Abstract
Background
Therapies that maintain remission for patients with Crohn's disease are essential. Stable remission rates have been demonstrated for up to 2 years in adalimumab-treated patients with moderately to severely active Crohn's disease enrolled in the CHARM and ADHERE clinical trials.
Aim
To present the long-term efficacy and safety of adalimumab therapy through 4 years of treatment.
Methods
Remission (CDAI <150), response (CR-100) and corticosteroid-free remission over 4 years, and maintenance of these endpoints beyond 1 year were assessed in CHARM early responders randomised to adalimumab. Corticosteroid-free remission was also assessed in all adalimumab-randomised patients using corticosteroids at baseline. Fistula healing was assessed in adalimumab-randomised patients with fistula at baseline. As observed, last observation carried forward and a hybrid nonresponder imputation analysis for year 4 (hNRI) were used to report efficacy. Adverse events were reported for any patient receiving at least one dose of adalimumab.
Results
Of 329 early responders randomised to adalimumab induction therapy, at least 30% achieved remission (99/329) or CR-100 (116/329) at year 4 of treatment (hNRI). The majority of patients (54%) with remission at year 1 maintained this endpoint at year 4 (hNRI). At year 4, 16% of patients taking corticosteroids at baseline were in corticosteroid-free remission and 24% of patients with fistulae at baseline had healed fistulae. The incidence rates of adverse events remained stable over time.
Conclusions
Prolonged adalimumab therapy maintained clinical remission and response in patients with moderately to severely active Crohn's disease for up to 4 years. No increased risk of adverse events or new safety signals were identified with long-term maintenance therapy. (http://clinicaltrials.gov number: NCT00077779).
Introduction
Crohn's disease (CD) is a relapsing and remitting intestinal inflammatory disorder. The pathogenesis of CD is not well characterised, although environmental, genetic and microbial factors leading to a dysregulated immune response are all thought to play key roles. Elevated levels of tumour necrosis factor alpha (TNFα) may be observed both at disease onset and during times of flare.1–3 Evolving treatment goals for CD include inducing and maintaining clinical and endoscopic remission (also termed ‘deep remission’),4 avoiding prolonged exposure to corticosteroids, improving patient quality of life, and reducing hospitalisations and surgeries.
The use of agents directed against TNFα has made a significant impact on the management of patients with CD. However, most efficacy data with these agents are limited to that collected during relatively short-term (up to 1 year) clinical trials. Due to the chronic relapsing and progressive nature of CD, clinical trial data over longer durations of treatment (i.e. several years) are desirable to demonstrate both long-term efficacy and safety of these agents.
Adalimumab, a fully human monoclonal antibody against TNFα, has been shown in randomised, placebo-controlled clinical trials to be effective for the induction and maintenance of remission and to achieve mucosal healing in patients with moderately to severely active CD.5–9 The Phase III adalimumab maintenance trial CHARM (Crohn's Trial of the Fully Human Antibody Adalimumab for Remission Maintenance) was followed by a long-term, open-label extension, ADHERE (Additional Long-Term Dosing With HUMIRA to Evaluate Sustained Remission and Efficacy in CD), which collected efficacy and safety data for up to 4 years of treatment with adalimumab. Previous reports from ADHERE demonstrated that 2 years of treatment with adalimumab was associated with maintenance of stable rates of clinical remission, fistula healing, decreased hospitalisations and improved patient quality of life.10,11 A later analysis of patients receiving corticosteroids at the start of adalimumab therapy in CHARM demonstrated stable rates of corticosteroid-free remission for up to 3 years.12 In this current report, we present the extended long-term efficacy (including maintenance of clinical response and remission, fistula healing and corticosteroid-free remission) and safety of adalimumab through 4 years of therapy.
Methods
CHARM and ADHERE trials
Detailed methods and patient demographics of the CHARM and ADHERE trials have been published previously.5,10 Briefly, CHARM was a 56-week, multicentre, phase III, double-blind, randomised, placebo-controlled trial to assess the efficacy and safety of adalimumab for the treatment of patients with moderately to severely active CD. Adult patients with a diagnosis of CD for at least 4 months and a Crohn's Disease Activity Index (CDAI) between 220 and 450 received open-label induction with adalimumab (80 mg at week 0, 40 mg at week 2). Patients enrolled in CHARM could have received previous treatment with another anti-TNF antagonist. CD-related medications were to remain stable during CHARM, with the exception of corticosteroids, which could be tapered at the investigator's discretion beginning at week 8. Patients who experienced a flare after they tapered their dosages of steroids could have their corticosteroid dosages increased to the dosage prior to the start of the taper. Adjustments (increases or decreases) in other CD-related concomitant treatments, including initiation of a treatment a patient was not taking previously, were allowed in ADHERE following at least 3 months of open-label adalimumab exposure. At week 4, patients were randomised to receive placebo, either 40 mg adalimumab weekly or 40 mg every other week. After week 12, patients who experienced a disease flare or nonresponse could move to open-label treatment with 40 mg every other week, and then to 40 mg weekly for continued flare or nonresponse. At the end of CHARM, patients could enter the open-label extension ADHERE trial. Enrolment in ADHERE was conducted on a rolling basis as subjects completed CHARM, so the duration of participation in ADHERE varied. Patients still on blinded therapy at the end of CHARM received adalimumab 40 mg every other week upon entry to ADHERE. Patients on open-label adalimumab every other week or weekly continued on the same regimen. During ADHERE, patients on every other week adalimumab could move to weekly dosing for disease flare or for nonresponse. Termination of the ADHERE study occurred at each study site upon country and local (if applicable) regulatory and reimbursement approval of adalimumab for CD, and opening of the adalimumab long-term registry (PYRAMID) for enrolment. All sites were terminated in December 2008.
Data analysis
The long-term effect of adalimumab treatment (CDAI over time, clinical remission and clinical response through week 212 of adalimumab treatment) was assessed in the all-adalimumab mITT population from CHARM, which consisted of patients who achieved at least a 70 point reduction in CDAI from baseline with open-label induction therapy and were randomised to any adalimumab every other week or weekly (randomised responders; n = 329). Patients who responded to open-label induction therapy who were randomised to 40 mg every other week (n = 172) were also analysed separately. Efficacy endpoints evaluated in CHARM and ADHERE (which initiated study enrolment in 2003) were primarily based on CDAI (i.e. clinical remission or clinical response). Reflective of the standard of care for CD at the time, mucosal healing was not assessed in either study. Clinical remission was defined as CDAI <150, whereas clinical response was defined as a decrease in CDAI of ≥100 points from baseline (CR-100). Maintenance of clinical remission and clinical response beyond 1 year was assessed in patients who enrolled in ADHERE and who were in clinical remission (n = 145) or CR-100 (n = 172) at week 56 of CHARM. Fistula healing (defined as complete healing of draining fistulae, assessed using gentle compression during physical examination) through 4 years was assessed for all adalimumab-randomised patients (regardless of response to induction therapy) with fistula(e) at the baseline of CHARM (n = 70). Corticosteroid-free remission (CDAI <150 without concomitant corticosteroids) was assessed in randomised responders regardless of baseline corticosteroid use (n = 329) and also for randomised patients taking corticosteroids at the baseline of CHARM, regardless of response to induction therapy (n = 206). Maintenance of corticosteroid-free remission beyond 1 year was determined for two groups of patients who were in corticosteroid-free remission at week 56 of CHARM: randomised responders (n = 147) and all randomised patients who used corticosteroids at CHARM baseline (n = 53). Initiation of corticosteroids or immunosuppressants (defined as azathioprine, 6-mercatopurine or methotrexate) in the randomised responder population (n = 329) was also analysed throughout the study period.
Clinical assessment
CDAI was collected at baseline and at weeks 2, 4, 6, 8, followed by every 4 weeks through week 56 of CHARM5 and at 12-week intervals during ADHERE.10 Adverse events were recorded for any patient receiving at least one dose of adalimumab (n = 854) (including open-label induction therapy in CHARM) through 4 years of treatment (or up to 70 days after the last dose of adalimumab, if treatment was prematurely discontinued) to assess long-term safety.
Statistical methods
The CDAI over time was analysed using a nonlinear model using four-parameter log-logistic function. Categorical endpoints were analysed using three methods, as-observed, last observation carried forward (LOCF) and a hybrid nonresponder imputation (hNRI). The time patients spent in ADHERE varied because of the rolling enrolment from CHARM, different timing of study site closure and movement of patients to commercial drug. As not all patients who completed CHARM participated in the study for the full 4 years, NRI was used to analyse results for the all-adalimumab mITT population through year 3, whereby patients with missing data (for any reason, including study termination) were assumed not to have achieved the endpoint. After year 3, missing data for patients who were documented to have moved to commercial adalimumab were analysed using the LOCF method, and patients with data missing for any other reason were assumed to not have efficacy. An additional analysis of clinical remission was conducted on the patients randomised to adalimumab every other week using three imputation methods: one as described above; one using the same imputation rules, except that patients who received dose escalation were also assumed to not have achieved remission from that point forward; and an as-observed analysis for patients who remained on every other week dosing throughout the study. NRI was used to assess corticosteroid-free remission through year 4 and maintenance of corticosteroid-free remission from week 56 of CHARM. LOCF was not used to analyse corticosteroid-free remission because it was possible for subjects to initiate corticosteroids between study visits when remission was assessed.
Results
Patient disposition
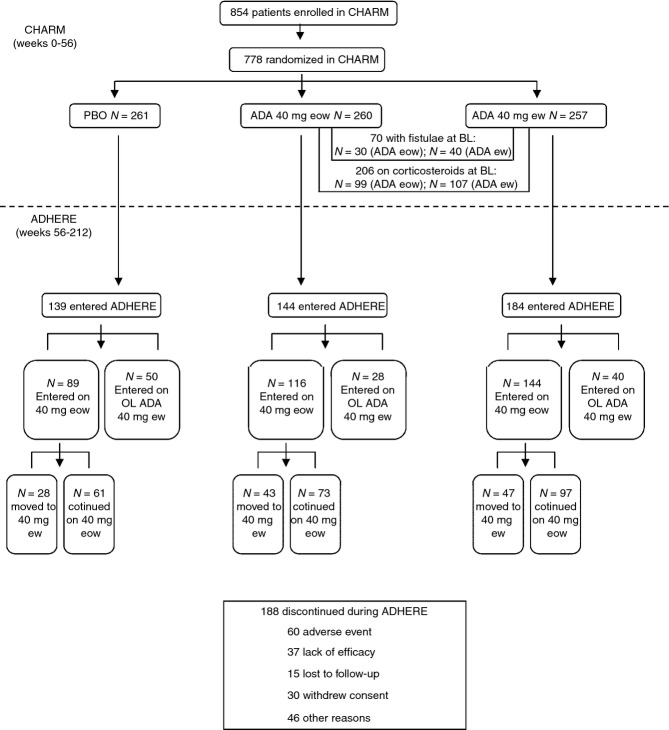
Detailed patient demographics from CHARM and disposition data from CHARM and ADHERE have been reported previously5,10 Briefly, patients were representative of patients with long-standing moderately to severely active CD, with a mean baseline CDAI of 313. Nearly 50% of enrolled patients had previously been treated with another anti-TNF antagonist. As of the December 2008 termination date, at least 50% of subjects who enrolled from CHARM remained in ADHERE through Week 156 (212 weeks from CHARM baseline). A diagram of the patient flow during CHARM and ADHERE is shown in Figure1. Of the patients randomised to adalimumab every other week during CHARM, 16.5% moved to weekly dosing in years 2 to 4 of treatment (Figure1).
Figure 1.
Disposition of CHARM patients entering ADHERE. The primary reason for discontinuation during ADHERE is also shown for all patients. ADA, adalimumab; PBO, placebo; eow, every other week; ew, weekly; OL, open-label.
Maintenance of clinical remission and response
CDAI over time from baseline to week 212 for the CHARM mITT population is shown in Figure2a. The CDAI was at the threshold for clinical remission (150) by week 8, and decreased steadily through 4 years from CHARM baseline. The proportions of the all-adalimumab mITT population achieving clinical remission during CHARM and ADHERE are shown in Figure2b. Ongoing adalimumab treatment was associated with maintained efficacy through 4 years, using as-observed, LOCF or the more conservative hNRI imputation method. Similar results were observed for patients achieving clinical response (CR-100) (Figure2c). Remission rates were also stable over 4 years in the subset of the mITT population randomised to adalimumab every other week (Figure3).
Figure 2.
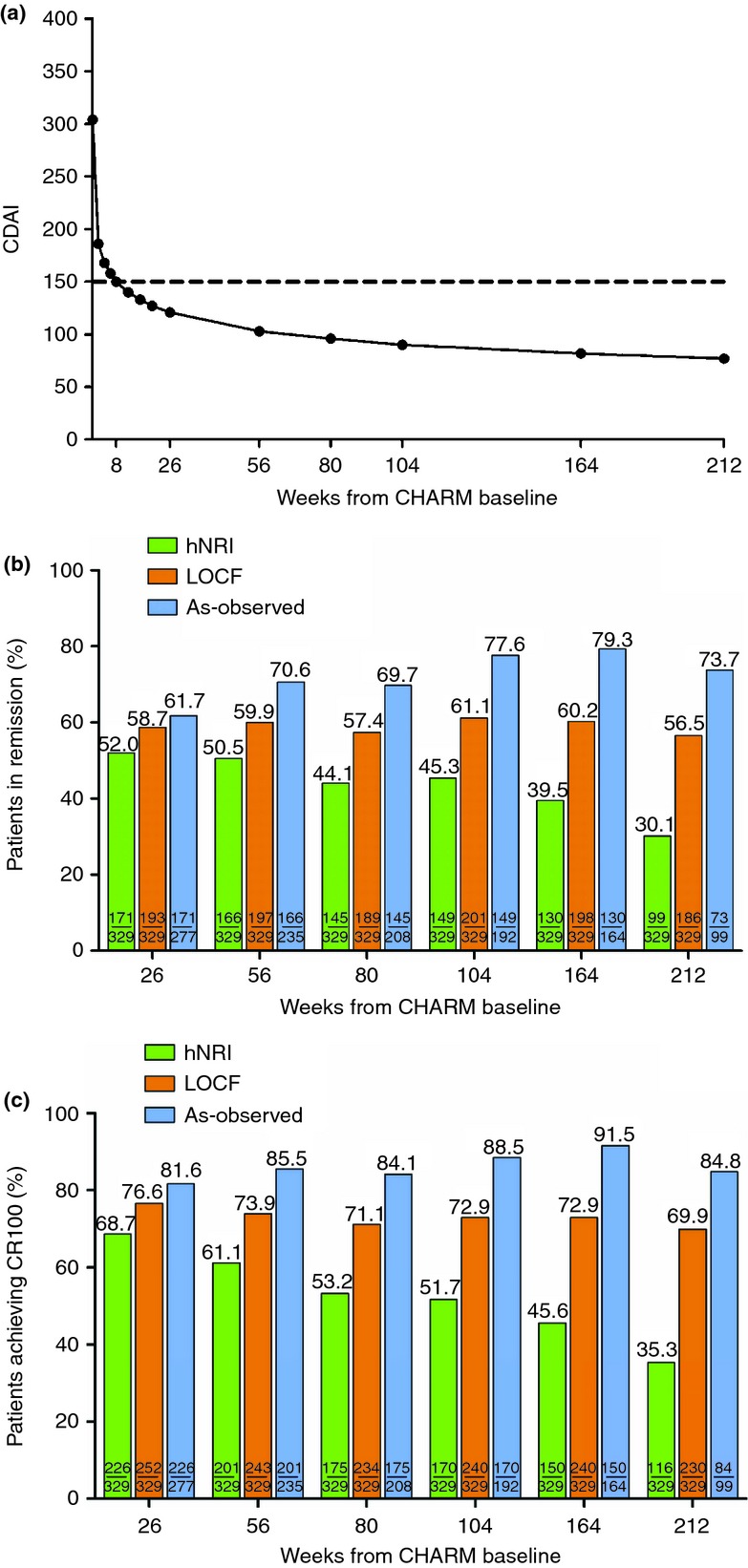
Maintenance of clinical remission and response in patients responding to adalimumab induction therapy and receiving any dose of ADA: mITT (n = 329). (a) Crohn's disease activity index (CDAI) over time: Nonlinear model using four-parameter log-logistic function. Dotted line represents the threshold for clinical remission (CDAI <150). (b) Percentage of patients in remission (CDAI <150) over time. (c) Percentage of patients achieving clinical response (CR-100) over time. Green bars, hNRI analysis; orange bars, LOCF analysis; blue bars, as-observed.
Figure 3.
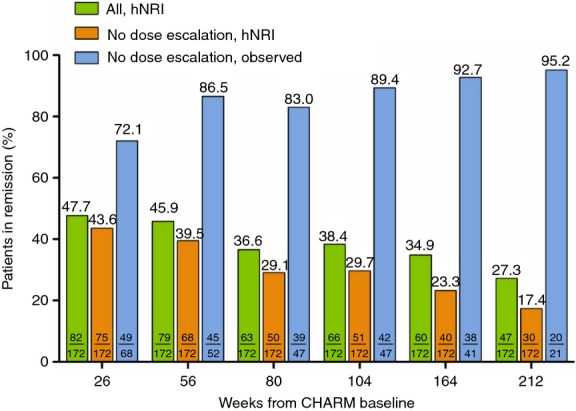
Long-term maintenance of remission in patients randomised to adalimumab 40 mg eow: mITT (n = 172). Percentage of patients in remission (CDAI <150) over time. Green bars, all patients randomised to adalimumab every other week (all, hNRI analysis); orange bars, patients randomised to adalimumab every other week excluding patients that escalated to weekly dosing (no dose escalation, hNRI analysis); blue bars, patients randomised to adalimumab every other week excluding patients that escalated to weekly dosing (no dose escalation, as-observed analysis).
The proportions of patients maintaining clinical remission and response to adalimumab therapy beyond week 56 of CHARM are shown in Figures4a,b respectively. Ongoing adalimumab therapy maintained response and remission in greater than half of patients at year 4, even with the conservative hNRI imputation method.
Figure 4.
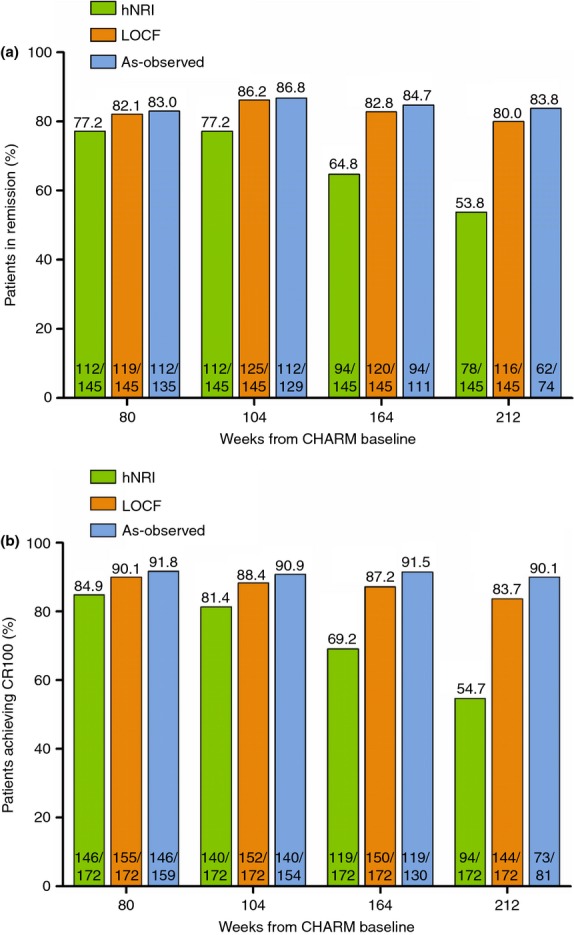
Long-term maintenance of clinical remission and response for patients in remission or response at week 56 of CHARM. (a) Percentage of patients who were in remission at the end of CHARM (CDAI <150) (n = 145) that maintained remission over time. (b) Percentage of patients who had a clinical response at the end of CHARM (CR-100) (n = 172) that maintained a response over time. Green bars, hNRI analysis; orange bars, LOCF analysis; blue bars, as-observed.
Maintenance of fistula healing
The data for fistula healing in patients randomised to adalimumab who had draining fistulas at baseline are shown in Figure5. Rates of fistula healing were maintained throughout the 4-year period of follow-up.
Figure 5.
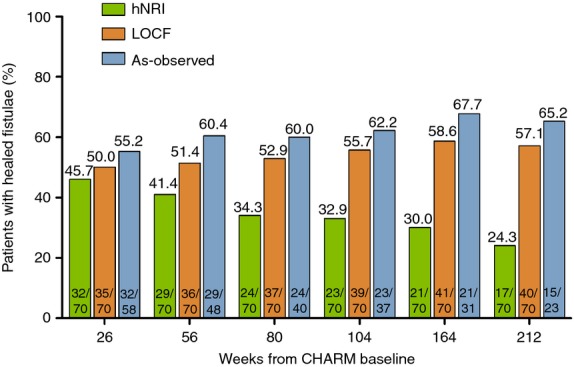
Long-term efficacy of fistula healing in patients randomised to adalimumab with fistulas at baseline (ITT, n = 70). Green bars, hNRI analysis; orange bars, LOCF analysis; blue bars, as-observed.
Initiation of concomitant medications
During CHARM and ADHERE, corticosteroid and immunomodulator use was initiated by 17.8% (35/197) and 2.3% (4/173), respectively, of randomised responders who were naïve to these therapies at the baseline of CHARM.
Maintenance of corticosteroid-free remission
The rates of corticosteroid-free remission for randomised responders (regardless of baseline corticosteroid use) and in patients randomised to adalimumab receiving corticosteroids at CHARM baseline are shown in Figures6A,B. Corticosteroid-free clinical remission rates remained stable through the 4-year treatment period, and greater than half of patients using corticosteroids at baseline and in corticosteroid-free remission at the end of CHARM maintained corticosteroid-free clinical remission throughout ADHERE.
Figure 6.
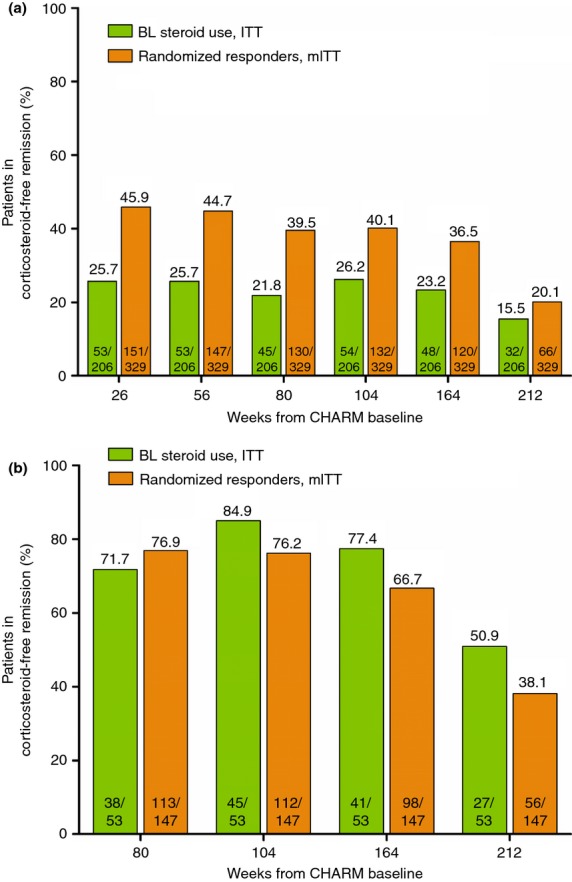
Maintenance of corticosteroid-free clinical remission in patients who were taking corticosteroids at CHARM baseline (ITT, n = 206) and in randomised responders, regardless of baseline corticosteroid use (mITT, n = 329). (a) Percentage of patients who discontinued corticosteroid use and achieved clinical remission (CDAI <150) over time (NRI analysis). (b) Percentage of patients who achieved corticosteroid-free clinical remission at the end of CHARM (n = 53 for the ITT population taking corticosteroids at baseline of CHARM and n = 147 for the randomised responder population) and maintained corticosteroid-free clinical remission over time (NRI analysis). Green bars, patients taking corticosteroids at baseline; orange bars, all randomised responders, regardless of baseline corticosteroid use.
Safety
An overview of rates of treatment-emergent adverse events of interest during adalimumab exposure, up to 212 weeks, in the safety population, which includes any patient exposed to adalimumab including the patients who did not respond to induction therapy, is outlined in Table 1. The safety database of 854 patients comprised 1669.7 patient-years of exposure to adalimumab. The exposure-adjusted rates of all categories of adverse events were consistent with the 2-year exposure report.10 The most common category of adverse events was infection. Abdominal and anal abscess were the most common serious infections reported, with incidence rates of 0.7 and 1.0 events/100PY respectively. Almost all opportunistic infections were candidiasis, with oral candidiasis occurring most frequently (0.9 events/100PY), and no new cases of tuberculosis were observed since the 2-year report. After 4 years of adalimumab treatment, the rate of demyelinating disease was 0.2 events/100PY, with no new events reported since the 2-year exposure report. No events of heart failure were observed. There were 53 liver-related AEs reported, the majority (n = 48) of which were abnormalities or increases in liver function tests. No cases of hepatic failure were observed. The overall incidence rate through year 4 of any malignancy was low and remained stable since the 2-year exposure report (1.6 events/100PY). The most commonly reported malignancy was basal cell carcinoma (0.4 events/100PYs). Additional malignancies observed since the 2-year report included one case each of colon cancer, hepatocellular carcinoma, lung adenocarcinoma, renal cell carcinoma and vulval cancer. Two treatment-emergent deaths occurred during the entire follow-up period, one during CHARM and one during ADHERE; the details of these have been previously reported.5,10 No cases of hepatosplenic T-cell lymphoma were observed.
Table 1.
Overview of treatment-emergent adverse events of interest: all adalimumab patients from first dose through year 4
| Any adalimumab n = 854, PY = 1669.7 Events (E/100PY) | |
|---|---|
| Any adverse event (AE) | 9736 (583.1) |
| Any AE at least possible drug related | 1933 (115.8) |
| Severe AE | 730 (43.7) |
| Serious AE | 517 (31.0) |
| Any AE leading to discontinuation of study drug | 252 (15.1) |
| Any infection | 1966 (117.7) |
| Serious infection* | 102 (6.1) |
| Tuberculosis† | 3 (0.2) |
| Malignancy‡ | 26 (1.6) |
| Injection site pain | 125 (7.5) |
| Opportunistic infection§ (excluding TB) | 22 (1.3) |
| Congestive heart failure | 0 |
| Demyelinating disorder | 3 (0.2) |
| Liver event¶ | 53 (3.2) |
| Deaths | 2 (0.1) |
AE, adverse event; PY, patient-years.
Serious infections since week 60 of ADHERE included abdominal abscess (two events), anal abscess (nine events), limb abscess (one event), gastroenteritis (three events), pyelonephritis (three events), sepsis (one event), abscess intestinal (one event), bronchitis (one event), lobar pneumonia (one event), lower respiratory tract infection (one event), sinusitis (one event), urinary tract infection (one event), cellulitis (four events), cervicitis (one event), infectious peritonitis (one event), muscle abscess (one event), perineal abscess (one event).
No new cases of tuberculosis were reported since week 60 of ADHERE.
Malignancies since week 60 of ADHERE included basal cell carcinoma (four events), skin cancer (one event), breast cancer (one event), squamous cell carcinoma (one event), colon cancer (one event), renal cell carcinoma (one event), hepatocellular carcinoma (three events), lung adenocarcinoma (one event), vulval cancer (one event).
Opportunistic infections included mostly candidiasis (21 events). One event of cytomegalovirus infection was reported.
Liver events included abnormal or increased liver function tests (48 events), hepatic steatosis (three events), hepatitis (one event) and hepatic neoplasm (one event).
Discussion
Data regarding the long-term efficacy and safety of anti-TNF therapy are largely limited to observational or retrospective reports of patient cohorts from single centres.13 This report extends the safety and efficacy data of adalimumab for the treatment of CD for up to 4 years of therapy, from the previously reported results in CHARM and ADHERE,10–12 and provides data from the longest duration of follow-up in a clinical trial for CD of any anti-TNF agent. Long-term maintenance of clinical remission (including corticosteroid-free remission) and response was achieved in clinically meaningful proportions of adalimumab-treated patients with CD. In addition, for patients presenting with draining fistulas or those using corticosteroids at baseline of the CHARM trial, long-term fistula healing and corticosteroid-free remission were maintained through 4 years of treatment. Taken together, these results underscore the long-term efficacy of adalimumab maintenance therapy.
One important observation is that three different analysis methods, as-observed, LOCF and the more stringent hybrid NRI, were used to measure various categorical endpoints, with each analysis method producing distinct results. Larger differences between LOCF and hNRI were observed at later time points. The lower percentages observed with hNRI analysis at later time points can be explained by classifying those patients who left the study for any reason (except those who were documented to move from study drug to commercially available adalimumab after week 156 of follow-up) as nonresponders. However, as noted in Figure1, it is important to mention that not all of these patients left the trial due to loss of response to adalimumab. The true estimate of potential efficacy at each time point is likely to lie somewhere between the values determined by the least conservative (as-observed) and the most conservative (hNRI) analyses.
Maintenance of remission was also evident in the subset of patients randomised to the approved 40 mg every other week maintenance dose of adalimumab, with slightly higher rates of remission observed when patients who escalated to weekly dosing were included in the analysis. This finding is consistent with a previously published report from CHARM that showed that patients who dose escalated were able to regain efficacy.14 A recently published analysis from CHARM suggested that weekly dosing may be associated with greater efficacy than every other week dosing in patients with elevated CRP who have lost response to treatment with a prior anti-TNF agent.15
In addition to efficacy, it is important to examine safety of prolonged anti-TNF therapy to provide clinicians with information to aid determination of the benefits and risks of this strategy. Adverse events of special interest with TNF antagonist therapy include serious infections and malignancies.16 The most commonly reported adverse event category over 4 years of adalimumab exposure was infections. The incidence rates of any infections, opportunistic infections and serious infections were stable over time. Similarly, the incidence rate of any malignancies remained low and was stable over time, suggesting no cumulative risk associated with ongoing adalimumab exposure. Furthermore, prolonged treatment with adalimumab for CD did not reveal any new safety signals. The overall safety data for 4 years of adalimumab therapy for CD were consistent with long-term safety data representing nearly 12 years of adalimumab exposure across multiple indications.16
Patient selection for biological therapy is an important consideration to gain the greatest benefit of therapy over a prolonged duration of time. The LOCF analysis found that 80% of patients who achieved remission with adalimumab therapy at 1 year continued to be in remission over the next 3 years. A previous analysis found that patients with disease duration of less than 2 years had the greatest likelihood of achieving remission at 1 year in CHARM and the higher rate of remission persisted through the 3 years of data evaluated.17 These patients also had a trend towards fewer adverse events. Additional factors associated with a greater likelihood of remission included elevated CRP at baseline and no prior treatment with an anti-TNF agent. Selection of patients with short disease duration and evidence of active inflammation for adalimumab treatment would likely lead to greater rates of long-term efficacy than those reported here.
The length of time that a clinician should continue maintenance therapy with TNF antagonists for patients with CD remains a key point of discussion. Although data from the STORI trial18 suggested that infliximab withdrawal was successful in approximately half of patients with CD in stable remission, given the progressive and lifelong nature of CD, physicians should carefully consider the risks and benefits of different approaches in the long-term use of biologics. It is possible that patients in deep remission are at lower risk of future relapse.4 Long-term treatment strategies, including withdrawal of either an anti-TNF agent or concomitant immunomodulator therapy, need further evaluation.
The long-term follow-up of this study provides beneficial information about remission and response rates over time. However, a few limitations of the study exist. First, the analysis of the long-term efficacy of adalimumab was complicated by the varying duration of follow-up, with not all patients reaching the week 212 visit due to study termination. We attempted to overcome this by analysing the data using different imputation methods for missing data. A second limitation of this long-term efficacy study is that assessment of mucosal appearance was not collected in CHARM and ADHERE. With the increasing interest in the concept of mucosal healing, data regarding the long-term efficacy of adalimumab on mucosal healing or disease progression using a digestive damage score (such as the recently developed Lemann score)19 are of interest, but could not be analysed using this data set as endoscopic or radiographic imaging data were not collected in CHARM and ADHERE. Finally, there is increasing interest in the correlation of serum drug levels of anti-TNF agents and antibodies against these agents and efficacy, but samples for assessment of pharmacokinetic parameters were not collected during CHARM or ADHERE.
In summary, these data suggest a substantial clinical benefit with long-term adalimumab therapy for patients with moderately to severely active CD. The rates of adverse events were stable with long-term treatment up to 4 years.
Acknowledgments
Declaration of personal interests: R Panaccione has received consultant and/or lecture fees from AbbVie, Amgen, AstraZeneca, Axcan Pharma (now Aptalis), Biogen Idec, Bristol-Myers Squibb, Centocor, ChemoCentryx, Eisai Medical Research Inc, Elan Pharmaceuticals, Ferring, Genetech, GlaxoSmithKline, Janssen, Merck Sharp and Dohme Corp, Millennium Pharmaceuticals Inc. (now Takeda), Ocera Therapeutics Inc., Otsuka America Pharmaceutical, Pfizer, Shire Pharmaceuticals, Prometheus Laboratories, Schering-Plough, Synta Pharmaceuticals Corp, Teva, UCB Pharma, and Warner Chilcott. J-F Colombel has served as consultant, advisory board member or speaker for AbbVie, Bristol Meyers Squibb, Ferring, Genentech, Giuliani SPA, Given Imaging, Merck & Co., Millenium Pharmaceuticals Inc., Pfizer Inc. Prometheus Laboatories, Sanofi, Schering Plough Corporation, Takeda, Teva Pharmaceuticals, UCB Pharma (previously named Celltech Therapeutics, Ltd). W Sandborn has served as a consultant for AbbVie, ActoGeniX NV, AGI Therapeutics, Inc., Alba Therapeutics Corporation, Albireo, Alfa Wasserman, Amgen, AM-Pharma BV, Anaphore, Astellas, Athersys, Inc., Atlantic Healthcare Limited, Aptalis, BioBalance Corporation, Boehringer-Ingelheim Inc, Bristol-Myers Squibb, Celgene, Celek Pharmaceuticals, Cellerix SL, Cerimon Pharmaceuticals, ChemoCentryx, CoMentis, Cosmo Technologies, Coronado Biosciences, Cytokine Pharmasciences, Eagle Pharmaceuticals, Eisai Medical Research Inc, Elan Pharmaceuticals, EnGene, Inc., Eli Lilly, Enteromedics, Exagen Diagnostics, Inc., Ferring Pharmaceuticals, Flexion Therapeutics, Inc., Funxional Therapeutics Limited, Genzyme Corporation, Genentech, Gilead Sciences, Given Imaging, GlaxoSmithKline, Human Genome Sciences, Ironwood Pharmaceuticals, Janssen, KaloBios Pharmaceuticals, Inc., Lexicon Pharmaceuticals, Lycera Corporation, Meda Pharmaceuticals, Merck Research Laboratories, MerckSerono, Merck & Co., Millennium, Nisshin Kyorin Pharmaceuticals Co., Ltd., Novo Nordisk A/S, NPS Pharmaceuticals, Optimer Pharmaceuticals, Orexigen Therapeutics, Inc., PDL Biopharma, Pfizer, Procter and Gamble, Prometheus Laboratories, ProtAb Limited, Purgenesis Technologies, Inc., Receptos, Relypsa, Inc., Salient Pharmaceuticals, Salix Pharmaceuticals, Inc., Santarus, Shire Pharmaceuticals, Sigmoid Pharma Limited, Sirtris Pharmaceuticals, Inc. (a GSK company), S.L.A. Pharma (UK) Limited, Targacept, Teva Pharmaceuticals, Therakos, Tillotts Pharma AG, TxCell SA, UCB Pharma, Viamet Pharmaceuticals, Vascular Biogenics Limited (VBL), Warner Chilcott UK Limited; has received speaker fees from AbbVie, Bristol-Myers Squibb and Janssen; and has received research funding from AbbVie, Bristol-Myers Squibb, Genentech, GlaxoSmithKline, Janssen, Millennium, Novartis, Pfizer, Procter and Gamble Pharmaceuticals, Shire Pharmaceuticals, and UCB Pharma. G D'Haens has served as a consultant for AbbVie, Actogenix, Amgen, Boehringer Ingelheim, Janssen, Cosmo Technologies, Elan, Engene, Ferring Pharmaceuticals, Giuliani, GlaxoSmithKline, Merck, MSD, Neovacs, Novonordisk, Otsuka, PDL Biopharma, Pfizer, Receptos, Salix Pharmaceuticals, SetPoint, Shire, Sigmoid Pharma, Takeda, Teva, Tillots, UCB; has served as a speaker for AbbVie, Tillotts, Tramedico, Ferring, MSD, UCB, Norgine, Shire; and has received research funding from AbbVie, Janssen Biologics, Given Imaging, MSD, DrFalk Pharma, Photopill. Q Zhou, R Thakkar and A Robinson are employees of AbbVie Inc, and own AbbVie stock and/or options. P Pollack is a former Abbott employee, and owns stocks in AbbVie Inc, and Abbott.
Declaration of funding interests: AbbVie Inc funded the study and the analysis, provided writing support and reviewed and approved the publication. The preparation of this study was funded by AbbVie Inc. Writing support was provided by Kristina Kligys, PhD, of AbbVie Inc. and was funded by AbbVie Inc.
Authorship
Guarantor of the article: Remo Panaccione.
Author contributions: RP, JFC, WJS and GD collected data. QZ performed statistical analyses. RP, JFC, WJS, GD, QZ, PFP, RBT and AMR contributed to the design of the study, interpretation of data and analyses, and writing and review of each draft of the publication. All authors approved the final version of the manuscript.
References
- 1.Cho JH. The genetics and immunopathogenesis of inflammatory bowel disease. Nat Rev Immunol. 2008;8:458–66. doi: 10.1038/nri2340. [DOI] [PubMed] [Google Scholar]
- 2.Carbonnel F, Jantchou P, Monnet E, Cosnes J. Environmental risk factors in Crohn's disease and ulcerative colitis: an update. Gastroenterol Clin Biol. 2009;33(Suppl. 3):S145–57. doi: 10.1016/S0399-8320(09)73150-1. [DOI] [PubMed] [Google Scholar]
- 3.van Deventer SJ. Review article: targeting TNF alpha as a key cytokine in the inflammatory processes of Crohn's disease–the mechanisms of action of infliximab. Aliment Pharmacol Ther. 1999;13(Suppl. 4):3–8. doi: 10.1046/j.1365-2036.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 4.Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn's disease. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.06.019. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 5.Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn's disease: the CHARM trial. Gastroenterology. 2007;132:52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Rutgeerts P, Enns R, et al. Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–38. doi: 10.7326/0003-4819-146-12-200706190-00159. [DOI] [PubMed] [Google Scholar]
- 7.Hanauer SB, Sandborn WJ, Rutgeerts P, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn's disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–33. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Sandborn WJ, Hanauer SB, Rutgeerts P, et al. Adalimumab for maintenance treatment of Crohn's disease: results of the CLASSIC II trial. Gut. 2007;56:1232–9. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutgeerts P, Van Assche G, Sandborn WJ, et al. Adalimumab induces and maintains mucosal healing in patients with Crohn's disease: data from the EXTEND trial. Gastroenterology. 2012;142:1102–11. doi: 10.1053/j.gastro.2012.01.035. [DOI] [PubMed] [Google Scholar]
- 10.Panaccione R, Colombel JF, Sandborn WJ, et al. Adalimumab sustains clinical remission and overall clinical benefit after 2 years of therapy for Crohn's disease. Aliment Pharmacol Ther. 2010;31:1296–309. doi: 10.1111/j.1365-2036.2010.04304.x. [DOI] [PubMed] [Google Scholar]
- 11.Colombel JF, Schwartz DA, Sandborn WJ, et al. Adalimumab for the treatment of fistulas in patients with Crohn's disease. Gut. 2009;58:940–8. doi: 10.1136/gut.2008.159251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamm MA, Hanauer SB, Panaccione R, et al. Adalimumab sustains steroid-free remission after 3 years of therapy for Crohn's disease. Aliment Pharmacol Ther. 2011;34:306–17. doi: 10.1111/j.1365-2036.2011.04717.x. [DOI] [PubMed] [Google Scholar]
- 13.Oussalah A, Danese S, Peyrin-Biroulet L. Efficacy of TNF antagonists beyond one year in adult and pediatric inflammatory bowel diseases: a systematic review. Curr Drug Targets. 2010;11:156–75. doi: 10.2174/138945010790309939. [DOI] [PubMed] [Google Scholar]
- 14.Sandborn WJ, Colombel JF, Schreiber S, et al. Dosage adjustment during long-term adalimumab treatment for Crohn's disease: clinical efficacy and pharmacoeconomics. Inflamm Bowel Dis. 2011;17:141–51. doi: 10.1002/ibd.21328. [DOI] [PubMed] [Google Scholar]
- 15.Sandborn WJ, Colombel JF, D'Haens G, et al. Association of baseline C-reactive protein and prior anti-tumor necrosis factor therapy with need for weekly dosing during maintenance therapy with adalimumab in patients with moderate to severe Crohn's disease. Curr Med Res Opin. 2013;29:483–93. doi: 10.1185/03007995.2013.779575. [DOI] [PubMed] [Google Scholar]
- 16.Burmester GR, Panaccione R, Gordon KB, McIlraith MJ, Lacerda AP. Adalimumab: long-term safety in 23 458 patients from global clinical trials in rheumatoid arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriatic arthritis, psoriasis and Crohn's disease. Ann Rheum Dis. 2013;72:517–24. doi: 10.1136/annrheumdis-2011-201244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn's disease. J Crohns Colitis. 2013;7:213–21. doi: 10.1016/j.crohns.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn's disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70. doi: 10.1053/j.gastro.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 19.Pariente B, Cosnes J, Danese S, et al. Development of the Crohn's disease digestive damage score, the Lemann score. Inflamm Bowel Dis. 2011;17:1415–22. doi: 10.1002/ibd.21506. [DOI] [PMC free article] [PubMed] [Google Scholar]