Abstract
Objective
Little is known about glycemic control in type 2 diabetes patients treated with insulin in the high-risk period between hospital discharge and follow-up. We sought to assess the impact of remote glucose monitoring on postdischarge glycemic control and insulin titration.
Methods
We randomly assigned 28 hospitalized type 2 diabetes patients who were discharged home on insulin therapy to routine specialty care (RSC) or RSC with daily remote glucose monitoring (RGM). We compared the primary outcome of mean blood glucose and exploratory outcomes of hypoglycemia/hyperglycemia rates, change in hemoglobin A1c and glycated albumin, and insulin titration frequency between groups.
Results
Mean blood glucose was not significantly different between the treatment arms (144 ± 34 mg/dL in the RSC group and 172 ± 41 mg/dL in the RGM group; not significant), nor were there significant differences in any of the other measures of glycemia during the month after discharge. Hypoglycemia (glucometer reading <60 mg/dL) was common, occurring in 46% of subjects, with no difference between groups. In as-treated analysis, insulin dose adjustments (29% with an increase and 43% with decrease in insulin dose) occurred more frequently in the patients who used RGM (average of 2.8 vs. 1.2 dose adjustments; P = .03).
Conclusion
In this pilot trial in insulin-treated type 2 diabetes, RGM did not affect glycemic control after hospital discharge; however, the high rate of hypoglycemia in the postdischarge transition period and the higher frequency of insulin titration in patients who used RGM suggest a safety role for such monitoring in the transition from hospital to home.
INTRODUCTION
Patients with diabetes mellitus have a 3-fold increased risk for hospitalization compared to the nondiabetic population. Although much has been written about insulin use, glycemic control, and safety during hospitalizations, little has been reported on posthospitalization glycemic control, particularly for the 25% of type 2 diabetes patients who are discharged home on insulin (1). Insulin is a challenging medication to prescribe at discharge because the dosing is dependent on many factors that change in the immediate postdischarge period, including diet, activity, medications, and physiologic stress. Glycemic control and safety during the convalescent period immediately after hospital discharge in type 2 diabetes patients treated with insulin in the hospital is unknown. A survey of glycemic control postdischarge that included a high proportion of patients discharged on glucocorticoids reported that 49% had blood glucose (BG) levels >300 mg/dL. Yet even with these very high glucose levels, none of the 47 patients called for assistance (2). Although patients with type 2 diabetes are generally thought to be at low risk for hypoglycemia (3), hypoglycemia may be more common and riskier in patients with multiple comorbidities (4,5). In the survey of glucocorticoid-treated patients, 30% of patients self-reported at least one BG level <70 mg/dL, but rates of severe hypoglycemia were not reported (2).
It is now possible to monitor patients’ glucometer, blood pressure, and weight data remotely without the need for telephone lines or even a computer. Remote monitoring, or telehealth for diabetes care, has been extensively studied in the outpatient setting as a way to improve glycemic control but less so in the transition from hospital to home (6,7). Postdischarge telemonitoring has been most extensively studied in the heart failure patient population, with various balances of human and technological resources and with mixed results (8).
We used a remote monitoring system and web-based communication portal to gain insight into glycemic control in the immediate postdischarge period and adjust insulin dosing, if needed. We chose a 1-month follow-up period to align with current efforts around 30-day readmissions and allow for adequate time for resumption of care by outpatient providers. We hypothesized that patients discharged on insulin therapy who had daily remote glucose monitoring (RGM) would have lower mean BG 1 month after discharge compared with routine specialty care (RSC) patients, verified by direct glucometer downloads in both groups.
METHODS
Trial Design/Participants
We performed a randomized, controlled pilot study of adult patients with type 2 diabetes admitted to Massachusetts General Hospital between September 2011 and March 2013 who had an inpatient diabetes service or endocrinology consultation, a planned discharge home, and were prescribed treatment with insulin at the time of discharge. Patients with limited life expectancy, lack of access to an email address within their household, or who did not speak English were excluded. Patients were approached for study enrollment during business hours Monday through Thursday. Randomization treatment assignments occurred by block randomization in a block of four.
Intervention
Prior to discharge, consenting participants were randomly assigned to routine posthospitalization diabetes-specialty care or additional RGM and web-portal access to glucose readings and diabetes care plan. All patients completed baseline questionnaires querying demographic data, diabetes self-care, diabetes distress (Problem Areas in Diabetes questionnaire), and overall perceptions of diabetes control and impact on quality of life. Patients had blood drawn for hemoglobin A1c (HbA1c) and glycated albumin and were provided with new OneTouch Ultra 2 glucometers (Lifescan, Milpitas, CA). Patients in the intervention arm received a MedApps HealthPAL (Alere, Scottsdale, AZ) device and a glucometer data transfer cable. They were briefly taught at the bedside how to connect the device and also how to access the self-monitoring website (Diabetes Connect, Partners Center for Connected Health, Boston, MA) just prior to discharge. Website functionality allowed patient and provider to comment asynchronously on blood sugars and diabetes medication adjustments, but no specific recommendations were made regarding the patient’s use of the web portal. Patients were asked to upload glucometer readings on a daily basis by connecting the glucometer to the HealthPAL device and were reminded via email and web portal message if more than 2 business days had elapsed since the last upload.
All patients received detailed written diabetes discharge instructions, including specific instructions on self-monitored BG (SMBG) timing and targets, medication dosing and timing, treatment of hypoglycemia, contact information for the consulting provider for any postdis-charge glycemia-related questions, and outpatient followup plan as per RSC by the inpatient diabetes team (medical doctor [MD]/nurse practitioner). In addition to insulin, the oral anti hyperglycemic agent, metformin, was continued at discharge if there were no contraindications, but all other oral agents were discontinued on admission, and none of the patients were on glucagon-like peptide 1 agonist therapy. Patients had study follow-up 1 month after discharge, at which time they completed questionnaires, blood was drawn for HbA1c and glycated albumin, and their glucometer data were directly downloaded. All patients also received a study retention phone call 1 week postdischarge, at which time their follow-up visit was scheduled. In the RSC group, other follow-up phone calls were made at the discretion of the inpatient diabetes team, with patients called within 1 week of discharge by the inpatient diabetes provider who saw them in the hospital. In the intervention group, the participant was called if there were no data uploaded for 72 hours or if extreme blood sugars were noted (<70 or >350 mg/dL). A study MD reviewed the remotely uploaded blood sugar readings through the web portal once each working day (Monday through Friday, excluding hospital holidays). Any adjustments to insulin dosing based on extreme blood sugar readings were made via phone call and immediately posted to the secure website. Insulin dose adjustments not related to extreme blood sugar readings were first posted to the secure website, with a follow-up phone call to the patient to confirm receipt and understanding of the medication changes.
Outcomes
The primary outcome of interest was postdischarge mean patient-day averaged glucose from direct glucometer downloads. As patients were asked to assess their glycemic control a variable number of times each day, patient-day weighted mean BG, the mean of all BG readings on a single day averaged over the course of the follow-up period, was calculated for each patient (9). Exploratory secondary outcomes, established a priori, included postdischarge occurrence of hypoglycemia (BG <60 mg/dL), hyperglycemia (BG >300 mg/dL), frequency of insulin titration after discharge, change in glycated albumin, and change in HbA1c. All HbA1c measurements were performed using a high-performance liquid chromatography method that is Diabetes Control and Complication Trial–aligned and serves as one of the primary reference methods for the National Glycohemoglobin Standardization Program, with intra- and interassay coefficients of variation less than 2% (10). Glycated albumin was measured using a LUCICA GA-L kit (Asahi Kasei Pharma Corporation, Tokyo, Japan) with a Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation) on stored, frozen (−70°C) serum samples (11).
Sample size was based on having 80% power to detect a 38-mg/dL difference in the patient-day weighted mean BG between groups, assuming a standard deviation of 35 mg/dL and 2-tailed confidence interval of 0.05.
Statistical Methods
We compared baseline characteristics, the primary outcome of patient-day weighted mean BG, and exploratory outcomes of occurrence of hypoglycemia and hyperglycemia, frequency of insulin titration, change in HbA1c, and change in glycated albumin after discharge, using t tests for continuous variables, Fisher tests for dichotomous variables, and χ2 tests for categorical variables. Primary and exploratory outcomes were assessed as both intention-to-treat and as-treated analyses.
RESULTS
A total of 240 patient charts were prescreened to assess for eligibility for the study based on daily census logs of the inpatient consultation services (Fig. 1; CONSORT diagram). A total of 202 patients were ineligible because they were discharged to somewhere other than home (30%); deemed an inappropriate candidate for the study by the primary team (21%); received blood products, thereby invalidating assessment of HbA1c (11%); were not prescribed insulin therapy (12%); or did not have access to email (6%). Ultimately, we approached 38 patients who met the eligibility criteria, and 29 consented to participate. One patient was lost to follow-up and 2 intervention participants failed to upload glucometer readings.
Fig. 1.
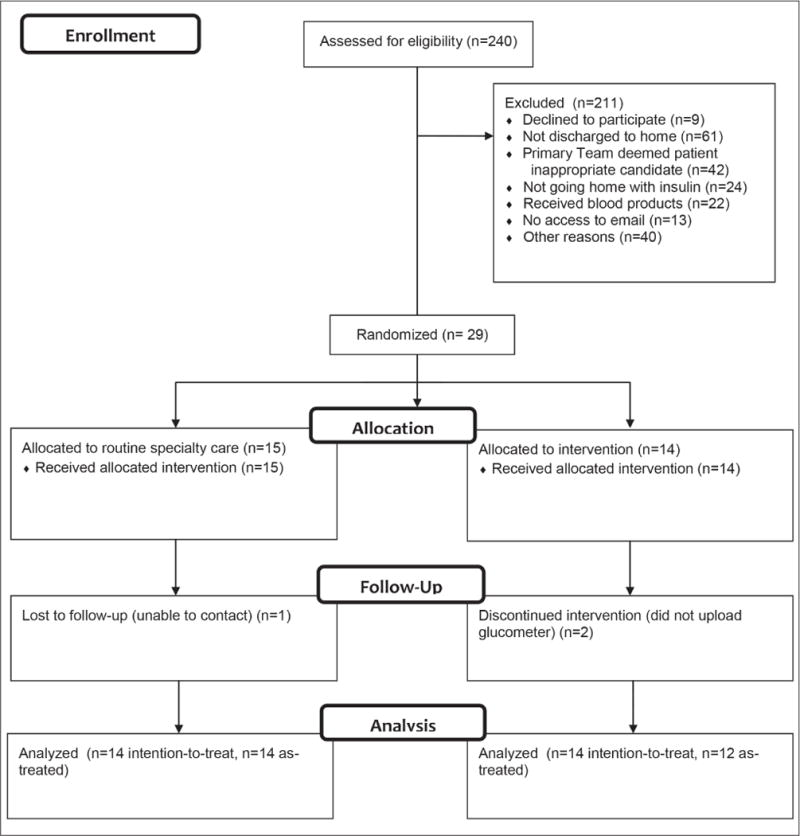
CONSORT diagram.
The 28 patients who completed the study were followed for an average (SD) of 32 (9) days after discharge. Their median age was 57 years (interquartile range [IQR], 45 to 63 years); 29% were female; and 57% were white, 14% non-white Hispanic, and 18% black. Median duration of type 2 diabetes was 10 years (IQR, 1 to 16 years), and average (SD) baseline HbA1c was 10.3% (2.2%), with average (SD) baseline glycated albumin of 28% (8.7%) (reference range, 11 to 16%). The patients were admitted for a variety of medical reasons, including 25% for uncontrolled diabetes and 29% for surgery. Hospital length-of-stay was a median of 4 days (IQR, 2 to 6 days). Forty-three percent of the cohort was insulin-naïve prior to admission. There were no significant differences in baseline characteristics between groups (Table 1).
Table 1.
Baseline Characteristics
| Routine specialty care (n = 14) |
RGM intervention (n = 14) |
|
|---|---|---|
| Age, years (SD) | 52 (14) | 54 (16) |
| Female, n (%) | 3 (21) | 5 (36) |
| White, n (%) | 8 (57) | 8 (57) |
| Hispanic, n (%) | 1 (7) | 3 (21) |
| Self-reported DM duration, years (SD) | 8 (11) | 12 (8) |
| Length of stay, days (SD) | 4.0 (2) | 4.9 (3) |
| Admission diagnosis, n (%) | ||
| Cardiovascular disease | 3 (21) | 3 (21) |
| DM | 5 (36) | 2 (14) |
| Pancreatitis | 1 (7) | 1 (7) |
| Surgery | 4 (29) | 4 (29) |
| Comorbidities, n (%) | ||
| Cardiovascular disease | 4 (29) | 3 (21) |
| Hypertension | 7 (50) | 10 (71) |
| Chronic kidney diseasea | 0 (0) | 4 (29) |
| Depression | 3 (21) | 1 (7) |
| Asthma/COPD | 3 (21) | 3 (21) |
| Baseline HbA1c, % (SD) | 10.2 (2.4) | 10.5 (1.9) |
| Baseline glycated albumin, % (SD)b | 30 (10) | 26 (7) |
| Insulin-naïve prior to admission, n (%) | 8 (57) | 4 (29) |
| Glucocorticoids at discharge, n (%) | 1 (7) | 2 (14) |
| Time to postdischarge follow-up, days (SD) | 30 (5) | 34 (11) |
| Baseline PAID score (SD) | 29 (15) | 26 (19) |
Abbreviations: COPD = chronic obstructive pulmonary disease; DM = diabetes mellitus; HbA1c = hemoglobin A1c; PAID = Problem Areas in Diabetes Scale [0–100]; RGM = remote glucose monitoring.
P = .01.
Reference range for glycated albumin enzyme-linked immunosorbent assay, 11–16%.
Feasibility
Of the 14 patients randomly assigned to RGM, 12 (86%) successfully uploaded their glucometer data at least once, with a median of 25 glucometer uploads during the 1-month follow-up. While most participants successfully and frequently uploaded their glucometer readings, fewer than half of the remote monitoring patients (43%) logged onto the web portal more than once during the study period.
Outcomes
In the intention-to-treat analysis, there were no significant differences between RGM intervention and RSC groups in postdischarge average daily glucose, change in HbA1c, or change in glycated albumin from baseline in hospital to 1 month after discharge; both groups improved (Table 2). Insulin dose changes occurred in 86% of patients in the RGM group and 57% of patients in the RSC group (P = .2). The RGM group trended towards more frequent insulin titration after discharge compared with the RSC group (average of 2.5 vs. 1.2 adjustments; P = .06), and fewer patients in the RGM compared to the RSC group reported that SMBG was bothersome (14% vs. 57%; P = .04). Patients in the intervention cohort received on average (SD) of 3.9 (1.7) postdischarge diabetes provider phone calls, whereas the RSC cohort reported an average (SD) of 1.3 (1.4) phone calls (P = .002). Similar results as those reported above were seen in the as-treated analysis (n = 12 for RGM, n = 14 for RSC), with frequency of insulin titration reaching statistical significance (average of 2.8 vs. 1.2 adjustments; P = .03).
Table 2.
Primary and Exploratory Outcomes
| Routine specialty care (n = 14) |
RGM intervention (n = 14) |
|
|---|---|---|
| Mean patient-day weight average glucose, mg/dL (SD)a | 144 (34) | 172 (41) |
| Any hypoglycemia, BG ≤60 mg/dL, n (%) | 7 (50) | 6 (43) |
| Any hyperglycemia, BG >300 mg/dL, n (%)a | 4 (29) | 10 (71) |
| Change in HbA1c from recruitment to 1 month follow-up, % (SD) | −2.7 (2.2) | −1.9 (1.6) |
| Change in glycated albumin, % (SD) | −12.4 (9.8) | −7.1 (6.5) |
| Mean number of insulin dose changes (SD)a | 1.2 (1.6) | 2.5 (1.9) |
| Increase in insulin dose, n (%) | 2 (14) | 6 (43) |
| Decrease in insulin dose, n (%) | 6 (43) | 6 (46) |
| SMBG bothersome, n (%)b | 8 (57) | 1 (14) |
Abbreviations: RMG = remote glucose monitoring; BG = blood glucose; SMBG = self-monitored blood glucose.
P = .06.
P = .04.
When glucometer data were downloaded and aggregated at the postdischarge visit, 13 of the 28 patients in this pilot study had at least one hypoglycemic (BG <60 mg/dL) episode and 14 had at least one hyperglycemic (BG >300 mg/dL) value captured on their glucometer, with no difference between groups. More than two-thirds (20 of 28) of patients had insulin medication changed in the month after discharge, 29% with an increase and 43% with a decrease in insulin dose. Whereas all patients with hypoglycemia in the intervention cohort (n = 6) had their insulin adjusted appropriately, 1 patient in the RSC cohort did not have his insulin adjusted in the setting of recurrent hypoglycemia (BG <60mg/dL). Routine postdischarge followup appointments were scheduled for 75% (n = 21) of the patients. Half (n = 14) of the patients reported being seen by an outpatient provider prior to the 1-month study visit, at a median of 22 days (IQR, 12 to 24 days) after discharge. Four patients were readmitted within 30 days of discharge, none for diabetes or hypoglycemia.
DISCUSSION
In this pilot and feasibility study, there were no demonstrable differences in glycemic outcomes in patients offered daily RGM, perhaps because both groups had substantial decreases in hyperglycemia (as measured by HbA1c and glycated albumin). Although all participants demonstrated improved glycemic control, extreme hyper- and hypoglycemia were still common postdischarge, and adjustments to insulin doses were made more often than not in both groups. Patients who were being remotely monitored had more frequent adjustments to their insulin, but the RSC group may have been monitored more closely than in true usual care, having been followed by an inpatient diabetes service and endocrine fellows who routinely call patients postdischarge for safety, biasing our results to the null.
Though a type 2 diabetes population is usually assumed to have low rates of hypoglycemia (12), there was a high frequency of hypoglycemia (46%) in these postdischarge subjects within a short follow-up period, suggesting that careful postdischarge monitoring is important for safety in this group. The higher mean BG in the RGM group may be related to the interventions in response to earlier detection of hypoglycemia. More patients needed a reduction, rather than an increase, in insulin dose after discharge, possibly reflecting improving insulin sensitivity due to resolution of the stress response of acute illness or glucotoxicity from prolonged hyperglycemia, and/or changes in activity and diet after hospitalization.
We found that remote glucometer monitoring was feasible and acceptable to patients offered enrollment in this study. The population of patients who were approached was potentially enriched to accept monitoring by the fact that the primary hospital providers were aware of the study design and deemed 17.5% of the prescreened patients inappropriate for the study. Feasibility data collected on patients who were approached to enroll in the study showed that <10% of eligible patients would be unable to participate in RGM due to lack of home resources, and <25% of patients approached declined to participate.
Among those who were enrolled, we found that subjects were able to use the plug-in remote monitoring device with their glucometer with minimal teaching prior to discharge. Whereas most participants successfully and frequently uploaded their glucometer readings, fewer than half of the remote monitoring patients (43%) logged onto the self-monitoring web portal more than once during the study period. This highlights the ease of use of the remote monitoring device compared to either the challenge or lack of perceived need for a web-based interface, which has implications for the many patient web portals being developed for diabetes care. Although only a little over one-third (36%) reported being “not comfortable” with computers and 60% reported being “very comfortable” with computers, 42% of patients did not have an email address of their own and instead shared an email address of someone with whom they lived. This is a significant consideration for future remote-monitoring web-portal designs, as they may need to be able to accommodate multiple users with the same email, though this was not a concern in this study because only one participant was enrolled per household. Barriers elicited in the exit questionnaire about use of the web portal were predominantly related to difficulty accessing the website (i.e., limited computer or internet access and difficulty with navigating the secure login system).
Our findings must be considered within the limitations of the study design. As all of the eligible patients were followed by inpatient endocrinology specialty teams, the population likely included relatively more uncontrolled and complicated type 2 diabetes cases. The 1-month followup period precluded meaningful interpretation of changes in HbA1c, and although glycated albumin may be useful in short-term follow-up, it is not a measurement currently used in clinical practice in the U.S. In addition, although recruiting from a limited pool reduced between-group differences in in-hospital management, it limited the pool of available participants, and the small sample size limited our ability to detect differences between groups. The size, single-center study, and specialty-treated population may limit generalizability; future work may seek to enroll a broader cross-section of patients discharged on insulin.
CONCLUSION
Changes in healthcare delivery models (i.e., accountable care organizations) putting providers and hospitals at risk for outcomes across the care continuum have led to increasing interest in the transition from inpatient to outpatient care, especially for high-risk medications and patient populations (13–15). Patients with diabetes on insulin therapy are both, and as such make a good test case for exploring new modalities in care across the care continuum. The current results demonstrate an unexpectedly high rate of hypoglycemia in recently discharged insulin-treated patients with type 2 diabetes, highlighting the utility of close monitoring in the transition from hospital for expedient insulin dose reductions. We found that although remote monitoring to send information to providers was easy for patients to incorporate into their routines, uptake of the web portal was low, which has implications for patient-centered solutions in this high-risk population.
Acknowledgments
We thank Dr. Michael Steffes, (Department of Laboratory Medicine and Pathology, University of Minnesota) for his assistance with the glycated albumin measurements. We would like to recognize Khinlei Myint U. and Dr. Joseph Kvedar, at the Partners Center for Connected Health for their continued technical support of our use of the remote monitoring devices and Diabetes Connect web-portal. Dr. Nancy J. Wei was supported by an NIDDK training grant (number T32 DK 007 028). Dr. Deborah J. Wexler was supported by an NIDDK Career Development Award (number K23 DK 080 228), which also supported this project in part.
Abbreviations
- BG
blood glucose
- HbA1c
hemoglobin A1c
- IQR
interquartile range
- RGM
remote glucose monitoring
- RSC
routine specialty care
Footnotes
To purchase reprints of this article, please visit: www.aace.com/reprints.
DISCLOSURE
The authors have no multiplicity of interest to disclose.
References
- 1.Wei NJ, Wexler DJ, Nathan DM, Grant RW. Intensification of diabetes medication and risk for 30-day readmission. Diabet Med. 2013;30:e56–e62. doi: 10.1111/dme.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimmel B, Sullivan MM, Rushakoff RJ. Survey on transition from inpatient to outpatient for patients on insulin: what really goes on at home? Endocr Pract. 2010;16:785–791. doi: 10.4158/EP10013.OR. [DOI] [PubMed] [Google Scholar]
- 3.Wright AD, Cull CA, Macleod KM, Holman RR. Hypoglycemia in Type 2 diabetic patients randomized to and maintained on monotherapy with diet, sulfonylurea, metformin, or insulin for 6 years from diagnosis: UKPDS73. J Diabetes Complications. 2006;20:395–401. doi: 10.1016/j.jdiacomp.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Miller ME, Bonds DE, Gerstein HC, et al. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ. 2010;340:b5444. doi: 10.1136/bmj.b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller CD, Phillips LS, Ziemer DC, Gallina DL, Cook CB, El-Kebbi IM. Hypoglycemia in patients with type 2 diabetes mellitus. Arch Intern Med. 2001;161:1653–1659. doi: 10.1001/archinte.161.13.1653. [DOI] [PubMed] [Google Scholar]
- 6.Jia H, Feng H, Wang X, Wu SS, Chumbler N. A longitudinal study of health service utilization for diabetes patients in a care coordination home-telehealth programme. J Telemed Telecare. 2011;17:123–126. doi: 10.1258/jtt.2010.100314. [DOI] [PubMed] [Google Scholar]
- 7.Verhoeven F, Tanja-Dijkstra K, Nijland N, Eysenbach G, van Gemert-Pijnen L. Asynchronous and synchronous teleconsultation for diabetes care: a systematic literature review. J Diabetes Sci Technol. 2010;4:666–684. doi: 10.1177/193229681000400323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black JT, Romano PS, Sadeghi B, et al. A remote monitoring and telephone nurse coaching intervention to reduce readmissions among patients with heart failure: study protocol for the Better Effectiveness After Transition - Heart Failure (BEAT-HF) randomized controlled trial. Trials. 2014;15:124. doi: 10.1186/1745-6215-15-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg PA, Bozzo JE, Thomas PG, et al. “Glucometrics” – assessing the quality of inpatient glucose management. Diabetes Technol Ther. 2006;8:560–569. doi: 10.1089/dia.2006.8.560. [DOI] [PubMed] [Google Scholar]
- 10.Little RR, Rohlfing CL, Sacks DB. Status of hemoglobin A1c measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011;57:205–214. doi: 10.1373/clinchem.2010.148841. [DOI] [PubMed] [Google Scholar]
- 11.Nathan DM, McGee P, Steffes MW, Lachin JM. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes. 2014;63:282–290. doi: 10.2337/db13-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 13.Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: a randomized trial. Ann Intern Med. 2012;157:1–10. doi: 10.7326/0003-4819-157-1-201207030-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138:161–167. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 15.Wu EQ, Zhou S, Yu A, Lu M, Sharma H, Gill J, Graf T. Outcomes associated with insulin therapy disruption after hospital discharge among patients with type 2 diabetes who had used insulin before and during hospitalization. Endocr Pract. 2012;18:651–659. doi: 10.4158/EP11314.OR. [DOI] [PubMed] [Google Scholar]


