Abstract
Background
Childhood cancer survivors treated with anthracycline chemotherapy are at an increased risk of long-term cardiac toxicity, and guidelines recommend that exposed survivors undergo echocardiography every 1–5 years. However, it is unclear whether survivors should undergo echocardiographic screening indefinitely, or if a period of echocardiographic stability indicates that screening is no longer necessary. The objective of this study was to evaluate the outcomes of echocardiographic screening to aid in the refinement of existing guidelines.
Methods
We retrospectively analyzed the results of echocardiographic screening in a cohort of adult survivors of childhood cancer treated with anthracyclines and/or cardiac radiation therapy. Interval regression analysis was performed to identify predictors of single-episode or sustained abnormal echocardiograms.
Results
The cohort constituted 333 survivors, with median follow-up time of 15.8 years post-treatment (range: 5.0–47.9), and median age at treatment of 8 years (range: 1.5–18). Forty-nine survivors had an abnormal echocardiogram (14.7%), and 29 (8.7%) had reproducible abnormal findings. An ongoing continual increase in the incidence of sustained echocardiographic abnormality was seen among patients treated with >250 mg/m2 doxorubicin at age <5 years, reaching 43% by 20 years of therapy. In contrast, no sustained abnormal echocardiographic findings arose after 10 years of therapy in survivors treated with <250 mg/m2 at age ≥5 years.
Conclusions
Single-episode echocardiographic abnormalities are often not reproduced in subsequent evaluations. The duration of echocardiographic screening for childhood cancer survivors should be reassessed for patients who received lower doses of anthracycline after age 5. Pediatr Blood Cancer © 2015 Wiley Periodicals, Inc.
Keywords: anthracycline, cardiotoxicity, pediatric cancer, survivorship
INTRODUCTION
As survival rates for childhood cancer improve, reducing the long-term toxicities of treatment has emerged as a priority in pediatric oncology. Among the most significant complications of pediatric cancer treatment is the occurrence of cardiac morbidity, occurring in up to 57% of survivors treated with anthracycline chemotherapy.[1] The receipt of radiation therapy (RT) to the heart is also strongly associated with cardiac morbidity in these patients.[2]
Recognizing these risks, expert groups have published consensus-based follow-up guidelines intended to detect early clinical indicators of cardiac dysfunction, with the aim of reducing long-term cardiac morbidity. However, significant limitations in existing echocardiographic screening data have resulted in discordance in surveillance recommendations.[3] The Children's Oncology Group (COG), for example, recommends lifelong echocardiographic screening beginning 2 years post-treatment for survivors treated with anthracyclines and/or mediastinal RT, at a frequency of every 1–5 years, depending on treatment age, anthracycline dose, and RT exposure.[4] In contrast, Scottish Intercollegiate Guidelines recommend screening every 2–3 years for patients at greater risk of anthracycline-induced cardiotoxicity, without recommending a screening duration.[5]
Challenges applying the findings of the existing literature to clinical practice arise for several reasons. Many studies have compared the average ventricular function measures from anthracycline-exposed patients to the average results among non-exposed survivors.[6–10] This approach does not reveal the proportion of anthracycline-exposed survivors who will develop abnormal ventricular function indices over time, for whom screening may be beneficial. Follow-up duration is limited in some studies, so that it is not known whether it is necessary to continue screening indefinitely in all patients, or whether a series of normal echocardiograms indicates that future tests are unlikely to be clinically useful. Consequently, guidelines are inconsistent on the optimal duration of screening. Finally, although high rates of echocardiographic abnormalities have been reported among survivors,[11] many studies report on one-time echocardiographic abnormalities. However, the reproducibility and clinical significance of single-episode abnormalities are uncertain, and the benefit of early detection is inferred largely from elderly patients with complex cardiac pathology.[12–14] Consequently, although routine echocardiographic screening has the appeal of potentially detecting pre-symptomatic heart disease at a time when intervention may prevent further deterioration, it is also possible that for some patients, it is unnecessary and detects abnormalities of no clinical consequence.[15,16]
The purpose of this study was to help establish optimal use of echocardiographic screening by identifying risk factors associated with sustained echocardiographic abnormalities among pediatric cancer survivors, and to describe the incidence of clinically significant late-onset cardiac morbidity among survivors with an extended post-treatment follow-up.
METHODOLOGY
Study Design and Setting
The study cohort comprised adult survivors of pediatric cancer managed in the Aftercare program at the Princess Margaret Cancer Centre in Toronto, Canada. Eligible patients were diagnosed with a malignancy before age 18, had attained age ≥18 years, were treated with anthracyclines and/or cardiac RT, had ≥5 years of follow-up post-treatment, and at least one echocardiogram after 1 year of completing therapy. Date of treatment completion was ascertained through patient chart review (i.e., date of last radiation treatment or final chemotherapy cycle). As the study focus was not acute congestive heart failure, patients with a history of acute ventricular dysfunction arising within 1 year of completing therapy were excluded. The institutional research ethics board of the Princess Margaret Cancer Centre approved the study.
Medical Record Abstraction
Demographic information, including age at diagnosis, sex, age at treatment completion and length of follow-up, was collected, along with patient data on history of hypertension, diabetes mellitus, and smoking status. Anthracyclines included doxorubicin, daunomycin, and epirubicin. Anthracycline doses were converted to doxorubicin-equivalent dose using a conversion factor of 0.83 for daunomycin and 0.67 for epirubicin.[17]
Echocardiography
All echocardiograms were performed at a tertiary cardiac care center according to the Ontario Cardiac Care Network Quality Standards.[18] Echocardiography was ordered using a risk-based frequency defined in the follow-up guidelines of the Children's Oncology Group.[4] Echocardiographic parameters, obtained from original reports, included ejection fraction (EF) and shortening fraction (SF). An echocardiogram was considered abnormal if EF <55% or SF <28%[19] or if valvular abnormalities were described as more than “trivial.” Patients with significant abnormalities were referred to a cardiologist for further management. Survivors were considered to have “any” abnormality when there was at least one abnormal echocardiogram, regardless of subsequent echocardiographic results. A “sustained” abnormality was defined as an abnormal echocardiogram followed by one or more consecutive abnormal echocardiograms.
Statistical Analysis
Chi-square tests were performed to compare categorical variables and two sample t-tests were used to assess continuous variables. Interval regression analyses were performed to identify predictors of time to any abnormal echocardiogram, and to sustained abnormal echocardiograms. This method fits a parametric model to failure time data that can be right, left, or interval-censored, and accounts for cases in which the initial abnormal echocardiogram was found at the first visit, and also for variation in timing and frequency of echocardiography among patients in different risk groups. The following variables were assessed through bivariate analysis: age, anthracycline dose, number and frequency of echocardiograms performed, RT receipt, smoking status and a history of hypertension described in the medical record. Variables ultimately included in multivariate analyses were selected on the basis of statistical significance, clinical importance, and confounding. The incidence of abnormal echocardiography was estimated using interval censoring and plotted based on a nonparametric maximum likelihood estimator using Turnbull's algorithm.[20] All analyses were performed using SAS (version 9.3: SAS Institute, Cary, NC). Plots were produced using R statistical software.[21]
RESULTS
Four hundred and twenty-one patients met inclusion criteria. Seventy-nine were excluded as they had no echocardiographic data available, and nine were excluded due to incomplete and/or missing data on treatment-related variables. Excluded patients were more likely to have leukemia than included patients (44% vs. 30%) and less likely to have lymphoma (36% vs. 47%, P = 0.02 for difference in diagnoses), were more likely to be ≥5 years old at diagnosis (85% vs. 67%, P = 0.001), but did not differ significantly with respect to cumulative anthracycline dose (P = 0.27). The remaining 333 survivors were analyzed.
Patient demographic and clinical characteristics are summarized in Table I. Overall, 224 (67.3%) of those included in the study were ≥5 years of age at the time of initial cancer treatment. The majority of patients (69.1%) were treated with a doxorubicin-equivalent dose <250 mg/m2 and 130 (39%) patients received thoracic RT (median prescribed dose = 15 Gy, range 10–45 Gy). The mean doxorubicin-equivalent dose was significantly higher in patients with sustained abnormal echocardiography at 295 mg/m2 (P < 0.001). The median number of echocardiograms performed was 2 (range: 1–13), with 96 patients having one echocardiogram. The median follow-up time post-treatment was 15.8 years (range: 5.0–47.9 years). The median interval between the end of treatment and the first echocardiogram was 5.7 years (range 0.9–48.0 years) and the median interval between echocardiograms was 2.2 years (range 0.1–19.4 years). Following an abnormal echocardiogram, the median interval to the next echocardiogram was 1.5 years.
TABLE I.
Demographics, Treatment and Follow-Up Characteristics
| % (N) or mean (SD) | |
|---|---|
| Age, mean (SD) | 8.47 (4.92) |
| 1–4 years, % (No.) | 32.73 (109) |
| ≥5 years, % (No.) | 67.27 (224) |
| Male sex, % (No.) | 49.25 (164) |
| Diagnosis, % (No.) | |
| Hodgkin lymphoma | 35.44 (118) |
| ALL | 43.54 (145) |
| Wilm tumor | 10.21 (34) |
| Ewing sarcoma | 3.90 (13) |
| Osteosarcoma | 3.30 (11) |
| Rhabdomyosarcoma | 2.10 (7) |
| Lymphoma | 0.60 (2) |
| Undifferentiated sarcoma | 0.60 (2) |
| Angiosarcoma | 0.30 (1) |
| Doxorubicin dose (mg/m2), mean (SD)a | 212.9 (103.52) |
| Radiotherapy receipt, % (No.)b | 39.04 (130) |
| Age x Anthracycline Dose, % (No.) | 23.12 (77) |
| 1–4 years + 1–250 mg/m2 | 9.61 (32) |
| 1–4 years + ≥250 mg/m2 | 52.55 (175) |
| ≥5 + 1–250 mg/m2 | 14.71 (49) |
| ≥5 + ≥ 250 mg/m2 | 10.51 (35) |
| Any age + no anthracycline | |
| Conventional cardiac risk factors | |
| Smoking (former) | 3.3 (11) |
| Smoking (current at last follow-up) | 10.2 (34) |
| Hypertension (ever) | 3.6 (13) |
| Diabetes (ever) | 2.1 (7) |
| No. echocardiograms, mean (SD)c | 2.86 (2.10) |
Anthracycline drugs included doxorubicin, daunomycin, and epirubicin. Anthracycline doses were converted to doxorubicin equivalent dose by using conversion factor 0.83 for daunomycin and 0.67 for epirubicin.
Radiation received by the heart.
Number of echocardiograms received.
Any Echocardiographic Abnormality
Forty-nine patients (14.7%) had at least one abnormal echocardiogram during the follow-up period. The median time to first abnormal echocardiogram was 11.7 years post-treatment (range: 1.8–42.0 years). The probability of having any echocardiographic abnormality steadily increased and reached 20% by 20 years (Fig. 1). Thirty-nine (79.5%) and 28 patients (57.1%) had an abnormality in EF and SF, respectively. Three patients (6.1%) had a valvular abnormality.
Fig. 1.
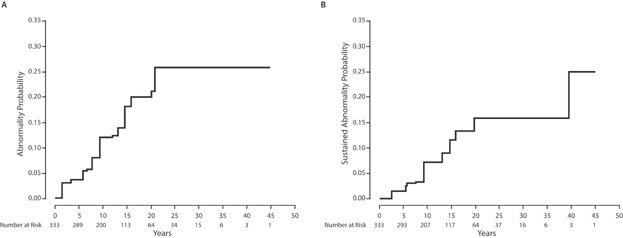
(A) Probability of producing an abnormal echocardiogram. (B) Probability of producing an abnormal sustained echocardiogram.
The 10-year probabilities of having an abnormal echocardiogram among patients, <250 mg/m2 and ≥250 mg/m2 were 8.3% (95% CI = 4.2–12.3%) and 23.3% (95% CI = 12.5–32.7%), respectively. By 20 years after treatment, these rates were 19.5% (95% CI = 8.3–29.2%) and 36.9% (95% CI = 22.8–48.4%), respectively (P < 0.001, Fig. 2). No echocardiographic abnormalities were noted in the first 20 years of follow-up among patients treated with thoracic RT without anthracyclines. The results of univariate interval regression analyses indicated that an anthracycline dose ≥250 mg/m2 was associated with a significantly greater risk of having any abnormal echocardiogram (P < 0.01; Table II). In multivariate analysis, this association remained statistically significant (P < 0.01; Table II). Receipt of mediastinal RT was not significantly associated with abnormal findings.
Fig. 2.
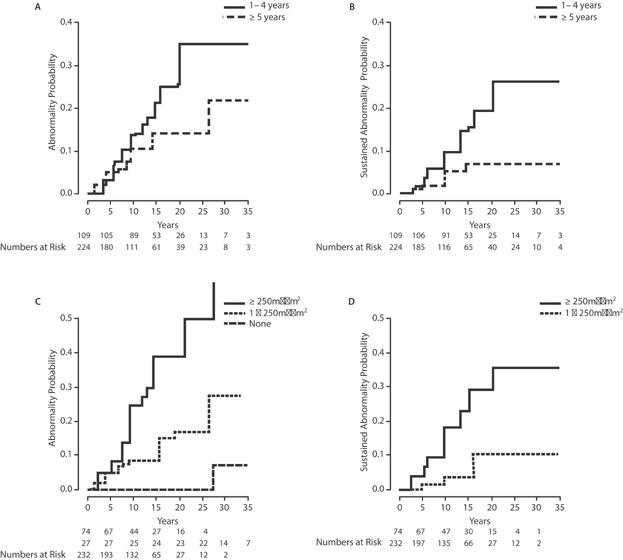
Cumulative incidence of abnormal echocardiography stratified by age at treatment (A,B) and by anthracycline dose (C,D).
TABLE II.
Univariate and Multivariate Interval Regression Analyses for any Abnormal Echocardiogram
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Coefficientb (95% CI) | P-value | Coefficient (95% CI)a | P-value | |
| Age category | ||||
| Age ≥5 years | Reference | — | Reference | — |
| Age <5 years | −0.54 (−1.13, 0.046) | 0.0711 | −0.34 (−0.85, 0.17) | 0.186 |
| Anthracycline dose | ||||
| None | Reference | − | Reference | − |
| 1–250 mg/m2 | −1.10 (−2.32, 0.12) | 0.0783 | 0.97 (−2.23, 0.29) | 0.130 |
| ≥250 mg/m2 | −2.06 (−3.34, −0.77) | <0.01 | −1.95 (−3.26, −0.64) | <0.01 |
| No. of echocardiogramsb | 0.15 (−0.059, 0.35) | 0.163 | — | — |
| Echocardiogram frequency | 0.041 (0.0085, 0.074) | 0.014 | —c | — |
| RT receipt | ||||
| No RT | Reference | — | Reference | — |
| RT received | 0.50 (−0.065, 1.06) | 0.083 | 0.27 (−0.26, 0.81) | 0.312 |
| Smoking status | ||||
| Never smoked | Reference | — | — | — |
| Ever smoked | −0.20 (−0.93, 0.53) | 0.590 | — | — |
| Hypertension | ||||
| Non-hypertensive | Reference | — | — | — |
| Hypertensive | −0.27 (−1.29, 0.75) | 0.6002 | — | — |
| Diabetes | ||||
| Non-diabetic | Reference | — | — | — |
| Diabetic | 0.40 (−1.47, 2.27) | 0.6733 | — | — |
A negative coefficient indicates an increased likelihood of having an abnormal echocardiogram (i.e., reduced time to abnormal echocardiography).
Number of normal echocardiograms prior to first abnormal echocardiogram.
Echocardiogram frequency was not significant after adjusting for age and treatment and was therefore omitted from the final model.
The 10-year probabilities of abnormal echocardiography among patients ages 1–4 years and ≥5 years at the time of treatment were 13.6% (95% CI = 6.6–20.1%) and 10.9% (95% CI = 5.9–15.5%), respectively. By 20 years after treatment, these rates had increased to 26.7% (95% CI = 15.7–36.2%) and 14.5% (95% CI = 7.9–20.7%), respectively (Fig. 2). Although the incidence of abnormal echocardiograms in the younger group increased steadily during this 20-year period, few abnormal echocardiograms were observed beyond 15 years in those receiving chemotherapy at ≥5 years of age. Age <5 years at treatment had a borderline association with developing an abnormal echocardiogram (P = 0.07), but this was not significant in multivariate analysis (Table II). When stratified by age and anthracycline dose, the 20-year rates abnormal echocardiography were 11% (≥5 years + <250 mg/m2), 18% (<5 + <250 mg/m2), 29% (≥5 years + ≥250 mg/m2), and 49% (<5 years + ≥250 mg/m2, P = 0.01).
Sustained Echocardiographic Abnormality
Twenty-nine patients (8.7%) had sustained abnormal echocardiography. For nine patients (2.7%), the results of only one echocardiogram were retrievable, and therefore, we were unable to determine whether abnormal echocardiography was sustained or not. This means there were, at minimum, 11 patients with non-sustained abnormal echocardiography, none of which received any cardiac pharmacologic treatment that could account for subsequent normal echocardiography. The 20-year probability of having a sustained echocardiographic abnormality was 15.7% (95% CI = 11–21%; Fig. 1). Younger treatment age and anthracycline dose ≥250 mg/m2 were significantly associated with the development of sustained echocardiographic abnormalities in both univariate and multivariate analyses (P = 0.032; Table III). The 20-year probabilities of having sustained abnormal echocardiography among patients receiving 0 mg/m2, <250 mg/m2, and ≥250 mg/m2 were 0%, 10.2%, and 35.5%, respectively. Over the entire follow-up duration, there was a consistent increase in the incidence of sustained echocardiographic abnormalities among individuals that received ≥250 mg/m2, whereas the probability of having a sustained abnormal echocardiogram may have plateaued by year 15 in the <250 mg/m2 group (Fig. 2).
TABLE III.
Univariate and Multivariate Interval Regression Analyses for Sustained Abnormal Echocardiograms Only
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Coefficientb (95% CI) | P-value | Coefficient (95% CI)a | p-value | |
| Age category | ||||
| Age ≥5 years | Reference | — | Reference | — |
| Age <5 years | −0.89 (−1.64, 0.15) | 0.019 | −0.72 (−1.37, −0.06) | 0.033 |
| Anthracycline dose | ||||
| None | Reference | — | — | — |
| <250 mg/m2 | −0.99 (−2.47, 0.50) | 0.194 | −0.96 (−2.53, 0.61) | 0.229 |
| ≥250 mg/m2 | −2.10 (−3.65, −0.56) | <0.01 | −2.10 (−3.72, −0.48) | 0.011 |
| No. of echocardiogramsb | −0.042 (−0.22, 0.13) | 0.64 | — | — |
| Echocardiogram frequency | 0.062 (0.019, 0.11) | <0.01 | —c | — |
| RT receipt | ||||
| No RT | Reference | — | Reference | — |
| RT received | 0.19 (−0.41, 0.79) | 0.53 | −0.028 (−0.60, 0.55) | 0.925 |
| Smoking status | ||||
| Never smoked | Reference | — | — | — |
| Ever smoked | −0.59 (−1.32, 0.14) | 0.114 | — | — |
| Hypertension | ||||
| Non-hypertensive | Reference | — | — | — |
| Hypertensive | −0.70 (−1.70, 0.30) | 0.172 | — | — |
| Diabetesd | ||||
| Non-diabetic | Reference | — | — | — |
| Diabetic | — | — | — | — |
A negative coefficient indicates an increased likelihood of having an abnormal echocardiogram or reduced time to abnormal echocardiography.
Number of normal echocardiograms prior to first abnormal echocardiogram.
Echocardiogram frequency was not significant in multivariable analysis with age and treatment exposures, and therefore was excluded from the final model.
A univariate analysis was not performed with the diabetes variable as no sustained echocardiogram abnormalities were present in individuals with a positive diabetes status.
The 20-year probabilities of having sustained abnormal echocardiograms among patients aged 1–4 and patients ≥5 years at treatment were 26.2% and 6.9%, respectively. Few events were observed in the older age group beyond 15 years (Fig. 2).
When stratified by age and dose, there was a significant (P = 0.01) difference in the risk of having sustained abnormal echocardiography, which was greatest in younger patients that received a high anthracycline dose (20-year risk = 54%, 95% CI = 27–70%; Fig. 3). In contrast, few events occurred among the 162 patients treated with <250 mg/m2 at age ≥5, where the probability of having an abnormal echocardiogram may have plateaued at 5% (95% CI = 1–9%) after 10 years, with no additional sustained abnormalities occurring among the 109 echocardiograms done beyond 10 years post-treatment in this group.
Fig. 3.
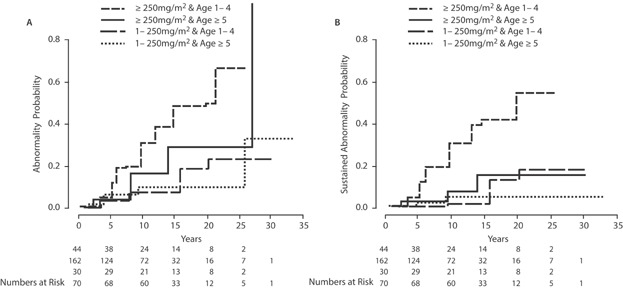
Cumulative incidence of abnormal echocardiography stratified by age at treatment (A) and by anthracycline dose (B).
Additional Investigations and Medical Interventions
Thirty-five patients (10.4% of all patients) with abnormal echocardiography were referred to a cardiologist. Twenty-four patients underwent additional investigations (e.g., stress echocardiography ± Holter monitoring) following abnormal echocardiography. Sixteen patients (32.6% of those with abnormal echocardiography, 4.8% of the entire cohort) received a medical intervention. Specifically, 13 patients were prescribed angiotensin converting enzyme inhibitors, 10 were prescribed beta blockers, and two were given diuretic medications. Calcium-channel blockers, statins, and digoxin were also prescribed. Nine (69.2%) of these patients were <5 years of age at the time of treatment. Two patients were admitted to hospital for cardiac-related complications. The median time to the initiation of any cardiac treatment was 12.5 years (range: 3.5–47.2 years) post-treatment. No patients died during the course of follow-up.
DISCUSSION
Non-invasive and widely available echocardiography remains the recommended screening instrument for cardiac abnormalities in childhood cancer survivors.[4] These recommendations are largely based on studies that report a decrement in the average echocardiographic indices of ventricular function among anthracycline-exposed survivors compared to non-exposed survivors.[6,7,22] Although this method of reporting is useful to elucidate factors associated with overall decline in average cardiac performance, it does not provide insight into the proportion of patients likely to benefit from periodic echocardiographic screening. Moreover, there are no data to establish appropriate cardiac screening duration, and existing guidelines make different recommendations on this issue. Our clinical impression is that for many anthracycline-treated patients, indefinite screening is of limited value, but there have been no clinical studies to elucidate the appropriate time to discontinue screening.
Our finding that young age at exposure and higher anthracycline doses (≥250 mg/m.2) were predictive of the risk of abnormal echocardiography is consistent with prior work,[2,23,24] as well as the risk stratification seen across various guidelines. These findings support the use of continued screening among long-term survivors, particularly those exposed to ≥250 mg/m2 prior to age 5, as the incidence of echocardiographic abnormalities continues to rise for at least 25 years post-treatment, with over half of survivors developing sustained evidence of ventricular dysfunction. In contrast, none of the patients treated with <250 mg/m2 after age 5 developed sustained evidence of ventricular dysfunction beyond 10 years of screening. In our cohort, this subgroup accounted for 51.6% of the study population. If these patients could be discharged from routine echocardiographic screening, it would produce a significant reduction in their burden of follow-up, in addition to saving the costs of unnecessary testing.
The findings of this clinical study are consistent with the results of recent economic simulation studies.[25,26] Wong et al. modeled the cost-effectiveness of different echocardiograph screening schedules for a variety of anthracycline-exposed patient risk-profiles, and estimated that echocardiographic screening at 2-year intervals among patients ≥5 years at diagnosis treated with an anthracycline dose <300 mg/m2, without RT, could be expected to increase per-person life expectancy by less than 3 months, at a cost of $235,000/QALY.[25] Yeh et al. reported similar findings, where the most cost-effective screening strategy in this relatively low-risk group was no assessment.[26] Taken together, it appears that there is an emerging body of evidence that beyond 10 years after exposure, echocardiography is not clinically useful or cost-effective in patients treated with less than 250 mg/m2 after age 5.
Studies in other clinical settings have also shown that as a diagnostic test, echocardiographic results can be user-dependent, and therefore not always reproducible.[27] Our findings further illustrate this limitation of echocardiography as a screening modality, where only 72% of patients with abnormal echocardiography had reproducible results. This illustrates a limitation of some studies that have reported high rates of single-episode echocardiographic abnormalities among survivors,[2,6,27,28] as a significant proportion of exposed patients with an abnormal echocardiogram at a single visit may be misclassified as cases, potentially causing an overestimation of ventricular dysfunction.
The clinical significance of asymptomatic echocardiographic abnormalities detected in survivors is often uncertain. Much of the assumed benefit of early detection and treatment are inferred from studies of elderly patients with complex cardiac pathology and additional comorbidities.[29–31] It seems possible, however, that many of the echocardiographic abnormalities detected in survivors are of no clear short-term clinical consequence, as with a median follow-up of 15.8 years, only 4.8% of the cohort ultimately required a pharmaceutical intervention. Similarly, a comparison of studies that report the rate of asymptomatic echocardiographic abnormalities to others reporting clinically apparent heart disease reveals the latter to be considerably less common.[32–38] This suggests that improving the value of screening will require increasing the positive-predictive value of the tests employed, and not simply identifying increasingly subtle abnormalities. Large collaborative case-control studies will likely be required evaluating alternative diagnostic tools, possibly in conjunction with assessment of cardiac biomarkers to develop screening protocols that are capable of identifying precursors of significant heart disease.[39,40]
This study has several limitations that should be considered. First, although the intent was to evaluate echocardiography based on the COG guidelines, irregular attendance among some patients meant that many underwent echocardiograms at inconsistent time intervals. This likely resulted in some delay in detecting cases that would have been considered “events” earlier had an echocardiogram been performed at a scheduled time. We addressed this issue statistically by performing a series of interval regression analyses to account for inconsistent screening schedules. Secondly, we did not distinguish different types of abnormalities in our analyses separately, and some features of ventricular remodeling (e.g., left ventricular posterior wall dimension) were not always specifically reported. Patients with normal ejection fraction may have other more subtle myocardial changes of uncertain clinical significance, and our study did not routinely capture these.[41] Finally, although patients with persistent or clinically significant echocardiographic abnormalities were referred to tertiary care cardiologists with survivorship experience, there was no formalized protocol for treatment. This reflects the lack of data regarding the optimal management for asymptomatic echocardiographic abnormalities for these survivors. Recognizing these issues, we believe our results reflect the likely outcomes of screening in a specialized follow-up care setting, given the inevitable variation in patient attendance and current uncertainty regarding the best echocardiographic parameter to identify clinically important ventricular dysfunction among survivors. We also did not identify RT as a risk factor for developing echocardiographic abnormalities, likely due to the fact that the median RT dose to our patients was low, and RT-associated valvular abnormalities typically occur >20 years after exposure, which is later than the follow-up of most of the patients on this study. Our results would likely not apply to patients with cardiac doses >15 Gy. Further follow-up among patients with higher RT doses would likely reveal the emergence of valvular abnormalities in some patients.
CONCLUSIONS
Childhood cancer survivors exposed to ≥250 mg/m2 of anthracyclines prior to age 5 had an ongoing risk of developing sustained echocardiographic abnormalities for up to 25 years following treatment. In contrast, patients receiving <250 mg/m2 after age 5 had no new sustained abnormalities detected after 10 years of follow-up, suggesting that prolonged screening for anthracycline-induced cardiomyopathy could be reconsidered in this group. Efforts should be made to improve not only screening sensitivity, but also the specificity of screening to identify clinically important findings.
Acknowledgments
This work was funded by the Canadian Cancer Society through the Pediatric Oncology Group of Ontario. The authors extend their thanks to senior graphic artist, Bruna Ariganello.
REFERENCES
- 1.Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol. 2013;12:697–710. doi: 10.1038/nrclinonc.2013.195. [DOI] [PubMed] [Google Scholar]
- 2.Abosoudah I, Greenberg ML, Ness KK, Benson L, Nathan PC. Echocardiographic surveillance for asymptomatic late-onset anthracycline cardiomyopathy in childhood cancer survivors. Pediatr Blood Cancer. 2011;57:467–472. doi: 10.1002/pbc.22989. [DOI] [PubMed] [Google Scholar]
- 3.Kremer LCM, Mulder RL, Oeffinger KC, Bhatia S, Landier W, Levitt G, Constine LS, Wallace HW, Caron HN, Armenian SH, Skinner R, Hudson MM. A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the international late effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. 2013;60:543–549. doi: 10.1002/pbc.24445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers. Children's Oncology Group; 2013. http://www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf, accessed March 2015.
- 5.Wallace W, Thompson L, Anderson R. Long term follow-up of survivors of childhood cancer: Summary of updated SIGN guidance. BMJ. 2013;346:f1190. doi: 10.1136/bmj.f1190. [DOI] [PubMed] [Google Scholar]
- 6.Hudson MM, Rai SN, Nunez C, Merchant TE, Marina NM, Zalamea N, Cox C, Phipps S, Pompeu R, Rosenthal D. Noninvasive evaluation of late anthracycline cardiac toxicity in childhood cancer survivors. J Clin Oncol. 2007;25:3635–3643. doi: 10.1200/JCO.2006.09.7451. [DOI] [PubMed] [Google Scholar]
- 7.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 8.Sorensen K, Levitt GA, Bull C, Dorup I, Sullivan ID. Late anthracycline cardiotoxicity after childhood cancer. Cancer. 2003;97:1991–1998. doi: 10.1002/cncr.11274. [DOI] [PubMed] [Google Scholar]
- 9.Dietz AC, Sivanandam S, Konety S, Kaufman CL, Gage RM, Kelly AS, Neglia JP, Mulrooney DA. Evaluation of traditional and novel measures of cardiac function to detect anthracycline-induced cardiotoxicity in survivors of childhood cancer. J Cancer Surviv. 2014;8:183–189. doi: 10.1007/s11764-013-0326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karakurt C, Kocak G, Ozgen U. Evaluation of the left ventricular function with tissue tracking and tissue Doppler echocardiography in pediatric malignancy survivors after anthracycline therapy. Echocardiography. 2008;25:880–887. doi: 10.1111/j.1540-8175.2008.00695.x. [DOI] [PubMed] [Google Scholar]
- 11.van der Pal HJ, van Dalen EC, Hauptmann M, Kok WE, Caron HN, van den Bos C, Oldenburger F, Koning CC, van Leeuwen FE, Kremer LC. Cardiac function in 5-year survivors of childhood cancer: A long-term follow-up study. Arch Intern Med. 2010;170:1247–1255. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 12.Dargie HJ. Effect of carvedilol on outcome after myocardial infarction in patients with left-ventricular dysfunction: The CAPRICORN randomised trial. Lancet. 2001;357:1385–1390. doi: 10.1016/s0140-6736(00)04560-8. [DOI] [PubMed] [Google Scholar]
- 13.Kober L, Torp-Pedersen C, Carlsen J, Bagger H, Eliasen P, Lyngborg K, Videbaek J, Cole DS, Auclert L, Pauly NC. For the Trandolapril Cardiac Evaluation (TRACE) Study Group. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 14.Investigators S. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 15.Croswell JM, Ransohoff DF, Kramer BS. Principles of cancer screening: Lessons from history and study design issues. Semin Oncol. 2010;37:202–215. doi: 10.1053/j.seminoncol.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grimes DA, Schulz KF. Uses and abuses of screening tests. Lancet. 2002;359:881–884. doi: 10.1016/S0140-6736(02)07948-5. [DOI] [PubMed] [Google Scholar]
- 17.Shankar SM, Marina N, Hudson MM, Adams JM, Landier W, Bhatia S, Meeske K, Chen MH, Kinahan KE. Monitoring for cardiovascular disease in survivors of childhood cancer: Report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121:e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 18. Standards for provision of echocardiography in Ontario. Toronto, ON, Canada: Cardiac Care Network, 2012.
- 19.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, St John Sutton M, Stewart WJ. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Turnbull BW. Nonparametric estimation of a survivorship function with doubly censored data. J Am Stat Assoc. 1974;69:169–173. [Google Scholar]
- 21. R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2013.
- 22.Rathe M, Carlsen NLT, Oxhøj H. Late cardiac effects of anthracycline containing therapy for childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;48:663–667. doi: 10.1002/pbc.20313. [DOI] [PubMed] [Google Scholar]
- 23.Andolina JR, Dilley K. Anthracycline-induced cardiac toxicity more likely in underweight childhood cancer survivors. J Pediatr Hematol Oncol. 2010;32:411–415. doi: 10.1097/MPH.0b013e3181dccd37. [DOI] [PubMed] [Google Scholar]
- 24.Galper SL, James BY, Mauch PM, Strasser JF, Silver B, LaCasce A, Marcus KJ, Stevenson MA, Chen MH, Ng AK. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117:412–418. doi: 10.1182/blood-2010-06-291328. [DOI] [PubMed] [Google Scholar]
- 25.Wong FL, Bhatia S, Landier W, Francisco L, Leisenring W, Hudson MM, Armstrong GT, Mertens A, Stovall M, Robison LL, Lyman GH, Lipshultz SE, Armenian SH. Cost-effectiveness of the children's oncology group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med. 2014;160:672–683. doi: 10.7326/M13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh JM, Nohria A, Diller L. Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: A model-based estimation of the clinical and economic effects. Ann Intern Med. 2014;160:661–671. doi: 10.7326/M13-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipshultz SE, Landy DC, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, French CA, Rovitelli AM, Proukou C, Adams JM. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012 doi: 10.1200/JCO.2010.33.7907. JCO.2010.2033.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hequet O, Le Q, Moullet I, Pauli E, Salles G, Espinouse D, Dumontet C, Thieblemont C, Arnaud P, Antal D. Subclinical late cardiomyopathy after doxorubicin therapy for lymphoma in adults. J Clin Oncol. 2004;22:1864–1871. doi: 10.1200/JCO.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 29.Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, Seward JB. Prediction of risk for first age-related cardiovascular events in an elderly population: The incremental value of echocardiography. J Am Coll Cardiol. 2003;42:1199–1205. doi: 10.1016/s0735-1097(03)00943-4. [DOI] [PubMed] [Google Scholar]
- 30.Pastore A, Geiger S, Baur D, Hausmann A, Tischer J, Horster S, Stemmler HJ. Cardiotoxicity after anthracycline treatment in survivors of adult cancers: Monitoring by USCOM, echocardiography and Serum biomarkers. World J Oncol. 2013;4:18–25. doi: 10.4021/wjon635w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, Rubino M, Veglia F, Fiorentini C, Cipolla CM. Anthracycline-induced cardiomyopathy clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 32.Vandecruys E, Mondelaers V, De Wolf D, Benoit Y, Suys B. Late cardiotoxicity after low dose of anthracycline therapy for acute lymphoblastic leukemia in childhood. J Cancer Surviv. 2012;6:95–101. doi: 10.1007/s11764-011-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, Leisenring WM. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Termuhlen AM, Tersak JM, Liu Q, Yasui Y, Stovall M, Weathers R, Deutsch M, Sklar CA, Oeffinger KC, Armstrong G, Robison LL, Green DM. Twenty-five year follow-up of childhood Wilms tumor: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57:1210–1216. doi: 10.1002/pbc.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kremer LC, van Dalen EC, Offringa M, Ottenkamp J, Voute PA. Anthracycline-induced clinical heart failure in a cohort of 607 children: Long-term follow-up study. J Clin Oncol. 2001;19:191–196. doi: 10.1200/JCO.2001.19.1.191. [DOI] [PubMed] [Google Scholar]
- 36.van Dalen EC, van der Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: A long-term follow-up study. Eur J Cancer. 2006;42:3191–3198. doi: 10.1016/j.ejca.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Green DM, Grigoriev YA, Nan B, Takashima JR, Norkool PA, D'Angio GJ, Breslow NE. Congestive heart failure after treatment for Wilms' tumor: A report from the National Wilms' Tumor Study group. J Clin Oncol. 2001;19:1926–1934. doi: 10.1200/JCO.2001.19.7.1926. [DOI] [PubMed] [Google Scholar]
- 38.Creutzig U, Diekamp S, Zimmermann M, Reinhardt D. Longitudinal evaluation of early and late anthracycline cardiotoxicity in children with AML. Pediatr Blood Cancer. 2007;48:651–662. doi: 10.1002/pbc.21105. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, Donovan FD, Metzger ML, Arevalo A, Durand JB. Screening adult survivors of childhood cancer for cardiomyopathy: Comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monsuez JJ. Detection and prevention of cardiac complications of cancer chemotherapy. Arch Cardiovasc Dis. 2012;105:593–604. doi: 10.1016/j.acvd.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Armenian SH, Gehlehter SK, Vase T, Venkatramani R, Landier W, Wilson KD, Herrera C, Reichman L, Menteer JD, Mascarenhas L, Freyer DR, Venkataraman K, Bhatia S. Screening for cardiac dysfunction in anthracycline-exposed childhood cancer survivors. Clin Cancer Res. 2014;20:6314–6323. doi: 10.1158/1078-0432.CCR-13-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]


