Abstract
Recent work suggests that analysis of the cortical thickness in key brain regions can be used to identify individuals at greatest risk for development of Alzheimer’s disease (AD). However, it is unclear to what extent this “signature” is a biological marker of normal memory function – the primary cognitive domain affected by AD. We examined the relationship between the AD signature biomarker and memory functioning in a group of neurologically healthy young and older adults. Cortical thickness measurements and neuropsychological evaluations were obtained in 110 adults (age range 21–78, mean=46) drawn from the Brain Resource International Database. The cohort was divided into young adult (n=64, age 21–50) and older adult (n=46, age 51–78) groups. Cortical thickness analysis was performed with FreeSurfer, and the average cortical thickness extracted from the eight regions that comprise the AD signature. We examined the relationship between AD-signature cortical thickness and memory, and tested whether this relationship varies as a function of age group. Mean AD-signature cortical thickness was positively associated with performance on the delayed free recall trial of a list learning task and this relationship did not differ between younger and older adults. Mean AD-signature cortical thickness was not associated with performance on a test of psychomotor speed, as a control task, in either group. The results suggest that the AD signature cortical thickness is a marker for memory functioning across the adult lifespan.
Keywords: Alzheimer’s Disease, Cortical Thickness, Structural MRI, Neuropsychology, Memory
INTRODUCTION
Alzheimer’s disease (AD) is characterized clinically by early decline in memory, followed by impairment in other cognitive domains and disrupted activities of daily living (Burns, Byrne, & Maurer, 2002; Forstl & Kurz, 1999). Despite the well characterized clinical progression of the disease, over the past several years there has been a tremendous push towards characterizing its biology and development of valid and reliable biological markers. Biological markers are critical for disease detection, disease staging, and to monitor and predict treatment response. Development of biological markers can also provide insight into the relevant pathological changes that contribute to the clinical presentation of disease. With the advancement of acquisition and analysis techniques, magnetic resonance imaging (MRI) has emerged as an obvious instrument for development of novel biological markers in AD.
The application of structural MRI has revealed a specific pattern of cortical thinning among older adults that appears to be associated with AD risk and progression. This pattern, known as the AD cortical “signature” (B.C. Dickerson et al., 2009), comprises the medial, inferior, and pole of the temporal lobe; the angular gyrus; the superior and inferior frontal lobe; the superior parietal lobule; the supramarginal gyrus; and the precuneus. There is prominent thinning in these areas among individuals with prevalent AD (Braak & Braak, 1991; Brun & Gustafson, 1976; Lerch et al., 2005) and among individuals at risk for AD by virtue of being diagnosed with mild cognitive impairment (MCI; (Bakkour, Morris, & Dickerson, 2009)). Asymptomatic individuals with evidence of fibrillar forms of amyloid, have cortical thinning in these regions compared with individuals without evidence of amyloid and the degree of cortical thinning in these areas predicts progression to clinical AD among cognitively normal individuals (Bakkour et al., 2009; B.C. Dickerson et al., 2009; Dickerson et al., 2001; Dickerson et al., 2011). Taken together, these findings have led investigators to conclude that this cortical signature is a valid and reliable biological marker for AD (Bakkour et al., 2009; Bakkour, Morris, Wolk, & Dickerson, 2013; Dickerson et al., 2011).
It is possible, however, that in addition to reflecting AD pathology per se, structures included in the cortical signature regions may simply mediate memory function, the primary cognitive domain affected in AD. In this scenario, individual differences in cortical signature thickness would be related to memory functioning irrespective or independent of risk for AD. Supportive of this hypothesis, several of the regions included in the cortical signature have been implicated in normal memory function (Rolls, 2000; Squire & Kowlton, 1999; Squire & Zola-Morgan, 1991). To test this possibility, we examined the relationship between cortical thickness in the AD cortical signature regions and memory function in healthy non-demented older adults as well as in young adults. The inclusion of the latter group was especially important as this age group targets a period that is ostensibly free of the presence of AD pathology. To test the specificity of the cortical signature regions with memory we also compared the extent to which variability in these regions are related to a non-memory cognitive domain.
METHODS
Subjects
Structural MRI and neuropsychological data for the current study came from BRAINnet Foundation (www.BRAINnet.net). Although the database includes neuropsychological data from 6 primary sites throughout the world (Clark et al., 2006; Gordon, Cooper, Rennie, Hermens, & Williams, 2005) structural MRI data were collected at two sites (Westmead and Adelaide) in Australia. For the current study, adults older than 21 years of age, with complete T1-weighted MRI data, and neuropsychological data were selected from the database. This selection approach yielded 110 individuals who ranged in age from 21 to 76. The subject group was divided into two age groups, comprising younger (ages 21–49) and older (ages 50–78) adults. Younger adults had slightly more years of formal education and the two groups were similar in sex distribution (see Table 1). Participants were screened with the Somatic and Psychological Health Report (Hickie, Davenport, Naismith, & Scott, 2001) and excluded if they had a significant psychiatric or neurological history, including clinical diagnosis of AD. Other exclusionary criteria included neurologic illness, history of brain injury, and/or drug or alcohol addiction. Individuals with first-degree family members with known diagnoses of schizophrenia, bipolar disorder, attention deficit hyperactivity disorder, or genetic disorder were excluded.
Table 1.
Demographic and clinical variables in study participants included in analyses.
| Younger | Older | Total | Statistic | |
|---|---|---|---|---|
| N | 64 | 46 | 110 | |
| Age, mean years (SD) | 35.6 (8.5) | 60.7 (7.1) | 46.1 (14.8) | t(108)=16.4, p<0.001 |
| Education, mean years (SD) | 15.0 (2.6) | 13.2 (2.7) | 14.2 (2.8) | t(101)=3.5, p=0.001 |
| % Female | 58 | 43 | 52 | χ2(1)=2.2, P=0.138 |
| Total intracranial volume, mm3 (SD) | 1580323 (192359) | 1564077 (149333) | 1573529 (175094) | t(108)=0.48, p=0.633 |
| Mean AD-signature cortical thickness, mm (SD) | 2.61 (0.193) | 2.47 (0.185) | 2.55 (0.201) | t(108)=3.829, p<0.001 |
| Delayed free recall, mean no. of words (SD) | 8.0 (2.0) | 6.7 (2.1) | 7.5 (2.1) | t(108)=3.4, p=0.001 |
| Mean no. finger taps (SD) | 172.6 (22.8) | 159.5 (21.3) | 167.7 (24.0) | t(108)=2.7, p=0.009 |
MRI
Structural brain image acquisition was conducted at two imaging sites, a 1.5 Tesla Siemens Vision Plus (Westmead Hospital, Sydney) and a 1.5 Tesla Siemens Sonata (Wakefield Imaging, Adelaide) with identical scanner protocols (Grieve, Clark, Williams, Peduto, & Gordon, 2005; Grieve, Korgaonkar, Clark, & Williams, 2011). The T1-weighted, magnetization prepared rapid acquisition gradient-echo (MPRAGE) MR sequence was acquired (Flip Angle = 12°, TI= 200msec, TR = 9.7ms, TE=4ms, 256×256 FOV, 1mm isotropic slice thickness) in the sagittal plane.
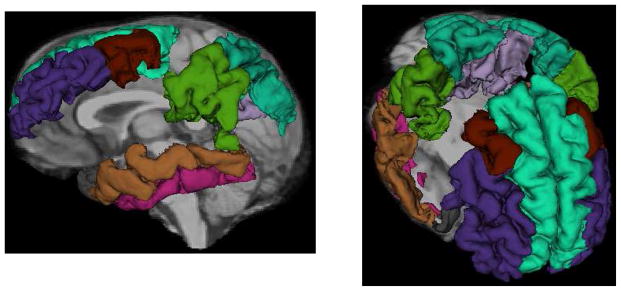
This study used archival MRI data that were analyzed previously. Surface-based estimation of cortical thickness was performed with the FreeSurfer image analysis suite (version 4.3). FreeSurfer is freely available for download online (http://sufer.nmr.mgh.harvard.edu/) and the technical details of these procedures are described in prior publications (Dale, Fischl, & Sereno, 1999; Fischl, Sereno, & Dale, 1999; Grieve et al., 2011). Individual whole brain 3D MPRAGE brain representations were anatomically labeled by the software (Desikan et al., 2006). Volumetric representations of cortical and subcortical brain regions were calculated through a series of image processing steps including image alignment to Talairach space, field intensity normalization, skull stripping, and surface alignment (Fischl et al., 2002). Cortical surface thickness was estimated through the calculation of the shortest distance between gray and white matter representation boundaries and voxels representing gray and cerebrospinal fluid on the tessellated surface (Fischl & Dale, 2000). We selected eight bilateral regions that beset overlapped with the AD-signature described by Dickerson and colleagues (B.C. Dickerson et al., 2009), including the medial temporal gyrus, temporal pole, inferior temporal gyrus, supramarginal gyrus, superior parietal lobule, precuneus, middle frontal gyrus, and superior frontal gyrus (Figure 1). We computed the average cortical thickness in these regions, across both hemispheres, so that each subject had a single value representing the AD-signature cortical thickness.
Figure 1.
FreeSurfer regions-of-interest representing the 8 regions comprising the AD cortical signature superimposed on a single T1-weighted MRI scan. Labeled regions include the medial temporal gyrus (brown), temporal pole (grey), inferior temporal gyrus (pink), supramarginal gyrus (dark green), superior parietal lobule (teal), precuneus (lavender), middle frontal gyrus (purple: rostral, rust: caudal), and superior frontal gyrus (light teal)
Neuropsychological assessment
Participants were evaluated with IntegNeuro™, a computerized assessment tool that comprises a series of automated, cognitive tests generated by a touch-screen computer (NEC MultiSync LCD 1530 V) (see (Brickman et al., 2012; Clark et al., 2006; Paul et al., 2005; Silverstein et al., 2010; Williams et al., 2005)for full description and psychometric properties). For the current study, we focused on declarative memory as the primary cognitive construct of interest. Participants were read a list of 12 words over 4 learning trials and asked to free recall the words after each trial administration and then again after a distractor list was read to them. Following a 20-minute interval, participants were asked to recall the words again. For the current study, the primary measure of interest was the number of words free recalled after the 20-minute delay. As a non-memory control task, participants were evaluated with a test of simple motor tapping. Participants tapped a circle on the touch-screen with their index finger as quickly as possible for a single 60-second trial. The total number of taps with their dominant finger was the primary variable of interest for this test.
Statistical analysis
Multiple regression analysis was used to examine the relationship between mean cortical thickness in the signature regions and performance on the free recall memory test. In addition to an Age Group variable, indicating membership in the younger versus older age group, we calculated an interaction term by multiplying the Age Group (0, 1) variable by the mean cortical thickness value. Main effects of Cortical Thickness and Age Group would indicate a relationship between mean cortical thickness and memory and between age group and memory, respectively. A significant interaction term would indicate that the relationship between cortical thickness and memory varies as a function of age group. To test the specificity of associations with memory functioning, we examined performance on the Finger Tapping Test as the dependent variable in separate models. We also re-ran the primary regression analysis after removing the Cortical Thickness term and its interaction with Age Group to determine the extent to which age-associated differences in memory are mediated by cortical thickness. All statistical models included subject education, and total intracranial volume as covariates. Even in the case of a non-significant interaction between age group and cortical thickness, we stratified the analysis to examine the relationship between cortical thickness and memory separately in younger and older individuals.
Demographic factors were compared between young and older groups with t-test and χ2 analyses.
RESULTS
Demographic, neuroimaging, and neuropsychological test performance data are presented in Table 1. Younger and older participants were similar in terms of intracranial volume and sex distribution. Older adults had fewer years of formal education, smaller AD signature cortical thickness values, and lower performance on memory and finger tapping tests.
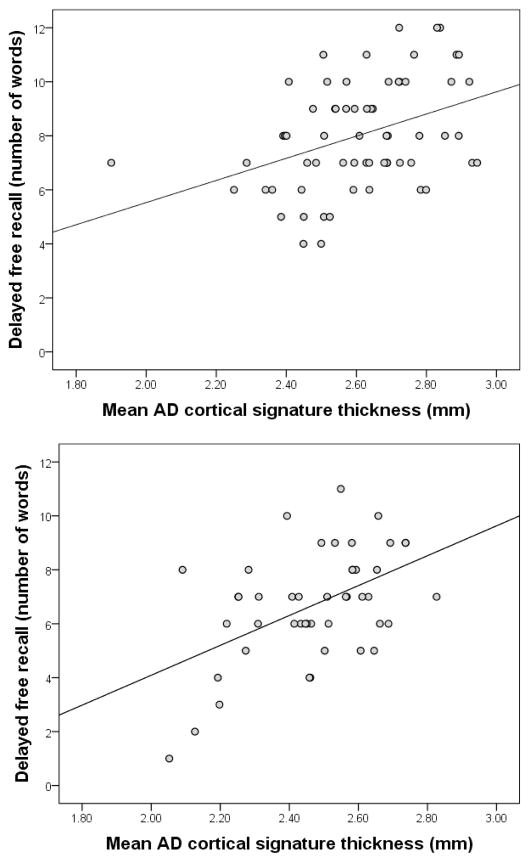
Results of the primary multiple regression analysis are presented in Table 2. Increased AD cortical signature thickness values were associated with better performance on memory testing (main effect of cortical thickness). However, the relationship between cortical thickness values and memory test performance was similar in younger and older adults (non-significant Cortical Thickness by Age Group interaction; Figure 2). In the multiple regression analysis, Age Group was not related to performance on memory testing (non-significant main effect of Age Group), but when the cortical thickness value and interaction terms were removed from the model, it was (main effect of Age Group, β=−1.04, p=0.016). In a stratified analysis, the relationship between cortical thickness and memory was similar in younger (β=3.45, p=0.011) and older (β=5.17, p=0.004) participants. Separate scatterplots displaying the relationship between cortical thickness and memory in younger and older groups are displayed in Figure 1. When the primary multiple regression analysis was repeated with finger tapping performance as the dependent variable, cortical thickness was not related to performance (main effect of Cortical Thickness, β=−4.53, p=0.781) nor did it interact with Age Group (Cortical Thickness by Age Group interaction, β=38.43, p=0.154). None of the covariates were associated with memory or finger tapping performance.
Table 2.
Results of the primary multiple regression analysis that examined the relationship between cortical thickness in the AD signature regions and performance on the delayed free recall trial of the list learning test (as the dependent variable). The overall model was statistically significant (F(5,102)=7.24, p<0.001).
| Predictor | β | 95% confidence interval | t | p |
|---|---|---|---|---|
| Age Group (0=Young 1=Older) | −4.87 | −14.73 – 5.00 | −0.979 | 0.330 |
| Mean cortical thickness AD signature | 3.47 | 0.92 – 6.02 | 2.703 | 0.008 |
| Age Group x AD Signature interaction | 1.74 | −2.16 – 5.63 | 0.885 | 0.378 |
| Intracranial volume | −8.54 × 10−7 | −3.00×10−7 – 1.00×10−7 | −0.767 | 0.445 |
| Years of education | 0.08 | −0.06 – 0.23 | 1.138 | 0.258 |
Figure 2.
The relationship between mean AD cortical signature thickness in younger adults (upper panel) and older adults (lower panel).
DISCUSSION
We found that mean cortical thickness in AD signature regions was associated with memory performance similarly in younger and older neurologically-healthy adults and that age group differences in memory performance were mediated partially by cortical thickness in the AD signature regions. The association between AD signature cortical thickness and cognition appeared to be specific for memory, as cortical thickness in these regions was not associated with performance on a finger tapping test, although it should be noted that the regions that comprise the cortical signature are likely involved in other higher order processes given their wide distribution in cortex. Our findings suggest that the AD signature cortical thickness measure supports memory functioning similarly in young and older adults.
The findings have important implications regarding the specificity of the AD cortical signature as a biological marker for AD. Participants in the current study were cognitively healthy adults without clinical evidence of AD and the cortical thickness in AD-specific regions correlated reliably with memory abilities across age groups and similarly within each age group. The association between age group and memory functioning was not reliable when the cortical thickness measure was in the statistical model, suggesting that cortical thickness in these regions partially mediates age-related memory differences. Further, individual differences in cortical thickness were similarly related to memory functioning in older and younger adults, suggesting that these regions support memory similarly across the adult lifespan. Because this cortical thickness pattern is associated with normal memory functioning in neurologically healthy younger and older adults, the extent to which it is a specific biological marker for AD needs to be considered. Low cortical thickness values in these regions in any given individual may reflect both normal individual and disease-specific thinning.
There are important clinical implications for our observations. Past work indicates that cortical thickness in the AD signature regions does indeed have some diagnostic and disease tracking utility. Individuals with prevalent AD and those who are non-demented, but progress to AD, both have more thinning in these regions than individuals who are non-demented and do not develop AD (Dickerson et al., 2011). But if cortical thickness in these regions primarily reflects individual differences related to memory performance, then a critical question is the extent to which the AD signature provides more sensitive and specific diagnostic and disease tracking information than what would be provided by comprehensive evaluation of memory. Indeed, myriad studies that have examined memory test performance in the context of AD show a similar pattern: older individuals with lower memory test performance are more likely to progress to AD; individuals with prevalent AD have poorer memory performance than individuals without AD; memory performance discriminates among different neurodegenerative conditions; and memory test performance tracks closely with progression of AD (Arnaiz & Almkvist, 2003; Cosentino, Brickman, & Manly, 2011; Flicker, Ferris, & Reisberg, 1991; Jacobs et al., 1995; Troster et al., 1993). Comprehensive evaluation of memory is more feasible, inexpensive, and convenient than neuroimaging studies and post-processing requirements necessary to derive cortical thickness variables for clinical purposes and future work should compare the two modalities explicitly to determine whether one has increased clinical utility over the other. Future studies need to compare the “value added” of MRI-derived cortical thickness measure as a diagnostic and prognostic tool over comprehensive evaluation of memory functioning.
Our study has a few limitations that should be pointed out. First, we did not use biological data, such as cerebrospinal fluid measures of amyloid and tau protein as a screening measure. Thus, it is possible that the older adult sample may have been “contaminated” by a few subjects with preclinical AD. This potential problem would bias our findings against the null hypothesis. That is, we would expect stronger relationships between AD cortical signature measures and memory functioning in older adults, presumably driven by those with preclinical disease. Because we did not observe this effect, we do not believe that this relative weakness is particularly operative in our data. Older adults did have poorer memory and thinner cortices than younger adults, but the similarity in the relationship between the two suggests a continuum of brain-behavior relationships reflecting individual differences in structure and function, not a relationship driven by an incipient neurodegenerative process. Second, Dickerson and colleagues derived the AD signature regions by exploring the entire cortical mantle and then validating these regions in independent samples after manually delimiting the identified regions of interest (B. C. Dickerson et al., 2009). In our study, which used archival FreeSurfer-derived data, we averaged cortical thickness in the regions-of-interest that contained those areas defined as the AD signature and thus somewhat overestimated the regions involved. The inclusion of larger areas of cortex may also help explain the effect sizes we observed when examining the relationship between cortical thickness and memory, which were relatively larger than what is typically observed in morphological studies that examine the relationship between single regions-of-interest and cognitive function. Because large areas of cortex were represented in our signature region, we likely captured individual differences in networks of regions that support memory function that extend beyond medial temporal lobe structures, classically associated with episodic memory.
In summary, our results suggest that cortical thickness in AD signature regions is a sensitive marker of memory functioning across the adult lifespan.
Acknowledgments
We acknowledge the data and support provided by BRAINnet; www.BRAINnet.net, under the governance of the BRAINnet Foundation. BRAINnet is the scientific network that coordinates access to the Brain Resource International Database for independent scientific purposes. We also thank the individuals who gave their time to participate in the database. This research was approved by local ethics committees. SMG acknowledges the Sydney Medical School Foundation for support.
Footnotes
Disclosures
Dr. Grieve previously received consulting fees from Brain Resource Ltd.
Dr. Williams previously received consulting fees and stock options from Brain Resource Ltd.
Mr. Busovaca, Dr. Zimmerman, Ms. Meier, Ms. Griffith, and Dr. Korgaonkar declare that they do not have financial and personal relationships that might bias this work.
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
References
- Arnaiz E, Almkvist O. Neuropsychological features of mild cognitive impairment and preclinical Alzheimer’s disease. Acta Neurol Scand Suppl. 2003;179:34–41. doi: 64 [pii] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Dickerson BC. The cortical signature of prodromal AD: regional thinning predicts mild AD dementia. Neurology. 2009;72(12):1048–1055. doi: 10.1212/01.wnl.0000340981.97664.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A, Morris JC, Wolk DA, Dickerson BC. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: specificity and differential relationships with cognition. Neuroimage. 2013;76:332–344. doi: 10.1016/j.neuroimage.2013.02.059. S1053-8119(13)00214-0 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brickman AM, Meier IB, Korgaonkar MS, Provenzano FA, Grieve SM, Siedlecki KL, Zimmerman ME. Testing the white matter retrogenesis hypothesis of cognitive aging. Neurobiol Aging. 2012;33(8):1699–1715. doi: 10.1016/j.neurobiolaging.2011.06.001. S0197-4580(11)00207-7 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A, Gustafson L. Distribution of cerebral degeneration in Alzheimer’s disease. A clinico-pathological study. Arch Psychiatr Nervenkr. 1976;223(1):15–33. doi: 10.1007/BF00367450. [DOI] [PubMed] [Google Scholar]
- Burns A, Byrne EJ, Maurer K. Alzheimer’s disease. Lancet. 2002;360(9327):163–165. doi: 10.1016/S0140-6736(02)09420-5. S0140-6736(02)09420-5 [pii] [DOI] [PubMed] [Google Scholar]
- Clark CR, Paul RH, Williams LM, Arns M, Fallahpour K, Handmer C, Gordon E. Standardized assessment of cognitive functioning during development and aging using an automated touchscreen battery. Arch Clin Neuropsychol. 2006;21(5):449–467. doi: 10.1016/j.acn.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Cosentino SA, Brickman AM, Manly JJ. Neuropsychological assessment of the dementias of late life. In: Schaie KW, Willis SL, editors. Handbook of the Psychology of Aging. 7. London: Academic Press; 2011. pp. 339–352. [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. S1053-8119(06)00043-7 [pii] [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Buckner RL. The Cortical Signature of Alzheimer’s Disease: Regionally Specific Cortical Thinning Relates to Symptom Severity in Very Mild to Mild AD Dementia and is Detectable in Asymptomatic Amyloid-Positive Individuals. Cerebral Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Buckner RL. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. bhn113 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Goncharova I, Sullivan MP, Forchetti C, Wilson RS, Bennett DA, deToledo-Morrell L. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22(5):747–754. doi: 10.1016/s0197-4580(01)00271-8. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, Detoledo-Morrell L. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76(16):1395–1402. doi: 10.1212/WNL.0b013e3182166e96. WNL.0b013e3182166e96 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology. 1991;41(7):1006–1009. doi: 10.1212/wnl.41.7.1006. [DOI] [PubMed] [Google Scholar]
- Forstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):288–290. doi: 10.1007/s004060050101. [DOI] [PubMed] [Google Scholar]
- Gordon E, Cooper N, Rennie C, Hermens D, Williams LM. Integrative neuroscience: the role of a standardized database. Clin EEG Neurosci. 2005;36(2):64–75. doi: 10.1177/155005940503600205. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Hum Brain Mapp. 2005;25(4):391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Clark CR, Williams LM. Regional heterogeneity in limbic maturational changes: evidence from integrating cortical thickness, volumetric and diffusion tensor imaging measures. Neuroimage. 2011;55(3):868–879. doi: 10.1016/j.neuroimage.2010.12.087. S1053-8119(11)00020-6 [pii] [DOI] [PubMed] [Google Scholar]
- Hickie IB, Davenport TA, Naismith SL, Scott EM. SPHERE: a national depression project. SPHERE National Secretariat. Med J Aust. 2001;175(Suppl):S4–5. doi: 10.5694/j.1326-5377.2001.tb143781.x. [DOI] [PubMed] [Google Scholar]
- Jacobs DM, Sano M, Dooneief G, Marder K, Bell KL, Stern Y. Neuropsychological detection and characterization of preclinical Alzheimer’s disease. Neurology. 1995;45(5):957–962. doi: 10.1212/wnl.45.5.957. [DOI] [PubMed] [Google Scholar]
- Lerch JP, Pruessner JC, Zijdenbos A, Hampel H, Teipel SJ, Evans AC. Focal decline of cortical thickness in Alzheimer’s disease identified by computational neuroanatomy. Cereb Cortex. 2005;15(7):995–1001. doi: 10.1093/cercor/bhh200. bhh200 [pii] [DOI] [PubMed] [Google Scholar]
- Paul RH, Lawrence J, Williams LM, Richard CC, Cooper N, Gordon E. Preliminary validity of “integneuro”: a new computerized battery of neurocognitive tests. Int J Neurosci. 2005;115(11):1549–1567. doi: 10.1080/00207450590957890. P53X6V0564155788 [pii] [DOI] [PubMed] [Google Scholar]
- Rolls ET. Memory systems in the brain. Annu Rev Psychol. 2000;51:599–630. doi: 10.1146/annurev.psych.51.1.599. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Jaeger J, Donovan-Lepore AM, Wilkniss SM, Savitz A, Malinovsky I, Dent G. A comparative study of the MATRICS and IntegNeuro cognitive assessment batteries. J Clin Exp Neuropsychol. 2010;32(9):937–952. doi: 10.1080/13803391003596496922037272. [pii] [DOI] [PubMed] [Google Scholar]
- Squire LR, Kowlton BJ. The medial temporal lobe, the hippcomapus, and the memory systems of the brain. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. Cambridge: The MIT Press; 1999. [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Troster AI, Butters N, Salmon DP, Cullum CM, Jacobs D, Brandt J, White RF. The diagnostic utility of savings scores: differentiating Alzheimer’s and Huntington’s diseases with the logical memory and visual reproduction tests. J Clin Exp Neuropsychol. 1993;15(5):773–788. doi: 10.1080/01688639308402595. [DOI] [PubMed] [Google Scholar]
- Williams LM, Simms E, Clark CR, Paul RH, Rowe D, Gordon E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromarker”. Int J Neurosci. 2005;115(12):1605–1630. doi: 10.1080/00207450590958475. T3844475V6R35Q27 [pii] [DOI] [PubMed] [Google Scholar]