Abstract
The synthesis of acyclic cucurbit[n]uril dendrimers G1 – G3 that bear four dendrons on their aromatic sidewalls via thiolate SN2 chemistry is reported. G1 – G3 are polycationic and can bind to pEGFP plasmid DNA as shown by dynamic light scattering (DLS), gel electrophoresis, and scanning electron microscopy (SEM). The gene delivery ability of G1 – G3 is presented.
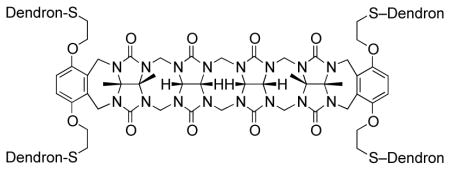
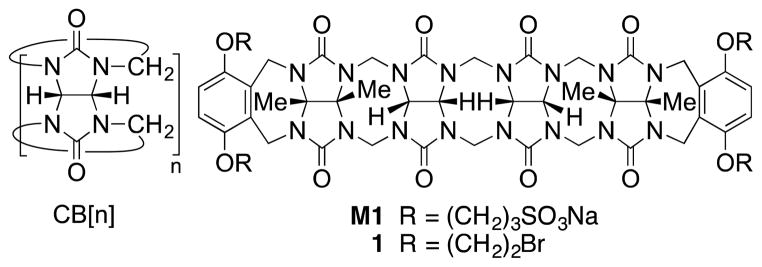
The cucurbit[n]uril family of molecular containers (CB[n], n = 5, 6, 7, 8, 10, 14; Figure 1) features a hydrophobic cavity that is shaped by n glycoluril rings and guarded by two symmetry equivalent ureidyl C=O portals.1 CB[n] compounds have attracted substantial interest from the supramolecular community in the past 15 years2 because of the ready availability of a homologous series of hosts that display high affinity and high selectivity toward hydrophobic cations and even neutral species in water.3 Even more importantly, CB[n] host-guest chemistry is highly stimuli responsive in that photochemistry, electrochemistry, pH changes, and chemical stimuli can be used to dictate changes in CB[n] host-guest constitutions.4 Accordingly, CB[n] compounds have been used to construct a variety of functional supramolecular systems, including molecular machines, chemical sensors, drug delivery systems, and materials for gas sorption and purification.4 Of high relevance to the work reported in this paper are previous reports of the use of CB[n] compounds to complex to the focal point of dendrons and the periphery of dendrimers in a non-covalent fashion. For example, Kim’s group constructed ternary complexes between poly(propyleneimine) (PPI) dendrimers, CB[6], and DNA and explored gene delivery efficiency.5 In contrast, Kaifer’s group used CB[n] host-guest chemistry to bind the focal points of dendritic wedges and electrochemically trigger dendrimer assembly.6 More recently, CB[7] non-covalent complexes of poly(amidoamine) (PAMAM) dendrimers formed the basis for a light harvesting system and a system capable of controlled release under redox or supramolecular control.7 Despite significant interest in the non-covalent CB[n] dendrimer chemistry, there have been no examples to date of CB[n] compounds serving as a covalent core for dendrimers. The main reason for the absence of covalent CB[n] dendrimer chemistry is that controlled functionalization of CB[n] remains challenging with synthetic schemes for the preparation of monofunctionalized CB[n] derivatives appearing only in the past few years.8 Dendrimers have broadly impacted science, especially the fields of drug and gene delivery.9 Polycationic dendrimers bind DNA efficiently by multivalent electrostatical interactions and deliver it inside cells. Accordingly, we sought to prepare covalent CB[n] dendrimers bearing cationic arms for transfection studies.
Figure 1.
Chemical structures of CB[n] (n = 5, 6, 7, 8, 10, 14) molecular containers and acyclic CB[n]-type molecular containers M1 and 1.
Recently, the groups of Isaacs10 and Sindelar11 have been studying the acyclic CB[n]-type receptors that consist of a central glycoluril oligomer capped with two aromatic walls as typified by M1 (Figure 1). Acyclic CB[n]-type receptors retain the essential molecular recognition features of the CB[n] family of molecular containers. For example, M1 and its derivatives have been shown to function as components of sensor arrays, as receptors for carbon nanotubes, as solubilizing agents for insoluble drugs, and as an in vivo reversal agent for the neuromuscular blocking agent rocuronium.10 Because acyclic CB[n] compounds are synthesized by a building block methodology, they are amenable to more straightforward synthetic modification which allows diversification. In this paper we report the preparation of three acyclic CB[n] cored dendrimers (G1 – G3) with peripheral ammonium functionality, their self-assembly properties with DNA, and investigation of their gene delivery ability. We envisioned that acyclic CB[n] compounds would enhance transfection efficiency because their C=O portals should promote intermolecular aggregation by ion-dipole interactions with the terminal NH3+ groups.
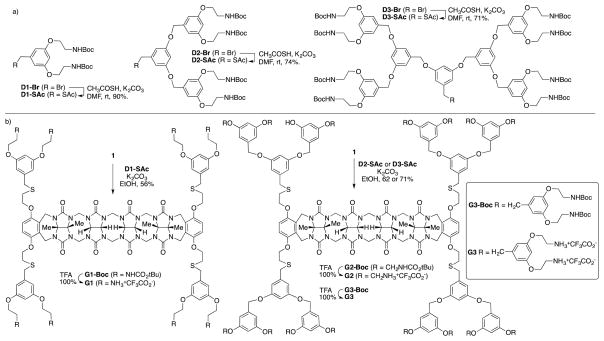
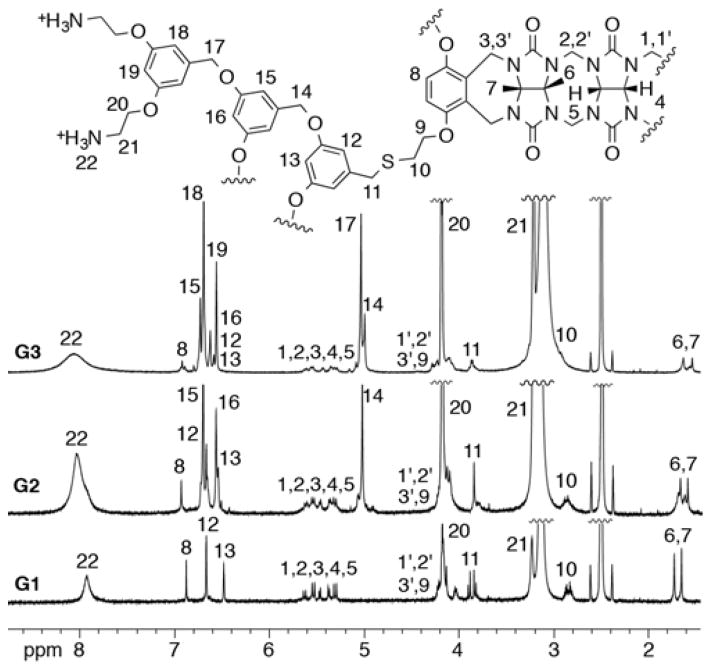
For the preparation of a series of acyclic CB[n] dendrimers, we decided to use a post-functionalization strategy to graft Frechet-type dendrons12 onto a central acyclic CB[n]-type receptor. As a starting material we selected the known acyclic CB[n]-type container 1 which contains four electrophilic primary alkyl bromide arms.10f Scheme 1 shows the structures of dendrons D1-Br, D2-Br, and D3-Br which were prepared according to the literature procedures.13 To transform D1-Br, D2-Br, and D3-Br from their electrophilic alkyl bromide forms into nucleophilic forms that would react with acyclic CB[n] container 1 we allowed them to react with thiolacetic acid under basic conditions to yield D1-SAc, D2-SAc, and D3-SAc in good yield (Scheme 1). The attachment of the dendrons to the core scaffold 1 was accomplished by the in situ deprotection of D1-SAc – D3-SAc using K2CO3 in EtOH to yield the thiolates which undergo efficient SN2 reaction with 1 to yield the corresponding 1st – 3rd generation dendrimers G1-Boc – G3-Boc in good yields (56 – 71%) after purification by precipitation. Deprotection of G1-Boc – G3-Boc into G1 – G3 was accomplished in quantitative yield by treatment with CF3CO2H at room temperature. Compounds G1 – G3 were characterized by 1H and 13C NMR spectroscopy and by electrospray mass spectrometry; the data was fully consistent with the depicted structures (Supporting Information). For example, the 1H NMR spectra recorded for G1 – G3 at 70 °C in DMSO (Figure 2) shows the expected number of aromatic C-H resonances (G1: 3; G2: 5; G3: 7) in the 6–7 ppm region and the expected 5 resonances for the glycoluril CH2 and CH groups in the 5.3 – 5.7 ppm region of the spectrum. 1H NMR spectra recorded at lower temperatures in DMSO or in water are broadened which is indicative of aggregation phenomena. Similarly, the 13C NMR spectra recorded for G1-Boc – G3-Boc in DMSO display the expected 3 C=O resonances along with aromatic resonances (G1-Boc: 7 expected, 7 found; G2-Boc: 11 expected, 11 found; G3-Boc: 15 expected, 14 found). The high resolution electrospray mass spectra recorded for G1 – G3 show multiply charged ions (+2 to +7) that match the calculated values derived from their free base molecular formulas.
Scheme 1.
Synthesis of: a) dendrons D1-SAc – D3-SAc, and b) 1st – 3rd generation dendrimers G1 – G3.
Figure 2.
1H NMR spectra recorded (600 MHz, DMSO-d6, 70 °C) for the G1, G2, and G3 dendrimers.
To further investigate the aggregation of G1 – G3 in water which was inferred based on the NMR experiments described above, we initially performed DLS measurements. DLS measurements of aqueous solutions of G1 – G3 show size distributions with maxima at 118 nm, 131 nm, and 100 nm, respectively which correspond to aggregated forms of the dendrimers (Supporting Information). Similarly, SEM of deposited aqueous solutions of G1 – G3 showed particles in the 20 nm – 1 μm range (Supporting Information). We posit that the amphiphilic nature of polycationic G1 – G3 with their large aromatic surfaces along with their ureidyl C=O portals which can bind to ammonium ions by ion-dipole interactions promotes the aggregation process.
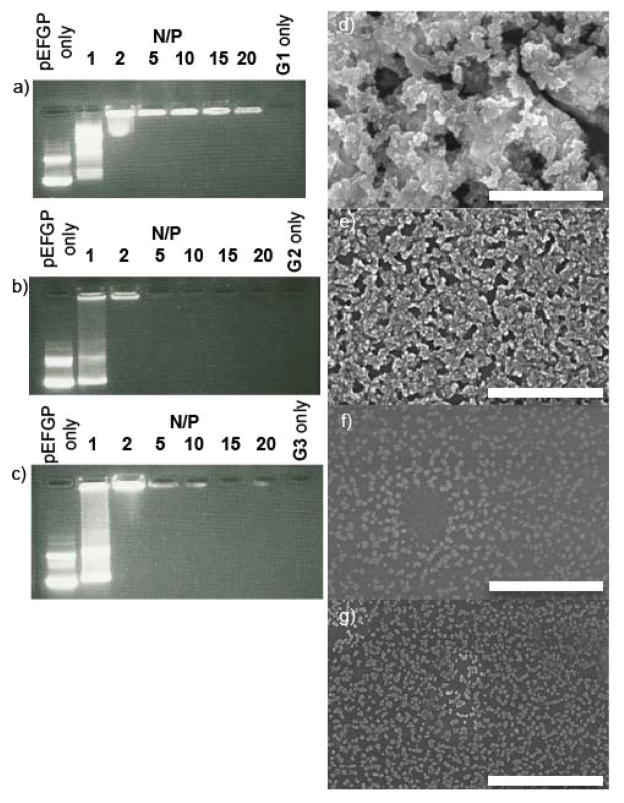
Next, we sought to determine the ability of the polycationic G1 – G3 to interact with plasmid DNA (pEGFP). Figure 3a–c shows the agarose gel electrophoresis images of pEGFP in the presence of ethidium bromide (EtBr) and an increasing concentration of G1, G2, or G3 defined by the N/P ratio (number of amine/number of phosphate). For G1, G2, and G3 free DNA remains visible at the electrically neutral ratio (N/P = 1); at higher N/P ratios (N/P ≥ 2) the bands migrate more slowly indicative of condensation of the plasmid. For G1, the fluorescence produced by the intercalation of EtBr into the DNA base pairs persists even at higher N/P ratios whereas for G2 and G3 the fluorescence becomes diminished (Figure 3a–c). This result suggests that the condensation of pEGFP is more efficient with G2 and G3 than with G1 and that displacement of EtBr occurs readily for G2 and G3. Our interpretation regarding the pEGFP condensing ability of G1 – G3 is supported by SEM measurements (Figure 3d–g). At N/P = 20, the plasmid-G1 complexes (Figure 3e) are structurally similar to that of pEGFP alone (Figure 3d). In sharp contrast, however, for the G2 and G3 plasmid DNA complexes, individual particles with diameters in the 20 to 100 nm range are observed (Figure 3f,g). Further support for the condensation of the pEGFP in solution in the presence of G1 – G3 was obtained by DLS measurements. At N/P = 20, the DLS measurement of particles formed by Gn-plasmid DNA complexes show size distributions (75, 66, 81 nm) that are significantly smaller than aggregates formed by dendrimers alone (118, 131, 100 nM) or pEGFP alone (49 and 471 nm) (Supporting Information). Peaks are also observed at ≈ 2 nm in the DLS for G2 and G3 alone that are at the detection limit of the instrument which may correspond to small amounts of unaggregated dendrimer. Finally, we measured the Zêta potential of the Gn-plasmid DNA polyplexes (G1: +38 mV; G2: +46 mV; G3: +49 mV) which confirmed that the Gn-plasmid DNA polyplexes are strongly positively charged (Supporting Information) and provides an explanation of the condensation that occurs upon polyplex formation.
Figure 3.
Agarose gel electrophoresis images of pEFGP (0.8 μg/well) and EtBr (0.5 μg mL−1) in the presence of: a) G1, b) G2, and c) G3 at different N/P ratios. SEM images of: d) pEGFP alone (3 μg/mL), e) pEGFP and G1, f) pEGFP and G2, and g) pEGFP and G3 at N/P 20. Scale bar: 5 μm.
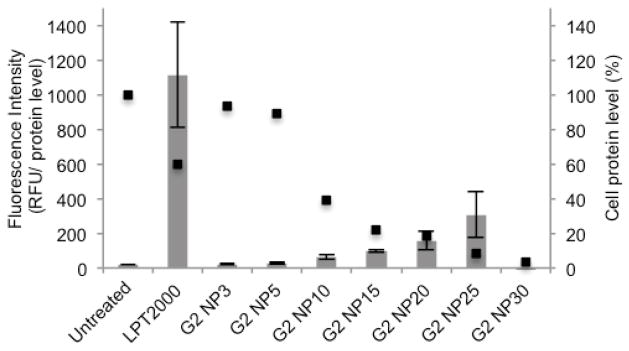
We were encouraged by the ability of G1 – G3 to condense pEGFP DNA and therefore proceeded to perform transfection experiments with HeLa cells. Experimentally, we treated HeLa cells (50,000 cells/well) with the Gn-plasmid DNA polyplexes for 24 h followed by cell lysis and measurement of EGFP fluorescence. Figure 4 shows a plot of fluorescence intensity as a function of N/P ratio for G2 in comparison to untreated cells and Lipofectamine 2000 (Life Technologies) as a positive control (G1 and G3, Supporting Information). Even at high N/P ratio (N/P = 20) only very modest increases in fluorescence intensity and therefore transfection was observed. Figure 4 also shows the cellular protein levels as measured using a commercial BCA protein assay kit (Pierce). At N/P > 5, the total amount of cellular protein decreases significantly which indicates that G2 is cytotoxic at these concentrations. Related observations were made during the transfection experiments performed with G1 and G3 (Supporting Information). Dendrimers with similar arms and C60 or pillararene cores displayed better transfection efficiency and lower cytotoxicity which suggests the acyclic CB[n] core may require modification.13–14 In addition to the observed cellular toxicity of G1 – G3, additional barriers will need to be surmounted to improve the gene delivery ability of acyclic CB[n] dendrimers including cell culture medium stability, cell membrane transport, and endosomal release.15
Figure 4.
Gene delivery experiments of pEGFP on HeLa cells. Fluorescence intensity (bars) and percentage of total cellular proteins (squares) are given for various N/P ratios with G2 (polyplexes prepared in 5% glucose solutions) and for negative (untreated HeLa cells) and positive (Lipofectamine 2000) controls. Means and standard deviation of triplicate measurements are given.
In summary, we have prepared acyclic CB[n] dendrimers G1 – G3 which are the first examples of covalent dendrimers incorporating cucurbiturils. Dendrimers G1 – G3 are shown to aggregate in water and cause condensation of pEGFP as shown by DLS, SEM, and gel electrophoresis. Very modest enhancements of gene delivery in HeLa cells were observed for G2-pEGFP at high N/P ratios, although this was accompanied by increased cytotoxicity. This paper further demonstrates the synthetic versatility of the acyclic CB[n] scaffold and stimulates the design of complex acyclic CB[n] compounds for diverse applications including transfection and drug delivery.
Supplementary Material
Acknowledgments
We thank the NIH (R01-CA168365 to L.I. and V.B.) and the University of Maryland (Millard and Lee Alexander Fellowship to B.V.) for financial support. We acknowledge using the Maryland NanoCenter NispLab and Prof. Muro and Ghaffarian (University of Maryland) for DLS access.
Footnotes
Notes
The authors declare no competing financial interests.
Experimental procedures, characterization data, 1H and 13C NMR spectra for new compounds; DLS data, SEM data, and transfection protocol. The Supporting Information is available free of charge on the ACS Publications website.
References
- 1.a) Freeman WA, Mock WL, Shih NY. J Am Chem Soc. 1981;103:7367. [Google Scholar]; b) Kim J, Jung IS, Kim SY, Lee E, Kang JK, Sakamoto S, Yamaguchi K, Kim K. J Am Chem Soc. 2000;122:540. [Google Scholar]; c) Day AI, Arnold AP, Blanch RJ, Snushall B. J Org Chem. 2001;66:8094. doi: 10.1021/jo015897c. [DOI] [PubMed] [Google Scholar]; d) Day AI, Blanch RJ, Arnold AP, Lorenzo S, Lewis GR, Dance I. Angew Chem, Int Ed. 2002;41:275. doi: 10.1002/1521-3773(20020118)41:2<275::aid-anie275>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]; e) Liu S, Zavalij PY, Isaacs L. J Am Chem Soc. 2005;127:16798. doi: 10.1021/ja056287n. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Cheng XJ, Liang LL, Chen K, Ji NN, Xiao X, Zhang JX, Zhang YQ, Xue SF, Zhu QJ, Ni XL, Tao Z. Angew Chem, Int Ed. 2013;52:7252. doi: 10.1002/anie.201210267. [DOI] [PubMed] [Google Scholar]
- 2.a) Lee JW, Samal S, Selvapalam N, Kim HJ, Kim K. Acc Chem Res. 2003;36:621. doi: 10.1021/ar020254k. [DOI] [PubMed] [Google Scholar]; b) Lagona J, Mukhopadhyay P, Chakrabarti S, Isaacs L. Angew Chem, Int Ed. 2005;44:4844. doi: 10.1002/anie.200460675. [DOI] [PubMed] [Google Scholar]; c) Nau WM, Florea M, Assaf KI. Isr J Chem. 2011;51:559. [Google Scholar]; d) Masson E, Ling X, Joseph R, Kyeremeh-Mensah L, Lu X. RSC Adv. 2012;2:1213. [Google Scholar]
- 3.a) Mock WL, Shih NY. J Org Chem. 1986;51:4440. [Google Scholar]; b) Liu S, Ruspic C, Mukhopadhyay P, Chakrabarti S, Zavalij PY, Isaacs L. J Am Chem Soc. 2005;127:15959. doi: 10.1021/ja055013x. [DOI] [PubMed] [Google Scholar]; c) Biedermann F, Uzunova VD, Scherman OA, Nau WM, De Simone A. J Am Chem Soc. 2012;134:15318. doi: 10.1021/ja303309e. [DOI] [PubMed] [Google Scholar]; d) Cao L, Šekutor M, Zavalij PY, Mlinarić-Majerski K, Glaser R, Isaacs L. Angew Chem, Int Ed. 2014;53:988. doi: 10.1002/anie.201309635. [DOI] [PubMed] [Google Scholar]; e) Shetty D, Khedkar JK, Park KM, Kim K. Chem Soc Rev. 2015;44:8747. doi: 10.1039/c5cs00631g. [DOI] [PubMed] [Google Scholar]
- 4.a) Ko YH, Kim E, Hwang I, Kim K. Chem Commun. 2007:1305. doi: 10.1039/b615103e. [DOI] [PubMed] [Google Scholar]; b) Loh XJ, del Barrio J, Toh PPC, Lee TC, Jiao D, Rauwald U, Appel EA, Scherman OA. Biomacromolecules. 2012;13:84. doi: 10.1021/bm201588m. [DOI] [PubMed] [Google Scholar]; c) Del Barrio J, Horton P, Lairez D, Lloyd G, Toprakcioglu C, Scherman O. J Am Chem Soc. 2013;135:11760. doi: 10.1021/ja406556h. [DOI] [PubMed] [Google Scholar]; d) Isaacs L. Acc Chem Res. 2014;47:2052. doi: 10.1021/ar500075g. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Ghale G, Nau WM. Acc Chem Res. 2014;47:2150. doi: 10.1021/ar500116d. [DOI] [PubMed] [Google Scholar]; f) Miyahara Y, Abe K, Inazu T. Angew Chem, Int Ed. 2002;41:3020. doi: 10.1002/1521-3773(20020816)41:16<3020::AID-ANIE3020>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; g) Kim E, Kim D, Jung H, Lee J, Paul S, Selvapalam N, Yang Y, Lim N, Park CG, Kim K. Angew Chem, Int Ed. 2010;49:4405. doi: 10.1002/anie.201000818. [DOI] [PubMed] [Google Scholar]; h) Walker S, Oun R, McInnes FJ, Wheate NJ. Isr J Chem. 2011;51:616. [Google Scholar]
- 5.a) Lee JW, Ko YH, Park SH, Yamaguchi K, Kim K. Angew Chem, Int Ed. 2001;40:746. doi: 10.1002/1521-3773(20010216)40:4<746::aid-anie7460>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]; b) Lim YB, Kim T, Lee JW, Kim SM, Kim HJ, Kim K, Park JS. Bioconjugate Chem. 2002;13:1181. doi: 10.1021/bc025581r. [DOI] [PubMed] [Google Scholar]
- 6.a) Ong W, Kaifer AE. Angew Chem, Int Ed. 2003;42:2164. doi: 10.1002/anie.200250214. [DOI] [PubMed] [Google Scholar]; b) Wang W, Kaifer AE. Angew Chem Int Ed. 2006;45:7042. doi: 10.1002/anie.200602220. [DOI] [PubMed] [Google Scholar]; c) Sobransingh D, Kaifer AE. Langmuir. 2006;22:10540. doi: 10.1021/la061188o. [DOI] [PubMed] [Google Scholar]
- 7.a) Zeng Y, Li Y, Li M, Yang G, Li Y. J Am Chem Soc. 2009;131:9100. doi: 10.1021/ja902998g. [DOI] [PubMed] [Google Scholar]; b) Zhang X, Zeng Y, Yu TJC, Yang G, Li Y. Langmuir. 2014;30:718. doi: 10.1021/la404349w. [DOI] [PubMed] [Google Scholar]
- 8.a) Lucas D, Minami T, Iannuzzi G, Cao L, Wittenberg JB, Anzenbacher P, Isaacs L. J Am Chem Soc. 2011;133:17966. doi: 10.1021/ja208229d. [DOI] [PubMed] [Google Scholar]; b) Zhao N, Lloyd G, Scherman O. Chem Commun. 2012;48:3070. doi: 10.1039/c2cc17433b. [DOI] [PubMed] [Google Scholar]; c) Cao L, Isaacs L. Org Lett. 2012;14:3072. doi: 10.1021/ol3011425. [DOI] [PubMed] [Google Scholar]; d) Vinciguerra B, Cao L, Cannon JR, Zavalij PY, Fenselau C, Isaacs L. J Am Chem Soc. 2012;134:13133. doi: 10.1021/ja3058502. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Ahn Y, Jang Y, Selvapalam N, Yun G, Kim K. Angew Chem, Int Ed. 2013;52:3140. doi: 10.1002/anie.201209382. [DOI] [PubMed] [Google Scholar]; f) Cao L, Hettiarachchi G, Briken V, Isaacs L. Angew Chem, Int Ed. 2013;52:12033. doi: 10.1002/anie.201305061. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Ayhan MM, Karoui H, Hardy M, Rockenbauer A, Charles L, Rosas R, Udachin K, Tordo P, Bardelang D, Ouari O. J Am Chem Soc. 2015;137:10238. doi: 10.1021/jacs.5b04553. [DOI] [PubMed] [Google Scholar]
- 9.a) Draghici B, Ilies MA. J Med Chem. 2015;58:4091. doi: 10.1021/jm500330k. [DOI] [PubMed] [Google Scholar]; b) Yang J, Zhang Q, Chang H, Cheng Y. Chem Rev. 2015;115:5274. doi: 10.1021/cr500542t. [DOI] [PubMed] [Google Scholar]
- 10.a) Ma D, Zavalij PY, Isaacs L. J Org Chem. 2010;75:4786. doi: 10.1021/jo100760g. [DOI] [PubMed] [Google Scholar]; b) Ma D, Hettiarachchi G, Nguyen D, Zhang B, Wittenberg JB, Zavalij PY, Briken V, Isaacs L. Nat Chem. 2012;4:503. doi: 10.1038/nchem.1326. [DOI] [PubMed] [Google Scholar]; c) Ma D, Zhang B, Hoffmann U, Sundrup MG, Eikermann M, Isaacs L. Angew Chem, Int Ed. 2012;51:11358. doi: 10.1002/anie.201206031. [DOI] [PubMed] [Google Scholar]; d) Shen C, Ma D, Meany B, Isaacs L, Wang Y. J Am Chem Soc. 2012;134:7254. doi: 10.1021/ja301462e. [DOI] [PubMed] [Google Scholar]; e) Minami T, Esipenko N, Akdeniz A, Zhang B, Isaacs L, Anzenbacher P. J Am Chem Soc. 2013;135:15238. doi: 10.1021/ja407722a. [DOI] [PubMed] [Google Scholar]; f) Zhang B, Zavalij PY, Isaacs L. Org Biomol Chem. 2014;12:2413. doi: 10.1039/c3ob42603c. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Gilberg L, Zhang B, Zavalij PY, Sindelar V, Isaacs L. Org Biomol Chem. 2015;13:4041. doi: 10.1039/c5ob00184f. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Zhang B, Isaacs L. J Med Chem. 2014;57:9554. doi: 10.1021/jm501276u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Stancl M, Hodan M, Sindelar V. Org Lett. 2009;11:4184. doi: 10.1021/ol9017886. [DOI] [PubMed] [Google Scholar]; b) Stancl M, Gilberg L, Ustrnul L, Necas M, Sindelar V. Supramol Chem. 2014;26:168. [Google Scholar]
- 12.Hawker CJ, Fréchet JMJ. J Am Chem Soc. 1990;112:7368. [Google Scholar]
- 13.Sigwalt D, Holler M, Iehl J, Nierengarten JF, Nothisen M, Morin E, Remy JS. Chem Commun. 2011;47:4640. doi: 10.1039/c0cc05783e. [DOI] [PubMed] [Google Scholar]
- 14.Nierengarten I, Nothisen M, Sigwalt D, Biellmann T, Holler M, Remy JS, Nierengarten JF. Chem Eur J. 2013;19:17552. doi: 10.1002/chem.201303029. [DOI] [PubMed] [Google Scholar]
- 15.Wiethoff CM, Middaugh CR. J Pharm Sci. 2003;92:203. doi: 10.1002/jps.10286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.