Abstract
BACKGROUND
Lung cancer screening recommendations are based on results from the National Lung Screening Trial (NLST). The authors determined how the screening-eligible US population differs from NLST participants in terms of characteristics that affect their ability to benefit from screening.
METHODS
The authors identified respondents to the 2012 Health and Retirement Study (HRS), a national survey of individuals aged ≥50 years who are eligible for screening based on US Preventive Services Task Force and Centers for Medicare and Medicaid Services criteria. Comorbidities, life expectancy, smoking history, and other characteristics were compared between the screening-eligible population and NLST participants.
RESULTS
The authors estimated that in 2013, 8.4 million individuals (95% confidence interval, 7.9–8.9 million individuals) would have met the eligibility criteria for lung cancer screening established by the US Preventive Services Task Force. Compared with NLST participants, HRS screening-eligible respondents were older, more likely to be current smokers, and more likely to have been diagnosed with comorbidities. The 5-year survival rate was 87% in the HRS screening-eligible individuals versus 93% in the NLST participants (P<.001, based on a 2-sided test). Life expectancy was 18.7 years in the HRS screening-eligible individuals versus 21.2 years in the NLST participants.
CONCLUSIONS
The US population eligible for lung cancer screening is probably less likely to benefit from early detection than NLST participants because they face a high risk of death from competing causes. The results of the current study highlight the need for smoking cessation interventions targeting those patients eligible for screening and tools to help clinicians determine the potential benefits of screening in individual patients.
Keywords: comorbidity, early detection of cancer, life expectancy, lung neoplasms, population characteristics, smoking
INTRODUCTION
In 2013, the US Preventive Services Task Force (USPSTF), a US government advisory panel, recommended that adults aged 55 to 80 years who have a smoking history of 30 pack-years and who currently smoke or have quit within the past 15 years undergo annual low-dose computed tomography (LDCT) scans to screen for lung cancer.1 In 2015, the Centers for Medicare and Medicaid Services (CMS) announced that Medicare, the government insurance program for disabled and elderly individuals, will cover lung cancer screening for asymptomatic Medicare beneficiaries aged 55 to 77 years who meet the USPSTF criteria for smoking status and history.2 These decisions, like much of the recent literature regarding the benefits of screening, rely heavily on results from the National Lung Screening Trial (NLST).3–6 The NLST was a US-based trial that randomized 53,454 adults enrolled between August 2002 and April 2004 at 33 US medical centers to receive 3 annual LDCT screens or chest x-rays. The characteristics and outcomes of NLST participants have been well-described in trial publications,3–6 but to the best of our knowledge little is known regarding the characteristics of individuals eligible for screening. In 2010, the NLST reported on the characteristics of NLST participants compared with those of respondents to the Tobacco Use Supplement of the 2002 to 2004 US Census Bureau Current Population Survey.4 They found that trial participants were similar to trial-eligible individuals in the general population based on sex and race/ethnicity, but more trial participants were aged <70 years, were former (vs current) smokers, and were college-educated.
The comparisons in the 2010 NLST report need to be updated because the characteristics of the US population aged ≥65 years have changed over the last decade. Although the percentage of adults aged ≥65 years who were current smokers remained approximately the same between 2005 and 2013, the size of the US population aged ≥65 years has increased by approximately 15% between 2000 and 2010.7,8 In addition, although several reports have estimated the US population meeting the NLST criteria, these reports were published before the release of the 2013 USPSTF or 2015 CMS eligibility criteria, and therefore do not specifically address those recommendations.9–11
The objective of the current study was to provide an update regarding how the characteristics of NLST participants compare with more recent information concerning the US screening-eligible population. The results will be useful for determining the generalizability of the NLST findings to community practice and assessing the need for smoking cessation interventions and decision aids to facilitate the translation of screening recommendations into practice. We applied the 2013 USPSTF and 2015 CMS eligibility criteria to respondents to the Health and Retirement Study (HRS).12 The HRS is a nationally representative, National Institute on Aging-sponsored panel survey designed to collect information regarding the US population of adults aged ≥50 years currently in or nearing retirement. At the time of the current analysis, 2012 was the most recent available year. In the current study, we compared the characteristics of NLST participants with those of our HRS-estimated screening-eligible population, including demographics, socioeconomic status, comorbidities, survival rates, and life expectancy.
MATERIALS AND METHODS
Data Source
We assessed the characteristics of NLST participants using the NLST participant file.13 We assessed the characteristics of the screening-eligible population using the HRS.12 Respondents are surveyed every 2 years in the HRS. The sample is replenished every 6 years to account for attrition. Only noninstitutionalized adults are eligible for inclusion initially, but respondents who move into nursing homes remain in the HRS panel. Response rates, which vary across survey waves and components, are mostly between 80% and 90%. Information regarding deceased respondents is collected from proxy subjects.
We categorized respondents’ smoking history using questions that asked if they ever smoked, whether they currently smoke, the number of cigarettes they smoke currently, the number of cigarettes they smoked when they smoked the most, the age or year they started smoking, and the age or year they stopped smoking. Table 1 presents unweighted counts of the number of respondents categorized by current smoking status and smoking history.
TABLE 1.
Unweighted Counts of Respondents to the 2012 HRS Survey
| Category | All Ages
|
Aged 55 to 80 Years
|
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Meets USPSTF criteria for lung cancer screening | 2223 | 9.4 | 1775 | 10.5 |
| Current smoker, ≥ 30 pack-y | 1332 | 5.6 | 1066 | 6.3 |
| Former smoker, ≥ 30 pack-y, quit within past 15 y | 891 | 3.8 | 709 | 4.2 |
| Other former or current smokers | 10,673 | 45.0 | 7654 | 45.3 |
| Current smoker, < 30 pack-y | 1828 | 7.7 | 1250 | 7.4 |
| Current smoker, missing pack-y | 153 | 0.6 | 113 | 0.7 |
| Former smoker, < 30 pack-y, quit within past 15 y | 1235 | 5.2 | 872 | 5.2 |
| Former smoker, missing pack-y, quit within past 15 y | 9 | 0.0 | 9 | 0.1 |
| Former smoker, quit > 15 y ago | 7043 | 29.7 | 5107 | 30.2 |
| Smoker, missing pack-y and current smoking status | 405 | 1.7 | 303 | 1.8 |
| Never smoked | 10,357 | 43.6 | 7190 | 42.5 |
| Smoking status missing | 477 | 2.0 | 282 | 1.7 |
| Total | 23,730 | 100.0 | 16,901 | 100.0 |
Abbreviations: HRS, Health and Retirement Study; USPSTF, United States Preventive Services Task Force.
Sample Selection
The main study sample included respondents who were in the 2012 HRS survey sample and alive at the beginning of 2012. In separate analyses, we focused on respondents to the 2000 HRS (to determine how the number of screening-eligible individuals has changed over time) and the 2006 HRS (to determine 5-year survival rates).
We considered a respondent to be eligible for lung cancer screening based on the USPSTF lung cancer screening recommendation if they: 1) were aged 55 to 80 years; 2) had at least a 30-pack-year smoking history (1 pack-year is equivalent to smoking 1 pack of cigarettes [20 cigarettes] a day for 1 year); and 3) currently smoked or had quit within the past 15 years. We classified respondents as eligible for lung cancer screening under fee-for-service Medicare if they met the USPSTF criteria for smoking history as well as: 1) self-reported that they were enrolled in Medicare Part B (which covers outpatient care); 2) self-reported that they were not enrolled in a Medicare Advantage Plan; and 3) were aged 55 to 77 years. In addition, we reported selected results for respondents who met the criteria described above but were enrolled in a Medicare Advantage Plan. Medicare Advantage Plans are offered as an alternative to the government-run fee-for-service plan and offer equivalent benefit levels. Medicare enrollees can choose whether to enroll in a Medicare Advantage Plan or the government-run plan.
Statistical Analysis
We calculated the number of individuals in each eligibility category using the 2012 HRS sample weights and the “svy: total” command in Stata statistical software (version 11; StataCorp LP, College Station, Tex). We used the weighted percentage of screening-eligible respondents in the 2012 HRS who reported that they received a flu shot within the past year as an upper bound estimate of patients’ compliance with preventive services recommendations. All statistical tests were 2-sided with an α level of 5%.
We compared age group, sex, race, ethnicity, educational attainment, smoking status, and comorbidities that are common and reported in both the NLST and HRS data (diabetes, heart disease, and stroke) between subjects in the NLST (including subjects in both the LDCT arm and the chest x-ray arm)3,4 and HRS screening-eligible respondents. We tested the significance of differences in age group using weighted logistic regressions. We ran separate regressions for each characteristic.
We measured survival time among HRS screening-eligible respondents in 2006, which aligns with approximately the midpoint of the NLST, beginning from the date they responded to the survey. Records were right-censored at December 31, 2012. We reported Kaplan-Meier curves for screening-eligible and non-screening-eligible respondents. Stata’s command for creating Kaplan-Meier curves does not permit the use of sample weights. As an alternative, we divided the sample weights, Wi for respondent i, by 1000; rounded the weights to the nearest integer; and created Wi/1000 replicates of each observation. We computed Kaplan-Meier curves using this modified sample. We compared 5-year survival rates between screening-eligible respondents in the HRS and participants in the control arm of the NLST using a large-sample Z-test for proportions.
We projected life expectancy for screening-eligible HRS respondents and, separately, NLST participants by: 1) estimating Weibull survival models, adjusting for age and sex; 2) using the estimated parameters of the model to calculate monthly age-specific and sex-specific mortality rates; 3) estimating age-specific and sex-specific survival curves using the maximum of the projected mortality rates from the Weibull model and US life table values14; 4) calculating age-specific and sex-specific life expectancies by summing the area under the survival curves; and 5) calculating the average life expectancy in the sample using these values.15 In step 3, survival at time t + 1 equals survival at time t multiplied by 1 minus the percentage of individuals dying, , in which is the mortality rate estimated form the Weibull model and is the age-specific and sex-specific US life table value. By incorporating US life table values, we assume that no one survives past age 100 years and ensure that life expectancy estimates are not unreasonably large. We used the survival curves to calculate age-specific and sex-specific life expectancy and calculated life expectancy (step 5 above) among various groups of HRS respondents and, separately, NLST participants by averaging across individuals in each group.
RESULTS
There were 1775 respondents to the 2012 HRS who would have met the 2013 USPSTF criteria for lung cancer screening and 770 who would have met the 2015 CMS criteria. The results presented below used sample weights to create nationally representative estimates.
Table 2 displays estimated counts and 95% confidence intervals (95% CIs) for the number of individuals in the United States based on the HRS data who met the criteria set forth for lung cancer screening in 2013 by the USPSTF and in 2015 by the CMS. We estimated that 8.4 million individuals in 2012 would have met the 2013 USPSTF criteria, and 3.8 million were aged 65 to 80 years, including individuals who were not enrolled in Medicare. In 2000, only 2.9 million individuals (95% CI, 2.7–3.2 million individuals) were aged 55 to 64 years and would have met the 2013 USPSTF criteria, whereas 4.6 million were aged 55 to 64 years in 2012. Overall for 2012, 58% of individuals had received a flu shot within the prior year.
TABLE 2.
Estimates of the Number of Smokers (in Millions) in the 2012 HRS Survey Who Met the 2013 USPSTF and 2015 Medicare Lung Cancer Screening Criteriaa
| Age Group | USPSTFb
|
Medicare FFSc
|
||
|---|---|---|---|---|
| No. | 95% CI | No. | 95% CI | |
| 55–64 | 4.6 | 4.2–5.0 | 0.6 | 0.42–0.69 |
| 65–74 | 3.1 | 2.8–3.4 | 1.7 | 1.49–1.91 |
| 75–77 | 0.3 | 0.22–0.35 | ||
| 75–80 | 0.7 | 0.6–0.8 | ||
| Total | 8.4 | 7.9–8.9 | 2.5 | 2.28–2.79 |
Abbreviations: 95% CI, 95% confidence interval; FFS, fee-for-service; HRS, Health and Retirement Study; LE, life expectancy; USPSTF, US Preventive Services Task Force.
Estimates were weighted to produce nationally representative results.
In 2013, the USPSTF recommended lung cancer screening with low-dose computed tomography in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years.
Medicare covers screening for beneficiaries aged 55 to 77 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years.
For respondents enrolled in fee-for-service Medicare in 2012, there were 2.5 million individuals aged 55 to 77 years who would have met the 2015 CMS criteria for lung cancer screening. Of these, 2.0 million were aged 65 to 77 years. Overall for 2012, 70% of CMS screening-eligible beneficiaries (of all ages) received a flu shot.
We estimated that there were 1.1 million Medicare Advantage members in 2012 who were aged 55 to 77 years and would have met the 2015 CMS criteria for lung cancer screening (95% CI, 0.9–1.3 million individuals). There were 3.5 million individuals aged 65 to 77 years, including individuals not enrolled in Medicare, who would have met the 2015 CMS criteria (95% CI, 3.2–3.8 million individuals).
Comparing the characteristics of NLST participants with those of 2012 HRS screening-eligible respondents who would have met the 2013 USPSTF screening criteria, NLST participants were younger; more likely to have a college degree; less likely to be a current smoker; and less likely to have been diagnosed with diabetes, heart disease, or stroke (Table 3). The results comparing characteristics of NLST participants with those of 2012 HRS screening-eligible respondents enrolled in fee-for-service Medicare and who would have met the 2015 CMS screening criteria were similar to those for the NLST participants versus the 2013 USPSTF criteria (Table 3).
TABLE 3.
Characteristics of the 53,456 NLST Participants and Individuals in the 2012 HRS Survey Who Met the 2013 USPSTF and 2015 Medicare Criteriaa
| Characteristic | NLST
|
USPSTFb
|
Medicare FFSc
|
||
|---|---|---|---|---|---|
| % | % | P | % | P | |
| Age group, y | |||||
| 55–59 | 42.8 | 29.4 | <.001 | 9.3 | <.001 |
| 60–64 | 30.6 | 25.3 | 12.5 | ||
| 65–69 | 17.8 | 22.3 | 36.2 | ||
| 70–74 | 8.8 | 14.2 | 30.8 | ||
| ≥75 | 0.0 | 8.8 | 11.1 | ||
| Male | 59.0 | 56.8 | .150 | 52.3 | .009 |
| Black | 4.4 | 7.0 | <.001 | 5.7 | .122 |
| Hispanic | 1.7 | 3.9 | <.001 | 3.8 | .002 |
| College degree | 31.5 | 12.9 | <.001 | 11.3 | <.001 |
| Current smoker | 48.2 | 60.8 | <.001 | 55.9 | .003 |
| Diabetes | 9.7 | 21.7 | <.001 | 24.1 | <.001 |
| Heart disease | 12.7 | 26.4 | <.001 | 33.2 | <.001 |
| Stroke | 2.8 | 8.2 | <.001 | 11.6 | <.001 |
Abbreviations: FFS, fee-for-service; HRS, Health and Retirement Study; NLST, National Lung Screening Trial; USPSTF, US Preventive Services Task Force.
Estimates were weighted to produce nationally representative results.
In 2013, the USPSTF recommended lung cancer screening with low-dose computed tomography in adults aged 55 to 80 years who have a 30 pack-year smoking history and currently smoke or have quit within the past 15 years.
Includes respondents who are covered by Medicare Part B and do not have lung disease. Estimates from the HRS were weighted to produce nationally representative estimates.
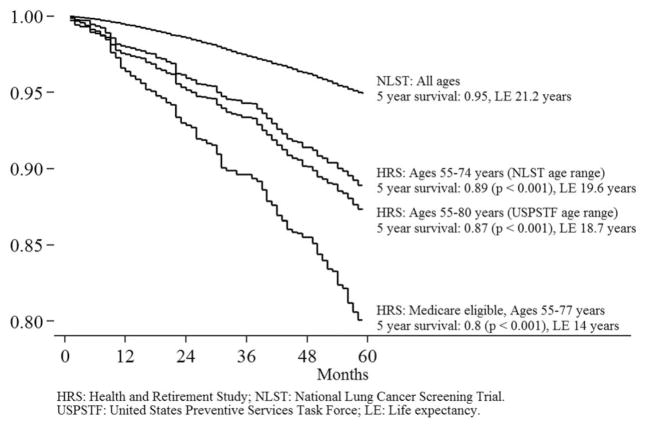
Figure 1 displays Kaplan-Meier survival curves for NLST participants (the top curve) and the HRS respondents who would have met the 2013 USPSTF lung cancer screening criteria. Estimates for HRS respondents were adjusted for sampling weights, as described earlier, but we refer to survival among “respondents” for ease of explication. Each curve shows results for a different group of HRS screening-eligible respondents. The 5-year survival rates among HRS screening-eligible respondents in 2006 were significantly lower than the 95% survival rate observed among participants in the NLST (P<.001 in each case).
Figure 1.
Survival among screening-eligible individuals and National Lung Screening Trial (NLST) participants. HRS indicates Health and Retirement Study; LE, life expectancy; USPSTF, US Preventive Services Task Force.
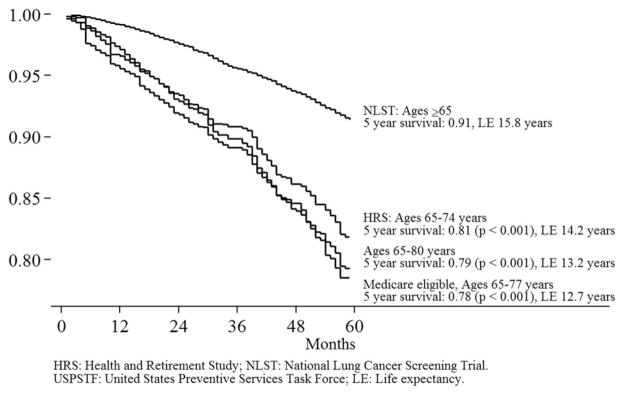
We estimated that life expectancy among NLST participants was 21.2 years. We estimated life expectancy among HRS screening-eligible respondents aged 55 to 74 to be 19.6 years. The difference reflects differences in the age and sex distribution between NLST participants and the US population (as captured by the HRS sample weights) as well as differences in survival within age and sex groups. Life expectancy among HRS screening-eligible respondents aged 55 to 80 years, the age range for which screening is recommended by the USPSTF, was 18.7 years. Life expectancy was found to be 14.0 years among HRS screening-eligible respondents enrolled in fee-for-service Medicare who were aged 55 to 77 years. Figure 2 shows survival and life expectancy estimates among respondents aged ≥65 years.
Figure 2.
Survival among screening-eligible individuals and National Lung Screening Trial (NLST) participants aged ≥ 65 years. HRS indicates Health and Retirement Study; LE, life expectancy.
DISCUSSION
Most of what we know about the benefits and harms of screening for lung cancer with LDCT is based on data from the NLST.5,6,16,17 The benefits and harms of screening vary based on the risk of lung cancer and the risk of death from competing causes. Compared with the characteristics of NLST participants, the results of the current study demonstrated that the screening-eligible US population differed in several respects. Individuals eligible for screening in the general population included a higher percentage of current smokers; faced a higher risk of death from lung cancer; and, all else being equal, were substantially more likely to benefit from screening. However, individuals eligible for screening in the United States were also found to be older, more likely to have comorbidities, and to have higher mortality rates and shorter life expectancies.
Pinsky et al reported that, in the NLST, the false-positive rate was higher in Medicare-eligible participants compared with those aged <65 years (27.7% vs 22.0%), invasive diagnostic procedures after false-positive screening results were more frequent in the older cohort compared with those aged <65 years (3.3% vs 2.7%), and complications from invasive procedures were in the range of 9% to 10% for both groups.6 Estimates of the overdiagnosis rate based on NLST data ranged from 9.9% to 18.5% of screen-detected cases.18,19 As lung cancer screening is implemented in the United States, surveillance will be needed to monitor whether community practice has higher false-positive results, overdiagnosis, or major complications from invasive procedures than observed in the NLST.
The findings of the current study are relevant for efforts to increase the capacity of the health system to deliver lung cancer screening in community practice. We found that 8.4 million individuals would have met the USPSTF criteria for lung cancer screening in 2012. Our estimate is close to estimates of the screening-eligible population aged 55 to 74 years based on NLST criteria and the 2010 National Health Interview Survey (approximately 8.7 million).10,11 Although the national landscape is likely to change the 2015 CMS coverage decision, studies conducted before 2015 have suggested additional lung cancer screening programs and radiologists would be needed to provide screening services to all the individuals who meet the USPSTF screening criteria.20,21 An additional resource-related question is whether adherence to annual lung cancer screening in community practice will be similar to the NLST. Adherence to lung cancer screening was >90% in the NLST, but this occurred within the context of a randomized trial.3 NLST participants volunteered for the trial and would not necessarily have the same characteristics or motivations as a random sample of all eligible patients in a community. We found that only 58% of the lung cancer screening-eligible population received a flu shot; thus, compliance with lung cancer screening in community practice might not be as high as observed in the NLST.
The projected benefits of lung cancer screening are highly dependent on how screening affects smoking rates.22 The Agency for Healthcare Research and Quality’s Treating Tobacco Use and Dependence clinical guidelines23 indicate that: 1) pharmacotherapy is effective in the treatment of nicotine dependence; 2) behavioral counseling is effective in the treatment of nicotine dependence; and 3) the combination of both is more effective than either alone. The CMS decision regarding final coverage requires that screening-eligible beneficiaries who are current smokers receive smoking cessation counseling and information regarding tobacco cessation interventions.2 The results of the current study indicate that the screening-eligible population will have higher smoking rates than those noted among NLST participants, thus creating a substantial demand for cessation services. The mandate to integrate smoking cessation into screening services capitalizes on a “teachable moment” that may coincide with an individual undergoing lung cancer screening.24 Full implementation of effective smoking cessation services will be an important addition to community practice; in 2010, < 50% of smokers in the United States had been advised by a physician to quit.25 A recent examination of smoking cessation among current smokers in the NLST demonstrated higher quit rates among those who reported their primary care providers had completed the more intensive components of the 5As (ask, advise, assess, assist [talk about quitting or recommend stop-smoking medications or counseling], and arrange follow-up): assist and arrange.26 Thus, greater efforts are needed to integrate cessation services into clinical practice, particularly given that physician involvement can play a critical role in smoking cessation.23
The current study is based on the HRS survey, which relies on respondent self-report for collecting data regarding smoking and health conditions. Health conditions among NLST participants also were assessed via self-report.2 Our estimates may be biased by systematic differences between survey respondents and nonrespondents and by misreporting by respondents. The estimates in the current study are based on 2012 HRS data, which were published before the 2013 USPSTF and 2015 CMS recommendations were released. These estimates do not reflect any changes in the size or composition of the screening-eligible population after 2012.
The results of the current study demonstrate that the screening-eligible US population differs from NLST participants in important respects that influence the potential benefits of screening vary so widely within the screening-eligible population, it is important for physicians to tailor screening recommendations to individual patients.17 CMS requires as a condition of coverage that clinicians engage in shared decision-making with beneficiaries before lung cancer screening. We found that < 12% of eligible patients have a college degree, suggesting that clinicians may find it challenging to communicate concepts such as “competing risks” and “false-positives” with screening-eligible patients. Patient decision support tools can help to bridge the communication gap,27–29 but they should incorporate information regarding the risks associated with invasive procedures and how the benefits of early detection are related to the risk of death from competing causes.
Acknowledgments
FUNDING SUPPORT
David Howard received support from the Centers for Disease Control and Prevention 12IPA17659.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Drs. Howard and Bach are members of the Medicare Evidence Development and Coverage Advisory Committee. Dr. Howard received funding through an Interagency Personnel Agreement from the Centers for Disease Control and Prevention.
References
- 1.Moyer VA U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 2.Syrek JT, Chin J, Ashby L, Hermansen J, Hutter L Center for Medicare & Medicaid Services. [Accessed February 11, 2015];Decision memo for screening for lung cancer with low dose computed tomography (LDCT) (CAG-00439N) http://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274.
- 3.National Lung Screening Trial Research Team. Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258:243–253. doi: 10.1148/radiol.10091808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Baseline characteristics of participants in the randomized National Lung Screening Trial. J Natl Cancer Inst. 2010;102:1771–1779. doi: 10.1093/jnci/djq434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Lung Screening Trial Research Team. Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinsky PF, Gierada DS, Hocking W, Patz EF, Jr, Kramer BS. National Lung Screening Trial findings by age: Medicare-eligible versus under-65 population. Ann Intern Med. 2014;161:627–633. doi: 10.7326/M14-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamal A, Agaku IT, O’Connor E, King BA, Kenemer JB, Neff L. Current cigarette smoking among adults–United States, 2005–2013. MMWR Morb Mortal Wkly Rep. 2014;63:1108–1112. [PMC free article] [PubMed] [Google Scholar]
- 8.Werner CA. The Older Population: 2010. Census Brief C2010BR-09. Washington, DC: US Census Bureau; 2011. [Google Scholar]
- 9.Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen. 2012;19:154–156. doi: 10.1258/jms.2012.012010. [DOI] [PubMed] [Google Scholar]
- 10.Doria-Rose VP, White MC, Klabunde CN, et al. Use of lung cancer screening tests in the United States: results from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21:1049–1059. doi: 10.1158/1055-9965.EPI-12-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J, Ward EM, Smith R, Jemal A. Annual number of lung cancer deaths potentially avertable by screening in the United States. Cancer. 2013;119:1381–1385. doi: 10.1002/cncr.27813. [DOI] [PubMed] [Google Scholar]
- 12.Institute for Social Research. [Accessed December 10, 2014];The Health and Retirement Study: an introduction. http://hrsonline.isr.umich.edu/index.php?p=intro.
- 13.National Cancer Institute Cancer Data Access System. [Access March 2, 2015];National Lung Screening Trial. https://biometry.nci.nih.gov/cdas/studies/nlst/
- 14.Arias E. United States life tables, 2006. Natl Vital Stat Rep. 2010;58:1–40. [PubMed] [Google Scholar]
- 15.Howard DH, Tangka FK, Seeff LC, Richardson LC, Ekwueme DU. The impact of detection and treatment on lifetime medical costs for patients with precancerous polyps and colorectal cancer. Health Econ. 2009;18:1381–1393. doi: 10.1002/hec.1434. [DOI] [PubMed] [Google Scholar]
- 16.Bach PB, Mirkin JN, Oliver TK, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307:2418–2429. doi: 10.1001/jama.2012.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bach PB, Gould MK. When the average applies to no one: personalized decision making about potential benefits of lung cancer screening. Ann Intern Med. 2012;157:571–573. doi: 10.7326/0003-4819-157-8-201210160-00524. [DOI] [PubMed] [Google Scholar]
- 18.de Koning HJ, Meza R, Plevritis SK, et al. Benefits and harms of computed tomography lung cancer screening strategies: a comparative modeling study for the US Preventive Services Task Force. Ann Intern Med. 2014;160:311–320. doi: 10.7326/M13-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patz EF, Jr, Pinsky P, Gatsonis C, et al. NLST Overdiagnosis Manuscript Writing Team. Overdiagnosis in low-dose computed tomography screening for lung cancer. JAMA Intern Med. 2014;174:269–274. doi: 10.1001/jamainternmed.2013.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smieliauskas F, MacMahon H, Salgia R, Shih YC. Geographic variation in radiologist capacity and widespread implementation of lung cancer CT screening. J Med Screen. 2014;21:207–215. doi: 10.1177/0969141314548055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eberth JM, Qiu R, Adams SA, et al. Lung cancer screening using low-dose CT: the current national landscape. Lung Cancer. 2014;85:379–384. doi: 10.1016/j.lungcan.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 22.McMahon PM, Kong CY, Bouzan C, et al. Cost-effectiveness of computed tomography screening for lung cancer in the United States. J Thorac Oncol. 2011;6:1841–1848. doi: 10.1097/JTO.0b013e31822e59b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agency for Healthcare Research and Quality. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services, Agency for Healthcare Research and Quality; 2015. [Google Scholar]
- 24.Pua BB, Dou E, O’Connor K, Crawford CB. Integrating smoking cessation into lung cancer screening programs [published online ahead of print May 16, 2015] Clin Imaging. doi: 10.1016/j.clinimag.2015.05.004. pii: S0899-707100116-3. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Quitting smoking among adults-United States, 2001–2010. MMWR Morb Mortal Wkly Rep. 2011;60:1513–1519. [PubMed] [Google Scholar]
- 26.Park ER, Gareen IF, Japuntich S, et al. Primary care provider-delivered smoking cessation interventions and smoking cessation among participants in the National Lung Screening Trial [published online ahead of print June 15, 2015] JAMA Intern Med. doi: 10.1001/jamainternmed.2015.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Memorial Sloan Kettering Cancer Center. [Accessed December 2, 2014];Lung Cancer Screening Decision Tool. http://nomograms.mskcc.org/Lung/Screening.aspx.
- 28.US Department of Veterans Affairs, National Center for Health Promotion and Disease Prevention. [Accessed March 10, 2015];Lung cancer screening. http://www.prevention.va.gov/Lung_Cancer_Screening.asp.
- 29.Volk RJ, Linder SK, Leal VB, et al. Feasibility of a patient decision aid about lung cancer screening with low-dose computed tomography. Prev Med. 2014;62:60–63. doi: 10.1016/j.ypmed.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]