Abstract
The role of angiogenesis in tumor growth has been studied continuously for over 45 years. It is now appreciated that angiogenesis is also essential for the dissemination and establishment of tumor metastases. In this review, we focus on the role of angiogenesis as a necessity for the escape of tumor cells into the bloodstream and for the establishment of metastatic colonies in secondary sites. We also discuss the role of tumor lymphangiogenesis as a means of dissemination of lymphatic metastases. Appropriate combination therapies may be used in the future to both prevent and treat metastatic disease through the rational use of anti-angiogenic and anti-lymphangiogenic therapies in ways that are informed by the current and future work in the field.
Keywords: metastasis, angiogenesis, malignant, endothelial cell, lymphangiogenesis, EMT, anti-angiogenesis, vessel, cancer, tumor
Angiogenesis and Tumor Growth
Normal epithelial cells multiply during development or embryogenesis, but adult epithelial cells divide more sparingly, such as in response to regular turnover and renewal or during tissue expansion and wound healing. Carcinoma cells originate from the transformation or mutation of normal epithelial cells. Their unrestricted cellular proliferation results in a mass of cells within the epithelial compartment either protruding out from the surface (i.e., in the epidermis), protruding into the lumen of the tube (e.g., in the colon) or filling the lumen of a gland (e.g., in the prostate). This stage is called “carcinoma in situ” or “intraepithelial neoplasia” and is considered a pre-cancerous neoplasm. If found, these lesions can be surgically removed quite easily. For example, polyps are routinely removed during colonoscopy examinations. Such benign tumors are localized with no chance of spreading, since the epithelial layer is not vascularized These confined tumors receive their oxygen and nutrients via diffusion from capillaries that lie under the basement membrane of the epithelial layer. Eventually equilibrium may be reached between tumor cell proliferation and apoptosis such that there is no net gain and the overall size of the mass does not increase. Visualization of these dormant yet viable lesions is more difficult in other tissues. For example, prostatic intraepithelial neoplasia (PIN) is found in approximately 16% of all men that undergo prostate biopsies each year (2015 Annual Report on Prostate Disease, Harvard). A diagnosis of PIN presents a major dilemma because such tumors may remain dormant for years, while others will progress.
In vitro, tumor cells grown in spheroids have an upper size limit based on the distance that nutrients can diffuse in the media into the core of the spheroid [1]. Similarly, tumors grown in organs ex vivo can only expand to ~1–2 mm [2]. In vivo, tumors cannot expand beyond the diffusion limit of nutrients from the nearest capillary, which is approximately 100–500 microns, unless new blood vessels grow toward the tumor. The process by which these tumor-associated neovessels sprout from existing blood vessels was first referred to as “tumor angiogenesis” by Dr. Judah Folkman in 1971 [2]. In his seminal paper in The New England Journal of Medicine in 1971, he hypothesized that “endothelial cells (EC) may limit tumor expansion” and that “for every increase in tumor diameter there must be an increase in tumor vascularization” [2]. Folkman proposed that the mitotic index of the tumor cells and the EC in the capillary were interdependent with a symbiotic relationship as in an ecosystem [2]. In fact, he hypothesized that tumors must secrete factors he called “tumor angiogenesis factor (TAF)” many years before the first one was purified [3].
We now appreciate that angiogenesis is a normal physiological process involving the proliferation, migration and morphogenesis of EC from existing vessels into new blood vessels. Angiogenesis is an active process during development and in physiological processes such as wound healing or thickening of the endometrium during the menstrual cycle. It is distinguished from vasculogenesis, which is the de novo formation of the first vessels from angioblasts in an embryo. From the point of view of the EC, tumor angiogenesis and normal angiogenesis are quite similar. They differ mainly in the source of the EC mitogen or chemoattractant. Notably, tumor neovascularization differs in tumor cells originating in non-vascularized epithelium (e.g., in transgenic mice overexpressing a tissue-specific oncogene) versus those in the vascularized dermis or lamina propria (e.g., tumor cells injected or implanted as a xenograft). The former requires an initial invasion of the epithelial basement membrane to gain access to underlying blood vessels, called the vertical growth phase. A second difference is that normal angiogenesis is time-limited, whereas tumor angiogenesis continues as long as the tumor is in place.
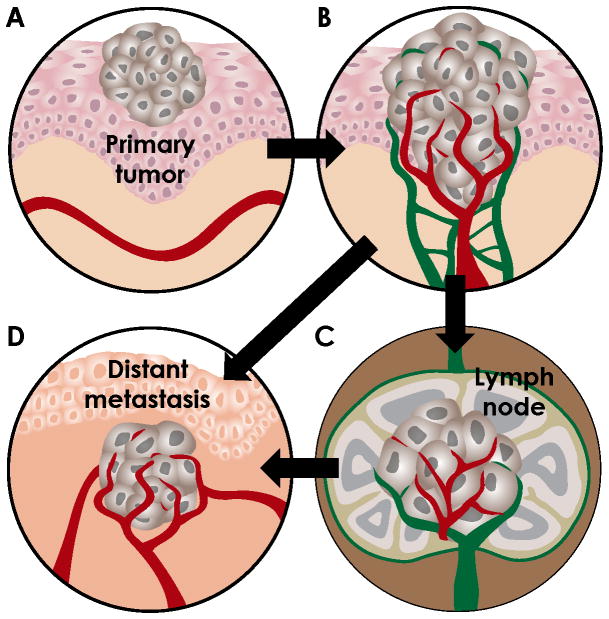
As tumor expansion occurs, the inner tumor cells get further from their blood supply and become relatively hypoxic. Hypoxia upregulates the expression of many angiogenic growth factors in tumor cells. (For a list of angiogenesis stimulators, see the following reviews [4–7]). Briefly, the process of tumor angiogenesis follows these sequential steps: 1) tumor cells release growth factors, such as VEGF and FGF, to attract EC toward the tumor mass; 2) EC (and other cells) secrete enzymes to degrade the proteins in the basement membrane of the capillary or post-capillary venule (never arteries); 3) EC (tip cells) begin to migrate or sprout toward the source of the stimulant, usually at right angles to the existing vessel; 4) EC continue to migrate and cells behind the leading tip cell, called stalk cells, proliferate and align in a single-file orientation; 5) the aligned EC then morph and create a lumen or tube in the center of the newly formed vessel; 6) blood is perfused into the lumen of the new sprout. New capillaries typically loop and interconnect to create a plexus within the tumor. This process is illustrated in Figure 1A-B.
Figure 1. Illustration of steps in the metastasis process.
A. Early carcinomas are confined to the epithelial compartment and receive their oxygen and nutrients by diffusion. B. To grow beyond 1mm3, tumors acquire neovascularization. Increased tumor-associated vascular and lymphatic density increases the propensity for tumor dissemination. Blood vessel, red; lymphatic vessel, green. C. Tumor cells can escape via lymphatic vessels and arrest in sentinel lymph nodes. Tumor cells in the lymph node may invade local blood vessels or remain in the lymphatic system to be recycled to the vascular system. D. Tumor cells may also invade blood vessels in the tumor (intravasation), travel in the circulation and exit in the new organ environment (extravasation). Tumor expansion again requires angiogenesis in the secondary site. Tumor cells can metastasize via the vascular system (B→D) or the lymphatic system (B→C→D).
Tumor-associated capillaries are notoriously abnormal. A detailed review of their pattern and structure is outlined by Dvorak and colleagues [8]. Briefly, tumor vessels are tortuous and misguided. They are malformed and hyperplastic. Due to the high expression of VEGF (and other factors) in the tumor environment, tumor vessels are also highly permeable and leaky. This leads to a high volume of fluid within the tumor microenvironment and high interstitial fluid pressures. Normal capillaries are stabilized by intermittent smooth muscle cells called pericytes that surround the capillary abluminally to support its structure and patency and to promote its survival and function [9]. In contrast, tumor vessels are immature, show rapid turnover and generally lack sufficient pericyte coverage.
The initiation of tumor angiogenesis is a pivotal point in tumor progression and has been called the “angiogenic switch” [10]. This hallmark of cancer denotes the shift from dormancy to progressive growth [11, 12]. Importantly, both benign neoplasms (such as a uterine fibroid cyst or vascular malformation) and malignant cancers gain angiogenic potential as they grow in size and acquire additional vascularization. Therefore, the angiogenic switch is often mistaken as a sign of malignancy. Rather, angiogenesis should be viewed as an ”organizing principle” that is required to obtain any significant gain in tissue mass in a variety of diseases [13].
Many cells within the tumor microenvironment, in addition to tumor cells, can secrete angiogenic factors that cause increased vascularization. Most notable are tumor-associated fibroblasts and macrophages. Tumor cells mixed with fibroblasts isolated from human breast carcinomas, called carcinoma-associated fibroblasts (CAF), can dramatically increase tumor angiogenesis and tumor growth rates [14, 15]. Specifically, CAFs secrete SDF1, which promotes neovessel formation and endothelial progenitor cell (EPC) recruitment [16]. Tumor-associated macrophages (TAM) deliver MMP9 to the tumor microenvironment, and MMP9 cleaves VEGF from matrix sequestration -- freeing it to stimulate vessel growth, motility and permeability [17]. It is worth mentioning that many growth factors are multi-potent within the tumor environment. For instance, EGF is a robust stimulator of tumor cells and EC. Tumor vessels upregulate EGFR, unlike normal vessels, and therefore respond to stimulation via EGF [18, 19].
Angiogenesis and Metastasis
Primary carcinomas rarely cause patient death (with the exception of lung and liver cancer). Rather, most carcinoma-related mortality is due to complications associated with metastasis. The term metastasis was first contrived by Joseph-Claude-Anthelme Récamier [20] (reviewed by [21]). Metastasis refers to the transfer of cancer cells from one part of the body to another. The spread of cancer cells from organ to organ is incredibly inefficient; only 0.01% of cells that leave the primary site go on to create a distant metastasis [22]. Why, then, do so many cancer patients succumb to metastatic disease? Because tumors are composed of billions of cancer cells, and 1–4 million cancer cells can be shed into the circulation each day from a one-gram tumor [23]. Additionally, hundreds of metastatic colonies can arise in an individual organ and the unrestricted growth of these colonies can lead to replacement of normal tissue by tumor in essential organs, such as the lung, liver, brain and bone marrow.
Unlike carcinomas and melanomas, sarcomas rarely metastasize. Likewise, small avascular dormant lesions do not metastasize. In general, metastasis is a late step in the progression of carcinoma; therefore small tumors found in the early stages can be cured by surgical excision. The metastatic process is described as a cascade of interrelated and sequential events that are each rate-limiting (reviewed by [7, 21, 24, 25]). The ordered steps of metastasis are similar for most malignant neoplasms, yet the route and destination may vary: 1) Angiogenesis, depicted in Figure 1B. Cancer cells must develop a vascular (or lymphatic) network [26]. Neovessels not only provide nutrients for the tumor to grow but also provide an escape route for the tumor cells to enter the circulation. In general, the larger the tumor and the more vascular density within the tumor, the greater the chance a tumor cell will escape. 2) Intravasation. Malignant tumor cells must detach from neighboring cells and matrix, invade through the capillary basement membrane and migrate through the endothelial barrier to enter the bloodstream. The transition to the mesenchymal phenotype is thought to aid in this migratory process. Alternately, detached tumor cells may be swept up in the mixture of fluid and cells that flow into lymphatic capillaries (described in more detail below). All lymphatic fluid is eventually recycled into the venous system, so either route may result in tumor cells in the circulatory system, depicted in Figure 1C–D. 3) Survival in circulation. The shear stress exerted by the pulsatile flow of blood and high serum concentration is an inhospitable environment for tumor cells. Most circulating tumor cells will die in the first 24 hours by either attrition or direct cytotoxicity and lysis by Natural Killer (NK) cells on immunosurveillance [27]. Platelets protect tumor cells in the initial hours after intravasation, and platelet-derived TGFβ may enhance tumor cell extravasation via EMT pathways [28]. 4) Extravasation. Tumor cells are thought to transmigrate through inter-endothelial junctions to extravasate in distant organs. Recent studies show that lymphocyte (and possibly tumor cell) diapedesis can also occur through transcellular routes [29]. Although tumor cells extravasate via venules, they often then migrate to arterioles where the oxygenation state of the tissue is higher. Cancer cells do not lodge in the first vascular bed they encounter; rather, tumor cell and host interactions dictate metastatic patterning. 5) Secondary tumor formation (depicted in Figure 1D). Extravasation does not necessarily result in a successful metastasis. Most single cancer cells that get out of the circulation either apoptose, get eliminated by immune cells, or just remain dormant [30]. To eventually form macrometastases, malignant tumor cells must proliferate and again undergo angiogenesis to result in a clinically relevant secondary tumor. Metastatic tumors can re-metastasize to additional secondary sites and can even re-implant in the primary tumor [25].
One of the strongest lines of evidence linking angiogenesis and metastasis is that tumor microvessel density correlates with increased metastatic potential and poor survival in nearly all forms of malignancy (discussed in detail below). Angiogenesis is essential for the growth of lung micrometastases. Some studies suggest that bone-marrow derived EPC contribute to the early angiogenic stages of metastatic growth [31], while others propose that cooption of normal vessels is a mechanism for metastasis vascularization [32]. Anti-angiogenic therapies in preclinical trials may reduce both the incidence and severity of metastasis (see Anti-Angiogenesis Therapy and Metastasis section below). Somewhat paradoxically, Folkman and colleagues showed that the surgical excision of a murine primary tumor could stimulate the growth of dormant metastases [33]. The mechanism for this angiogenic switch in the metastatic site was due to the loss of an endogenous angiogenesis inhibitor, angiostatin, being secreted systemically by the primary tumor. Similarly, the knockout of other endogenous angiogenesis suppressors promotes tumor vascularization and metastasis [34, 35]. These data further strengthen the link between angiogenesis and metastasis.
Interestingly, pericytes also play a role in metastasis. Pericytes help to control the patency of capillary lumens and are therefore essential for tumor cell dissemination. MMP9 knockout mice lack pericytes and have collapsed morphology in vessels and diminished metastasis [17].
Besides extravasation, circulating tumor cells (CTCs) may also lodge in the microvessels of distant organs and begin to grow intraluminally. These cells could eventually rupture the vessel wall and gain access to the underlying tissue [36]. CTCs also represent “metastatic intermediates,” as they indicate one step of the metastatic process. As such, these cells are now highly valued as surrogate markers of therapeutic efficacy in preclinical and clinical trials [37, 38]. In other words, if a drug is effective at shrinking a tumor, then fewer CTCs should be released into the circulation. High CTC numbers correlate with poor clinical outcome, and drugs that reduce CTC levels may be candidates for clinical development.
The seed and soil hypothesis, postulated by Paget [39] and popularized by Fidler [24, 40, 41], describes the tropism of certain tumors for specific organs. This may be due to the fact that certain tumors have receptors for specific growth factors preferentially secreted from a specific organ. For instance, colon cancer cells expressing cMET receptor homed to the liver, which secretes HGF [42]. Alternately, tissue specific vascular beds may have “zipcodes” or surface markers recognized by tumor cells [43]. In fact, certain primary tumors are found to prepare a pre-metastatic niche in the secondary location prior to tumor cell arrival [44]. Conversely, primary tumor cells secreting prosaposin inhibited metastatic colonization by systemically inducing the expression of thrombospondin-1 in lung stromal cells [45].
Lymphangiogenesis and Metastasis
Lymphatic vessels originally sprout from veins in mid-gestation (E9-9.5 in mice) [46]. However, in the adult, the lymphatic system is structurally and functionally quite different than the blood vascular system [47]. The vascular system is a closed, circulatory system in which blood is pumped through arteries, arterioles, capillaries, venules and veins to deliver oxygen and nutrients to all of the cells in the body. Lymphatic vessels, on the other hand, comprise a unidirectional, fluid recycling system. Fluid (and cells) are taken up into lymphatic capillaries, and fluid propulsion is channeled down lymphatic collecting ducts (containing valves) toward lymph nodes (LN). Fluid may be filtered through several LNs; but, ultimately, all lymphatic fluid is emptied back into the venous system [48].
At the microvessel level, lymphatic capillaries are structurally dissimilar to vascular capillaries. Lymphatic capillaries are lined with a thin endothelium that lacks or has minimal basement membrane and lacks pericyte coverage. Anchoring filaments function to open small gaps at button junctions between neighboring lymphatic EC (LEC) when interstitial fluid pressure rises [49]. These intercellular LEC gaps are essential to regulate fluid dynamics and to prevent tissue edema by increasing drainage. They also allow the infiltration of immune cells, such as dendritic and Langerhans cells, to gain access to the LN. Most notably, these openings offer direct and passive access of tumor cells to the lymphatic system. The LN has a filter function that initially protects the host but may act as a breeding ground or hub for tumor cell pooling that eventually spreads to other sites. Entrance to the lymphatic system may not guarantee that tumor cells will reach the LN, as some tumor cells were required to have the “key” (expression of CCR8) in order to unlock the “gate” (CCL1 expression by LEC) at the LN sinus [50].
Malignant carcinomas of virtually every type are reported to metastasize to regional LNs [51]. Some types of cancer spread via the vascular and lymphatic systems simultaneously, while others seem to metastasize in a metachronous manner with a tumor in the sentinel (draining) LN preceding those metastases in distant organs. In these sequential cases, lymphadenectomy can be curative [52]. The TNM staging system used for tumor classification is based on the assumption that most tumors progress sequentially from a primary tumor to lymph node metastasis and then to distant metastasis.
Tumor-associated LEC can secrete chemotactic factors to attract malignant tumor cells [53]. The process of tumor lymphangiogenesis is similar to that of tumor angiogenesis in that it is growth factor-mediated, results in ill-formed hyperplastic capillaries and increases the microvascular density and area through which tumor cells could escape. Yet, tumor lymphangiogenesis differs from angiogenesis in that mainly the peritumoral lymphatic vessels are enlarged and few lymphatic capillaries sprout inside the tumor. In histological human melanoma patient samples, the parameter of the lymphatic vessel area was more predictive of metastasis than the gold standard, tumor thickness [54]. Tumors engineered to overexpress the lymphangiogenic factor, VEGFC, stimulated tumor-associated lymphangiogenesis inside and around the tumor periphery and increased LN metastasis [55]. Conversely, silencing VEGFC in another model ablated only the intratumoral LEC, and LN metastasis was unchanged, suggesting that peritumoral lymphatic vessels are sufficient for LN metastasis [56].
Once tumor cells are in the LN, they essentially become a secondary tumor and can either invade blood capillaries in the LN or keep traveling in the lymph to eventually enter the circulation. The lymphatic system is now recognized as a significant source of CTCs in the blood. Lymph node metastasis is correlated with an increased risk of distant metastasis and a poor clinical outcome in most cancers. An unresolved question is the extent to which LN metastases do themselves populate metastases in local or distant non-lymphatic organs.
Vascular Density and Metastasis
Angiogenesis is a hallmark of cancer and is linked to metastasis. Therefore, the ability to accurately and reliably quantify the vasculature within a tumor is essential. In fact, Folkman discussed tumor capillary density in his first angiogenesis paper and ranked brain tumors as the most highly vascular, followed by carcinomas, sarcomas and chondrosarcomas, in that order [2]. Later, he and Dr. Noel Weidner published the methodologies for obtaining and calculating the microvessel density (MVD) within tumors [57]. Typically, MVD is calculated as the number of vessels stained positive by immunostaining for specific EC markers per high-power microscope field (200x or 0.74mm2). Since it is not feasible to count every field, Weidner popularized the notion of counting “hot spots” or areas of high vascular density. As MVD is heterogeneous throughout the tumor but tends to increase towards the periphery of the tumor, choosing the best field to count is a topic of debate. Additionally, which marker of tumor blood vessels to choose and which antibodies to use for staining is controversial, with popular options being CD31, CD34, and vWF [58].
Regardless of the technical details, MVD is a reproducible prognostic indicator of metastasis for certain cancers. A meta-analysis combining the data from 87 articles regarding MVD in human breast cancer patients found that high MVD predicted poor survival [59]. In a review by Kerbel, >50 publications (>8000 patients in total) reported prognostic value for MVD measurements [60]. Taken together, these studies suggest that MVD can aid in staging, metastatic potential, recurrence and survival predictions.
Although increased MVD is associated with increased metastasis, most tumor MVD is still less than its normal counterpart. For instance, the MVD in normal breast is higher than in breast carcinoma, and the MVD in normal lung is higher than in lung carcinoma [61]. One exception is in melanoma; the MVD in melanoma is usually much higher than in surrounding skin samples [62]. MVD more accurately reflects the metabolic burden of a tissue rather than its angiogenic dependence [63].
MVD is not an accurate indicator of therapeutic efficacy. A decrease in MVD during treatment with an angiogenesis inhibitor may indicate that the drug is active but the lack of MVD inhibition should not be equated to drug failure [63]. In fact, MVD may remain the same or even increase in the presence of an active and potent anti-angiogenic drug. For instance, a drug may decrease overall tumor size while the MVD in each high-power field remains constant — as was the case in tumors treated with a soluble neuropilin-2 B domain [64]. If the rate of tumor cell apoptosis is greater than that of EC apoptosis, then the drug may decrease overall tumor size while the MVD increases per high-power field. In the tumor “normalization” paradigm put forth by Jain, increases in tumor MVD (or at least perfusion) may be needed prior to chemotherapy to adequately shrink tumor burden [65].
Additionally, a patient’s tumor MVD should not be used as a predictor of potential response to therapy [63]. A common misconception is that tumors with low MVD, such as pancreatic carcinoma, will not respond to anti-angiogenic therapy. Experimental evidence suggests the reverse is true [66]. Tumors with low vascular density are highly susceptible to anti-angiogenic therapy, presumably because it is easier to shift the tumor toward an ischemic environment. Highly vascularized tumors are also amenable to therapy but may require higher doses of drug [63].
Newer techniques, especially for the assessment of anti-angiogenic therapeutic efficacy, have used proliferative capillary index (CD31+/Ki67+ vessels) or microvessel pericyte coverage index (MPI) to measure vessel maturity [61]. Additionally, lymphatic vascular density (LVD), lymphatic vessel infiltration (LVI), vessel caliber using MRI [67], FACS analysis [68], and perfusion with fluorescent lectins [17] have been used to accurately calculate and predict vascular phenotypes. The use of automated counting programs and new software has greatly enhanced the standardization of these methods [69].
Anti-Angiogenesis Therapy and Metastasis
If angiogenesis is a critical and rate-limiting step in tumor progression, then it follows that blocking angiogenesis should inhibit cancer progression. Indeed, Folkman postulated in 1971 that drugs could be used to inhibit angiogenesis years before the first angiogenesis inhibitor was found [2]. In 1980, interferon α/β was shown to inhibit capillary EC motility, and therefore represented the first experimental evidence for angiogenesis inhibition [70]. Now the list of angiogenesis inhibitors is too numerous to mention individually here but includes classes of molecules such as: endogenous proteins (eg., thrombospondin), soluble receptors, receptor tyrosine kinase inhibitors (small molecule inhibitors), siRNA to angiogenic factors, and antibodies to individual growth factors and receptors (reviewed in [71–74]).
It is important to mention that malignant tumors secrete multiple angiogenic growth factors at the same time. VEGF has been the most well-studied angiogenic factor. It is apparent that the majority of angiogenesis inhibitors currently in clinical trials target the VEGF signaling pathway. Many tumors initially respond to VEGF-targeted therapies only to relapse due to tumor evasion. Tumor evasion refers to a tumor cell upregulating a different angiogenic factor in response to one pathway being blocked and is caused by the redundancy of angiogenic factors produced by tumor cells [73]. For example, tumors exposed to anti-VEGF strategies may upregulate FGF or other angiogenic factors. Combination therapies are now being explored. Evasion from anti-angiogenesis drugs is often misinterpreted as EC resistance. In the case of anti-VEGF drugs, it is actually the tumor cells that are the target since the tumor cell secretes the VEGF. Tumor cells exposed to an hypoxic and acidic environment, due to the pruning of blood vessels during anti-angiogenic therapy, may respond to the stress in a multitude of ways, such as by switching to anaerobic metabolism, undergoing EMT, or altering its secreted factors which affect inflammation and fibrosis (reviewed by [75]).
Angiogenesis inhibitors have been divided into two main classes depending on their mode of action and their target cell. Direct angiogenesis inhibitors act directly on the endothelial cell, but may have off-target effects on tumor cells as well. These inhibitors include endostatin, angiostatin, thalidomide, TNP470, and Semaphorin 3F [48]. Direct angiogenesis inhibitors typically block EC proliferation or motility regardless of the stimulator. Their mode of action may be downstream of the growth factor-induced signal transduction cascades, and therefore incubation of EC with these direct inhibitors trumps the action of growth factors like VEGF or FGF. Evasion or resistance is less likely to occur from direct angiogenesis inhibitors. Alternately, indirect angiogenesis inhibitors block growth factors or pathways of cells other than EC. Examples of indirect inhibitors include antibodies to growth factors like anti-VEGF, soluble receptors of VEGFR, or inhibitors of pericyte recruitment. Since indirect angiogenesis inhibitors block factors emanating from tumor cells they are likely to result in resistance.
Chemotherapies are cytotoxic drugs that inhibit the growth of any rapidly dividing cell, which in a cancer patient means the tumor cell. These anti-mitotic drugs are typically given at maximum tolerated doses and are very toxic to the host cells as well as the tumor cells. Therefore, to prevent major bone marrow suppression, the patient is given the drug for a set period of time and then given a break or “holiday” from the drug to recover their bone marrow cells. When given at low doses on a continuous schedule, chemotherapy can target the EC and not the tumor cell. Anti-angiogenic chemotherapy was successful at inhibiting the expansion of multiple tumor types in vivo [76]. This type of regularly-scheduled or continually-dosed chemotherapy is also called “metronomic chemotherapy” [77, 78].
In 2009, two independent laboratories reported that anti-angiogenic therapies stimulated metastasis [79, 80]. More recently a similar response was found with the same drug in liver cancer [81]. Overinterpretation of these results should be cautioned. One of the drugs used was sunitinib, which is a receptor tyrosine kinase inhibitor of the VEGFR and PDGFR that targets multiple cell types in the tumor microenvironment in addition to tumor-associated EC and pericytes. Sunitinib was given at a high dose of 120 mg/kg. The experiment was designed such that the sunitinib was given prior to tumor injection as a “conditioning” [79]. Under these circumstances, there is no tumor at the time of drug delivery, therefore there is no angiogenesis to inhibit. Rather, the drug was actually used to treat normal EC in the lung, which may then be called anti-vascular therapy. Regardless, when tumor cells were injected intravenously into the mouse pre-treated with sunitinib, greater numbers of tumor cells were able to colonize the lungs and establish experimental metastases. Our interpretation of these results is that high doses of a kinase inhibitor of the VEGF pathway in normal mice is likely to result in the blocking of VEGF survival signals and subsequent EC drop-out. The “damaged” lung vessels may have been more easily traversed by the tumor cells, resulting in lung colonization. Increased metastasis was not found when sunitinib was given at later time points [79]. These types of experimental conditions are unlikely to occur in the clinic, as patients are not given this drug prophylactically.
Future Directions
Cancer therapy, as used today, is most commonly used to treat metastatic disease and not primary tumors. Little work has been done to distinguish the differences between the causes, types and patterns of angiogenesis in secondary versus primary tumors. It is critical that these studies be undertaken, where possible, in patient-derived samples or in G.E.M. models of metastatic cancers. Similarly, pre-clinical studies of anti-angiogenesis therapy and evasion should always be conducted in animal models where established metastases are present. Until such studies are the norm, we are unlikely to develop angiogenesis inhibitors that are highly effective in the metastatic setting.
Acknowledgments
The authors would like to acknowledge Kristin Johnson for the graphic illustration. We thank Melissa Anderson for editing and administrative assistance. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under award numbers R01CA037393 (BRZ) and R21CA155728 (DRB). These studies were also supported by the Vascular Biology Program at Boston Children’s Hospital.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Tannock IF. The relation between cell proliferation and the vascular system in a transplanted mouse mammary tumour. British journal of cancer. 1968;22(2):258–273. doi: 10.1038/bjc.1968.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folkman J. Tumor angiogenesis: therapeutic implications. The New England journal of medicine. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 3.Shing Y, Folkman J, Sullivan R, Butterfield C, Murray J, Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- 4.Klagsbrun M, D’Amore PA. Regulators of angiogenesis. Annual review of physiology. 1991;53:217–239. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. The role of angiogenesis in tumor growth. Seminars in cancer biology. 1992;3(2):65–71. [PubMed] [Google Scholar]
- 6.Iruela-Arispe ML, Dvorak HF. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thrombosis and haemostasis. 1997;78(1):672–677. [PubMed] [Google Scholar]
- 7.Fidler IJ, Kumar R, Bielenberg DR, Ellis LM. Molecular determinants of angiogenesis in cancer metastasis. The cancer journal from Scientific American. 1998;4(Suppl 1):S58–66. [PubMed] [Google Scholar]
- 8.Nagy JA, Chang SH, Shih SC, Dvorak AM, Dvorak HF. Heterogeneity of the tumor vasculature. Seminars in thrombosis and hemostasis. 2010;36(3):321–331. doi: 10.1055/s-0030-1253454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Amore PA. Capillary growth: a two-cell system. Seminars in cancer biology. 1992;3(2):49–56. [PubMed] [Google Scholar]
- 10.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86(3):353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 11.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 12.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Folkman J. Is angiogenesis an organizing principle in biology and medicine? Journal of pediatric surgery. 2007;42(1):1–11. doi: 10.1016/j.jpedsurg.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 14.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5(15):1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 15.Watnick RS. The role of the tumor microenvironment in regulating angiogenesis. Cold Spring Harbor perspectives in medicine. 2012;2(12):a006676. doi: 10.1101/cshperspect.a006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 17.Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix biology : journal of the International Society for Matrix Biology. 2015 doi: 10.1016/j.matbio.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amin DN, Bielenberg DR, Lifshits E, Heymach JV, Klagsbrun M. Targeting EGFR activity in blood vessels is sufficient to inhibit tumor growth and is accompanied by an increase in VEGFR-2 dependence in tumor endothelial cells. Microvascular research. 2008;76(1):15–22. doi: 10.1016/j.mvr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Amin DN, Hida K, Bielenberg DR, Klagsbrun M. Tumor endothelial cells express epidermal growth factor receptor (EGFR) but not ErbB3 and are responsive to EGF and to EGFR kinase inhibitors. Cancer research. 2006;66(4):2173–2180. doi: 10.1158/0008-5472.CAN-05-3387. [DOI] [PubMed] [Google Scholar]
- 20.Recamier JCA. Recherches sur le Traitment du Cancer. Paris, France: Gabon; 1829. [Google Scholar]
- 21.Talmadge JE, Fidler IJ. AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer research. 2010;70(14):5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fidler IJ. Metastasis: guantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. Journal of the National Cancer Institute. 1970;45(4):773–782. [PubMed] [Google Scholar]
- 23.Butler TP, Gullino PM. Quantitation of cell shedding into efferent blood of mammary adenocarcinoma. Cancer research. 1975;35(3):512–516. [PubMed] [Google Scholar]
- 24.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nature reviews Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 25.Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147(2):275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Folkman J. Role of angiogenesis in tumor growth and metastasis. Seminars in oncology. 2002;29(6 Suppl 16):15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 27.Guillerey C, Smyth MJ. NK Cells and Cancer Immunoediting. Current topics in microbiology and immunology. 2015 doi: 10.1007/82_2015_446. [DOI] [PubMed] [Google Scholar]
- 28.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer discovery. 2012;2(12):1091–1099. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinelli R, Zeiger AS, Whitfield M, Sciuto TE, Dvorak A, Van Vliet KJ, Greenwood J, Carman CV. Probing the biomechanical contribution of the endothelium to lymphocyte migration: diapedesis by the path of least resistance. Journal of cell science. 2014;127(Pt 17):3720–3734. doi: 10.1242/jcs.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hedley BD, Chambers AF. Tumor dormancy and metastasis. Advances in cancer research. 2009;102:67–101. doi: 10.1016/S0065-230X(09)02003-X. [DOI] [PubMed] [Google Scholar]
- 31.Gao D, Nolan D, McDonnell K, Vahdat L, Benezra R, Altorki N, Mittal V. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochimica et biophysica acta. 2009;1796(1):33–40. doi: 10.1016/j.bbcan.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong HS, Jones D, Liao S, Wattson DA, Cui CH, Duda DG, Willett CG, Jain RK, Padera TP. Investigation of the Lack of Angiogenesis in the Formation of Lymph Node Metastases. Journal of the National Cancer Institute. 2015;107(9) doi: 10.1093/jnci/djv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79(2):315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 34.Lee YJ, Koch M, Karl D, Torres-Collado AX, Fernando NT, Rothrock C, Kuruppu D, Ryeom S, Iruela-Arispe ML, Yoon SS. Variable inhibition of thrombospondin 1 against liver and lung metastases through differential activation of metalloproteinase ADAMTS1. Cancer research. 2010;70(3):948–956. doi: 10.1158/0008-5472.CAN-09-3094. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Hawighorst T, Velasco P, Streit M, Hong YK, Kyriakides TR, Brown LF, Bornstein P, Detmar M. Thrombospondin-2 plays a protective role in multistep carcinogenesis: a novel host anti-tumor defense mechanism. The EMBO journal. 2001;20(11):2631–2640. doi: 10.1093/emboj/20.11.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nature medicine. 2000;6(1):100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- 37.Beije N, Jager A, Sleijfer S. Circulating tumor cell enumeration by the CellSearch system: the clinician’s guide to breast cancer treatment? Cancer treatment reviews. 2015;41(2):144–150. doi: 10.1016/j.ctrv.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 38.Okegawa T, Itaya N, Hara H, Tambo M, Nutahara K. Circulating tumor cells as a biomarker predictive of sensitivity to docetaxel chemotherapy in patients with castration-resistant prostate cancer. Anticancer research. 2014;34(11):6705–6710. [PubMed] [Google Scholar]
- 39.Paget S. The Distribution of Secondary Growths in Cancer of the Breast. The Lancet. 1889;133(3421):571–573. [PubMed] [Google Scholar]
- 40.Fidler IJ, Poste G. The “seed and soil” hypothesis revisited. The Lancet Oncology. 2008;9(8):808. doi: 10.1016/S1470-2045(08)70201-8. [DOI] [PubMed] [Google Scholar]
- 41.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. The Lancet Oncology. 2002;3(1):53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 42.Radinsky R, Risin S, Fan D, Dong Z, Bielenberg D, Bucana CD, Fidler IJ. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 1995;1(1):19–31. [PubMed] [Google Scholar]
- 43.Ruoslahti E. Vascular zip codes in angiogenesis and metastasis. Biochemical Society transactions. 2004;32(Pt3):397–402. doi: 10.1042/BST0320397. [DOI] [PubMed] [Google Scholar]
- 44.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nature reviews Cancer. 2009;9(4):285–293. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kang SY, Halvorsen OJ, Gravdal K, Bhattacharya N, Lee JM, Liu NW, Johnston BT, Johnston AB, Haukaas SA, Aamodt K, et al. Prosaposin inhibits tumor metastasis via paracrine and endocrine stimulation of stromal p53 and Tsp-1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(29):12115–12120. doi: 10.1073/pnas.0903120106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oliver G. Lymphatic vasculature development. Nature reviews Immunology. 2004;4(1):35–45. doi: 10.1038/nri1258. [DOI] [PubMed] [Google Scholar]
- 47.Kaipainen A, Bielenberg DR. Hemangiogenesis versus Lymphangiogenesis. Vol. 2. Oxford: Elsevier Ltd; 2010. [Google Scholar]
- 48.Migliozzi MT, Mucka P, Bielenberg DR. Lymphangiogenesis and metastasis-A closer look at the neuropilin/semaphorin3 axis. Microvascular research. 2014;96C:68–76. doi: 10.1016/j.mvr.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. The Journal of experimental medicine. 2007;204(10):2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Das S, Sarrou E, Podgrabinska S, Cassella M, Mungamuri SK, Feirt N, Gordon R, Nagi CS, Wang Y, Entenberg D, et al. Tumor cell entry into the lymph node is controlled by CCL1 chemokine expressed by lymph node lymphatic sinuses. The Journal of experimental medicine. 2013;210(8):1509–1528. doi: 10.1084/jem.20111627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zwaans BM, Bielenberg DR. Potential therapeutic strategies for lymphatic metastasis. Microvascular research. 2007;74(2–3):145–158. doi: 10.1016/j.mvr.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ward PM, Weiss L. Metachronous seeding of lymph node metastases in rats bearing the MT-100-TC mammary carcinoma: the effect of elective lymph node dissection. Breast cancer research and treatment. 1989;14(3):315–320. doi: 10.1007/BF01806303. [DOI] [PubMed] [Google Scholar]
- 53.Shields JD, Emmett MS, Dunn DB, Joory KD, Sage LM, Rigby H, Mortimer PS, Orlando A, Levick JR, Bates DO. Chemokine-mediated migration of melanoma cells towards lymphatics--a mechanism contributing to metastasis. Oncogene. 2007;26(21):2997–3005. doi: 10.1038/sj.onc.1210114. [DOI] [PubMed] [Google Scholar]
- 54.Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2005;18(9):1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 55.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nature medicine. 2001;7(2):192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 56.Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer research. 2005;65(21):9789–9798. doi: 10.1158/0008-5472.CAN-05-0901. [DOI] [PubMed] [Google Scholar]
- 57.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. The New England journal of medicine. 1991;324(1):1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 58.Nico B, Benagiano V, Mangieri D, Maruotti N, Vacca A, Ribatti D. Evaluation of microvascular density in tumors: pro and contra. Histology and histopathology. 2008;23(5):601–607. doi: 10.14670/HH-23.601. [DOI] [PubMed] [Google Scholar]
- 59.Uzzan B, Nicolas P, Cucherat M, Perret GY. Microvessel density as a prognostic factor in women with breast cancer: a systematic review of the literature and meta-analysis. Cancer research. 2004;64(9):2941–2955. doi: 10.1158/0008-5472.can-03-1957. [DOI] [PubMed] [Google Scholar]
- 60.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nature reviews Cancer. 2002;2(10):727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 61.Eberhard A, Kahlert S, Goede V, Hemmerlein B, Plate KH, Augustin HG. Heterogeneity of angiogenesis and blood vessel maturation in human tumors: implications for antiangiogenic tumor therapies. Cancer research. 2000;60(5):1388–1393. [PubMed] [Google Scholar]
- 62.Valencak J, Heere-Ress E, Kopp T, Schoppmann SF, Kittler H, Pehamberger H. Selective immunohistochemical staining shows significant prognostic influence of lymphatic and blood vessels in patients with malignant melanoma. Eur J Cancer. 2004;40(3):358–364. doi: 10.1016/j.ejca.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 63.Hlatky L, Hahnfeldt P, Folkman J. Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn’t tell us. Journal of the National Cancer Institute. 2002;94(12):883–893. doi: 10.1093/jnci/94.12.883. [DOI] [PubMed] [Google Scholar]
- 64.Geretti E, van Meeteren LA, Shimizu A, Dudley AC, Claesson-Welsh L, Klagsbrun M. A mutated soluble neuropilin-2 B domain antagonizes vascular endothelial growth factor bioactivity and inhibits tumor progression. Molecular cancer research : MCR. 2010;8(8):1063–1073. doi: 10.1158/1541-7786.MCR-10-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 66.Beecken WD, Fernandez A, Joussen AM, Achilles EG, Flynn E, Lo KM, Gillies SD, Javaherian K, Folkman J, Shing Y. Effect of antiangiogenic therapy on slowly growing, poorly vascularized tumors in mice. Journal of the National Cancer Institute. 2001;93(5):382–387. doi: 10.1093/jnci/93.5.382. [DOI] [PubMed] [Google Scholar]
- 67.Emblem KE, Farrar CT, Gerstner ER, Batchelor TT, Borra RJ, Rosen BR, Sorensen AG, Jain RK. Vessel caliber--a potential MRI biomarker of tumour response in clinical trials. Nature reviews Clinical oncology. 2014;11(10):566–584. doi: 10.1038/nrclinonc.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Adini A, Fainaru O, Udagawa T, Connor KM, Folkman J, D’Amato RJ. Matrigel cytometry: a novel method for quantifying angiogenesis in vivo. Journal of immunological methods. 2009;342(1–2):78–81. doi: 10.1016/j.jim.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 69.Iakovlev VV, Gabril M, Dubinski W, Scorilas A, Youssef YM, Faragalla H, Kovacs K, Rotondo F, Metias S, Arsanious A, et al. Microvascular density as an independent predictor of clinical outcome in renal cell carcinoma: an automated image analysis study. Laboratory investigation; a journal of technical methods and pathology. 2012;92(1):46–56. doi: 10.1038/labinvest.2011.153. [DOI] [PubMed] [Google Scholar]
- 70.Brouty-Boye D, Zetter BR. Inhibition of cell motility by interferon. Science. 1980;208(4443):516–518. doi: 10.1126/science.6154315. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y, Adjei AA. Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor. The oncologist. 2015;20(6):660–673. doi: 10.1634/theoncologist.2014-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Husein B, Abdalla M, Trepte M, Deremer DL, Somanath PR. Antiangiogenic therapy for cancer: an update. Pharmacotherapy. 2012;32(12):1095–1111. doi: 10.1002/phar.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abdollahi A, Folkman J. Evading tumor evasion: current concepts and perspectives of anti-angiogenic cancer therapy. Drug resistance updates : reviews and commentaries in antimicrobial and anticancer chemotherapy. 2010;13(1–2):16–28. doi: 10.1016/j.drup.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 74.Folkman J. Angiogenesis inhibitors: a new class of drugs. Cancer biology & therapy. 2003;2(4 Suppl 1):S127–133. [PubMed] [Google Scholar]
- 75.Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer cell. 2014;26(5):605–622. doi: 10.1016/j.ccell.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer research. 2000;60(7):1878–1886. [PubMed] [Google Scholar]
- 77.Kerbel RS, Klement G, Pritchard KI, Kamen B. Continuous low-dose anti-angiogenic/metronomic chemotherapy: from the research laboratory into the oncology clinic. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2002;13(1):12–15. doi: 10.1093/annonc/mdf093. [DOI] [PubMed] [Google Scholar]
- 78.Hanahan D, Bergers G, Bergsland E. Less is more, regularly: metronomic dosing of cytotoxic drugs can target tumor angiogenesis in mice. The Journal of clinical investigation. 2000;105(8):1045–1047. doi: 10.1172/JCI9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer cell. 2009;15(3):232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15(3):220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu XD, Sun HC, Xu HX, Kong LQ, Chai ZT, Lu L, Zhang JB, Gao DM, Wang WQ, Zhang W, et al. Antiangiogenic therapy promoted metastasis of hepatocellular carcinoma by suppressing host-derived interleukin-12b in mouse models. Angiogenesis. 2013;16(4):809–820. doi: 10.1007/s10456-013-9357-6. [DOI] [PubMed] [Google Scholar]