Abstract
Attentional problems in patients with attention deficit hyperactivity disorder (ADHD) have often been linked with deficits in cognitive control. Whether these deficits are associated with increased sensitivity to external salient stimuli remains unclear. To address this issue, we acquired functional brain images (fMRI) in 38 boys with and without ADHD (age: 11–16 years). To differentiate the effects of item novelty, contextual rareness and task relevance, participants performed a visual oddball task including four stimulus categories: a frequent standard picture (62.5%), unique novel pictures (12.5%), one repeated rare picture (12.5%), and a target picture (12.5%) that required a specific motor response. As a main finding, we can show considerable overlap in novelty‐related BOLD responses between both groups, but only healthy participants showed neural deactivation in temporal as well as frontal regions in response to novel pictures. Furthermore, only ADHD patients, but not healthy controls, engaged wide parts of the novelty network when processing the rare but familiar picture. Our results provide first evidence that ADHD patients show enhanced neural activity in response to novel but behaviorally irrelevant stimuli as well as reduced habituation to familiar items. These findings suggest an inefficient use of neuronal resources in children with ADHD that could be closely linked to increased distractibility. Hum Brain Mapp 36:2049–2060, 2015. © 2015 The Authors Human Brain Mapping Published by Wiley Periodicals, Inc.
Keywords: attention deficit hyperactivity disorder, distraction, fMRI, novelty, oddball
INTRODUCTION
With an estimated worldwide prevalence of approximately 5.3% [Polanczyk et al., 2007], attention deficit hyperactivity disorder (ADHD) is one of the most common childhood psychiatric disorders. Children and adolescents with ADHD show age‐inappropriate levels of inattention, hyperactivity, or restlessness and impulsivity leading to persisting social and academic impairments [Biederman et al., 2006]. Maintaining and regulating attention is a particular challenge for ADHD patients, as they are easily distracted by external stimuli and have severe difficulties to organize sensory and cognitive information according to their relevance [Satterfield et al., 1994]. It is unclear whether these problems are solely associated with diminished cognitive control (top‐down) or whether there is also an increased sensitivity to external sensory stimuli (bottom‐up).
Deficits in cognitive control have been repeatedly demonstrated in tasks that require the detection of a target stimulus among a frequently presented standard stimulus (oddball paradigm). Event‐related potential (ERP) studies revealed that the target‐related P3b was reduced in children with ADHD relative to healthy comparison groups [Jonkman et al., 2000; Kemner et al., 1996]. Additionally, recent fMRI studies reported reduced neural activity in patients with ADHD during target processing in a wide range of areas involved in cognitive control including temporal, parietal, and cingulate regions [Rubia et al., 2007; Tamm et al., 2006].
Beside intentional shifts of attention toward task‐relevant stimuli, attention can also be involuntarily captured by salient stimuli for instance due to their novelty or unexpectedness. This mechanism is crucial for adaptive behavior, because disruption of an ongoing task might be necessary when rare or particularly novel stimuli signal that something threatening or potentially rewarding is happening. However, enhanced bottom‐up processing of external novel stimuli can also contribute to increased distractibility (orienting costs) [SanMiguel et al., 2010]. So far, surprisingly few studies have addressed this issue in ADHD and have systematically investigated the neural correlates of novelty processing in this patient group. To our knowledge, only one study included novel stimuli in an auditory fMRI oddball task [Stevens et al., 2007]. Stevens and colleagues showed diminished activity in ADHD patients compared to a healthy comparison group in response to novel tones in the left parietal lobule and the posterior part of the left superior temporal gyrus. Moreover, participants with ADHD showed no activation in half of the expected novelty‐related regions of interests indicating impaired processing of novel stimuli in these patients.
Conversely, ERP studies have suggested normal orienting responses to novel stimuli in ADHD, as children with and without the disorder did not differ in parameters of novelty‐associated ERP components (P3a) [Jonkman et al., 2000; Kemner et al., 1996]. Some studies have even shown a particular significance or beneficial influence of novelty for ADHD patients, as, for example, the presentation of task‐unrelated novel tones in comparison to standard tones improved their performance accuracy in a visual attention task [van Mourik et al., 2007]. Furthermore, motor symptoms are reduced when children and adolescents with ADHD are exposed to a novel environment [Antrop et al., 2000].
Summarizing, even though novelty appears to be behaviorally important in ADHD, it is unresolved how novelty affects perceptual and attentional processes in this patient group. In the current fMRI‐study, we therefore aimed to characterize the neural representation of novelty in children and adolescents with ADHD in more detail. We used a modified visual oddball paradigm [Bunzeck and Düzel, 2006] to isolate effects of novelty from effects of mere rareness and relevance in an event‐related functional magnetic imaging (fMRI) study. Considering previous studies, we predicted that children and adolescents with ADHD would show activation differences during target processing but not during the processing of novel stimuli.
METHOD
Participants
Thirty eight boys between the age of 11 and 16 participated in the study. They were recruited via the Department of Child and Adolescent Psychiatry and Psychotherapy and through advertisements in a local newspaper. All participants and their parents were interviewed with the Revised Schedule for Affective Disorders and Schizophrenia for School‐Age Children: Present and Lifetime Version [K‐SADS‐PL, Kaufmann et al., 1997]. Nineteen boys met the diagnostic criteria for ADHD according to the DSM‐IV, 13 for the combined subtype, and six for the primarily inattentive subtype. Two patients additionally fulfilled diagnostic criteria for oppositional defiant disorder. In the comparison group, no participant was diagnosed with a current or previous psychiatric disorder.
As Table 1 shows, the groups did not differ in age nor intelligence [Culture Fair Test—Revised Version, Weiss, 2008], but the comparison group scored significantly higher in a standardized measure of selective attention [d2, Brickenkamp, 2002]. Furthermore, the ADHD group as well as their parents reported significantly more attentional problems than the comparison group as assessed by the Youth Self Report [Achenbach, 1991a] and the Child Behavior Checklist [Achenbach, 1991b]. Four patients and one participant of the comparison group were left handed, all others were right handed. ADHD patients who currently used stimulant medication (n = 10) discontinued intake at least 48 h before the experiment.
Table 1.
Group characteristics
| Variable | ADHD (N = 19) | CG (N = 19) | t |
|---|---|---|---|
| Age (years) | 13.32 | 13.58 | 0.52 |
| Diagnoses: | |||
| ADHD—combined | 13 | — | |
| ADHD—inattentive | 6 | — | |
| Oppositional defiant disorder | 2 | — | |
| IQ (CFT) | 104.89 | 108.63 | 0.96 |
| Attentional performance (d2; PR)* | 55.23 | 77.79 | 2.5 |
| Attentional problems—self rating (YSR; T)** | 53.48 | 60.5 | −3.52 |
| Attentional problems—parental rating (CBCL; T)*** | 54.26 | 68.06 | −9.28 |
*P < 0.05, **P < 0.01, ***P < 0.001.
All participants and their parents gave written informed assent/consent. The study was approved by the local ethics committee and followed the ethical standards of the Helsinki declaration. As a reimbursement for their participation, children and adolescents received vouchers for a local shopping center (5€ per hour).
fMRI Task/Experimental Design and Task
For the visual oddball task, black‐and‐white pictures of landscape scenes were used. For each participant, one picture was randomly selected as a frequently presented standard picture (62.5%), one as the task‐relevant target, and one as a task‐irrelevant neutral oddball (12.5%). Furthermore, 50 novel pictures were interspersed (12.5%). In each run, the standard picture was presented 50 times intermixed with 10 target and 10 neutral oddballs as well as 10 novel pictures (total of 80 pictures per run). The experiment contained five runs with short intermediate breaks.
Participants were instructed to respond to every appearing picture as quickly and accurately as possible. A button press with the right index finger indicated target detection, whereas the left index finger button was associated with all nontargets. In contrast to previous studies, every stimulus required a button press to assure that participants attended to all items. To familiarize the participants with the task and their standard, target and neutral oddball picture, they performed a training block outside the MRI scanner. As Figure 1 illustrates, each picture was presented on a gray background for 600 ms followed by a white fixation cross with a duration randomly sampled from an exponential distribution with a mean of 3 s (range of 1.4–5.4 s). The last button press within the intertrial interval was counted as the final answer. Before each experimental run started, the target picture was presented again as a reminder for 5 s.
Figure 1.
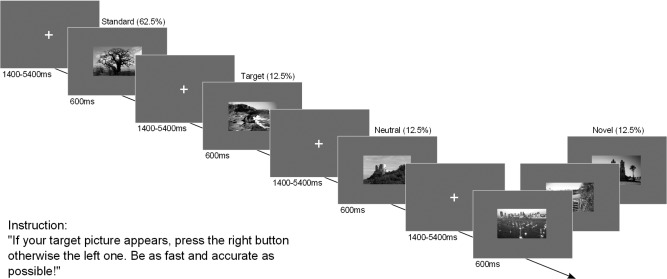
Modified visual oddball task.
fMRI image acquisition and processing
Imaging data were acquired on a 3T Siemens Magnetom Trio whole‐body MRI scanner equipped with an eight‐channel head coil. Structural images were collected by a T1‐weighted magnetization‐prepared rapid acquisition gradient echo sequence (192 sagittal slices, voxel size of 1 × 1 × 1 mm, field of view of 256 mm). Functional images were obtained in 32 slices by a whole‐brain T2*‐weighted echo planar imaging (EPI) sequence in interleaved order with a repetition time of 2 s (voxel size of 3.5 × 3.5 × 3.5 mm, field of view of 224 mm). The echo time was 30 ms and the flip angle 80°. The initial two images were not included in further processing.
In total, 725 EPI ‐ images (145 per run) of each participant were analyzed using statistical parametric mapping software (SPM8, Wellcome Trust Centre for NeuroImaging, London) running with MatlabR2009b (The MathWorks, Natick, MA). During preprocessing, they were corrected for slice‐time differences with regard to the first slice, realigned (reference = mean functional image) and spatially normalized to Montreal Neurological Institute (MNI) space. Furthermore a 6‐mm Gaussian kernel was applied for spatial smoothing and a high‐pass filter of 1/128 Hz for removal of low frequency confounds.
fMRI statistics
For each subject, an event‐related statistical model was computed by creating a “stick function” for each event onset (duration = 0 s), which was convolved with the canonical hemodynamic response function. Modeled conditions included standard, neutral oddball, target and novel images as well as errors (incorrect, multiple, and no responses). To capture residual movement‐related artifacts, six covariates were included (the three rigid‐body translation and three rotations resulting from realignment) as regressors of no interest. Regionally, specific condition effects were tested using linear contrasts for each subject and each condition (first‐level analysis). The resulting contrast images were entered into a second‐level random‐effects analysis separately for each group with age as a covariate (one‐sample t‐test). Activations were thresholded at P < 0.05 using cluster‐wise false discovery rate (FDR). Differences between groups were analyzed by two‐sample t‐tests using a threshold of P < 0.001 (uncorrected) for increased sensitivity and an extend range of k = 10 voxel.
To investigate the nature of group differences, individual beta weights were extracted by rfxplot [Gläscher, 2009] from the maximum peak voxel of the cluster differentiating between children and adolescents with and without ADHD. These beta weights were also subsequently used to assess potential relationships between brain activity and behavioral measures/group characteristics. The influence of mean reaction time, reaction time variability to the standard, mean accuracy, performance in the d2 test, age, and IQ on brain activation was assessed via Pearson's product moment correlation and the effects of medication usage and subtype in ADHD were investigated by point‐biserial correlations. Statistical significance was only assumed when Bonferroni corrected thresholds were exceeded.
We also explored whether age modulated activation during processing of novel and rare neutral stimuli. Activation differences between both groups were separately assessed in a young (11–13 years, N = 11) and an old (14–16 years, N = 8) ADHD and comparison group (young: N = 10, old: N = 9) by two‐sample t‐tests.
Moreover, we examined common activation patterns to novel (novel > standard) as well as rare familiar (neutral oddball > standard) stimuli in children and adolescents with and without ADHD by applying inclusive masking. Overlapping activation between rare familiar and novel pictures was also assessed by inclusive masking separately for each group. Based on the conjunction null hypothesis, a threshold of P < 0.05 FDR corrected and k > 10 voxel was applied to all contrast images used [Nichols et al., 2005].
Behavioral statistics
Error rates, reaction times, and number of trials with multiple button presses (as indicator of impulsivity) were analyzed in R (version 2.14.1, 2011) by two‐way repeated‐measures analyses of variance (ANOVAs) with the factors stimulus category (standard vs. target vs. neutral oddball vs. novel) and group (ADHD vs. healthy comparison group). Trials with multiple button presses or with errors were not considered for reaction time analysis.
RESULTS
Behavioral Data
The analysis of error rates revealed a very high accuracy in both groups. The average error rate was 0.88% over the whole experiment with a range between 0 and 4.5%. Stimulus category significantly affected the error rate (F (3,108) = 10.03, P < 0.0001), the number of trials with multiple button presses (F (3,108) = 65.4, P < 0.0001) and reaction times (F (3,108) = 72.97, P < 0.0001). No main effect of group or interaction of stimulus category and group were found (all P > 0.11). Post hoc paired t‐tests revealed that the responses to the target picture were associated with significantly higher error rates compared to all other stimulus categories (all t (37) > 3.1, P < 0.0005). Reaction times were fastest for the standard picture followed by the neutral oddball, the novel pictures and the target picture (all paired comparisons P < 0.001).
fMRI Results
Rare novel (novel > standard)
Novel compared to standard images evoked activity in a bilateral network comprising the parahippocampal gyrus and fusiform gyrus extending to the hippocampus as well as middle temporal gyrus and reaching into the inferior and middle occipital gyrus in both groups (Fig. 2A, Table 2). The right thalamus was activated only in the comparison group, but not in patients with ADHD. Inclusive masking revealed common activation in both groups in most of these areas (Table 2).
Figure 2.
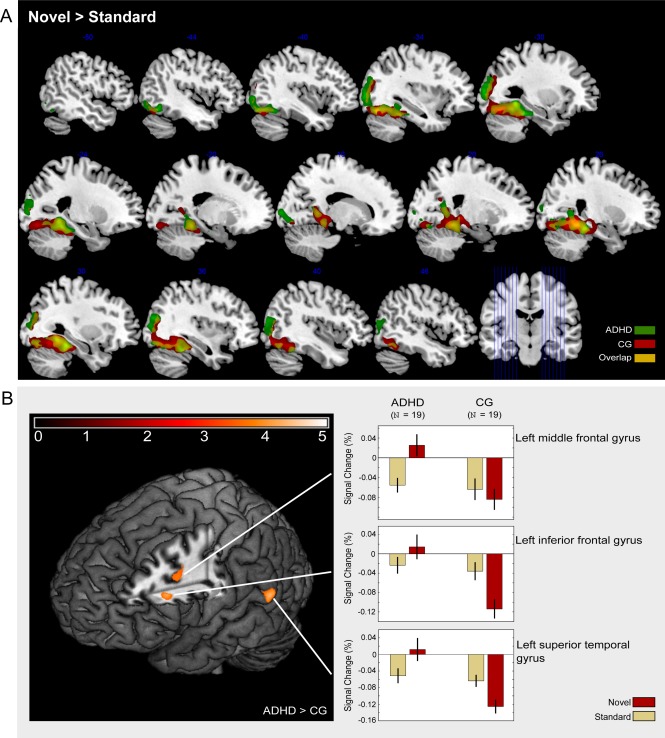
Processing of novel visual stimuli. (A) Activation overlap between participants with attention deficit hyperactivity disorder (ADHD) and a healthy comparison group (CG). (B) Areas showing stronger activation in ADHD patients than in the healthy comparison group (CG).
Table 2.
Activated brain regions in children and adolescents with ADHD and a healthy comparison group for three oddball categories against a standard picture
| ADHD group (N = 19) | Comparison group (N = 19) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Local maxima | Voxel per cluster | t | Local maxima | Voxel per cluster | t | |||||
| Activation Cluster | x | y | z | x | y | z | ||||
| Novel > Standard | ||||||||||
| R parahippocampal gyrusa (extending to hippocampus) | 28 | −36 | −16 | 10694 | 13.30 | 24 | −50 | −8 | 10828 | 18.92 |
| R fusiform gyrusa | 36 | −44 | −22 | 10.87 | 34 | −38 | −24 | 10.95 | ||
| R inferior temporal gyrusa | 50 | −56 | −18 | 9.01 | ||||||
| R middle temporal gyrusa | 36 | −80 | 20 | 8.89 | ||||||
| L parahippocampal gyrusa (extending to hippocampus) | −28 | −48 | −6 | 12.50 | ||||||
| L fusiform gyrusa | −30 | −36 | −20 | 10.81 | −30 | −68 | −14 | 12.62 | ||
| L middle occipital gyrusa | −36 | −72 | −16 | 10.86 | −34 | −72 | −16 | 12.20 | ||
| R middle occipital gyrusa | 38 | −72 | −12 | 10.03 | ||||||
| L inferior occipital gyrusa | −44 | −60 | −14 | 9.19 | ||||||
| R inferior occipital gyrusa | 44 | −62 | −16 | 9.59 | ||||||
| R thalamus | 20 | −30 | −2 | 10.90 | ||||||
| L precuneus | −16 | −74 | 50 | 233 | 5.33 | |||||
| R superior parietal lobule (extending to angularis) | 28 | −68 | 46 | 125 | 5.06 | |||||
| Neutral > Standard | ||||||||||
| R fusiform gyrus/parahippocampal gyrusb | 30 | −60 | −10 | 777 | 6.47 | |||||
| L fusiform gyrus/parahippocampal gyrusb | −32 | −52 | −18 | 725 | 7.95 | |||||
| R cuneus | 18 | −90 | 30 | 184 | 5.66 | |||||
| R precuneus | 1 | −68 | 24 | 503 | 5.05 | |||||
| L posterior cingulate | −8 | −60 | 10 | 95 | 4.47 | |||||
| L middle occipital gyrusb | −32 | −80 | 14 | 237 | 5.66 | |||||
| Target > Standard | ||||||||||
| L postcentral gyrusa | −48 | −28 | 56 | 995 | 11.45 | −46 | −24 | 56 | 2474 | 11.33 |
| L medial frontal gyrus (reaching in anterior cingulate) | −2 | −2 | 52 | 1007 | 7.66 | |||||
| R medial frontal gyrus | 6 | 10 | 50 | 706 | 7.35 | |||||
| R anterior cingulated (extending to posterior region) | 6 | −18 | 28 | 268 | 6.30 | |||||
| R posterior cingulate | 6 | −32 | 24 | 510 | 7.72 | |||||
| R precuneus | 4 | −72 | 40 | 242 | 5.06 | 8 | −72 | 40 | 454 | 7.21 |
| L thalamus | −10 | −18 | 8 | 127 | 6.80 | −12 | −24 | 8 | 555 | 7.05 |
| L insula | −46 | −4 | 4 | 472 | 6.60 | −34 | 12 | 4 | 99 | 5.41 |
| R insula | 32 | 16 | 4 | 490 | 6.91 | |||||
| R superior temporal gyrus (cluster includes right insula) | 46 | 12 | 4 | 268 | 6.50 | |||||
| R inferior parietal lobule | 48 | −44 | 44 | 143 | 6.25 | |||||
| L middle occipital gyrus/fusiform gyrus | −36 | −82 | −16 | 299 | 6.15 | |||||
| R anterior cerebellum/culmen | 28 | −46 | −20 | 1047 | 11.15 | 18 | −52 | −28 | 1139 | 9.36 |
| L culmen | −38 | −52 | −26 | 216 | 5.84 | |||||
P < 0.05, cluster‐wise FDR corrected; ADHD = attention deficit hyperactivity disorder; L = left; R = right.
Areas that showed common activity in both groups (inclusive masking).
Areas that showed common activity for novel and neutral pictures in ADHD (inclusive masking).
The direct comparison of the groups (ADHD > comparison group) showed significant differences in the left superior temporal gyrus, the left middle, and the inferior frontal gyrus (Table 3). The analysis of beta weights in these three regions revealed that activation differences were based on a deactivation following novel pictures in the comparison group but not in the patient group (Fig. 2B).
Table 3.
Activated brain regions: ADHD > Comparison Group
| Local maxima | |||||
|---|---|---|---|---|---|
| Brain region | x | y | z | Voxel per cluster | t |
| Novel > Standard | |||||
| L superior temporal gyrus | −58 | −50 | 10 | 68 | 4.44 |
| L inferior frontal gyrus | −44 | 20 | 22 | 50 | 4.31 |
| L middle frontal gyrus | −34 | 8 | 30 | 32 | 3.74 |
| Neutral > Standard | |||||
| L parahippocampal gyrus | −24 | −48 | −10 | 114 | 5.10 |
| L middle occipital gyrus | −32 | −80 | 12 | 19 | 4.35 |
| R fusiform gyrus | 32 | −58 | −12 | 30 | 4.03 |
| 22 | −70 | −12 | 15 | 3.84 | |
| L fusiform gyrus | −40 | −54 | −20 | 28 | 3.93 |
| R precuneus | 20 | −72 | 28 | 29 | 3.91 |
| R cuneus | 8 | −88 | 2 | 41 | 3.73 |
| L lingual gyrus | −16 | −72 | −10 | 14 | 3.69 |
| R middle temporal gyrus | 36 | −76 | 18 | 11 | 3.57 |
P < 0.001, uncorrected, k > 10; ADHD = attention deficit hyperactivity disorder; L = left; R = right.
The beta weights were not modulated by medication usage, ADHD subtype, age, or any of the behavioral measures (RT, accuracy, IQ, and d2) except for reaction time variability. For the superior temporal and middle frontal gyri, the product‐moment correlations were r = 0.39 (P < 0.05) and r = 0.4 (P < 0.05). Activity in the inferior frontal gyrus correlated with reaction time variability at r = 0.58 (P < 0.01) (Fig. 3). Separate group comparisons in the younger and the older group did not yield different results.
Figure 3.
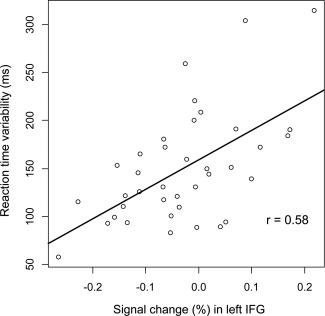
Relationship between individual novelty‐related activation and reaction time variability in standard trials in the left inferior frontal gyrus.
Rare familiar (neutral oddball > standard)
For the comparison group, there were no significant activation differences between the neutral oddball and the standard picture at the applied threshold. Children and adolescents with ADHD, however, showed activation bilaterally in the fusiform and parahippocampal gyrus, right cuneus, right precuneus, left posterior cingulate, and left middle occipital gyrus (Table 2). Significant group differences (ADHD > comparison group) were found in the bilateral lingual and fusiform gyrus, the left parahippocampal and middle occipital gyrus as well as the right middle temporal gyrus, right cuneus and precuneus (Fig. 4A, Table 3). Conjunction analysis in the form of inclusive masking revealed no common activity in both groups at the chosen threshold for the contrast rare versus standard, but it revealed an overlap between novelty and rareness‐related activity in children and adolescents with ADHD: Figure 4B shows the common activity within the bilateral fusiform and parahippocampal gyri and the left middle occipital gyrus.
Figure 4.
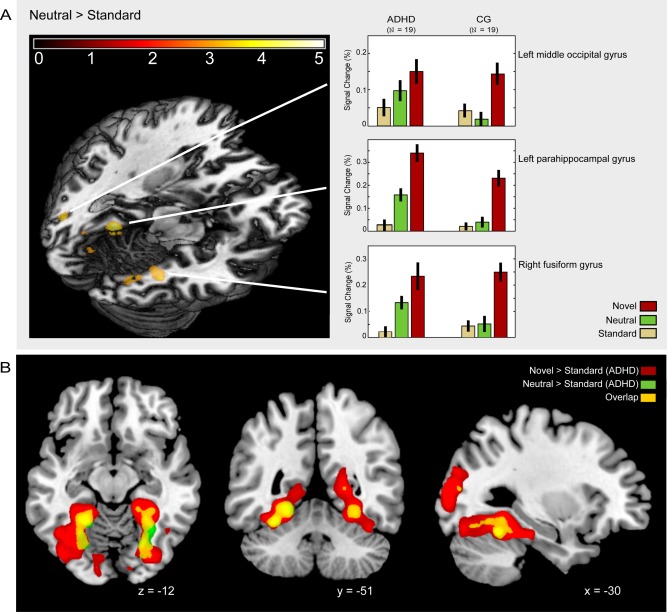
Processing of the neutral visual oddball. (A) Activation differences between the attention deficit hyperactivity group (ADHD) and the healthy comparison group (CG). (B) Overlapping activation for novel pictures and the neutral oddball in ADHD.
Focusing on the areas differentiating between ADHD and control participants, we found no correlation between the beta weights in the areas differentially activated by the groups and any performance measure, ADHD subtype, medication use, or age. Yet, when the groups were split halfway by age differences in the two sample t‐tests between young children with and without ADHD clearly reached significance (P < 0.001, uncorrected) in the bilateral fusiform gyri, the left middle occipital gyrus, the right precuneus, and both lingual gyri, whereas the effect in the same areas was less pronounced in the older subsamples (P < 0.01, uncorrected).
Rare target (target > standard)
In both groups, the correct detection of the rare target stimulus was associated with activation in the left postcentral gyrus, bilateral medial frontal gyrus, cingulate gyrus, and right precuneus. Furthermore, activation was found in the insula bilaterally, the left thalamus as well as the right anterior cerebellum and the right culmen (Table 2). The comparison group also activated the left culmen and left middle occipital gyrus extending into the fusiform gyrus. ADHD patients showed activation in the right superior temporal gyrus reaching into the right inferior parietal lobule. However, the direct comparison did not reveal any statistically significant differences between the groups.
DISCUSSION
We investigated the neural representation of novelty in children and adolescents with and without ADHD during a modified visual oddball task. As a main finding, we could show consistent neural responses to target and novel items in both groups. However, compared to healthy children and adolescents, participants with ADHD did not show neural deactivation in frontal and temporal areas in response to novel stimuli. Moreover, only the patient group significantly activated a network of brain regions in response to rare but familiar pictures that showed a great overlap with novelty‐associated areas. These findings seem to be unrelated to medication usage and subtype of ADHD as exploratory analyses revealed.
Novelty
In this study, novel stimuli activated a comparable network of brain regions in children and adolescents with and without ADHD. This network included the bilateral parahippocampal and fusiform gyrus, the hippocampus, temporal as well as occipital gyri, which is consistent with findings of novelty processing areas in visual oddball tasks in healthy adults [Kiehl et al., 2001]. The medial temporal areas and especially the hippocampus are known to detect deviance from expectation and novelty by matching incoming information with memory content [Kumaran and Maguire, 2009; Menon et al., 2000]. Increased activation in visual association cortices such as the fusiform gyrus and occipital regions has been associated with attention shifts driven by novel stimuli [Downar et al., 2000]. The involvement of the parahippocampal area has been previously reported in association with visual oddball tasks and could also be attributed to the landscape scenes used as stimulus material [Epstein et al., 1999].
Our results are also in line with ERP studies that did not show differences between ADHD patients and healthy comparison groups in the amplitude of the novelty related P3a component [Jonkman et al., 2000; Kemner et al., 1996] indicating intact detection of novelty in ADHD patients.
However, we also observed alterations in novelty‐related BOLD modulation between both groups. The healthy comparison group showed stronger deactivation of the neural signal within the left middle and inferior frontal gyrus as well as in the left superior temporal gyrus in response to novel pictures in comparison to the ADHD group. The middle frontal gyrus as well as the superior temporal gyrus are usually also activated during target processing [Linden et al., 1999; Stevens et al., 2000], whereas the left inferior frontal gyrus has previously been associated with extraction of meaning and semantic analysis [Bookheimer, 2002; Friedman et al., 2009]. The bilateral superior temporal gyrus and the right inferior and medial frontal gyrus are also part of the ventral attention network which is involved in bottom‐up attentional reorienting to salient and behaviorally relevant external stimuli [Corbetta and Shulman, 2002]. Interestingly, a recent meta‐analysis of functional MRI studies in ADHD [Cortese et al., 2012] has found evidence for both hypoactivation as well as hyperactivation in these areas. However, the hyperactivation we found in this study is based on missing deactivation in the ADHD patients and this deactivation has been discussed to be associated with preventing shifts to irrelevant stimuli [Corbetta and Shulman, 2002].
In our experiment, the novel pictures were not of particular behavioral relevance, as they required the frequent response of the left button. Accordingly, an efficient use of neural capacities in the current task could have entailed the suppression of a further analysis of the novel picture. Children and adolescents with ADHD failed to do so and allocated significantly more neural resources than the comparison group. This interpretation is further supported by moderate positive correlations between brain activity in these regions and the individual reaction time variability in standard trials which can be seen as a proxy of vigilance during the task. Therefore, in our study a general higher alertness was associated with higher deactivation.
Corbetta et al., [2008] argue that the ventral attention network is influenced by sustained top‐down signaling (possibly by the dorsal attention network) which enables the control of stimulus‐driven orienting and reorienting. The finding of both hyperactivation and hypoactivation in the ventral network in ADHD [Cortese et al., 2012] suggests that difficulties may lie in the top‐down modulation of this network rather than an impaired orienting response per se. It is conceivable that hypoactivation in ADHD occurs in tasks that require activation of the ventral network to plan and maintain appropriate behavior whereas suppression of the same network is needed to avoid distraction during other tasks. A deficit in adaptive regulation of bottom‐up processing would explain both findings.
Conversely, the lack of deactivation could involve a more elaborate processing of the novel pictures. Buckner et al. [2001] showed that enhanced left frontal activation along the inferior frontal gyrus was linked to successful incidental encoding of novel information in healthy adults. Thus, it would be interesting to assess in further studies whether children with ADHD show better subsequent recognition of unrepeated pictures than control children.
In contrast to our results, Stevens et al. found diminished activity for ADHD patients in the left parietal lobule and a posterior part of the left superior temporal gyrus in response to novel auditory stimuli [Stevens et al., 2007]. Our findings might result from the different sensory modality we used, as novelty processing networks have been shown to differ for distinct stimulus modalities. Although visual novels engage posterior brain regions more strongly, auditory novel oddballs elicit responses in the superior temporal plane [Halgren et al., 1995] and the inferior parietal lobule [Kiehl et al., 2001]. Moreover, in the study of Stevens et al. [2007], novel oddball tones did not require a response as they did in our experiment. Responding to every stimulus probably assured similar levels of attendance for novel pictures and the target picture. Thus, the observed activity in our study might be more clearly associated with pure novelty detection and less modulated by greater inattention in the ADHD group.
Rareness
Contrasting the contextually rare neutral oddball and the standard picture did not yield significant activation differences in the healthy comparison group. If a stimulus is repeatedly presented without further behavioral significance, the initial automatic novelty response associated with its first appearance declines throughout further processing [Cycowicz and Friedman, 1998]. This habituation reflects an efficient use of limited neural capacity and enables to focus on an ongoing task. We assume that the lack of activation differences between the standard picture and the neutral oddball in healthy children and adolescents could be due to the previously described process.
In contrast to the comparison group, ADHD patients activated the parahippocampal and fusiform gyrus, the cuneus, the precuneus, and bilateral middle occipital gyrus in response to the familiar oddball. Inclusive masking revealed that this activity pattern widely overlapped with the formerly identified novelty network (Fig. 4B) indicating that participants with ADHD did not differentiate between novel and familiar items to the same extent as the comparison group.
Although no correlation with age could be found, the difference between the groups seemed to be more pronounced in the younger group (11–13 years) when the age groups were analyzed separately in an exploratory analysis. Thus, the involvement of novelty‐related structures in the processing of a familiar picture might decrease with brain maturation which is decelerated in ADHD [Shaw et al., 2007].
However, effects similar to our results have been also reported in other domains. For instance, ADHD patients did not show differences between novel and familiar rare stimuli in theta activity [Fallahpour et al., 2010] or between familiar and unfamiliar (abstract) novel pictures in the P3a component [Marzinzik et al., 2012]. Furthermore, Jansiewicz et al., [2004] revealed that the habituation to visual stimuli in the peripheral hemifield was slowed for children and adolescents with ADHD. These results point to deficits in the processing of stimulus relevance in ADHD which might already involve early categorization and habituation processes. Our study complements these findings by showing for the first time that rare but familiar pictures activate the novelty network in children and adolescents with ADHD. We assume that the lack of differentiation between novel and familiar stimuli in ADHD could significantly contribute to their increased distractibility as the involuntary attention shift usually caused by novelty also appears after the rare but familiar stimulus.
Task Relevance
Target stimuli in our study elicited activity in medial frontal areas, the thalamus, insula, and precuneus as well as in occipital and temporal areas which is consistent with similar investigations of visual oddball tasks using fMRI [Ardekani et al., 2002; Clark et al., 2000; Kiehl et al., 2001; Stevens et al., 2000]. Additional neural activity within the left postcentral gyrus and right anterior cerebellum might be associated with the required motor response using the right index finger.
Surprisingly, we did not find any differences in neural activation in the group contrast. This contradicts other reports showing diminished activity for ADHD patients compared to a healthy comparison group in parietal association cortices, right precuneus, and thalamus [Tamm et al., 2006], as well as within the left middle frontal and the right superior temporal gyrus [Stevens et al., 2007] or basal ganglia, left and right superior temporal lobes and posterior cingulated [Rubia et al., 2007]. However, these differences might be accounted for by differences in the experimental setup. Our analysis included only trials with correct responses and without multiple button presses. Thus, only neural activity associated with correct task performance was extracted which might be similar in children and adolescents with and without ADHD. Alternatively, the more appealing stimulus material of landscape scenes compared to letters [Tamm et al., 2006], arrows [Rubia et al., 2007], or sine tones [Stevens et al., 2007] as well as the required button press for every picture could have improved the performance of participants with ADHD. It has been shown, that ADHD patients are able to show unimpaired performance when a task is more intriguing or when the frequency of target stimuli is high [Corkum and Siegel, 1993; Friedman‐Hill, 2010].
SUMMARY
To summarize, children and adolescents with ADHD and a healthy comparison group showed similar activation patterns in response to novel scene images, indicating intact novelty processing in ADHD. However, compared to the healthy comparison group, ADHD patients additionally engaged frontal and temporal areas associated with further processing when task‐irrelevant novel pictures were presented and activated the novelty network also in response to rare but familiar pictures.
In terms of a network approach as a framework to understand ADHD pathology, this study contributes to the existing literature by showing that missing deactivation in the ventral attention network can be linked to ADHD. The lack of deactiviation is probably related to a deficient top‐down dorsal network modulation but further studies have to investigate this relationship more closely. Furthermore, the reported novelty processing network overlaps widely with the orienting network proposed as one of three relevant attention networks by Posner and Petersen [Fan et al., 2005] which shows once more that novelty is a highly salient feature that attracts attention and induces an orienting response. Hyperactivation of this network in ADHD has been shown before during reorientation in a flanker task [Konrad et al., 2006] but not during the processing of familiar rare stimuli. Again, we argue that this altered bottom‐up processing of familiar stimuli might be modulated by dysfunctional top‐down processes because the contribution of the orienting network only appeared in children with ADHD but not in a healthy comparison group.
In conclusion, our findings on the processing of novel but also rare familiar stimuli suggest an inefficient use of neuronal resources in children with ADHD that might be closely linked to their increased distractibility.
ACKNOWLEDGMENTS
The authors thank Claus Tempelmann, Denise Scheermann, and Renate Blobel as well as Beato Suwa, Coraline Metzger, and Martin Walter for their support during the fMRI measurements. Furthermore, the authors like to thank Anna‐Maria Klopp, Emily Bauske, Annette Lederer, Laura Schares, and Andrea Simon for their help during the diagnostic procedures. Finally, the authors are indebted to all children and adolescents as well as their parents who participated in the study.
Correction added on 6 November 2015, after first online publication.
REFERENCES
- Achenbach TM (1991a): Manual of the Youth Self‐Report and 1991 Profile. Burlington: Department of Psychiatry, University of Vermont. [Google Scholar]
- Achenbach TM (1991b): Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington: Department of Psychiatry, University of Vermont. [Google Scholar]
- Antrop I, Roeyers H, Van Oost P, Buysse A (2000): Stimulation seeking and hyperactivity in children with ADHD. J Child Psychol Psychiatry 41:225–231. [PubMed] [Google Scholar]
- Ardekani BA, Choi SJ, Hossein‐Zadeh GA, Porjesz B, Tanabe JL, Lim KO, Bilder R, Helpern JA, Begleiter H (2002): Functional magnetic resonance imaging of brain activity in the visual oddball task. Cogn Brain Res 14:347–356. [DOI] [PubMed] [Google Scholar]
- Biederman J, Monuteaux MC, Mick E, Spencer T, Wilens TE, Silva JM, Snyder LE, Faraone SV (2006): Young adult outcome of attention deficit hyperactivity disorder: A controlled 10‐year follow‐up study. Psychol Med 36:167–179. [DOI] [PubMed] [Google Scholar]
- Bookheimer S (2002): Functional MRI of language: New approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25:151–188. [DOI] [PubMed] [Google Scholar]
- Brickenkamp R (2002): Test d2 – Aufmerksamkeits‐Belastungs‐Test. Göttingen: Hogrefe. [Google Scholar]
- Buckner RL, Wheeler ME, Sheridan MA (2001): Encoding processes during retrieval tasks. J Cogn Neurosci 13:406–415. [DOI] [PubMed] [Google Scholar]
- Bunzeck N, Düzel E (2006): Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron 51:369–379. [DOI] [PubMed] [Google Scholar]
- Clark VP, Fannon S, Lai S, Benson R, Bauer L (2000): Responses to rare visual target and distractor stimuli using event‐related fMRI. J Neurophysiol 83:3133–3139. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2002): Control of goal‐directed and stimulus‐driven attention in the brain. Nat Rev Neurosci 3:201–215. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL (2008): The reorienting system of the human brain: From environment to theory of mind. Neuron 58:306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkum PV, Siegel LS (1993): Is the continuous performance task a valuable research tool for use with children with attention‐deficit‐hyperactivity disorder? J Child Psychol Psychiatry 34:1217–1239. [DOI] [PubMed] [Google Scholar]
- Cortese S, Kelly C, Chabernaud C, Proal E, di Martino A, Milham MP, Castellanos FX (2012): Toward systems neuroscience of ADHD: A meta‐analysis of 55 fMRI studies. Am J Psychiatry 169:1038–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D (1998): Effect of sound familiarity on the event‐related potentials elicited by novel environmental sounds. Brain Cogn 36:30–51. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD (2000): A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci 3:277–283. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N (1999): The parahippocampal place area: Recognition, navigation, or encoding? Neuron 23:115–125. [DOI] [PubMed] [Google Scholar]
- Fallahpour K, Clarke SD, Goldberg E, Hermens DF, Falconer EM, Gordon E (2010): Alterations in theta activity associated with novelty and routinization processing in ADHD. Clin Neurophysiol 121:1336–1342. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI (2005): The activation of attentional networks. Neuroimage 26:471–479. [DOI] [PubMed] [Google Scholar]
- Friedman D, Goldman R, Stern Y, Brown TR (2009): The brain's orienting response: An event‐related functional magnetic resonance imaging investigation. Hum Brain Mapp 30:1144–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman‐Hill SR (2010): What does distractibility in ADHD reveal about mechanisms for top‐down attentional control? Cognition 115:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gläscher J (2009): Visualization of group inference data in functional neuroimaging. Neuroinformatics 7:73–82. [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Clarke JM, Heit G, Liégeois C, Chauvel P, Musolino A (1995): Intracerebral potentials to rare target and distractor auditory and visual stimuli. I. Superior temporal plane and parietal lobe. Electroencephalogr Clin Neurophysiol 94:191–220. [DOI] [PubMed] [Google Scholar]
- Jansiewicz EM, Newschaffer CJ, Denckla MB, Mostofsky SH (2004): Impaired habituation in children with attention deficit hyperactivity disorder. Cogn Behav Neurol 17:1–8. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Kemner C, Verbaten MN, Van Engeland H, Camfferman G, Buitelaar JK, Koelega HS (2000): Attentional capacity, a probe ERP study: Differences between children with attention‐deficit hyperactivity disorder and normal control children and effects of methylphenidate. Psychophysiology 37:334–346. [PubMed] [Google Scholar]
- Kaufmann J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N (1997): Schedule for affective disorders and schizophrenia for school‐age children ‐ Present and lifetime version (K‐SADS‐PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988. [DOI] [PubMed] [Google Scholar]
- Kemner C, Verbaten MN, Koelega HS, Buitelaar JK, van der Gaag RJ, Camfferman G, van Engeland H (1996): Event‐related brain potentials in children with attention‐deficit and hyperactivity disorder: Effects of stimulus deviancy and task relevance in the visual and auditory modality. Biol Psychiatry 40:522–534. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Laurens KR, Duty TL, Forster BB, Liddle PF (2001): Neural sources involved in auditory target detection and novelty processing: An event‐related fMRI study. Psychophysiology 38:133–142. [PubMed] [Google Scholar]
- Konrad K, Neufang S, Hanisch C, Fink GR, Herpertz‐Dahlmann B (2006): Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: Evidence from an event‐related functional magnetic resonance imaging study. Biol Psychiatry 59:643–651. [DOI] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA (2009): Novelty signals: A window into hippocampal information processing. Trends Cogn Sci 13:47–54. [DOI] [PubMed] [Google Scholar]
- Linden DE, Prvulovic D, Formisano E, Völlinger M, Zanella FE, Goebel R, Dierks T (1999): The functional neuroanatomy of target detection: An fMRI study of visual and auditory oddball tasks. Cereb Cortex 9:815–823. [DOI] [PubMed] [Google Scholar]
- Marzinzik F, Wahl M, Krüger D, Gentschow L, Colla M, Klostermann F (2012): Abnormal distracter processing in adults with attention‐deficit‐hyperactivity disorder. PLoS One 7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, White CD, Eliez S, Glover GH, Reiss AL (2000): Analysis of a distributed neural system involved in spatial information, novelty, and memory processing. Hum Brain Mapp 11:117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB (2005): Valid conjunction inference with the minimum statistic. Neuroimage 25:653–660. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA (2007): The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry 164:942–948. [DOI] [PubMed] [Google Scholar]
- Rubia K, Smith AB, Brammer MJ, Taylor E (2007): Temporal lobe dysfunction in medication‐naïve boys with attention‐deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry 62:999–1006. [DOI] [PubMed] [Google Scholar]
- SanMiguel I, Linden D, Escera C (2010): Attention capture by novel sounds: Distraction versus facilitation. Eur J Cogn Psychol 22:481–515. [Google Scholar]
- Satterfield JH, Schell AM, Nicholas T (1994): Preferential neural processing of attended stimuli in attention‐deficit hyperactivity disorder and normal boys. Psychophysiology 31:1–10. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL (2007): Attention‐deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA 104:19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AA, Skudlarski P, Gatenby JC, Gore JC (2000): Event‐related fMRI of auditory and visual oddball tasks. Magn Reson Imaging 18:495–502. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Pearlson GD, Kiehl KA (2007): An FMRI auditory oddball study of combined‐subtype attention deficit hyperactivity disorder. Am J Psychiatry 164:1737–1749. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL (2006): Parietal attentional system aberrations during target detection in adolescents with attention deficit hyperactivity disorder: Event‐related fMRI evidence. Am J Psychiatry 163:1033–1043. [DOI] [PubMed] [Google Scholar]
- van Mourik R, Oosterlaan J, Heslenfeld DJ, Konig CE, Sergeant JA (2007): When distraction is not distracting: A behavioral and ERP study on distraction in ADHD. Clin Neurophysiol 118:1855–1865. [DOI] [PubMed] [Google Scholar]
- Weiss RH (2008): Grundintelligenztest Skala 2 – Revision (CFT 20‐R). Göttingen: Hogrefe. [Google Scholar]


