Abstract
Introduction
Therapeutic potential of β-emitting cytotoxic radionuclides 90Y and 177Lu have been demonstrated in numerous preclinical and clinical trials. A bifunctional chelate that can effectively complex with the radioisotopes is a critical component for molecular targeted radiotherapy 90Y and 177Lu. A new bifunctional chelate 5p-C-NETA with a relatively long alkyl spacer between the chelating backbone and the functional unit for conjugation to a tumor targeting moiety was synthesized. 5p-C-NETA was conjugated to a model targeting moiety, a cyclic Arg-Gly-Asp-D-Tyr-Lys (RGDyK) peptide binding integrin αvβ3 protein overexpressed on various cancers. 5p-C-NETA was conjugated to c(RGDyK) peptide and evaluated for potential use in molecular targeted radiotherapy of 90Y and 177Lu.
Methods
5p-C-NETA conjugated with c(RGDyK) was evaluated in vitro for radiolabeling, serum stability, binding affinity, and the result of the in vitro studies of 5p-C-NETA-c(RGDyK) was compared to that of 3p-CNETA-c(RGDyK). 177Lu-5p-C-NETA-c(RGDyK) was further evaluated for in vivo biodistribution using gliobastoma bearing mice.
Result
The new chelate rapidly and tightly bound to a cytotoxic radioisotope for cancer therapy, 90Y or 177Lu with excellent radiolabeling efficiency and maximum specific activity under mild condition (>99%, RT, <1 min). 90Y- and 177Lu-radiolabeled complexes of the new chelator remained stable in human serum without any loss of the radiolanthanide for 14 days. Introduction of the tumor targeting RGD moiety to the new chelator made little impact on complexation kinetics and stability with 90Y or 177Lu. 177Lu-radiolabeled 5p-C-NETA-c(RGDyK) conjugate was shown to target tumors in mice and produced a favorable in vivo stability profile.
Conclusion
The results of in vitro and in vivo evaluation suggest that 5p-C-NETA is an effective bifunctional chelate of 90Y and 177Lu that can be applied for generation of versatile molecular targeted radiopharmaceuticals.
Keywords: Bifunctional Chelate, Targeted radiotherapy, Lu-177, Y-90, Radiolabeling, Serum Stability, Biodistribution
1. Introduction
α-or β-emitting cytotoxic radionuclides have been successfully applied to targeted therapy of cancers.1–3 A pure β-emitting 90Y (t½ = 64.1 h, Emax = 2.3 MeV) is a radionuclide component of a radioimmunotherapeutic (RIT) drug, Zevalin® in clinical use for treatment of B-cell non-Hodgkins lymphoma (NHL).1–3 90Y (t1/2 = 64.1 h) has the advantage of a longer range of penetration and homogeneous dose distribution at optimal therapeutic range.1–3 Less energetic β-emitting radionuclides 177Lu (t1/2 = 6.7 d, Emax = 0.5 MeV) with shorter half-lives relative to highly energetic 90Y have been investigated for radiotherapy of cancers.1–3 In addition to being a therapeutic β−-emitter, 177Lu possesses an imageable γ-emission, and its less energetic emission relative to 90Y was proposed to provide more selective tumor targeting and lower normal tissue damage.1 177Lu bound to CC49 antibody was evaluated for clinical RIT of ovarian cancer.4 A bifunctional chelate suitable for use in targeted radiotherapy is required to effectively hold the cytotoxic β-emitting radiolanthanide and contain a functional group for conjugation to a tumor targeting biomolecule. Since the radiolanthanides can be very toxic when deposited into normal tissue, it is critical to employ an optimal bifunctional chelate that can tightly hold the radiolanthanide and thus minimize toxic side effects related to dissociation of a radiolabeled complex during radiotherapy.5 Rapid complexation of a bifunctional chelate conjugated to a biomolecule with the radiolanthanide is required to preserve biological activity of a sensitive tumor targeting moiety.
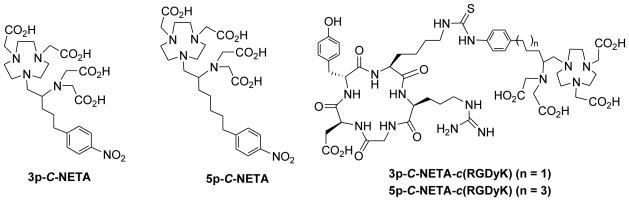
Research effort has been made on development of better bifunctional chelating agents that allow for practical preparation of less toxic radiopharmaceuticals for targeted radiotherapy using the radiolanthanides.1,3,7 We previously reported 3p-C-NETA ({4-[2-(bis-carboxy-methylamino)-5-(4-nitrophenyl)pentyl]-7-carbo-xymethyl-[1,4,7]triazanonan-1-yl} acetic acid, Figure 1) as a promising bifunctional chelator for use in targeted radiotherapy using 90Y, 177Lu, 212Bi, and 213Bi.7–9 3p-C-NETA was shown to rapidly form a stable complex with the radioisotopes, presumably by bimodal and cooperative binding of the macrocylic and acyclic moieties in the chelating backbone.
Figure 1.
Structure of 3p-C-NETA, 5p-C-NETA, 5p-C-NETA-c(RGDyK), and 3p-C-NETA-c(RGDyK) conjugate
Herein, we report synthesis and evaluation of a new bifunctional chelate 5p-C-NETA (2-({1-[4,7-bis(carboxymethyl)-1,4,7-triazanonan-1-yl]-7-(4-nitrophenyl)heptan-2-yl}(carbo-xymethyl) amino)acetic acid, Figure 1). As compared to the known bifunctional version of NETA (3p-C-NETA), the new chelate 5p-C-NETA contains a relatively longer alkyl spacer that connects a NETA chelating backbone with a functional group for conjugation to a biologically active molecule. It was reported that a linker in a peptide conjugate play a critical role in binding affinity of a receptor-targeted peptide. The design of the new chelator was intended for providing a sufficient spacing between a peptide and chelating NETA backbone and thereby minimizing steric hindrance in binding of the NETA-peptide conjugate to the receptor and maintaining high binding affinity to the receptor.
The new bifunctional chelate 5p-C-NETA was evaluated for radiolabeling kinetics and complex stability with 90Y and 177Lu in vitro. The chelation chemistry was applied to facile preparation of 90Y or 177Lu-radiolabeled peptide conjugates for targeted radiotherapy. We selected a cyclic peptide RGDyK (Arg-Gly-Asp-D-Tyr-Lys) as a model targeting vector that is known to bind to integrin αvβ3 over-expressed on many cancers including breast and prostate cancers and glioblastomas.10–12 Integrin αvβ3 has been investigated as a target protein of therapeutic and diagnostic radiopharmaceuticals.13–15 5p-C-NETA was conjugated to the cyclic RGD peptide, and the corresponding 5p-C-NETA-c(RGDyK) conjugate was evaluated for complexation kinetics, stability, and binding affinity with 90Y and 177Lu. The result of the in vitro complexation kinetics and stability of 5p-C-NETA-c(RGDyK) was compared to that of 3p-C-NETA-c(RGDyK). 177Lu-labeled 5p-C-NETA-c(RGDyK) conjugate were further evaluated for in vitro complex stability and tumor uptake using gliobastoma (U87MG) bearing mice.
2. Material and methods
2.1. Instruments and methods
1H, 13C, and DEPT NMR spectra were obtained using a Bruker 300 MHz NMR instrument, and chemical shifts are reported in ppm on the δ scale relative to TMS. Electro spray ionization (ESI) high resolution mass spectra (HRMS) were obtained on JEOL double sector JMS-AX505HA mass spectrometer (University of Notre Dame, IN). Analytical HPLC was performed on Agilent 1200 (Agilent, Santa Clara, CA) equipped with a diode array detector (λ = 254 and 280 nm), themostat set at 35 °C and a Zorbax Eclipse XDB-C18 column (4.6×150 mm, 80Å, Agilent, Santa Clara, CA). The mobile phase of a binary isocratic and gradient (2%B/2 min and 40%B/2–40min; solvent A = 0.1% TFA in H2O; solvent B = 0.1% TFA in CH3CN for method 1), or a binary gradient (0–100% B/15 min; solvent A, 0.1% TFA in H2O; solvent B, 0.1% TFA in CH3CN for method 2) at a flow rate of 1 mL/min was used. Semi-prep HPLC was performed on a Zorbax Eclipse XDB-C18 column (9.4×250 mm, 80Å). The mobile phase of a binary isocratic and gradient (2%B/2 min and 40%B/2–40min; solvent A = 0.1% TFA in H2O; solvent B = 0.1% TFA in CH3CN, flow rate of 3 mL/min for method 3) was used. All reagents were purchased from Sigma-Aldrich (St. Louis, MO) and used as received unless otherwise noted. c(RGDyk) peptide was purchased from Peptide International (#PCI-3662-PI, Louisville, KY). 90Y (0.05M HCl) and 177Lu (0.05M HCl) were purchased from Perkin Elmer (Waltham, MA).
2.2 Synthesis of 5p-C-NETA and 3p-C-NETA-c(RGDyK) and 5p-C-NETA-c(RGDyK) conjugatse
5-(4-nitrophenyl)pentan-1-ol (2).18
To a flask containing compound 119 (900 mg, 3.38 mmol) and 10 mL THF at 0 °C was added 1M BH3 in THF (6.76 mL, 6.76 mmol) dropwise over 15 min. The reaction mixture was allowed to room temperature and stirred for 4 h. The reaction mixture was quenched by 10 mL 7% K2CO3 and evaporated to dryness. The resulting residue was added H2O (20 mL) and extracted with ethyl acetate (20 mL×3). The combined organic layers were dried over MgSO4 and concentrated in vacuo to provide 2 (760 mg, 100%) as a yellow oil that was used for the next step without further purification. 1H NMR (CDCl3, 300 MHz) δ 1.36–1.45 (m, 2H), 1.50–1.72 (m, 4H), 1.83 (br, 1H), 2.71 (t, J = 7.8 Hz, 2H), 3.62 (t, J = 6.6 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H), 8.11 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 75 MHz) δ 25.4 (t), 30.8 (t), 32.4 (t), 35.8 (t), 62.7 (t), 123.6 (d), 129.2 (d), 146.2 (s), 150.6 (s).
1-(5-bromopentyl)-4-nitrobenzene (3).20
To a solution of 2 (700 mg, 3.35 mmol) and PPh3 (1.32 g, 5.02 mmol) in CHCl3 (10 mL) at 0 °C was added portionwise NBS (893 mg, 5.02 mmol) over 10 min. The reaction was stirred in 0 °C for 1 h and room temperature for 1 h. The reaction mixture was evaporated to dryness and purified via column chromatography on silica gel (60–230 mesh) eluting with 5% ethyl acetate in hexanes to afford pure 3 (780 mg, 86%). 1H NMR (CDCl3, 300 MHz) δ 1.39–1.54 (m, 2H), 1.56–1.74 (m, 2H), 1.81–1.94 (m, 2H), 2.72 (t, J = 7.8 Hz, 2H), 3.39 (t, J = 6.6 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H), 8.10 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 75 MHz) δ 27.7 (t), 30.1 (t), 32.5 (t), 33.7 (t), 35.6 (t), 123.6 (d), 129.2 (d), 146.3 (s), 150.3 (s).
1,3-diethyl 2-acetamido-2-[5-(4-nitrophenyl)pentyl]propanedio-ate (5)
To a flask containing anhydrous ethanol (10 mL) at room temperature was added portionwise Na (0.75 g, 32.6 mmol) over 30 min and the reaction mixture was stirred until all sodium disappeared. To a clear solution of NaOEt was added dropwise a solution of diethyl acetamidomalonate 4 (7.08 g, 32.6 mmol) in ethanol (30 mL) over 30 min. The resulting mixture was then heated at 50 °C for 1.5 h and then refluxed for 10 min. The solution became cloudy and light brownish indicating formation of deprotonated diethyl acetamidomalonic ester. To the reaction mixture at reflux was added dropwise 3 (8.9 g, 32.6 mmol) in ethanol (30 mL) over 30 min. The reaction mixture was maintained at reflux for 3 days while monitoring the reaction progress using TLC. The reaction mixture was allowed to cool to room temperature and then concentrated to dryness. To the residue, deionized water (100 mL) and extracted with diethyl ether (3 × 150 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo to the dryness. The residue was purified via column chromatography on silica gel (60–220 mesh) eluting with 30% ethyl acetate/hexanes to afford pure 5 (6.8 g, 51.1%) as a light yellow solid. MP = 126.0–127.1 °C. 1H NMR (CDCl3, 300 MHz) δ 1.06–1.18 (m, 2H), 1.24 (t, J = 7.1 Hz, 6H), 1.28–1.42 (m, 2H), 1.55–1.68 (m, 2H), 2.02 (s, 3H), 2.25–2.36 (m, 2H), 2.67 (t, J = 7.6 Hz, 2H), 4.23 (q, J = 7.1 Hz, 4H), 6.77 (s, 1H), 7.29 (d, J = 8.6 Hz, 2H), 8.12 (d, J = 8.6 Hz, 2H); 13C NMR (CDCl3, 75 MHz) δ 14.0 (q), 23.1 (q), 23.4 (t), 28.8 (t), 30.7 (t), 32.0 (t), 35.7 (t), 62.5 (t), 66.5 (s), 123.6 (d), 129.2 (d), 146.3 (s), 150.4 (s), 168.2 (s), 169.0 (s). HRMS (Positive ion FAB) Calcd for C20H29N2O7: [M + H]+ m/z 409.1969. Found: [M + H]+ m/z 409.1961.
2-amino-7-(4-nitrophenyl)heptanoic acid (6)
Compound 5 (6.52 g, 15.96 mmol) was dissolved in the mixture of acetic acid (16 mL) and conc. HCl (48 mL), and the resulting solution was maintained at reflux for 13 h. The reaction was allowed to room temperature and evaporated. The resulting residue was filtered while washing with isopropanol and dried in vacuo to provide pure 6 (4.5 g, 93%) as a yellow solid. M.P. 147.0–148.5 °C. 1H NMR (D2O, 300 MHz) δ 1.00–1.12 (m, 2H), 1.14–1.37 (m, 4H), 1.64–1.78 (m, 2H), 2.34 (t, J = 7.4 Hz, 2H), 3.85 (t, J = 6.1 Hz, 1H), 7.00 (d, J = 8.5 Hz, 2H), 7.71 (d, J = 8.5 Hz, 2H); 13C NMR (D2O, 75 MHz) δ 24.1 (t), 28.1 (t), 29.8 (t), 30.2 (t), 34.9 (d), 123.2 (d), 129.1 (d), 145.4 (s), 151.2 (s), 172.2 (s). HRMS (Positive ion FAB) Calcd for C13H18N2O4: [M + H]+ m/z 267.1339. Found: [M + H]+ m/z 267.1345.
methyl 2-amino-7-(4-nitrophenyl)heptanoate (7)
A solution of 6 (120 mg, 0.451 mmol) in MeOH (3 mL) at 0–5 °C was added thionyl chloride (0.5mL) dropwise, at which time the mixture was allowed to room temperature and then was stirred for 24 h. The resulting mixture was concentrated in vacuo to provide technically pure product 5c (140 mg, 98%) as an acidic salt. A slurry of the amino ester salt (170 mg, 0.537 mmol) in dry methanol (0.5 mL) was treated with Et3N (74 mg, 0.728 mmol). To the stirred slurry was then added anhydrous ether (30 mL), and the solution was cooled at −10 °C for 1 h. The resulted triethylamine hydrochloride salt was filtered off, and the filtrate was concentrated to provide amino ester 7 as an yellow oil which was directly used for the next step. 1H NMR (CDCl3, 300 MHz) δ 1.30–1.49 (m, 4H), 1.50–1.75 (m, 6H), 2.16 (br, 2H), 2.70 (t, J = 9.0 Hz, 2H), 3.42 (t, J = 5.9 Hz, 1H), 3.70 (s, 3H), 7.30 (d, J = 8.6 Hz, 2H), 8.13 (d, J = 8.6 Hz, 2H); 13C NMR (CDCl3, 75 MHz) δ 26.3 (t), 28.8 (t), 30.7 (t), 34.7 (t), 35.6 (t), 51.9 (q), 54.3 (d), 123.5 (d), 129.1 (d), 146.2 (s), 150.5 (s), 176.5 (s). HRMS (Positive ion FAB) Calcd for C14H20N2O4: [M + H]+ m/z 281.1496. Found: [M + H]+ m/z 281.1507.
2-amino-7-(4-nitrophenyl)heptan-1-ol (8)
To a solution of 7 (190 mg, 0.678 mmol) in anhydrous methanol (5 mL) was added portionwise NaBH4 (205 mg, 5.4 mmol) over 1 h. The mixture was then warmed to room temperature and stirred for 18 h. The reaction mixture was evaporated to dryness and quenched by H2O (80 mL) and extracted with ethyl acetate (120 mL × 3). The combined organic layers were dried over MgSO4 and concentrated in vacuo to provide pure 8 (170 mg, 99%) as a orange oil that was used for the next step without further purification. 1H NMR (CDCl3, 300 MHz) δ 1.10–1.45 (m, 6H), 1.46–1.68 (m, 2H), 2.30–2.80 (m, 6H), 3.22 (t, J = 8.0 Hz, 1H), 3.42–3.57 (m, 1H), 7.25 (d, J = 8.4 Hz, 2H), 8.05 (d, J = 8.4 Hz, 2H); 13C NMR (CDCl3, 75 MHz) δ 25.8 (t), 30.1 (t), 31.1 (t), 33.9 (t), 35.7 (t), 52.8 (d), 66.5 (t), 123.5 (d), 129.1 (d), 146.2 (s), 150.6 (s). HRMS (Positive ion FAB) Calcd for C13H20N2O3: [M + H]+ m/z 253.1547. Found: [M + H]+ m/z 253.1562.
tert-butyl 2-{[2-(tert-butoxy)-2-oxoethyl][1-hydroxy-7-(4-nitrophenyl)heptan-2-yl]amino}acetate (9)
To a solution of 8 (169.7 mg, 0.67 mmol) and K2CO3 (207 mg, 1.5 mmol) in CH3CN (2 mL) at 0–5 °C was added dropwise a solution of tert-butyl bromoacetate (288.6 mg, 1.5 mmol) in CH3CN (1 mL) over 10 min while maintaining the temperature at 0 °C. The resulting mixture was allowed to room temperature and stirred for 19 h. The reaction mixture was filtered and evaporated to dryness. The residue was purified via column chromatography on silica gel (60–220mesh) and eluted with 25% ethyl acetate in hexane to provide pure 9 (236.9 mg, 73.6%). 1H NMR (CDCl3, 300 MHz) δ 1.12–1.34 (m, 6H), 1.40 (s, 18H), 1.55–1.66 (m, 2H), 2.66 (t, J = 7.8 Hz, 2H), 2.70–2.72 (m, 1H), 3.19 (t, J = 10.3 Hz, 1H), 3.38 (dd, J = 17.5, 23.6 Hz, 4H), 3.39 (br, 1H), 4.27 (d, J = 8.7 Hz, 1H), 7.27 (d, J = 8.5 Hz, 2H), 8.08 (d, J = 8.5 Hz, 2H); 13C NMR (CDCl3, 75 MHz) δ 26.6 (t), 28.0 (q), 28.6 (t), 29.3 (t), 30.8 (t), 35.7 (t), 53.17 (t), 62.6 (t), 65.3 (d), 81.3 (s), 123.5 (d), 129.1 (d), 146.2 (s), 150.5 (s), 172.3 (s). HRMS (Positive ion FAB) Calcd for C25H40N2O7: [M + H]+ m/z 481.2908. Found: [M + H]+ m/z 481.2859.
tert-butyl 2-{[2-(tert-butoxy)-2-oxoethyl][2-iodo-7-(4-nitrophen-yl)heptyl]amino} acetate (10)
To a solution of 9 (320 mg, 0.66 mmol) and PPh3 (262 mg, 1.0 mmol) and imidazole (68.1 mg, 1.0 mmol) in CH2Cl2 (5 mL) at 0 °C was added portionwise I2 (254 mg, 1.0 mmol) over 10 min. The reaction was stirred in 0 °C for 2h and room temperature for 1h. The reaction mixture was evaporated to dryness and purified via column chromatography on silica gel (60–230 mesh) eluting with 10% ethyl acetate in hexanes to afford pure 10 (266 mg, 68.3%). 1H NMR (CDCl3, 300 MHz) δ 1.30–1.40 (m, 2H), 1.44 (s, 18H), 1.52–1.77 (m, 5H), 1.89–1.96 (m, 1H), 2.71 (t, J = 7.5 Hz, 2H), 2.96 (dd, J = 8.4, 14.2 Hz, 1H), 3.27 (dd, J = 6.2, 14.1 Hz, 1H), 3.45 (dd, J = 2.8, 15.2 Hz, 4H), 4.06–4.15 (m, 1H), 7.32 (d, J = 8.6 Hz, 2H), 8.12 (d, J = 8.6 Hz, 2h); 13C NMR (CDCl3, 75 MHz) δ 28.2 (q), 28.3 (t), 29.3 (t), 30.7 (t), 35.7 (t), 36.8 (t), 37.2 (d), 56.9 (t), 64.1 (t), 81.2 (s), 123.6 (d), 129.2 (d), 146.2 (s), 150.6 (s), 170.5 (s). HRMS (Positive ion FAB) Calcd for C25H39N2O6I: [M – I + H2O]+ m/z 481.2908. Found: [M – I + H2O]+ m/z 481.2906.
tert-butyl 2-[(1-{4,7-bis[2-(tert-butoxy)-2-oxoethyl]-1,4,7-triazanonan-1-yl}-7-(4-nitrophenyl) heptan-2-yl)[2-(tert-butoxy)-2-oxoethyl]amino]acetate (13)
To a solution of 1217 (50 mg, 0.085 mmol) and DIPEA (32.9 mg, 0.26 mmol) in CH3CN (1.5 mL) was added portionwise compound 10 (30.3 mg, 0.085 mmol). The resulting mixture was stirred for overnight at room temperature while monitoring the reaction progress using TLC. The reaction mixture was evaporated to dryness. To the residue, 0.1M HCl solution (5 mL) was added and extracted with CHCl3 (2 × 10 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo to the dryness. The residue was purified via column chromatography on silica gel (220–440 mesh) eluting with 3% CH3OH in CH2Cl2 to afford pure 13 (51.7 mg, 74.2%). 1H NMR (CD3OD, 300 MHz) δ 1.33–1.61 (m, 42H), 1.62–1.81 (m, 4H), 2.70–2.90 (m, 6H), 3.07–3.92 (m, 17H), 7.45 (d, J = 7.5 Hz, 2H), 8.15 (d, J = 7.5 Hz, 2H); 13C NMR (CD3OD, 75 MHz) 26.3 (t), 27.1 (q), 27.1 (q), 27.8 (t), 28.9 (t), 30.5 (t), 35.1 (t), 50.6 (t), 51.2 (t), 52.8 (t), 54.3 (t), 55.4 (t), 57.8 (d), 58.5 (t), 81.0 (s), 81.1 (s), 81.6 (s), 123.1 (d), 129.2 (d), 146.3 (s), 150.7 (s), 171.3 (s), 171.4 (s), 172.0 (s). HRMS (Positive ion FAB) Calcd for C43H74N5O10: [M + H]+ m/z 820.5436. Found: [M + H]+ m/z 820.5402.
2-({1-[4,7-bis(carboxymethyl)-1,4,7-triazanonan-1-yl]-7-(4-nitrophenyl)heptan-2-yl}(carboxy-methyl)amino)acetic acid (14, 5p-C-NETA)
Compound 13 (20 mg, 0.024 mmol) at room temperature was treaeted dropwise with 6M HCl (aq. 3 mL) over 5 min. The resulting mixture was allowed to reflux. After 15 min, chloroform (~5 mL) was added to wash the aqueous layer. The aqueous solution was concentrated in vacuo to provide 14 (16.7 mg, 93.9%). 1H NMR (CD3OD, 300 MHz) δ 1.32–1.54 (m, 5H), 1.67–1.79 (m, 3H), 2.78 (t, J = 6.9 Hz, 2H), 3.38–3.94 (m, 19H), 4.12 (s, 4H), 7.46 (d, J = 7.8 Hz, 2H), 8.15 (d, J = 8.1 Hz, 2H). HRMS (Positive ion FAB) Calcd for C27H41N5O10: [M + H]+ m/z 596.2532. Found: [M + H]+ m/z 596.2928. Analytical HPLC (tR = 12.7 min, method 2).
2-{[7-(4-aminophenyl)-1-[4,7-bis(carboxymethyl)-1,4,7-triazanonan-1-yl]heptan-2-yl](carboxymethyl)amino}acetic acid (15)
To a solution of 14 (13.5 mg, 0.018 mmol) in H2O (5 mL) at room temperature was added dry 10% Pd/C (4 mg) under argon. The reaction mixture was placed under hydrogenation apparatus for 14 h. The resulting mixture was filtered via celite bed and washed thoroughly with water. The filtrate was concentrated to provide 15 (10.9 mg, 81%). 1H NMR (CD3OD, 300 MHz) δ 1.32–1.57 (m, 6H), 1.62–1.78 (m, 4H), 2.64–2.77 (m, 2H), 3.41–3.95 (m, 17H), 4.05 (s, 4H), 7.20–7.40 (m, 4H). HRMS (Negative ion FAB) Calcd for C27H43N5O8: [M – H]+ m/z 564.3039. Found: [M– H]+ m/z 564.3071. Analytical HPLC (tR = 9.7 min, method 2).
2-({1-[4,7-bis(carboxymethyl)-1,4,7-triazanonan-1-yl]-7-(4-isothiocyanatophenyl)-heptan-2-yl}(carboxymethyl)amino) acetic acid (16)
To a solution of 15 (2 mg, 0.0035 mmol) in water (0.1 mL) was added dropwise thiophosgene in CHCl3 (3.0 μL, 0.003 mmol, 1M solution). The resulting mixture was stirred at room temperature for 3 h. The aqueous layer was concentrated in vacuo to provide 16 (2 mg, 94.1%). 1H NMR (D2O, 300 MHz) δ 1.00–1.28 (m, 5H), 1.39–1.53 (m, 3H), 2.45–2.54 (m, 2H) 2.91–3.88 (m, 22H), 4.05–4.16 (m, 1H), 7.12 (s, 4H). HRMS (Negative ion FAB) Calcd for C28H41N5O8S: [M – OH]+ m/z 590.2654. Found: [M – OH]+ m/z 590.2647. Analytical HPLC (tR =11.5 min, method 2).
5p-C-NETA-c(RGDyK) (17)
To a solution of c(RGDyk) (0.55 mg, 0.000884 mmol) in 0.1 M NaHCO3 aqueous solution (0.5 mL), 5p-C-NETA-NCS (1.9 mg, 0.00265 mmol) was added. The resulting mixture was stirred at room temperature for 27 h and was evaporated to dryness. The residue was treated with CH3CN/H2O = 1/3 solution containing 0.1% TFA and purified by semi-preparative HPLC (method 1) to provide c(RGDyK)-5p-C-NETA (0.65 mg, 59.8%). Analytical HPLC (tR = 27.8 min, method 1). MALDI-TOF/MS calcd for C53H82N14O16S m/z 1227.389. Found: m/z 1227.731.
3p-C-NETA-c(RGDyK) (18)
To a solution of c(RGDyk) (0.57 mg, 0.000919 mmol) in 0.1 M NaHCO3 aqueous solution (0.5 mL), 3p-C-NETA-NCS9 (2.0 mg, 0.00276 mmol) was added. The resulting mixture was stirred at room temperature for 44 h and was evaporated to dryness. The residue was treated with CH3CN/H2O = 1/3 solution containing 0.1% TFA and purified by semi-preparative HPLC (method 1) to provide c(RGDyK)-3p-C-NETA (0.87 mg, 78.9%). Analytical HPLC (tR = 21.9 min, method 1). MALDI-TOF/MS calcd for C53H82N14O16S m/z 1199.336. Found: m/z 1199.833.
2.3. Radiolabeling of 5p-C-NETA, 5p-C-NETA-c(RGDyK) and 3p-C-NETA-c(RGDyK) peptide conjugate with 90Y or 177Lu
All HCl solutions were prepared from ultra pure HCl (Fisher Scientific, #A466-500). For metal-free radiolabeling, plasticware including pipette tips, tubes, and caps was soaked in 0.1M HCl overnight and washed thoroughly with Milli-Q (18.2 MΩ-cm) water, and air-dried overnight. Ultra pure ammonium acetate (Aldrich, #372331) was used to prepare buffer solutions (0.25 M, pH 5.5). After adjusting pH using HCl solution, 0.25 M NH4OAc buffer solution was treated with Chelex-100 resin (Bio-Rad, #142-2842, 1 g/100 mL buffer solution), shaken overnight at room temperature, and filtered through a 0.22μm filter (Corning, #430320) prior to use. 90Y and 177Lu were purchased from Perkin Elmer and the University of Missouri Research Reactor. TLC plates (6.6 × 2 cm or 1 cm, Silica gel 60 F254, EMD Chemicals Inc., #5554-7) with the origin line drawn at 0.6 cm from the bottom were prepared. To a buffer solution (0.25 M NH4OAc, pH 5.5) in a capped microcentrifuge tube (1.5 mL, Fisher Scientific, #05-408-129) was sequentially added a solution of 5p-C-NETA or NETA-RGD conjugates (20 μg) in water and 90Y (60 μCi) or 177Lu (60 μCi). The total volume of the resulting mixture was 40 μL. To a buffer solution (0.25 M NH4OAc, pH 5.5) in a capped microcentrifuge tube (1.5 mL, Fisher Scientific, #05-408-129) was sequentially added a solution of 5p-C-NETA or NETA-RGD conjugates (20 μg) in water and 90Y (60 μCi) or 177Lu (60 μCi). The total volume of the resulting mixture was 40 μL. 5p-C-NETA (20 μg) was also reacted with 90Y or 177Lu at high activity (6 mCi). The total volume of the reaction mixture was 120 μL. The reaction mixture was agitated on a thermomixer (Eppendorf, #022670549) set at 1,000 rpm at room temperature for 1 h. The radiolabeling efficiency was determined by ITLC eluted with acetonitrile/water (3:2 v/v) as the mobile phase for 5p-C-NETA and 20mM EDTA in 0.15 M NH4OAc for NETA-RGD conjugates. A solution of radiolabeled complexes (2.0 μL) was withdrawn at the designated time points (1 min, 5 min, 10 min, 20 min, 30 min, and 60 min), spotted on a TLC plate, and then eluted with the mobile phase. In case of radiolabeling of NETA-RGD conjugates, a solution of the radiolabeled complex that was prepared from reaction of NETA-RGD conjugate (1 mM, 0.6 μL) with the radioisotope was quenched by DTPA solution (1mM, 0.6 μL) at a 1.2-fold molar excess, and the resulting mixture was incubated for 20 min at RT and then eluted on TLC. After completion of elution, the TLC plate was warmed and dried on the surface of a heater maintained at 35 °C and scanned using a TLC scanner (Bioscan, #FC-1000). Unreacted radioisotope and radiolabeled complex has the respective Rf values of 0.5 and 0.8 on TLC eluted with acetonitrile/water (3:2 v/v) system. The respective Rf value of unreacted radioisotope and radiolabeled complex on TLC eluted with 20 mM EDTA in 0.15 M NH4OAc was 0.8 and 0.5.
2.4. Determination of maximum specific activity (MSA)
A solution of 5p-C-NETA, 5p-C-NETA-c(RGDyK), or 3p-C-NETA-c(RGDyK) in 0.25M NH4OAc containing different amount of each chelate (0.001 to 1.2 μg) was prepared by dilution. 90Y or 177Lu (50 μCi) was added to a solution of each chelate, and the final volume of the solution (pH 5.5) was adjusted to 10 μL. The reaction mixture was agitated on the thermomixer set at 1,000 rpm at room temperature for 1 h. The radiolabeling efficiency (%) was determined by ITLC as described above. The data were plotted as radiolabeling efficiency (%) vs. amount of chelator used in the reaction and fitted using sigmoidal dose response equation in GraphPad Prism (La Jolla, CA). The amount of mass required to achieve 50% labeling was determined, and this mass was multiplied by 2 to obtain the minimal mass for 100% labeling to determine the maximum specific activity.
2.5. In vitro stability of 90Y or 177Lu radiolabeled complexes
Human serum was purchased from Gemini Bio Products (#100110). 90Y- or 177Lu-radiolabeled 5p-C-NETA was prepared by reaction of 5p-C-NETA (83 μg) with 90Y (250 μCi) and 177Lu (250 μCi) in 0.25M NH4OAc buffer (pH 5.5, 80.2 μL for 90Y and 75.6 μL for 177Lu) at room temperature and 1,000 rpm for 2 h and 5 h, respectively. 5p-C-NETA-c(RGDyK) (83 μg for 90Y and 100 μg for 177Lu) was reacted with 90Y (300 μCi) or 177Lu (300 μCi) in 0.25 M NH4OAc buffer (pH 5.5, 86.6 μL for 90Y and 83.7 μL for 177Lu) at RT and 300 rpm overnight (13–14 h). 3p-C-NETA-c(RGDyK) (100 μg) was reacted with 90Y (300 μCi) or 177Lu (300 μCi) in 0.25 M NH4OAc buffer (pH 5.5, 86.6 μL for 90Y and 90.0 μL or 88.7 μL for 177Lu) at RT and 300 rpm overnight (13–14 h). The radiolabeled complexes prepared from the reactions were used for serum stability studies without further purification. The freshly prepared 177Lu-radiolabeled or 90Y-radiolabeled 5p-C-NETA (~240 μCi) was added to human serum (0.8 mL) in a microcentrifuge tube. 177Lu-radiolabeled or 90Y-radiolabeled NETA-RGD conjugates (~260–290 μCi) was added to human serum (1.0 mL) in a microcentrifuge tube. The stability of the pure radiolabeled complexes in human serum was evaluated at 37 °C for 14 days using ITLC (acetonitrile/water = 3:2 v/v for 5p-C-NETA or 20 mM EDTA in 0.15 M NH4OAc for 5p-C-NETA-c(RGDyK) and 3p-C-NETA-c(RGDyK) conjugates. A solution of the radiolabeled complex in serum was withdrawn at the designated time point, and the percentage of 90Y or 177Lu released from each of the radiolabeled complexes into serum was assessed by ITLC using the eluent as described above. For radiolabeled 5p-C-NETA-c(RGDyK) and 3p-C-NETA-c(RGDyK) conjugates, a sample of the mixture in the serum was quenched by adding DTPA solution (1 mM, 0.6 μL) and incubated for 20 min at RT prior to TLC analysis as described above.
90Y- or 177Lu-radiolabeled complexes of 5p-C-NETA, 5p-C-NETA-c(RGDyK), and 3p-C-NETA-c(RGDyK) were separately prepared for evaluation of in vitro serum stability by HPLC analyses. Each chelate (50 μg) was reacted with 90Y or 177Lu (150 μCi) in 0.25M NH4OAc buffer (pH 5.5), and the total volume was adjusted to 100 μL. The resulting mixture was reacted on the thermomixer set at 300rpm and RT for 13 h. The radiolabeled complexes prepared from the reactions were used for serum stability studies without further purification. The freshly prepared 90Y-radiolabeled or 177Lu-radiolabeled complex (120–140 μCi) was added to human serum (0.5 mL) in a microcentrifuge tube. A solution of the radiolabeled complex in serum was withdrawn at the designated time point over 14 days and quenched by adding EDTA solution (1 mM, 0.2 μL) prior to HPLC analysis (method: 0–100%B/15 min, solvent A = 0.1% TFA in H2O; solvent B = 0.1% TFA in CH3CN).
2.6. In vitro cell binding assay
The IC50 values for c(RGDyK) (Anaspec, #61183) and 5p-C-NETA-c(RGDyK) and 3p-C-NETA-c(RGDyK) conjugates were determined by a competitive displacement cell binding assay using 125I-echistatin (Perkin Elmer #NEX439, specific activity 2200 Ci/mmol). The human glioblastoma cell line U87MG was used as a model for αvβ3 expression. Cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% fetal bovine serum and 50 μg/mL gentamicin at 37 °C in a humidified incubator containing 5% CO2. Briefly, 1 × 106 U87MG cells suspended in binding buffer (20 mM Tris, pH7.4, 150 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, 0.1% bovine seine serum albumin), were incubated at 37 °C for 1 h in the presence of approximately 20,000 cpm 125I-echistatin and increasing concentrations of the RGD peptide or the 5p-C-NETA-RGD and 3p-C-NETA-RGD conjugates. After incubation, the reaction binding buffer was aspirated, and cells were washed 2 times with ice-cold binding buffer. The radioactivity bound to the cells was counted in a Packard Riastar gamma counting system. Three separate in vitro cell binding experiments were performed for statistical analysis, and IC50 values were calculated using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA).
2.7. Biodistribution study
All animal experiments were conducted in accordance with the guidelines established by the Animal Care and Use Committee of the University of Missouri and the Harry S. Truman Memorial Veterans’ Hospital Subcommittee for Animal Studies. Five- to six-week old female athymic nu/nu nude mice were obtained from Harlan Laboratories (Indianapolis, IN) and housed one week prior to subcutaneous implantation of 1 × 107 U87MG tumor cells in 0.1 mL of phosphate-buffered saline (PBS) in the right hind flank. When tumors reached an average size of 0.4–0.9 g, the mice were injected intravenously via the tail vein with 2.22 MBq of 177Lu-labeled 5p-C-NETA-c(RGDyK) in 100 μL of PBS. Major tissues, organs and tumors were excised from animals sacrificed at 1 h, 4 h and 24 h post-injection, weighed, and counted in a gamma counter. For the receptor blocking group, mice were injected 2.22 MBq of the 177Lu-labeled radiopharmaceutical containing 100 μg of the unlabeled RGD peptide and sacrificed at 4 h post-injection for tissue and organ collection. The radioactivity in each tissue/organ was decay-corrected by a known aliquot of the injected dose, and the percent-injected dose per gram of tissue (% ID/g) was calculated. Values were expressed as mean ± SD for each group of 4 mice.
3. Result and Discussion
3.1. Synthesis
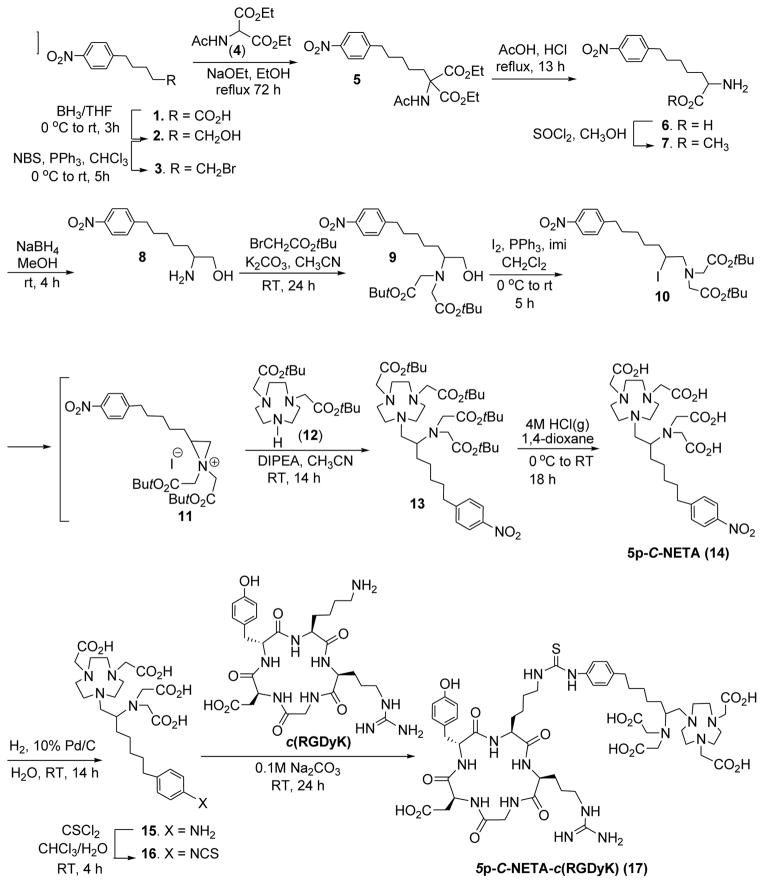
The new bifunctional chelate (5p-C-NETA, Scheme 1) contains the functional group (p-NO2-Bn) for conjugation to a peptide that is connected to the NETA chelating backbone16 via a pentyl chain. The key step in the synthesis of 5p-C-NETA (14) is the regiospecific ring opening of aziridinium ion (11) by bisubstituted 1,4,7-triazacyclononane (12)17 to provide compound 13 (Scheme 1). We previously reported the synthesis of secondary β-haloamines from primary β-amino alcohols via formation and regiospecific ring opening of aziridinium ions.7 The efficient synthetic method was applied for preparation of 5p-C-NETA as shown in Scheme 1. A functionalized alcohol 218 was prepared by reduction of carboxylic acid 119 using BH3/THF. Bromination of 2 using NBS and PPh3 produced 320 in 86% isolated yield. Compound 3 was reacted with sodium salt of diethyl acetamido malonate (4) for 14 h at RT to afford compound 5 in 43% isolated yield. Acidic hydrolysis of 5 followed by decarboxylation and removal of the acetyl protection group in 5 provided racemic p-nitrophenylpentylalanine 6 in excellent isolated yield (93%). Amino acid 6 was further converted to amino methyl ester 7. Reduction of 7 with NaBH4 followed by alkylation of 8 with t-butyl bromoacetate provided 9 in 74% isolated yield. Iodination of 9 using I2/PPh3/imidazole provided the secondary β-iodoamine 10. Intramolecular rearrangement7 of β-iodoamine 10 for formation of aziridinium ion 11 followed by regiospecific ring opening of 11 with 12 in a SN2 pathway provided the desired product 13 in a good isolated yield (74%). The t-butyl groups in 13 were removed by the treatment of 13 with 4M HCl (g) in 1,4-dioxane to produce 5p-C-NETA (14). The nitro group in 14 was reduced to afford 15 which was subsequently reacted with thiophosgene to provide the desired bifunctional chelate 16 containing an isothiocyanate group for conjugation to a peptide. 5p-C-NETA-c(RGDyK) conjugate 17 was prepared from base-promoted reaction of 16 with the cyclic peptide c(RGDyK) in 60% yield after purification of the reaction mixture by semi-prep HPLC. 3p-C-NETA-c(RGDyK) conjugate was isolated in 79% yield from reaction of 3p-C-NETA-NCS9 with the cyclic RGD peptide.
Scheme 1.
Synthesis of 5p-C-NETA and 5p-C-NETA-c(RGDyK)
3.2. Radiolabeling kinetics of 5p-C-NETA and 5p-C-NETA-c(RGDyK) and 3p-C-NETA-c(RGDyK)
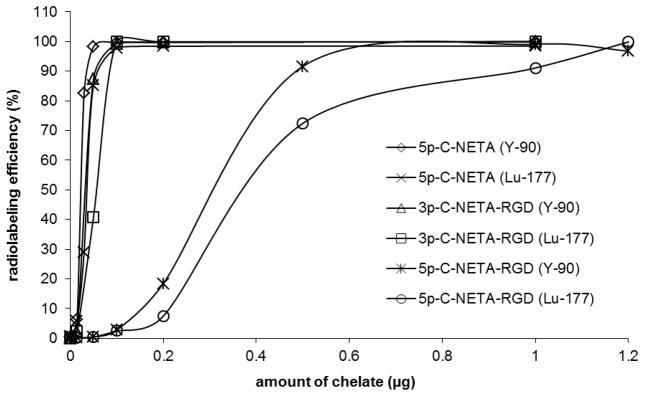
The new chelate 5p-C-NETA and 5p-C-NETA-c(RGDyK) and 3p-C-NETA-c(RGDyK) conjugates (20 μg) were evaluated for radiolabeling efficiency with the β-emitting radioisotopes, 90Y and 177Lu (60 μCi, pH 5.5, RT). Radiolabeling efficiency (mean ± standard deviation%) was measured in triplicate using ITLC (Table 1, and Supporting Information). The bifunctional chelator 5p-C-NETA instantly bound to 90Y or 177Lu at pH 5.5 (> 99%, 1 min, RT). It should be noted that radiolabeling of 5p-C-NETA with 90Y was significantly faster relative to C-DOTA (~84%, 1 h, RT).7 C-DOTA forms a stable complex with radiolanthanides but its slow complexation kinetic limits its application in the radiotherapy. 3p-C-NETA-c(RGDyK) was extremely fast in binding 90Y or 177Lu and nearly completely bound to the metals at the starting point of the radiolabeling reaction as expected from the previous result on complexation of 3p-C-NETA with the radioisotopes.7 Radiolabeling of 5p-C-NETA-c(RGDyK) conjugate with 90Y or 177Lu was slightly slower than that of 5p-C-NETA. However, 5p-C-NETA-c(RGDyK) almost completely bound to 90Y or 177Lu at 10 min time point (>99% radiolabeling efficiency, Table 1). 5p-C-NETA and NETA-RGD conjugates (20 μg) were also reacted with 90Y or 177Lu in higher activity (200 μCi) and instantly and almost completely bound to the radionuclides at 5 min with high specific activity (9.9 mCi/mg) and radiolabeling efficiency (>99%, Supporting Information). 5p-C-NETA (20 μg) remained highly efficient in binding 90Y or 177Lu at the highest activity (6 mCi), and radiolabeling was nearly complete in 1 min with the specific activity of 298.5 mCi/mg.
Table 1.
Radiolabling efficiency (%) of 5p-C-NETA, or 5p-C-NETA-c(RGDyK), and 3p-C-NETA-c(RGDyK) with 90Y or 177Lu (RT, 0.25M NH4OAC, pH 5.5, ITLC).#
| 90Y | 177Lu | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Time (min) | 5p-C-NETA | 3p-C-NETA-c(RGDyK) | 5p-C-NETA-c(RGDyK) | 5p-C-NETA | 3p-C-NETA-c(RGDyK) | 5p-C-NETA-c(RGDyK) |
| 1 | 99.3 ± 0.1 (99.5)* | 100.0 ± 0.0 | 70.8 ± 7.0 | 99.6 ± 0.1 (99.6)* | 100.0 ± 0.0 | 90.0 ± 4.0 |
| 5 | 99.7 ± 0.3 (99.5)* | 100.0 ± 0.0 | 98.5 ± 0.5 | 99.7 ± 0.3 (99.7)* | 100.0 ± 0.1 | 99.9 ± 0.2 |
| 10 | 99.8 ± 0.1 (99.7)* | 100.0 ± 0.0 | 99.6 ± 0.1 | 99.6 ± 0.1 (99.8)* | 100.0 ± 0.0 | 99.9 ± 0.0 |
| 20 | 99.8 ± 0.1 (99.7)* | 100.0 ± 0.0 | 99.8 ± 0.1 | 99.7 ± 0.1 (99.7)* | 100.0 ± 0.0 | 99.9 ± 0.0 |
| 30 | 99.9 ± 0.1 | 100.0 ± 0.0 (99.7)* | 99.9 ± 0.1 | 99.8 ± 0.2 | 100.0 ± 0.0 (99.7)* | 100.0 ± 0.0 |
| 60 | 100.0 ± 0.0 (99.7)* | 100.0 ± 0.0 | 100.0 ± 0.6 | 99.9 ± 0.1 (99.8)* | 100.0 ± 0.0 | 100.0 ± 0.1 |
Radiolabeling efficiency (mean ± standard deviation) was measured from the reaction of 5p-C-NETA, 5p-C-NETA-RGD, or 3p-C-NETA-RGD with 177Lu (20 μCi) in triplicate.
Radiolabeling efficiency was measured in single run from reaction of 5p-C-NETA with 90Y (6 mCi).
3.3. Maximum specific activity
The new chelate 5p-C-NETA and 5p-C-NETA-c(RGDyK) and 3p-C-NETA-c(RGDyK) conjugates were evaluated for radiolabeling with 90Y and 177Lu to determine the maximum specific activity. Each chelate in a series of concentration (0.001 – 1 μg) was labeled with 90Y or 177Lu (50 μCi) at RT for 1 h. The result of the maximum specific activity is shown in Figure 2. 5p-C-NETA, 5p-C-NETA-c(RGDyK), and 3p-C-NETA-c(RGDyK) bound to 90Y and 177Lu with high labeling efficiency (> 98%, 1 h). The NETA backbone in the chelate and conjugates was more efficient in binding 90Y than 177Lu. Among the chelate and conjugates tested, 5p-C-NETA bound to 90Y and 177Lu with the highest maximum specific activity (1,071 mCi/mg and 701 mCi/mg, respectively). All chelates (~1 μg) were efficiently radiolabeled with 90Y or 177Lu (50 μCi) in excellent radiolabeling efficiency. Conjugation of the NETA backbone to RGD peptide was shown to have no significant impact on radiolabeling kinetics with 90Y and 177Lu. 5p-C-NETA-RGDyK has much lower maximum specific activity relative to 5p-C-NETA in binding 90Y (87.8 mCi/mg) and 177Lu (64.6 mCi/mg). The respective maximum specific activity (mCi/mg) of 754 and 482 was determined in radiolabeling of 3p-C-NETA-RGDyK with 90Y and 177Lu. It seems that the presence of the longer pentyl chain in 5p-C-NETA-RGDyK affected radiolabeling efficiency of the NETA backbone with the metals, possibly due to its enhanced tendency for aggregation.
Figure 2.
Radiolabeling of 5p-C-NETA, 5p-C-NETA-c(RGDyK), and 3p-C-NETA-c(RGDyK) in different concentration with 90Y and 177Lu (50 μCi).
3.4. In vitro serum stability of 5p-C-NETA and 5p-C-NETA-c(RGDyK) and 3p-C-NETA-c(RGDyK) radiolabeled with 90Y and 177Lu
In vitro serum stability of 5p-C-NETA and NETA-RGD conjugates radiolabeled with 90Y or 177Lu was performed to determine if 5p-C-NETA chelate or NETA-RGD conjugates radiolabeled with 90Y or 177Lu remained stable without loss of 90Y or 177Lu in human serum (37 °C, pH 7). This was assessed by measuring the transfer of 90Y or 177Lu from the complex to serum proteins over the course of 14 days using ITLC (Supporting Information). Both 90Y-5p-C-NETA and 177Lu-5p-C-NETA remained extremely stable in human serum without releasing the radioactivity into the serum. The radiolabeled RGD conjugates of both 3p-C-NETA and 5p-C-NETA remained quite stable in human serum over 2 week period (Supporting Information). Even after the challenge of 90Y- or 177Lu-radiolabeled NETA-RGD conjugates in serum with 1 mM DTPA solution, only a small amount of the radioactivity (< 5%) was released from the complex. The serum stability data indicate that conjugation of NETA chelator with RGD peptide via the alkyl spacer has little impact on complexation kinetics and stability of the NETA chelator with 90Y and 177Lu. Dissociation of 90Y or 177Lu from the radiolabeled 5p-C-NETA and the NETA-RGD conjugates was also measured using RP-HPLC (the Supporting Information). The peak related to 90Y-EDTA or 177Lu-EDTA (tR = 2.5 min) was clearly separated from 90Y- or 177Lu-radiolabeled complex of 5p-C-NETA and the NETA-RGD conjugates. 90Y- or 177Lu-radiolabeled complex of 5p-C-NETA have the retention time at 8.7 min and remained inert in human serum over 2 weeks. No measurable amount of 90Y-EDTA or 177Lu-EDTA was detected on RP-HPLC over the period of 14 days. 90Y- or 177Lu-radiolabeled complexes of 3p-C-NETA-RGD and 5p-C-NETA-RGD conjugates have the respective retention time of ~7 min and ~7.5 min, and difference in retention time is expected from additional alkyl chain present in 5p-C-NETA-RGD conjugates. The RGD conjugates were quite stable in human serum for 14 days, although a tiny amount of radioactivity (<2%) was detected on RP-HPLC over the time period of measurement. It is interesting to note that when the NETA-RGD conjugates was mixed with serum, an unknown species was detected at the retention time of ~ 9 min. 90Y and 177Lu complexes of the NETA-RGD conjugates in serum were shown to be present in two different forms. It seems that the RGD moiety attached to NETA is bound to serum to produce the new peak detected by HPLC. To ensure that the new peak is not related to the activity released from the NETA-RGD conjugates, a sample of the radiolabeled NETA-RGD complexes was challenged with EDTA at 10-fold molar excess by incubating the mixture of the metal complex and EDTA for 1 h at 37 °C prior to HPLC run. No radioactivity related to formation of a radiolabeled EDTA complex was detected on HPLC (supporting information).
3.5. Binding affinity of 5p-C-NETA-c(RGDyK) and 3p-C-NETA-c(RGDyK) conjugates
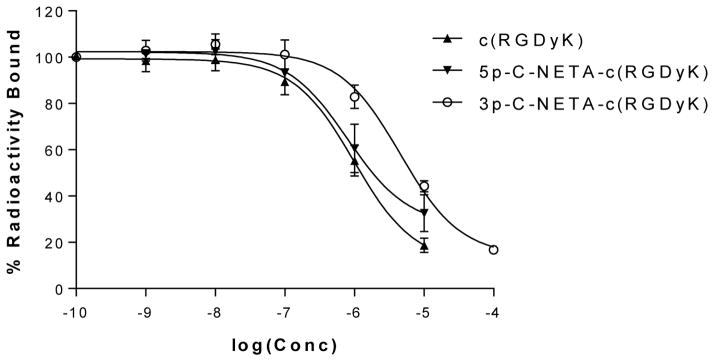
The in vitro binding affinities of c(RGDyK) and the 5p-C-NETA-c(RGDyK) and 3p-C-NETA-c(RGDyK) conjugates were compared in competitive binding assays using U87MG human glioblastoma cells and 125I-labeled echistatin as the standard. The IC50 value (1.40 ± 1.09 μM) of 5p-C-NETA-c(RGDyK) was comparable to 1.17 ± 0.46 μM for the unmodified c(RGDyK) peptide, while 3p-C-NETA-c(RGDyK) has a lower affinity in binding to the receptor (IC50 = 4.51 ± 0.65 μM) compared to 5p-C-NETA-c(RGDyK) (Figure 3). The result indicates that conjugation of the NETA chelating backbone had no significant effect on the binding affinity (P < 0.05) as shown in 5p-C-NETA-c(RGDyK). However, alkyl spacer in the NETA chelators (propyl vs pentyl) affected binding of the RGD to the receptor.
Figure 3.
IC50 analysis of c(RGDyK) peptide (▲), 5p-C-NETA-c(RGDyK) conjugate (▼) and 3p-C-NETA-c(RGDyK) (○) using human U87MG gliaoblastoma cells.
3.6. Biodistribution of 5p-C-NETA and 5p-C-NETA-c(RGDyK) conjugate
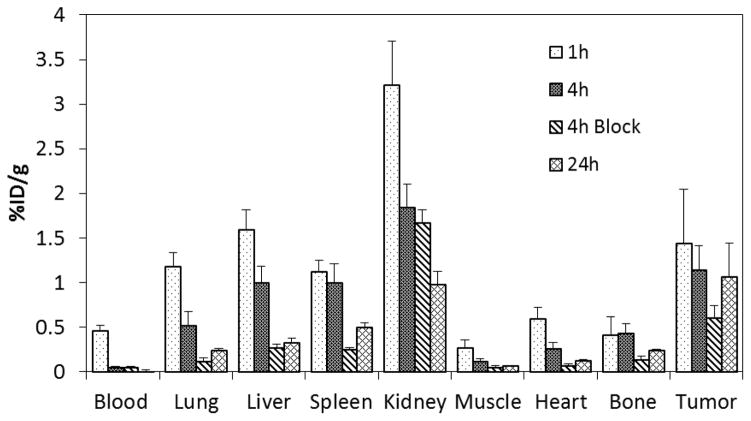
The in vivo stability and tumor targeting of 177Lu-radiolabeled 5p-C-NETA-c(RGDyK) were evaluated by a biodistribution study using nude mice bearing U87MG gliobastoma tumors as model for αvβ3 (Figure 4). The highest tumor uptake (1.44 ± 0.61%) of 177Lu-5p-C-NETA-c(RGDyK) was observed at 1 h post-injection. Uptake of the radiolabeled conjugate at 4 h and 24 h was 1.15 ± 0.27% and 10.7 ± 0.38% ID/g, respectively. Mice were co-injected with 100 μg of the unlabeled RGD peptide and the radiolabeled NETA-RGD conjugate for a blocking experiment for confirmation of receptor-specific binding of the NETA-RGD conjugate to integrin αvβ3 in the tumor. Significant decrease of the radioactivity in the tumor at 4 h post-injection (1.15 ± 0.27% ID/g vs. 0.60 ± 0.14% ID/g) was observed when mice were blocked with the unlabeled peptide. This result suggests the receptor-mediated targeting of 177Lu-5p-C-NETA-c(RGDyK). Radioactivity in the blood was very low at 1 h (0.46 ± 0.06% ID/g) and completely cleared at 24 h. The radiolabeled conjugate displayed high tumor-to-blood radioactivity ratio over the course of the experiment and showed the highest ratio at 24 h (74.2). The radiolabeled conjugate exhibited the highest radioactivity level in the kidney at 1 h (3.21 ± 0.49% ID/g), but the level decreased significantly at 24 h (0.98 ± 0.14% ID/g). Liver uptake (1.59 ± 0.22% ID/g) of the radiolabeled complex at 1 h was similar to that of tumor, but the radioactivity was well cleared from the organ at 24 h (0.32 ± 0.06% ID/g). Accumulation of the radioactivity in the heart, bone, and muscle was low at all the time points (<0.6%). The radioactivity in the lung and spleen (<1.2%) at 1 h decreased over the time. The biodistribution data indicate that 177Lu-5p-C-NETA-c(RGDyK) conjugate is stable in vivo and its uptake in the tumor is specific.
Figure 4.
Biodistribution of 177Lu-5p-C-NETA-c(RGDyK) conjugate in Nude Mice Bearing U87MG Tumor Xenografts.
4. Conclusion
The new bifunctional chelator 5p-C-NETA containing the NETA chelating backbone and the functional group linked via an extended alkyl spacer was efficiently prepared and evaluated for complexation with 90Y and 177Lu. The new chelate displayed excellent complexation kinetics and stability with 90Y and 177Lu. Conjugation of the chelator to the tumor targeting cyclic RGDyK peptide had no significant impact on radiolabeling efficiency with 90Y or 177Lu, binding affinity, and in vitro serum stability. 5p-C-NETA-c(RGDyK) conjugate radiolabeled with 177Lu was stable and shown to target tumors in mice and produced a favorable biodistribution profile. The results indicate that 5p-C-NETA is a promising bifunctional chelator of 90Y and 177Lu that can be employed for preparation of various radiophparmaceuticals for molecular targeted radiotherapy of cancers.
Supplementary Material
Acknowledgments
Financial Support: Supported by National Institutes of Health (2R01CA112503) and the Department of Veterans Affairs and the Harry S. Truman Memorial Veterans’ Hospital in Columbia, MO.
We acknowledge the financial support from the National Institutes of Health (2R01CA112503 to H. S. Chong). We also thank the Department of Veterans Affairs, for providing resources and use of facilities at the Harry S. Truman Memorial Veterans’ Hospital in Columbia, MO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Milenic DE, Brady ED, Brechbiel MW. Antibody-targeted radiation cancer therapy. Nature Rev. 2004;3:488–98. doi: 10.1038/nrd1413. [DOI] [PubMed] [Google Scholar]
- 2.Knox SJ, Meredith RF. Clinical radioimmunotherapy. Sem Radiat Oncol. 2000;10:73–93. doi: 10.1016/s1053-4296(00)80045-4. [DOI] [PubMed] [Google Scholar]
- 3.Volkert WA, Hoffman TJ. Therapeutic radiopharmaceuticals. Chem Rev. 1999;99:2269–2292. doi: 10.1021/cr9804386. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez RD, Partridge EE, Khazaeli MB, Plott G, Austin M, Kilgore L, Russell CD, Liu T, Grizzle WE, Schlom J, LoBuglio AF, Meredith RF. Intraperitoneal radioimmuno-therapy of ovarian cancer with 177Lu-CC49: a phase I/II study. Gynecol Oncol. 1997;65:94–101. doi: 10.1006/gyno.1996.4577. [DOI] [PubMed] [Google Scholar]
- 5.Harrison A, Walker CA, Parker D, Jankowski KJ, Cox JP. The in vivo release of Y-90 from cyclic and acyclic ligand-antibody conjugates. Int J Rad Appl Instrum B. 1991;18:469–76. doi: 10.1016/0883-2897(91)90107-v. [DOI] [PubMed] [Google Scholar]
- 6.Liu S. Radiolabeled Cyclic RGD Peptides as Integrin αvβ3-Targeted Radiotracers: Maximizing Binding Affinity via Bivalency. Bioconjugate Chem. 2009;20:2199–2213. doi: 10.1021/bc900167c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chong HS, Song HA, Kang CS, Le T, Sun X, Dadwal M, Lee HB, Lan X, Chen Y, Dai A. A highly effective bifunctional ligand for radioimmunotherapy of cancer. Chem Commun. 2011;47:5584–5586. doi: 10.1039/c0cc05707j. [DOI] [PubMed] [Google Scholar]
- 8.Kang CS, Song HA, Milenic DE, Baidoo KE, Brechbiel MW, Chong HS. Preclinical evaluation of NETA-based bifunctional ligand for radioimmunotherapy applications using 212Bi and 213Bi: radiolabeling, serum stability, and biodistribution and tumor uptake studies. Nucl Med Biol. 2013;40:600–605. doi: 10.1016/j.nucmedbio.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang CS, Sun X, Fang Jia, Song HA, Chen Y, Lewis M, Chong HS. Synthesis and preclinical evaluation of bifunctional ligands for improved chelation chemistry of 90Y and 177Lu for targeted radioimmunotherapy. Bioconjugate Chem. 2012;23:1775–1782. doi: 10.1021/bc200696b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sesgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horton MA. The alpha v beta 3 integrin “vitronectin receptor”. Int J Biochem Cell Biol. 1997;29:721–725. doi: 10.1016/s1357-2725(96)00155-0. [DOI] [PubMed] [Google Scholar]
- 12.Clezardin P. Integrins in bone metastasis formation and potential therapeutic implications. Curr Cancer Drug Targets. 2009;9:801–806. doi: 10.2174/156800909789760348. [DOI] [PubMed] [Google Scholar]
- 13.Wadas TJ, Deng H, Sprague JE, Zheleznyak A, Weilbaecher KN, Anderson CJ. Targeting the αvβ3 Integrin for small-animal PET/CT of osteolytic bone metastases. J Nucl Med. 2009;50:1873–1880. doi: 10.2967/jnumed.109.067140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z, Jia B, Shi J, Jin X, Zhao H, Li F, Liu S, Wang F. Tumor uptake of the RGD dimeric probe 99mTc-G3-2P4-RGD2 is correlated with Integrin αvβ3 expressed on both tumor cells and neovasculature. Bioconjugate Chem. 2010;21:548–555. doi: 10.1021/bc900547d. [DOI] [PubMed] [Google Scholar]
- 15.Janssen ML, Oyen WJ, Dijkgraaf I, Massuger LF, Frielink C, Edwards DS, Rajopadhye M, Boonstra H, Corstens FH, Boerman OC. Tumor Targeting with Radiolabeled αvβ3 Integrin Binding Peptides in a Nude Mouse Model. Cancer Res. 2002;62:6146–6151. [PubMed] [Google Scholar]
- 16.Chong HS, Gamestani K, Ma D, Milenic DE, Overstreet T, Brechbiel MW. Synthesis and biological evaluation of novel macrocyclic ligands with pendent donor groups as potential yttrium chelators for radioimmunotherapy with improved complex formation kinetics. J Med Chem. 2002;45:3458–3464. doi: 10.1021/jm0200759. [DOI] [PubMed] [Google Scholar]
- 17.Chong HS, Song HA, Birch N, Le T, Lim SY, Ma X. Efficient synthesis and evaluation of bimodal ligand NETA. Bioorg Med Chem Lett. 2008;18:3436–3439. doi: 10.1016/j.bmcl.2008.03.084. [DOI] [PubMed] [Google Scholar]
- 18.Palmer BD, Lee HH, Johnson P, Baguley BC, Wickham G, Wakelin LPG, McFadyen WD, Denny WA. J Med Chem. 1990;33:3008–3014. doi: 10.1021/jm00173a015. [DOI] [PubMed] [Google Scholar]
- 19.Kline SJ, Betebenner DA, Johnson DK. Bioconjugate Chem. 1991;2:26–31. doi: 10.1021/bc00007a005. [DOI] [PubMed] [Google Scholar]
- 20.Ashley JN, Collins RF, Davis M, Sirett NE. J Chem Soc. 1958:3298–3313. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.