2-adrenergic receptor regulation. J Biol Chem. 1999;274:10677–80. doi: 10.1074/jbc.274.16.10677. [DOI] [PubMed] [Google Scholar]
- 24.Song X, Raman D, Gurevich EV, Vishnivetskiy SA, Gurevich VV. Visual and both non-visual arrestins in their “inactive” conformation bind JNK3 and Mdm2 and relocalize them from the nucleus to the cytoplasm. J Biol Chem. 2006;281:21491–9. doi: 10.1074/jbc.M603659200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song X, Gurevich EV, Gurevich VV. Cone arrestin binding to JNK3 and Mdm2: conformational preference and localization of interaction sites. J Neurochem. 2007;103:1053–62. doi: 10.1111/j.1471-4159.2007.04842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDonald PH, et al. β-Arrestin 2: A Receptor-Regulated MAPK Scaffold for the Activation of JNK3. Science. 2000;290:1574–1577. doi: 10.1126/science.290.5496.1574. [DOI] [PubMed] [Google Scholar]
- 27.Miller WE, et al. Identification of a motif in the carboxyl terminus of beta - arrestin2 responsible for activation of JNK3. J Biol Chem. 2001;276:27770–7. doi: 10.1074/jbc.M102264200. [DOI] [PubMed] [Google Scholar]
- 28.Scott MG, et al. Differential nucleocytoplasmic shuttling of beta-arrestins. Characterization of a leucine-rich nuclear export signal in beta-arrestin2. J Biol Chem. 2002;277:37693–701. doi: 10.1074/jbc.M207552200. [DOI] [PubMed] [Google Scholar]
- 29.Zhan X, Kaoud TS, Kook S, Dalby KN, Gurevich VV. JNK3 Enzyme Binding to Arrestin-3 Differentially Affects the Recruitment of Upstream Mitogen-activated Protein (MAP) Kinase Kinases. The Journal of Biological Chemistry. 2013;288:28535–28547. doi: 10.1074/jbc.M113.508085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, et al. A scanning peptide array approach uncovers association sites within the JNK/beta arrestin signalling complex. FEBS Lett. 2009;583:3310–6. doi: 10.1016/j.febslet.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 31.Zhan X, Kook S, Gurevich EV, Gurevich VV. Arrestin-dependent activation of JNK family kinases. Handb Exp Pharmacol. 2014;219:259–80. doi: 10.1007/978-3-642-41199-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farooq A, et al. Activation of N-methyl-d-aspartate receptor downregulates inflammasome activity and liver inflammation via a beta-arrestin-2 pathway. Am J Physiol Gastrointest Liver Physiol. 2014;307:G732–40. doi: 10.1152/ajpgi.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luttrell LM, Miller WE. Arrestins as regulators of kinases and phosphatases. Prog Mol Biol Transl Sci. 2013;118:115–47. doi: 10.1016/B978-0-12-394440-5.00005-X. [DOI] [PubMed] [Google Scholar]
- 34.DeFea KA, et al. β-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–81. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luttrell LM, et al. Activation and targeting of extracellular signal-regulated kinases by beta-arrestin scaffolds. Proc Natl Acad Sci U S A. 2001;98:2449–54. doi: 10.1073/pnas.041604898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tohgo A, Pierce KL, Choy EW, Lefkowitz RJ, Luttrell LM. beta-Arrestin scaffolding of the ERK cascade enhances cytosolic ERK activity but inhibits ERK-mediated transcription following angiotensin AT1a receptor stimulation. J Biol Chem. 2002;277:9429–36. doi: 10.1074/jbc.M106457200. [DOI] [PubMed] [Google Scholar]
- 37.DeFea KA, et al. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a β-arrestin-dependent scaffolding complex. Proc Natl Acad Sci U S A. 2000;97:11086–91. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tohgo A, et al. The stability of the G protein-coupled receptor-beta-arrestin interaction determines the mechanism and functional consequence of ERK activation. J Biol Chem. 2003;278:6258–67. doi: 10.1074/jbc.M212231200. [DOI] [PubMed] [Google Scholar]
- 39.DeFea KA. β-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell Signal. 2011;23:621–9. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Bourquard T, et al. Unraveling the molecular architecture of a G protein-coupled receptor/beta-arrestin/Erk module complex. Sci Rep. 2015;5:10760. doi: 10.1038/srep10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong K, et al. A novel protein kinase A-independent, beta-arrestin-1-dependent signaling pathway for p38 mitogen-activated protein kinase activation by beta2-adrenergic receptors. J Biol Chem. 2008;283:29028–36. doi: 10.1074/jbc.M801313200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Cheng Z, Ma L, Pei G. β-Arrestin2 Is Critically Involved in CXCR4-mediated Chemotaxis, and This Is Mediated by Its Enhancement of p38 MAPK Activation. Journal of Biological Chemistry. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 43.Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–9. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller WE, Houtz DA, Nelson CD, Kolattukudy PE, Lefkowitz RJ. G-protein-coupled receptor (GPCR) kinase phosphorylation and beta-arrestin recruitment regulate the constitutive signaling activity of the human cytomegalovirus US28 GPCR. J Biol Chem. 2003;278:21663–71. doi: 10.1074/jbc.M303219200. [DOI] [PubMed] [Google Scholar]
- 45.Witherow DS, Garrison TR, Miller WE, Lefkowitz RJ. beta-Arrestin inhibits NF-kappaB activity by means of its interaction with the NF-kappaB inhibitor IkappaBalpha. Proc Natl Acad Sci U S A. 2004;101:8603–7. doi: 10.1073/pnas.0402851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao H, et al. Identification of β-arrestin2 as a G protein-coupled receptor-stimulated regulator of NF-kappaB pathways. Mol Cell. 2004;14:303–17. doi: 10.1016/s1097-2765(04)00216-3. [DOI] [PubMed] [Google Scholar]
- 47.Luan B, Zhang Z, Wu Y, Kang J, Pei G. Beta-arrestin2 functions as a phosphorylation-regulated suppressor of UV-induced NF-kappaB activation. Embo j. 2005;24:4237–46. doi: 10.1038/sj.emboj.7600882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun J, Lin X. Beta-arrestin 2 is required for lysophosphatidic acid-induced NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:17085–90. doi: 10.1073/pnas.0802701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cianfrocca R, et al. Life Sciences. 2014. β-Arrestin 1 is required for endothelin-1-induced NF-βB activation in ovarian cancer cells. [DOI] [PubMed] [Google Scholar]
- 50.Kang J, et al. A nuclear function of beta-arrestin1 in GPCR signaling: regulation of histone acetylation and gene transcription. Cell. 2005;123:833–47. doi: 10.1016/j.cell.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 51.Shi Y, et al. Critical regulation of CD4+ T cell survival and autoimmunity by beta-arrestin 1. Nat Immunol. 2007;8:817–24. doi: 10.1038/ni1489. [DOI] [PubMed] [Google Scholar]
- 52.Hu Z, et al. β-Arrestin 1 modulates functions of autoimmune T cells from primary biliary cirrhosis patients. J Clin Immunol. 2011;31:346–55. doi: 10.1007/s10875-010-9492-4. [DOI] [PubMed] [Google Scholar]
- 53.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–13. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 54.Wang P, et al. Beta-arrestin 2 functions as a G-protein-coupled receptor-activated regulator of oncoprotein Mdm2. J Biol Chem. 2003;278:6363–70. doi: 10.1074/jbc.M210350200. [DOI] [PubMed] [Google Scholar]
- 55.Zoudilova M, et al. β-Arrestins Scaffold Cofilin with Chronophin to Direct Localized Actin Filament Severing and Membrane Protrusions Downstream of Protease-activated Receptor-2. Journal of Biological Chemistry. 2010;285:14318–14329. doi: 10.1074/jbc.M109.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharma D, Malik A, Lee E, Britton RA, Parameswaran N. Gene Dosage-Dependent Negative Regulatory Role of β-Arrestin-2 in Polymicrobial Infection-Induced Inflammation. Infection and Immunity. 2013;81:3035–3044. doi: 10.1128/IAI.00653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma D, Packiriswamy N, Malik A, Lucas PC, Parameswaran N. Nonhematopoietic beta-Arrestin-1 Inhibits Inflammation in a Murine Model of Polymicrobial Sepsis. Am J Pathol. 2014 doi: 10.1016/j.ajpath.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lattin JE, et al. Beta-arrestin 2 is required for complement C1q expression in macrophages and constrains factor-independent survival. Molecular Immunology. 2009;47:340–347. doi: 10.1016/j.molimm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Aragay AM, et al. Monocyte chemoattractant protein-1-induced CCR2B receptor desensitization mediated by the G protein-coupled receptor kinase 2. Proceedings of the National Academy of Sciences. 1998;95:2985–2990. doi: 10.1073/pnas.95.6.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richardson RM, Marjoram RJ, Barak LS, Snyderman R. Role of the cytoplasmic tails of CXCR1 and CXCR2 in mediating leukocyte migration, activation, and regulation. J Immunol. 2003;170:2904–11. doi: 10.4049/jimmunol.170.6.2904. [DOI] [PubMed] [Google Scholar]
- 61.Fan GH, Yang W, Wang XJ, Qian Q, Richmond A. Identification of a motif in the carboxyl terminus of CXCR2 that is involved in adaptin 2 binding and receptor internalization. Biochemistry. 2001;40:791–800. doi: 10.1021/bi001661b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orsini MJ, Parent JL, Mundell SJ, Marchese A, Benovic JL. Trafficking of the HIV coreceptor CXCR4. Role of arrestins and identification of residues in the c-terminal tail that mediate receptor internalization. J Biol Chem. 1999;274:31076–86. doi: 10.1074/jbc.274.43.31076. [DOI] [PubMed] [Google Scholar]
- 63.Cheng ZJ, et al. β-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. J Biol Chem. 2000;275:2479–85. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- 64.Décaillot FM, et al. CXCR7/CXCR4 Heterodimer Constitutively Recruits β-Arrestin to Enhance Cell Migration. Journal of Biological Chemistry. 2011;286:32188–32197. doi: 10.1074/jbc.M111.277038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Su Y, et al. Altered CXCR2 signaling in beta-arrestin-2-deficient mouse models. J Immunol. 2005;175:5396–402. doi: 10.4049/jimmunol.175.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Basher F, et al. β-Arrestin 2: a Negative Regulator of Inflammatory Responses in Polymorphonuclear Leukocytes. Int J Clin Exp Med. 2008;1:32–41. [PMC free article] [PubMed] [Google Scholar]
- 67.Gaffal E, et al. beta-arrestin 2 inhibits proinflammatory chemokine production and attenuates contact allergic inflammation in the skin. J Invest Dermatol. 2014;134:2131–7. doi: 10.1038/jid.2014.117. [DOI] [PubMed] [Google Scholar]
- 68.Barlic J, et al. Regulation of tyrosine kinase activation and granule release through beta-arrestin by CXCRI. Nat Immunol. 2000;1:227–33. doi: 10.1038/79767. [DOI] [PubMed] [Google Scholar]
- 69.Fong AM, et al. Defective lymphocyte chemotaxis in β-arrestin2- and GRK6-deficient mice. Proceedings of the National Academy of Sciences. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Walker JKL, et al. β-Arrestin-2 regulates the development of allergic asthma. The Journal of Clinical Investigation. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheung R, et al. An arrestin-dependent multi-kinase signaling complex mediates MIP-1β/CCL4 signaling and chemotaxis of primary human macrophages. Journal of Leukocyte Biology. 2009;86:833–845. doi: 10.1189/jlb.0908551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ge L, Shenoy SK, Lefkowitz RJ, DeFea K. Constitutive protease-activated receptor-2-mediated migration of MDA MB-231 breast cancer cells requires both beta-arrestin-1 and -2. J Biol Chem. 2004;279:55419–24. doi: 10.1074/jbc.M410312200. [DOI] [PubMed] [Google Scholar]
- 73.Ahamed J, Haribabu B, Ali H. Cutting edge: Differential regulation of chemoattractant receptor-induced degranulation and chemokine production by receptor phosphorylation. J Immunol. 2001;167:3559–63. doi: 10.4049/jimmunol.167.7.3559. [DOI] [PubMed] [Google Scholar]
- 74.Vibhuti A, Gupta K, Subramanian H, Guo Q, Ali H. Distinct and shared roles of beta-arrestin-1 and beta-arrestin-2 on the regulation of C3a receptor signaling in human mast cells. PLoS One. 2011;6:e19585. doi: 10.1371/journal.pone.0019585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bamberg CE, et al. The C5a receptor (C5aR) C5L2 is a modulator of C5aR-mediated signal transduction. Journal of Biological Chemistry. 2010;285:7633–7644. doi: 10.1074/jbc.M109.092106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bohm SK, et al. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. J Biol Chem. 1996;271:22003–16. doi: 10.1074/jbc.271.36.22003. [DOI] [PubMed] [Google Scholar]
- 77.Jacob C, et al. Mast Cell Tryptase Controls Paracellular Permeability of the Intestine: ROLE OF PROTEASE-ACTIVATED RECEPTOR 2 AND β-ARRESTINS. Journal of Biological Chemistry. 2005;280:31936–31948. doi: 10.1074/jbc.M506338200. [DOI] [PubMed] [Google Scholar]
- 78.Loniewski K, Shi Y, Pestka J, Parameswaran N. Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages. Mol Immunol. 2008;45:2312–22. doi: 10.1016/j.molimm.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 79.Kizaki T, et al. Beta2-adrenergic receptor regulates Toll-like receptor-4-induced nuclear factor-kappaB activation through beta-arrestin 2. Immunology. 2008;124:348–56. doi: 10.1111/j.1365-2567.2007.02781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kizaki T, et al. Beta2-adrenergic receptor regulate Toll-like receptor 4-induced late-phase NF-kappaB activation. Mol Immunol. 2009;46:1195–203. doi: 10.1016/j.molimm.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Wang W, Xu M, Zhang YY, He B. Fenoterol, a beta(2)-adrenoceptor agonist, inhibits LPS-induced membrane-bound CD14, TLR4/CD14 complex, and inflammatory cytokines production through beta-arrestin-2 in THP-1 cell line. Acta Pharmacol Sin. 2009;30:1522–8. doi: 10.1038/aps.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang W, Zhang Y, Xu M, Zhang YY, He B. Fenoterol inhibits LPS-induced AMPK activation and inflammatory cytokine production through beta-arrestin-2 in THP-1 cell line. Biochem Biophys Res Commun. 2015;462:119–23. doi: 10.1016/j.bbrc.2015.04.097. [DOI] [PubMed] [Google Scholar]
- 83.Parameswaran N, et al. Arrestin-2 and G protein-coupled receptor kinase 5 interact with NFkappaB1 p105 and negatively regulate lipopolysaccharide-stimulated ERK1/2 activation in macrophages. J Biol Chem. 2006;281:34159–70. doi: 10.1074/jbc.M605376200. [DOI] [PubMed] [Google Scholar]
- 84.Fan H, et al. β-arrestins 1 and 2 differentially regulate LPS-induced signaling and pro-inflammatory gene expression. Mol Immunol. 2007;44:3092–9. doi: 10.1016/j.molimm.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, et al. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2006;7:139–47. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 86.Fan H, et al. β-arrestin 2 negatively regulates sepsis-induced inflammation. Immunology. 2010;130:344–51. doi: 10.1111/j.1365-2567.2009.03185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li H, et al. beta-arrestin 2 regulates Toll-like receptor 4-mediated apoptotic signalling through glycogen synthase kinase-3beta. Immunology. 2010;130:556–63. doi: 10.1111/j.1365-2567.2010.03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiao N, Li H, Mei W, Cheng J. SUMOylation attenuates human beta-arrestin 2 inhibition of IL-1R/TRAF6 signaling. J Biol Chem. 2015;290:1927–35. doi: 10.1074/jbc.M114.608703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seregin SS, et al. beta-Arrestins modulate Adenovirus-vector-induced innate immune responses: differential regulation by beta-arrestin-1 and beta-arrestin-2. Virus Res. 2010;147:123–34. doi: 10.1016/j.virusres.2009.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Appledorn DM, Patial S, Godbehere S, Parameswaran N, Amalfitano A. TRIF, and TRIF-interacting TLRs differentially modulate several adenovirus vector-induced immune responses. J Innate Immun. 2009;1:376–88. doi: 10.1159/000207194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Appledorn DM, et al. Adenovirus vector-induced innate inflammatory mediators, MAPK signaling, as well as adaptive immune responses are dependent upon both TLR2 and TLR9 in vivo. J Immunol. 2008;181:2134–44. doi: 10.4049/jimmunol.181.3.2134. [DOI] [PubMed] [Google Scholar]
- 92.Kawamata Y, et al. Tumor necrosis factor receptor-1 can function through a G alpha q/11-beta-arrestin-1 signaling complex. J Biol Chem. 2007;282:28549–56. doi: 10.1074/jbc.M705869200. [DOI] [PubMed] [Google Scholar]
- 93.Chen W, et al. β-arrestin 2 mediates endocytosis of type III TGF-beta receptor and down-regulation of its signaling. Science. 2003;301:1394–7. doi: 10.1126/science.1083195. [DOI] [PubMed] [Google Scholar]
- 94.Mythreye K, Blobe GC. The type III TGF-beta receptor regulates epithelial and cancer cell migration through beta-arrestin2-mediated activation of Cdc42. Proc Natl Acad Sci U S A. 2009;106:8221–6. doi: 10.1073/pnas.0812879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y, Liu C, Wei B, Pei G. Loss of beta-arrestin 2 exacerbates experimental autoimmune encephalomyelitis with reduced number of Foxp3+ CD4+ regulatory T cells. Immunology. 2013;140:430–40. doi: 10.1111/imm.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mo W, et al. Nuclear beta-arrestin1 functions as a scaffold for the dephosphorylation of STAT1 and moderates the antiviral activity of IFN-gamma. Mol Cell. 2008;31:695–707. doi: 10.1016/j.molcel.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 97.Heim MH, Moradpour D, Blum HE. Expression of hepatitis C virus proteins inhibits signal transduction through the Jak-STAT pathway. J Virol. 1999;73:8469–75. doi: 10.1128/jvi.73.10.8469-8475.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yang PL, Althage A, Chung J, Chisari FV. Hydrodynamic injection of viral DNA: a mouse model of acute hepatitis B virus infection. Proc Natl Acad Sci U S A. 2002;99:13825–30. doi: 10.1073/pnas.202398599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bari R, et al. Significant functional heterogeneity among KIR2DL1 alleles and a pivotal role of arginine 245. Blood. 2009;114:5182–90. doi: 10.1182/blood-2009-07-231977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yu MC, et al. An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells. Nat Immunol. 2008;9:898–907. doi: 10.1038/ni.1635. [DOI] [PubMed] [Google Scholar]
- 101.Li M, et al. T-cell Immunoglobulin and ITIM Domain (TIGIT) Receptor/Poliovirus Receptor (PVR) Ligand Engagement Suppresses Interferon-gamma Production of Natural Killer Cells via beta-Arrestin 2-mediated Negative Signaling. J Biol Chem. 2014;289:17647–57. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bjorgo E, et al. Cross talk between phosphatidylinositol 3-kinase and cyclic AMP (cAMP)-protein kinase a signaling pathways at the level of a protein kinase B/beta-arrestin/cAMP phosphodiesterase 4 complex. Mol Cell Biol. 2010;30:1660–72. doi: 10.1128/MCB.00696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y, Liu C, Wei B, Pei G. Loss of β-arrestin 2 exacerbates experimental autoimmune encephalomyelitis with reduced number of Foxp3+ CD4+ regulatory T cells. Immunology. 2013;140:430–440. doi: 10.1111/imm.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Y, et al. beta-arrestin2 stimulates interleukin-17 production and expression of CD4+ T lymphocytes in a murine asthma model. Iran J Allergy Asthma Immunol. 2011;10:171–82. [PubMed] [Google Scholar]
- 105.Wang G, et al. Effects of beta-arrestin 2 on cytokine production of CD4+ T lymphocytes of mice with allergic asthma. Indian J Exp Biol. 2011;49:585–93. [PubMed] [Google Scholar]
- 106.Li J, et al. Deficiency of beta-arrestin1 ameliorates collagen-induced arthritis with impaired TH17 cell differentiation. Proc Natl Acad Sci U S A. 2013;110:7395–400. doi: 10.1073/pnas.1221608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li H, et al. Chronic stress promotes lymphocyte reduction through TLR2 mediated PI3K signaling in a beta-arrestin 2 dependent manner. J Neuroimmunol. 2011;233:73–9. doi: 10.1016/j.jneuroim.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Man SM, Kanneganti TD. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shaw PJ, McDermott MF, Kanneganti TD. Inflammasomes and autoimmunity. Trends Mol Med. 2011;17:57–64. doi: 10.1016/j.molmed.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mao K, et al. beta-arrestin1 is critical for the full activation of NLRP3 and NLRC4 inflammasomes. J Immunol. 2015;194:1867–73. doi: 10.4049/jimmunol.1401989. [DOI] [PubMed] [Google Scholar]
- 111.Ohguro H, et al. Beta-arrestin and arrestin are recognized by autoantibodies in sera from multiple sclerosis patients. Proc Natl Acad Sci U S A. 1993;90:3241–5. doi: 10.1073/pnas.90.8.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Forooghian F, Cheung RK, Smith WC, O'Connor P, Dosch HM. Enolase and arrestin are novel nonmyelin autoantigens in multiple sclerosis. J Clin Immunol. 2007;27:388–96. doi: 10.1007/s10875-007-9091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bittner S, Afzali AM, Wiendl H, Meuth SG. Myelin oligodendrocyte glycoprotein (MOG35-55) induced experimental autoimmune encephalomyelitis (EAE) in C57BL/6 mice. J Vis Exp. 2014 doi: 10.3791/51275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsutsui S, et al. Glucocorticoids regulate innate immunity in a model of multiple sclerosis: reciprocal interactions between the A1 adenosine receptor and beta-arrestin-1 in monocytoid cells. Faseb j. 2008;22:786–96. doi: 10.1096/fj.07-9002com. [DOI] [PubMed] [Google Scholar]
- 115.Coureuil M, et al. Meningococcus Hijacks a β2-adrenoceptor/β-Arrestin pathway to cross brain microvasculature endothelium. Cell. 2010;143:1149–60. doi: 10.1016/j.cell.2010.11.035. [DOI] [PubMed] [Google Scholar]
- 116.Xia R, et al. Overexpression of beta-arrestin 2 in peripheral blood mononuclear cells of patients with cryptococcal meningitis. J Interferon Cytokine Res. 2010;30:155–62. doi: 10.1089/jir.2009.0017. [DOI] [PubMed] [Google Scholar]
- 117.Nials AT, Uddin S. Mouse models of allergic asthma: acute and chronic allergen challenge. Dis Model Mech. 2008;1:213–20. doi: 10.1242/dmm.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hollingsworth JW, et al. Both Hematopoietic-Derived and Non Hematopoietic-Derived β-Arrestin Regulates Murine Allergic Airway Disease. American Journal of Respiratory Cell and Molecular Biology. 2010;43:269–275. doi: 10.1165/rcmb.2009-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nichols HL, et al. β-Arrestin-2 mediates the proinflammatory effects of proteinase-activated receptor-2 in the airway. Proc Natl Acad Sci U S A. 2012;109:16660–5. doi: 10.1073/pnas.1208881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen M, et al. Genetic Deletion of beta-arrestin-2 Mitigates Established Airway Hyperresponsiveness in a Murine Asthma Model. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2014-0231OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Khachigian LM. Collagen antibody-induced arthritis. Nat Protoc. 2006;1:2512–6. doi: 10.1038/nprot.2006.393. [DOI] [PubMed] [Google Scholar]
- 122.Li P, et al. Increased expression of beta-arrestin 1 and 2 in murine models of rheumatoid arthritis: isoform specific regulation of inflammation. Mol Immunol. 2011;49:64–74. doi: 10.1016/j.molimm.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Campo GM, et al. Beta-arrestin-2 negatively modulates inflammation response in mouse chondrocytes induced by 4-mer hyaluronan oligosaccharide. Mol Cell Biochem. 2015;399:201–8. doi: 10.1007/s11010-014-2246-5. [DOI] [PubMed] [Google Scholar]
- 124.Low D, Nguyen DD, Mizoguchi E. Animal models of ulcerative colitis and their application in drug research. Drug Des Devel Ther. 2013;7:1341–57. doi: 10.2147/DDDT.S40107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee T, et al. beta-Arrestin-1 deficiency protects mice from experimental colitis. Am J Pathol. 2013;182:1114–23. doi: 10.1016/j.ajpath.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zeng LX, et al. [beta]-Arrestin2 encourages inflammation-induced epithelial apoptosis through ER stress/PUMA in colitis. Mucosal Immunol. 2014 doi: 10.1038/mi.2014.104. [DOI] [PubMed] [Google Scholar]
- 127.Chuang YH, Ridgway WM, Ueno Y, Gershwin ME. Animal models of primary biliary cirrhosis. Clin Liver Dis. 2008;12:333–47. ix. doi: 10.1016/j.cld.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu Z, et al. beta-Arrestin 1 modulates functions of autoimmune T cells from primary biliary cirrhosis patients. J Clin Immunol. 2011;31:346–55. doi: 10.1007/s10875-010-9492-4. [DOI] [PubMed] [Google Scholar]
- 129.Lymperopoulos A. GRK2 and beta-arrestins in cardiovascular disease: Something old, something new. Am J Cardiovasc Dis. 2011;1:126–37. [PMC free article] [PubMed] [Google Scholar]
- 130.Wisler JW, et al. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci U S A. 2007;104:16657–62. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Most P, et al. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J Clin Invest. 2004;114:1550–63. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Koch WJ. Adrenal beta-arrestin 1 inhibition in vivo attenuates post-myocardial infarction progression to heart failure and adverse remodeling via reduction of circulating aldosterone levels. J Am Coll Cardiol. 2011;57:356–65. doi: 10.1016/j.jacc.2010.08.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bathgate-Siryk A, et al. Negative impact of beta-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension. 2014;63:404–12. doi: 10.1161/HYPERTENSIONAHA.113.02043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Watari K, Nakaya M, Nishida M, Kim KM, Kurose H. beta-arrestin2 in infiltrated macrophages inhibits excessive inflammation after myocardial infarction. PLoS One. 2013;8:e68351. doi: 10.1371/journal.pone.0068351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Selman M, et al. Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches. Drugs. 2004;64:405–30. doi: 10.2165/00003495-200464040-00005. [DOI] [PubMed] [Google Scholar]
- 136.King TE, Jr., Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–61. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 137.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–60. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 138.Lovgren AK, et al. beta-arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3001564. 74ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rieber N, Hector A, Carevic M, Hartl D. Current concepts of immune dysregulation in cystic fibrosis. Int J Biochem Cell Biol. 2014;52:108–12. doi: 10.1016/j.biocel.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 140.White NM, et al. Altered cholesterol homeostasis in cultured and in vivo models of cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2007;292:L476–86. doi: 10.1152/ajplung.00262.2006. [DOI] [PubMed] [Google Scholar]
- 141.Manson ME, Corey DA, White NM, Kelley TJ. cAMP-mediated regulation of cholesterol accumulation in cystic fibrosis and Niemann-Pick type C cells. Am J Physiol Lung Cell Mol Physiol. 2008;295:L809–19. doi: 10.1152/ajplung.90402.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Manson ME, Corey DA, Rymut SM, Kelley TJ. beta-arrestin-2 regulation of the cAMP response element binding protein. Biochemistry. 2011;50:6022–9. doi: 10.1021/bi200015h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manson ME, Corey DA, Bederman I, Burgess JD, Kelley TJ. Regulatory role of beta-arrestin-2 in cholesterol processing in cystic fibrosis epithelial cells. J Lipid Res. 2012;53:1268–76. doi: 10.1194/jlr.M021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tunaru S, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9:352–5. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 145.Benyo Z, et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest. 2005;115:3634–40. doi: 10.1172/JCI23626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Walters RW, et al. β-Arrestin1 mediates nicotinic acid–induced flushing, but not its antilipolytic effect, in mice. The Journal of Clinical Investigation. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lauring B, et al. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3003877. 148ra115. [DOI] [PubMed] [Google Scholar]
- 148.Villa P, Ghezzi P. Animal models of endotoxic shock. Methods Mol Med. 2004;98:199–206. doi: 10.1385/1-59259-771-8:199. [DOI] [PubMed] [Google Scholar]
- 149.Porter KJ, et al. Regulation of lipopolysaccharide-induced inflammatory response and endotoxemia by β-arrestins. Journal of Cellular Physiology. 2010;225:406–416. doi: 10.1002/jcp.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li H, et al. beta-Arrestin 2 Negatively Regulates TLR4-Triggered Inflammatory Signaling via Targeting p38 MAPK and IL-10. J Biol Chem. 2014 doi: 10.1074/jbc.M114.591495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146:1763–74. doi: 10.1053/j.gastro.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hubbard WJ, et al. Cecal ligation and puncture. Shock. 2005;24(Suppl 1):52–7. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 153.Ayala A, Song GY, Chung CS, Redmond KM, Chaudry IH. Immune depression in polymicrobial sepsis: the role of necrotic (injured) tissue and endotoxin. Crit Care Med. 2000;28:2949–55. doi: 10.1097/00003246-200008000-00044. [DOI] [PubMed] [Google Scholar]
- 154.Povsic TJ, Kohout TA, Lefkowitz RJ. Beta-arrestin1 mediates insulin-like growth factor 1 (IGF-1) activation of phosphatidylinositol 3-kinase (PI3K) and anti-apoptosis. J Biol Chem. 2003;278:51334–9. doi: 10.1074/jbc.M309968200. [DOI] [PubMed] [Google Scholar]
- 155.Zhao M, Wimmer A, Trieu K, Discipio RG, Schraufstatter IU. Arrestin regulates MAPK activation and prevents NADPH oxidase-dependent death of cells expressing CXCR2. J Biol Chem. 2004;279:49259–67. doi: 10.1074/jbc.M405118200. [DOI] [PubMed] [Google Scholar]
- 156.Revankar CM, Vines CM, Cimino DF, Prossnitz ER. Arrestins block G protein-coupled receptor-mediated apoptosis. J Biol Chem. 2004;279:24578–84. doi: 10.1074/jbc.M402121200. [DOI] [PubMed] [Google Scholar]
- 157.Zhang Z, et al. β-Arrestins facilitate ubiquitin-dependent degradation of apoptosis signal-regulating kinase 1 (ASK1) and attenuate H2O2-induced apoptosis. Cellular Signalling. 2009;21:1195–1206. doi: 10.1016/j.cellsig.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 158.Ahn S, Kim J, Hara MR, Ren XR, Lefkowitz RJ. β-Arrestin-2 Mediates Anti-apoptotic Signaling through Regulation of BAD Phosphorylation. J Biol Chem. 2009;284:8855–65. doi: 10.1074/jbc.M808463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhao M, et al. beta-arrestin2 inhibits opioid-induced breast cancer cell death through Akt and caspase-8 pathways. Neoplasma. 2009;56:108–13. doi: 10.4149/neo_2009_02_108. [DOI] [PubMed] [Google Scholar]
- 160.Moorman J, et al. HIV-1 gp120 primes lymphocytes for opioid-induced, β-arrestin 2-dependent apoptosis. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2009;1793:1366–1371. doi: 10.1016/j.bbamcr.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qi S, et al. HSP27 phosphorylation modulates TRAIL-induced activation of Src-Akt/ERK signaling through interaction with beta-arrestin2. Cell Signal. 2014;26:594–602. doi: 10.1016/j.cellsig.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 162.Sun X, et al. Beta-arrestin 2 modulates resveratrol-induced apoptosis and regulation of Akt/GSK3ss pathways. Biochim Biophys Acta. 2010;1800:912–8. doi: 10.1016/j.bbagen.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 163.Zhu Z, Reiser G. PAR-1 activation rescues astrocytes through the PI3K/Akt signaling pathway from chemically induced apoptosis that is exacerbated by gene silencing of beta-arrestin 1. Neurochem Int. 2014;67:46–56. doi: 10.1016/j.neuint.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 164.Rojanathammanee L, et al. The 27-kDa heat shock protein confers cytoprotective effects through a beta 2-adrenergic receptor agonist-initiated complex with beta-arrestin. Mol Pharmacol. 2009;75:855–65. doi: 10.1124/mol.108.053397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Quoyer J, et al. GLP-1 mediates antiapoptotic effect by phosphorylating Bad through a beta-arrestin 1-mediated ERK1/2 activation in pancreatic beta-cells. J Biol Chem. 2010;285:1989–2002. doi: 10.1074/jbc.M109.067207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wu JX, Shan FX, Zheng JN, Pei DS. beta-arrestin promotes c-Jun N-terminal kinase mediated apoptosis via a GABA(B)R.beta-arrestin.JNK signaling module. Asian Pac J Cancer Prev. 2014;15:1041–6. doi: 10.7314/apjcp.2014.15.2.1041. [DOI] [PubMed] [Google Scholar]
- 167.Vines CM, et al. N-formyl peptide receptors internalize but do not recycle in the absence of arrestins. J Biol Chem. 2003;278:41581–4. doi: 10.1074/jbc.C300291200. [DOI] [PubMed] [Google Scholar]
- 168.Barlic J, et al. ?-Arrestins Regulate Interleukin-8-induced CXCR1 Internalization. Journal of Biological Chemistry. 1999;274:16287–16294. doi: 10.1074/jbc.274.23.16287. [DOI] [PubMed] [Google Scholar]
- 169.Aramori I, et al. Molecular mechanism of desensitization of the chemokine receptor CCR5: receptor signaling and internalization are dissociable from its role as an HIV co-receptor. 1997;16:4606–4616. doi: 10.1093/emboj/16.15.4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huttenrauch F, Pollok-Kopp B, Oppermann M. G protein-coupled receptor kinases promote phosphorylation and beta-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J Biol Chem. 2005;280:37503–15. doi: 10.1074/jbc.M500535200. [DOI] [PubMed] [Google Scholar]
- 171.Dery O, Thoma MS, Wong H, Grady EF, Bunnett NW. Trafficking of proteinase-activated receptor-2 and β-arrestin-1 tagged with green fluorescent protein. β-Arrestin-dependent endocytosis of a proteinase receptor. J Biol Chem. 1999;274:18524–35. doi: 10.1074/jbc.274.26.18524. [DOI] [PubMed] [Google Scholar]