Abstract
Background and Objective:
Due to the evolution of multidrug-resistant strains, screening of natural resources, especially actinomycetes, for new therapeutic agents discovery has become the interests of researchers. In this study, molecular, chemical and biological screening of soil actinomycetes was carried out in order to search for peptide-producing actinomycetes.
Materials and Methods:
60 actinomycetes were isolated from soils of Iran. The isolates were subjected to molecular screening for detection NRPS (non-ribosomal peptide synthetases) gene. Phylogenic identification of NRPS containing isolates was performed. Chemical screening of the crude extracts was performed using chlorine o-dianisidine as peptide detector reagent and bioactivity of peptide producing strains was determined by antimicrobial bioassay. High pressure liquid chromatography- mass spectrometry (HPLC-MS) with UV-visible spectroscopy was performed for detection of the metabolite diversity in selected strain.
Results:
Amplified NRPS adenylation gene (700 bp) was detected among 30 strains. Phylogenic identification of these isolates showed presence of rare actinomycetes genera among the isolates and 10 out of 30 strains were subjected to chemical screening. Nocardia sp. UTMC 751 showed antimicrobial activity against bacterial and fungal test pathogens. HPLC-MS and UV-visible spectroscopy results from the crude extract showed that this strain has probably the ability to produce new metabolites.
Conclusion:
By application of a combined approach, including molecular, chemical and bioactivity analysis, a promising strain of Nocardia sp. UTMC 751 was obtained. This strain had significant activity against Staphylococcus aureus and Pseudomonas aeruginosa. Strain Nocardia sp. UTMC 751 produce five unknown and most probably new metabolites with molecular weights of 274.2, 390.3, 415.3, 598.4 and 772.5. This strain had showed 99% similarity to Nocardia ignorata DSM 44496 T.
Keywords: Actinomycetes, Molecular screening, Non-ribosomal peptide synthetases, Peptide
INTRODUCTION
In recent years multi-drug resistant strains of pathogens has emereged because of extensive use of antimicrobials in medicine, animal husbandry and agriculture (1). Also, prevalence of cancer and emergence of new diseases provoked scientists and pharmaceutical companies to discover novel therapeutic agents (2).
Small bioactive peptides are a well-known group of therapeutic agents, including β-lactams, tyrocidin, gramicidins, cyclosporine , polymyxins, lipopeptides such as daptomycin and surfactin (1). Peptides are divided into two groups based on their biosynthesis routs: non-ribosomal and ribosomal. Ribosomal peptides are synthesized by all living creatures including mammals, amphibians, insects, plants, bacteria, and even viruses (3). Non-ribosomal peptides are synthesized by special and well-organized multi-enzymes, non-ribosomal peptide synthetases (NRPS) in restricted organisms. Bioactive peptides comprise a wide range of biological activities including, antiviral, antiprotozoal, hypocholesterolemic, antifungal, siderophore (4), antimicrobial (5;6), antitumor (7), immunosuppression (8), antioxidant (9), anti-hypertensive (10) and immunomodulatory activities (11;12). Actinomycetes are a major group of soil inhabitant bacteria. Up to now, more than 22 thousands microbial bioactive compounds are known, that half of them are produced by actinomycetes, (13). High frequencies of NRPS biosynthetic systems (58%) is reported among actinomycetes (14). Due to more than 80 years researches on actinomycetes, the chances of finding novel bioactive molecules through the isolation and screening of predominant actinomycetes, the members of Streptomyces genus has dramatically decreased (15;16). Thus, it is crucial that new groups of non-Streptomyces actinomycetes be pursued as sources of novel pharmaceutically active metabolites (17). It is shown that rare actinomycetes, which are difficult to isolate and culture may be a unique source for future drug discovery (18).
Because of the significant importance of nonribosomal peptides in modern medicine, finding NRPS gene containing strains is of great value. The presence of NRPS gene in isolates can be proved by molecular screening but it alone cannot show production of non-ribosomal peptides. Therefore, expression of NRPS clusters was tracked by conducting chemical screening of the strains. Chemical screening is an easy and rapid approach that recruits specific reagents to visualize the biosynthetic capacity of strain in production of different skeleton of secondary metabolites, including sugars, lipids, flavonoids, phenols, arylamines, steroids, terpenes, chloroamines, carbamates, antibiotics, surfactants, peptides, sulfonamides (19;20).
With the aim of attaining sources of bioactive peptides and due to the high diversity of nonribosomal peptides in actinomycetes, recovered soil actinomycets were subjected to molecular screening in order to find NRPS gene containing strains. Strains encompassing NRPS clusters were chemically screened and evaluation of their bioactivity was determined by antibacterial bioassay. HPLC (high pressure liquid chromatography) measurements in combination with mass spectrometry, providing an impression of the metabolite diversity produced by selected strain (21) which was used in metabolite analysis of the selected peptide producing strain.
MATERIALS AND METHODS
Soil sampling and isolation of actinomycetes.
The soil samples were collected aseptically from 20 cm depth of the various habitats of Iran. The samples were immediately taken to the laboratory and stored at 4 °C. The soil samples were dried at room temperature for 1 week, then they crushed and sieved using 2 mm sieve (22). Then, the soil samples were pretreated at 50 °C for 24h before isolation of actinomycetes. One gram of each soil sample was suspended in 10 ml sterile distilled water and vortexed for 45 seconds. The mixtures were allowed to settle, and various concentrations of the spore suspensions were inoculated and spread on the surface of ISP2 agar (malt extract 10 g, yeast extract 4 g, glucose 4 g, agar 15 g in one liter of tap water) and incubated at 28 °C for 7–21 days. The actinomycete putative colonies were selected and transferred to fresh ISP2 agar. The actinomycete isolates were purified by repeated subculturing on ISP2 medium and the pure cultures were maintained at −20 °C for further use.
Molecular screening.
The isolates were revived on ISP2 agar at 28 °C for 7 days. For genomic DNA isolation, one single colony was cultured in LB medium and was incubated at 28 °C at 220 rpm. After 48 h, the biomass were harvested and stored in −20 °C. DNA extraction was performed according to standard protocols (23).
PCR amplification of NRPS gene and DNA sequencing.
PCR amplification of NRPS gene in the isolated strains was done using A3F, A7R degenerate primers (A3F, GCS TAC SYS ATS TAC ACS TCS GG; A7R, SASGTCVCCSGTSCGGTAS) (24).
Each PCR reaction contained 2 μl of genomic DNA, 0.5 μM of primers, 1.5 mM MgCl2, 0.15 μM dNTPs, 5% DMSO, 1X standard Taq reaction buffer, 2 units of Taq DNA polymerase (Genet Bio) and dH2O up to 50 μl. PCR was performed according to the following condition: 96 °C for 3 min, 35 cycles of (96°C for 30s, 59–54 °C for 40s, 68°C for 140–190 s), 72 °C for 10 min. PCR products were observed through 1% agarose gel electrophoresis and ethidium bromide staining. The PCR products were sequenced by Macrogen (South Korea) and compared with that of other validated species in EzTaxon database.
Molecular characterization of selected isolates.
The isolates containing NRPS gene were subjected to PCR amplification of 16S rRNA gene with two universal primers, 9F (AAG AGT TTG ATC ATG GCT CAG) and 1541R (AGG AGG TGA TCC AAC CGC A) (25). PCR condition was same as past PCR (for NRPS gene) except annealing temperature which was 54°C. After detection of PCR products via 1% agarose gel electrophoresis, the product was sequenced by Macrogen (South Korea) (26;27).
Fermentation and extraction of metabolites.
Production of bioactive compound of the strains containing NRPS gene was done by fermentation in flasks. Actinomycete isolates were inoculated in 50 ml of ISP2 medium as seeding medium into 250 ml-Erlenmeyer flasks under aseptic conditions and incubated at 28°C for 48h at 220 rpm. Appropriate seeding material (5% v/v) was inoculated into 250-ml of ISP2 medium as fermentation broth medium into 1000ml–Erlenmeyer flasks and was incubated in a rotary shaker at 220 rpm, 28°C, for 7 days (28). After fermentation, the media were centrifuged at 4000 rpm for 20 min to remove the cell and debris. The supernatant was extracted by equal volume of ethyl acetate. The organic solvent was evaporated at low temperature and pressure to obtain metabolite extract (29;30). The extract of uninoculated fermentation medium was obtained as described above and used as negative control during the experiments.
Chemical screening of crude extracts.
The crude extracts were dissolved in dichloromethane-methanol (CH2Cl2/MeOH) 92:8 before analysis. TLC screening performed on a silica gel 60 F254 plate (Mecherey-Nagel). After drying, the silica plates were sprayed with chlorine o-dianisidine as peptide detection reagent. Green colored zones produced on a plate background were the indication of positive results. Ninhydrin reacting with free amines was used in order to detect the linear or cyclic nature of the peptides. Yellow colored zone formation was the indication of positive results.
Retention factor (Rf) of the compound was also determined after running thin layer chromatography. Virginamycin was used as a positive control.
Bioassay of antimicrobial activity.
Antimicrobial bioassay was carried out by agar well diffusion method against Bacilus subtilis UTMC 1416, Pseudomonas aeruginosa UTMC 1404, MRSA (methicillin resistant staphylococcus aureus) UTMC 1401, Aspergillus niger UTMC 1431 and Candida albicans UTMC 1430. After fermentation, the media were centrifuged at 4000 rpm for 20 min to remove cell and debris. The wells were aseptically bored in plates seeded with pathogenic strains and filled with 100–250 μl of culture filtrates and incubated for 72 hours at 28°C and 24 hours at 35°C for fungi and bacteria, respectively. The diameter (mm) of the inhibition zone around each well was measured (31–33). The bioassays were done in triplicates.
Chromatographic conditions for dereplication using HPLC-MS analysis.
Crude extract of the selected isolate was subjected to mass analysis and HRESIMS data were recorded on a Maxis ESI TOF mass spectrometer (Bruker Daltonics), and molecular formulas were calculated including the isotopic pattern (Smart Formula algorithm).
Analytical RP HPLC was carried out with an Agilent 1260 HPLC system equipped with a diode-array UV detector (DAD) at 200–600 nm and a Corona Ultra detector (Dionex) or a Maxis ESI TOF mass spectrometer. HPLC conditions: Waters Acquity UPLC BEH C18 column 50 × 2 mm, 1.7 μm, solvent A: H2O, 0.1% HCOOH; solvent B: acetonitrile, 0.1% HCOOH; gradient system: 5% B for 1 min, increasing to 95% B in 20 min; flow rate 0.6 mL/min; 40 °C.
RESULTS
Isolation of actinomycete strains.
From 15 soil samples of different habitats, 60 putative actinomycetes strains were isolated based on Gram staining and colony morphology. The isolates were observed to have the colonies with white, brown, yellow, cream, orange, surfaces. The isolates strains were deposited in University of Tehran Microorganisms Collection (UTMC).
Screening of actinomycetes with NRPS genes.
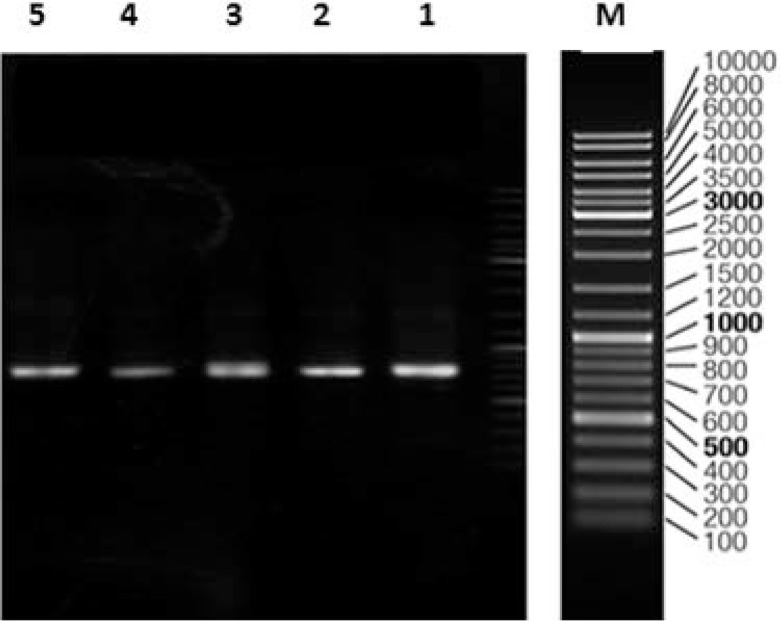
All 60 strains were subjected to DNA extraction and PCR using degenerate specific NRPS primers. A band with about 700 bp length was detected in 30 isolates by PCR screening of the A domain (Fig 1). Analysis of the NRPS sequences with registered genes in the NCBI database confirmed the 60–80% similarity of 10 among 30 investigated isolates. Afterwards phylogenic identification of the strains containing NRPS gene were done using 16SrRNA gene amplification. Sequences were compared with that of other validated species in EzTaxon to database. All of the 30 bacteria show high similarity (>98%) to the relatives in GenBank (34). Phylogenic identification of these 30 isolates shows presence of NRPS gene in rare actinomycetes such as Nocardia, Sacchrothrix, Micromonospora in addition to Streptomyces genus. The sequenced genes were submitted to the GenBank and their accession numbers are listed in Table 1. Finally 10 out of 30 investigated isolates were selected for chemical screening.
Fig. 1.
PCR amplification of NRPS gene sequences using primers A3F/A7R on agarose gel 0.8%. Lines, M: Marker, 1: Micromonospora sp. UTMC 509, 2: Micromonospora sp. UTMC 555, 3: Micromonospora sp. UTMC 736, 4: Nocardia sp. UTMC 827 and 5: Nocardiasp. UTMC 751.
Table 1.
Phylogenetic features of 10 selected actinomycete strains and similarity with registered NRPS gene in GenBank.
| UTMC Code | Length (bp) | Accession number | Closest relative and its accession number | % Identity |
|---|---|---|---|---|
| UTMC 751 | 936 | KF770971 | Nocardia ignorata DSM 44496(T) | 99% |
| UTMC 1046 | 900 | KF770976 | Streptomyces griseorubiginosus NBRC 13047(T) | 100% |
| UTMC 428 | 933 | KF770978 | Streptomyces rectiviolaceus NRRL B-16374(T) | 99.8% |
| UTMC 728 | 939 | KF356151.1 | Streptomyce skasugaensis M338-M1(T) | 99.6% |
| UTMC 736 | 934 | KF356152.1 | Micromonospora endolithica DSM 44398(T) | 99.9% |
| UTMC 537 | 886 | KF770972 | Saccharothrix yanglingensis Hhs.015(T) | 99.3% |
| UTMC 555 | 811 | KF770973 | Micromonospora aurantiaca ATCC 27029(T) | 100% |
| UTMC 509 | 929 | KF770974 | Micromonospora sediminicola SH2-13(T) | 99.3% |
| UTMC 827 | 941 | KF770975 | Nocardia ignorata DSM 44496(T) | 99% |
| UTMC 875 | 941 | KF770977 | Micromonospora tulbaghiae TVU1(T) | 100% |
Primary detection of peptide compounds by chemical screening.
Results of primary chemical screening using absorption measurement at 254 nm and 366 and reaction with chlorine o-dianisidine were revealed that strains UTMC 751, 827, 736, 509 and 555 were recognized as peptide producing strains. None of the spots react with ninhydrin reagent.
Antimicrobial bioassay of selected strains.
According to Table 2, the antimicrobial assays against five microbial pathogens showed that strain Nocardia sp. UTMC 751 has a broad-spectrum antimicrobial activity against tested bacterial and fungal pathogens.
Table 2.
Antimicrobial activities of selected isolates.
| Strain |
Microbial test strains |
||||
|---|---|---|---|---|---|
| C. albicans | S. aureus | A. niger | P. aeruginosa | B. subtilis | |
| Micromonospora sp. UTMC509 | - | - | - | - | - |
| Micromonospora sp. UTMC 736 | - | - | - | 11 | - |
| Micromonospora sp. UTMC 555 | - | 12 | - | - | - |
| Nocardia sp. UTMC 751 | - | 19 | 14 | 18 | - |
| Nocardia sp. UTMC 827 | - | 12 | - | 12 | - |
(−) no activity, weak activity (5–10 mm), mediocre activity (10–15 mm) and high activity (15–20 mm).
Detection of Nocardia sp. UTMC 751 secondary metabolites.
Analysis of produced secondary metabolites by Nocardia sp UTMC 751 in ISP2 medium revealed a number of compounds which are produced in enough amounts which are retrievable from the crude extract after separation process. Some compounds were attributed to insostamycin or monensin (656.4), mycolacton (714.5) and miamycin (615.3) based on their molecular weight values and UV absorption spectra.
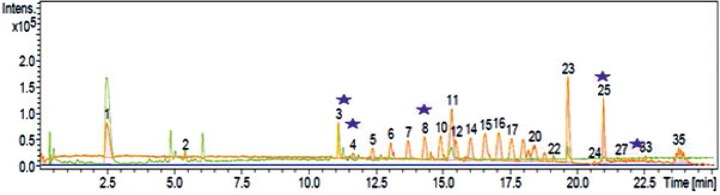
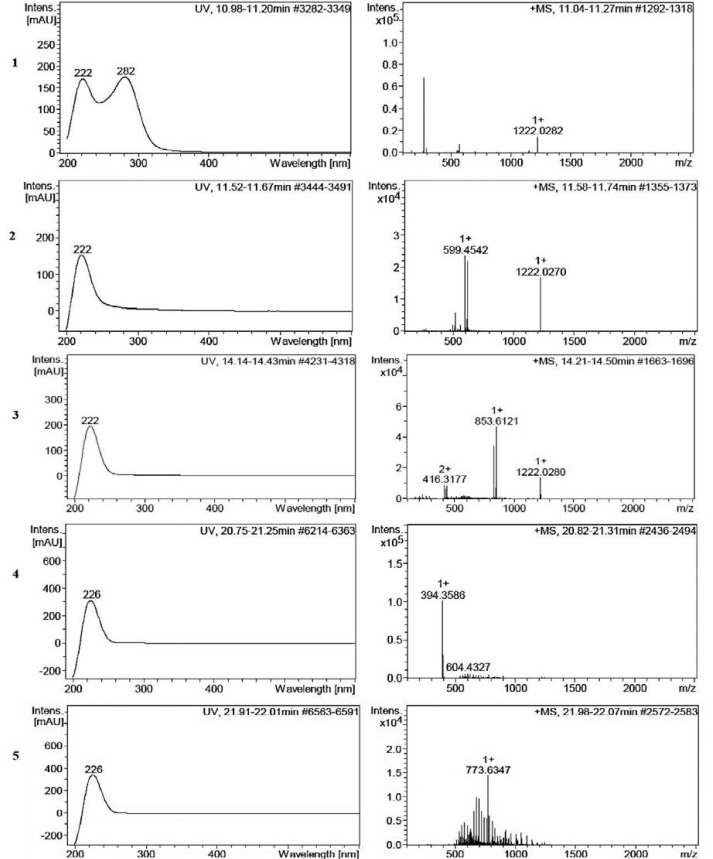
Also, Nocardia sp. UTMC 751 showed the ability to produce five new metabolites with molecular weights of 274.2, 390.3, 415.3, 598.4 and 772.5 probably, based on their molecular weight values and UV absorption spectra which did not matched with the natural compounds recorded in natural product database (Figs. 2 and 3).
Fig. 2.
Total ion chromatogram of major secondary metabolites of Nocardia sp. UTMC 751 crude extract. The compounds are assigned based on retention time and intensity of compounds in crude extract (Y: Intensity and X: Retention time). Metabolites with probable new structure features are indicated by star: Cmpd 3 11.27 min: 274.2 D, Cmpd 4, 11.74 min: 598.4D, Cmpd 8, 14.50 min: 415.3 D, Cmpd 25 21.31 min: 390.3 D, Cmpd 30, 22.07 min: 772.5D
Fig. 3.
Molecular mass and UV-Vis absorption spectra of probable new compounds. 1: Cmpd 3 11.27 min: 274.2 D, 2: Cmpd 4, 11.74 min: 598.4D, 3: Cmpd 8 14.50 min: 415.3 D 4: Cmpd 25 21.31 min: 390.3 D, 5: Cmpd 30, 22.07 min: 772.5D.
DISCUSSION
Most of the known peptide compounds in actinomycetes, including penicillins, cephalosporins, glycopeptides, vancomycin and actinomycins are non-ribosomal peptides which are synthesized by NRPS enzymes. The high detection levels (50%) of NRPS biosynthetic systems observed in our isolates confirmed the wide distribution of these sequences in this bacterial group.
In addition, the chance of finding novel peptides through screening of actinomycetes especially the genus Streptomyces has decreased. Therefore, the probability of discovering a novel peptide can be increased via screening of rare actinomycetes containing NRPS genes. In the present study phylogenic identification of NRPS genes containing isolates shows presence of NRPS genes in rare actinomycetes such as Nocardia, Sacchrothrix, Micromonospora in addition to Streptomyces genus. Since molecular screening by itself cannot prove production of non-ribosomal peptides, chlorine o-dianisidine that reacts with peptides was used to find peptide-producing the strains. Selection of the strains for chemical screening was performed based on phylogenetic analysis and percent identity of NRPS gene to their closest neighbors in GenBank. Due to the existence of –NH–CO– groups in peptides, chlorine o-dianisidine has greater ability to detect peptides. Positive reaction of TLC spots with chlorine o-dianisidine reagent suggests they are peptides and the absence of coloration of the TLC spots with ninhydrin suggests they are cyclic (35).
Easy to perform, speed, and low cost of TLC has made it a widely used technique. Using chemical screening, we were able to determine to some extent the peptide-producing strains and diversity of metabolites of the strains before dereplication (rapid identification of known compounds) and large-scale production. Peptides have a wide range of biological activity (36). When a peptide component shows a kind of biological activity, the probability of finding more activities can be increased. Since short peptides (1–50 amino acids) with cationic and hydrophobic properties, react with anionic components of the cell membrane through electrostatic interactions and providing activity against a wide variety of pathogenic microorganisms such as Gram-negative and Gram-positive bacteria, fungi, viruses, and parasites (37). In this study, in order to decrease the risk of selection peptide-producing strain, antibacterial bioassay against microbial pathogens was carried out to find strain with broad antimicrobial spectra and strain Nocardia sp. UTMC 751 was obtained based on molecular screening and chemical screening.
Strain Nocardia sp. UTMC 751 showed activity against P. aeruginosa and S. aureus which are quickly increasing as antibiotic resistant strains and the efficacy of many antibiotics against these pathogens has become limited. P. aeruginosa as a Gram-negative bacteria are often isolated from patients with MRSA infections (38). Hence monotherapy with active drugs against Gram-positive bacteria is not effective against polymicrobial infections by S. aureus and P. aeruginosa (39).
Different antimicrobial spectra exhibited the potential chemical diversity of its bioactive metabolites of Nocardia sp. UTMC 751 and represent the production of diverse peptides by this strain. Since many species of the genus Nocardia can cause serious infections in humans and animals, they are considered to be biologically more active than non-pathogenic species and so they can produce a variety of pharmaceutical agents.
HPLC-MS analysis of the crude extract produced by this strain gave a complete picture of its metabolic diversity. The molecular masses resulting from HPLC-MS measurements were searched in natural product database. The results indicate that five compounds with molecular weights of 274.2, 390.3, 415.3, 598.4 and 772.5 D have probably not been isolated from microorganisms before.
CONCLUSION
In summary, concerning actinomycetes importance in producing numerous natural compounds, we screened NRPS gene among actinomycetes isolated from soils of Iran. Consequently, 10 strains of actinomycetes containing NRPS genes were phylogenetically characterized which showed that that most of them were rare actinomycetes and were subjected for chemical and biological assay. In fact, the combined strategy of molecular and chemical analysis in parallel to bioactivity, was found as an effective approach in screening of bioactive peptide producing strains. Accordingly, strain Nocardia sp. UTMC 751 is introduced as valuable candidates of strain harboring the capacity of producing bioactive peptides. The bioactive peptide produced by this strain probably is among these five unknown compounds which will be subjected to structural elucidation after large scale fermentation and separation process that will be accomplished in further work.
REFERENCES
- 1. Dubin A, Mak P, Dubin G, Rzychon MG, Stec-Niemczyk J, Wladyka B, et al. New generation of peptide antibiotics. Acta Biochimica Polonica 2005; 52: 633. [PubMed] [Google Scholar]
- 2. Mkrtchyan H, Gibbons S, Heidelberger S, Zloh M, Limaki HK. Purification, characterisation and identification of acidocin LCHV, an antimicrobial peptide produced by Lactobacillus acidophilus nv Er 317/402 strain Narine. Int J Antimicrob agents 2010; 35: 255– 260 . [DOI] [PubMed] [Google Scholar]
- 3. Dashper SG, Liu SW, Reynolds EC. Antimicrobial peptides and their potential as oral therapeutic agents. Int J Pept Res Ther 2007; 13: 505– 516 . [Google Scholar]
- 4. Challis GL, Naismith JH. Structural aspects of non-ribosomal peptide biosynthesis. Curr Opin Struct Biol 2004; 14: 748– 756 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reddy KVR, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. Int J Antimicrob agents 2004; 24: 536– 547 . [DOI] [PubMed] [Google Scholar]
- 6. Rajanbabu V, Chen JY. Applications of antimicrobial peptides from fish and perspectives for the future. Peptides 2011; 32: 415– 420 . [DOI] [PubMed] [Google Scholar]
- 7. Olano C, Méndez C, Salas JA. Antitumor compounds from marine actinomycetes. Mar Drugs 2009; 7 (2): 210– 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mann J. Natural products as immunosuppressive agents. Nat Prod Rep 2001; 18: 417– 430 . [DOI] [PubMed] [Google Scholar]
- 9. Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides 2010; 31: 1949– 1956 . [DOI] [PubMed] [Google Scholar]
- 10. Erdmann K, Cheung BW, Schröder H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J Nutr Biochem 2008; 19: 643– 654 . [DOI] [PubMed] [Google Scholar]
- 11. Gauthier SF, Pouliot Y, Saint-Sauveur D. Immunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. Int Dairy J 2006; 16: 1315– 1323 . [Google Scholar]
- 12. St Georgiev V. Immunomodulatory activity of small peptides. Trends Pharmacol Sci 1990; 11: 373– 378 . [DOI] [PubMed] [Google Scholar]
- 13. Jiang Y, Han L, Chen X, Yin M, Zheng D, Wang Y, et al. Diversity and Bioactivity of Cultivable Animal Fecal Actinobacteria. Adv Microbiol 2013; 3: 1. [Google Scholar]
- 14. González I, Ayuso Sacido A, Anderson A, Genilloud O. González. Actinomycetes isolated from lichens: evaluation of their diversity and detection of biosynthetic gene sequences. FEMS microbiol Ecol 2005; 54: 401– 415 . [DOI] [PubMed] [Google Scholar]
- 15. Busti E, Monciardini P, Cavaletti L, Bamonte R, Lazzarini A, Sosio M, et al. Antibiotic-producing ability by representatives of a newly discovered lineage of actinomycetes. Microbiology 2006; 152 (3): 675– 683. [DOI] [PubMed] [Google Scholar]
- 16. Grünewald J, Marahiel MA. Chemoenzymatic and template-directed synthesis of bioactive macrocyclic peptides. Microbiol Mol Biol Rev 2006; 70: 121– 146 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dharmaraj S. Marine Streptomyces as a novel source of bioactive substances. World J Microbiol Biotechnol 2010; 26: 2123–2139. [Google Scholar]
- 18. Bredholdt H, Galatenko OA, Engelhardt K, Fjærvik E, Terekhova LP, Zotchev SB. Rare actinomycete bacteria from the shallow water sediments of the Trondheim fjord, Norway: isolation, diversity and biological activity. Environ Microbiol 2007; 9: 2756–2764. [DOI] [PubMed] [Google Scholar]
- 19. Jork H, Funk W, Fischer W, Wimmer H, Burns DT. (1990). Thin-layer chromatography. Reagents and detection methods. Volume 1a: VCH, Weinheim. [Google Scholar]
- 20. Taddei A, Valderrama M, Giarrizzo J, Rey M, Castelli C. Chemical screening: A simple approach to visualizing Streptomyces diversity for drug discovery and further research. Res Microbiol 2006; 157: 291– 297 . [DOI] [PubMed] [Google Scholar]
- 21. Wilson ID, Plumb R, Granger J, Major H, Williams R, Lenz EM. HPLC-MS-based methods for the study of metabonomics. J Chromatogr A 2005; 817: 67– 76 . [DOI] [PubMed] [Google Scholar]
- 22. Arifuzzaman M, Khatun MR, Rahman H. Isolation and screening of actinomycetes from Sundarbans soil for antibacterial activity. Afr J Biotechnol 2010; 9: 4615– 4619 . [Google Scholar]
- 23. Nikodinovic J, Barrow KD, Chuck JA. High yield preparation of genomic DNA from Streptomyces. Biotechniques 2003; 35: 932– 936 . [DOI] [PubMed] [Google Scholar]
- 24. Ayuso-Sacido A, Genilloud O. New PCR primers for the screening of NRPS and PKS-I systems in actinomycetes: detection and distribution of these biosynthetic gene sequences in major taxonomic groups. Microb Ecol 2005; 49: 10– 24 . [DOI] [PubMed] [Google Scholar]
- 25. Kumar V, Bharti A, Gusain O, Bisht GS. An improved method for isolation of genomic DNA from filamentous actinomycetes. J Sci Engg Tech Mgt 2010; 2: 10– 13 . [Google Scholar]
- 26. Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, et al. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 2007; 57: 2259– 2261 . [DOI] [PubMed] [Google Scholar]
- 27. Kieser T, Bibb MJ, Buttner MJ, Chater KF, Hopwood DA. (2000). Practical Streptomyces genetics. John Innes foundation, UK. [Google Scholar]
- 28. Ding D, Chen G, Wang B, Wang Q, Liu D, Peng M, et al. Culturable actinomycetes from desert ecosystem in northeast of Qinghai-Tibet Plateau. Annals Microbiol 2013; 63: 259– 266 . [Google Scholar]
- 29. Deepika L, Kannabiran K. Antibacterial and antifungal activity of Streptomyces sp. VITDDK3 isolated from Ennore coast, Tamil Nadu, India. Pharma. Res. Health Care 2010; 2: 188– 196 . [Google Scholar]
- 30. Hemashenpagam N. Purification of secondary metabolites from soil actinomycetes. Intl J Microbio Res 2011; 3: 148–156. [Google Scholar]
- 31. Nedialkova D, Naidenova M. Screening the antimicrobial activity of actinomycetes strains isolated from Antarctica. J Culture Collections 2005; 4: 29– 35 . [Google Scholar]
- 32. Anibou M, Chait A, Zyad A, Taourirt M, Ouhdouch Y, Benherref A. Actinomycetes from Moroccan habitats: isolation and screening for cytotoxic activities. World J Microbiol Biotechnol 2008; 24: 2019– 2025 . [Google Scholar]
- 33. Okudoh VI, Wallis FM. Antimicrobial activity of rare actinomycetes isolated from natural habitats in KwaZulu-Natal, South Africa. S Afr J Sci 2007; 103 (5–6): 216– 222. [Google Scholar]
- 34. Chun J, Lee JH, Jung Y, Kim M, Kim S, Kim BK, et al. EzTaxon: a web-based tool for the identification of prokaryotes based on 16S ribosomal RNA gene sequences. Int J Syst Evol Microbiol 2007; 57: 2259– 2261 . [DOI] [PubMed] [Google Scholar]
- 35. Wélé A, Zhang Y, Caux C, Brouard JP, Dubost L, Guette C, et al. Isolation and structure of cyclosenegalins A and B, novel cyclopeptides from the seeds of Annona senegalensis. J. Chem Soc Perkin Trans 1 2002; : 2712–2718. [Google Scholar]
- 36. Agyei D, Danquah MK. Industrial-scale manufacturing of pharmaceutical-grade bioactive peptides. Biotechnol Advances 2011; 29: 272– 277 . [DOI] [PubMed] [Google Scholar]
- 37. Hancock RE, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nature Biotechnol 2006; 24: 1551– 1557 . [DOI] [PubMed] [Google Scholar]
- 38. Konno M. Nosocomial infections caused by methicillin resistant Staphylococcus aureus in Japan. J Infect Chemother 1995; 1: 30–39 [Google Scholar]
- 39. Ahmed Z, Saeed Khan S, Khan M. Invitro trials of some antimicrobial combinations against Staphylococcus aureus and Pseudomonas aeruginosa. Saudi J Biol Sci 2013; 20: 79– 83 . [DOI] [PMC free article] [PubMed] [Google Scholar]