Abstract
Purpose
We examined the effects of an enhanced informal caregiver training (Enhanced-CT) protocol in cancer symptom and caregiver stress management to caregivers of hospitalized cancer patients.
Methods
We recruited adult patients in oncology units and their informal caregivers. We utilized a two-armed, randomized controlled trial design with data collected at baseline, post-training, and at 2 and 4 weeks after hospital discharge. Primary outcomes were self-efficacy for managing patients' cancer symptoms and caregiver stress, and preparedness for caregiving. Secondary outcomes were caregiver depression, anxiety, and burden. The education comparison (EDUC) group received information about community resources. We used general linear models to test for differences in the Enhanced-CT relative to the EDUC group.
Results
We consented and randomized 138 dyads: Enhanced-CT = 68 and EDUC = 70. The Enhanced-CT group had a greater increase in caregiver self-efficacy for cancer symptom management and stress management, and preparation for caregiving at the post-training assessment compared to the EDUC group but not at 2 and 4-week post-discharge assessments. There were no intervention group differences in depression, anxiety, and burden.
Conclusion
An Enhanced-CT protocol resulted in short-term improvements in self-efficacy for managing patients' cancer symptoms and caregiver stress, and preparedness for caregiving but not in caregivers' psychological well-being. The lack of sustained effects may be related to the single-dose nature of our intervention and the changing needs of informal caregivers after hospital discharge.
Keywords: family caregivers, symptom management, caregiver training, hospital discharge
Background
Living with cancer generates distress for patients and their caregivers [1]. Patients suffer from the disease burden, and their caregivers struggle to ease that suffering while enduring the stress of a having a loved one with a life-threatening illness. By 2030, 2.3 million Americans will receive a new diagnosis of cancer every year [2] and the majority of them will live more than 5 years after their diagnosis [3]. Many will rely on their family and friends (hereafter, “caregivers”) for assistance in their care.
Cancer caregivers report physical and emotional strain in caring for their loved ones [4]. Additionally, many caregivers feel ill prepared in managing cancer symptoms [5,6]. Lack of confidence and preparedness to provide the necessary complex care may intensify caregiver distress [7,8]. When caregivers' psychological well-being is impaired, patients' well-being also worsens [9].
Among the most stressful times for caregivers are the days and weeks following a hospital discharge [10,11]. Patients tend to be discharged quickly with caregiver teaching mostly ad hoc, provider-determined, and conducted on the day of discharge [12,13]. Further, caregivers are expected to assume clinical tasks traditionally performed by healthcare professionals [14]. Despite these enormous challenges, no consensus exists on how to best prepare and support caregivers for home discharge [12,13]. Patients are hospitalized for administration of cancer treatment and management of acute symptoms making the time of discharge a teachable moment.
In a previous study, we established the feasibility of providing a theoretically-derived, individualized, experiential caregiver training in symptom management during a cancer patient's hospitalization [15]. Additionally, the training led to improved cancer caregivers' self-efficacy in symptom management [16,17]. Self-efficacy is an important concept in caregiving because it is a prerequisite for the actual delivery of action [18]. However, the training did not affect levels of depression, anxiety, or quality of life among caregivers. This previous training neither addressed stress management needs nor taught strategies to promote self-care.
This study examined the effects of an enhanced caregiver training (Enhanced-CT) protocol that taught caregivers knowledge and skills for managing patient symptoms and strategies for managing their own psychological distress. This study was one of three projects in the Center for Self-Management in Life-Limiting Illness, a P01 Center funded by the National Institute of Nursing Research [19]. We compared the effects of the Enhanced-CT protocol to an education condition (EDUC) on caregiver self-efficacy in cancer symptom management and stress management, and preparedness as primary outcomes, and psychological well-being (depression, anxiety, and burden) as secondary outcomes. We also explored the 30-day emergency department (ED) visit and readmission rates of participating patients.
Method
We designed a two-armed, randomized controlled trial with data collection at baseline, immediately after training, and at 2 and 4 weeks after hospital discharge. After obtaining approval from the Duke University Health System Institutional Review Board, we conducted the study on oncology units at Duke University Hospital in Durham, North Carolina. Eligible patients were: admitted to oncology unit for treatment or cancer-related complications; 18 years or older; oriented to place, person, and time; and being discharged home with care needs. Patients under hospice care were ineligible. We also recruited patients' caregivers. Eligible caregivers were at least 18 years old and expected to care for patients after discharge; able to hear, read, and write in English; and willing to spend at least 2 hours in the hospital for the training. Patients without available or interested caregivers were ineligible to participate.
Study staff reviewed weekly patient rosters in participating oncology units. Using Duke University's secure server, study staff emailed a list of eligible patients to attending physicians for signoff. Study staff verified with unit nurses the plans to discharge patients to home before approaching patients for recruitment. If caregivers were not present during recruitment rounds, patients were asked about the presence of home caregivers after their hospital discharge. If patients responded affirmatively, study staff inquired on when their caregivers may visit and recruitment rounds were planned on that day. If multiple caregivers were identified by patients, they were asked to identify the caregiver who would be most involved in providing their home caregiving needs.
Study staff obtained written consent and completed baseline assessments from dyads. After completing baseline assessments, the study staff scheduled one-on-one sessions with the caregivers for training. Scheduling was only done when discharge appeared imminent based on progress notes or information provided by caregivers. After training schedules were established, dyads were randomized by the study nurse interventionist to either the Enhanced-CT or EDUC group using an a priori blocked randomization sequence stratified by study oncology units. The nurse interventionist was trained to deliver both arms of the intervention. Randomization tables and assignments were housed in a database that was separate from study tracking information. Study staff who collected data after hospital discharge were blinded to dyad intervention assignment. The random allocation sequence was generated by the study statisticians. A block size of four was used in the randomization sequence, and all study staff other than the statisticians were blinded to the block size number.
Immediately after the training, caregivers completed questionnaires on self-efficacy for managing patients' cancer symptoms and self-efficacy for stress management as well as preparedness for caregiving. Research staff members, who were blinded to group assignment, collected follow-up data by phone at 2 and 4 weeks after hospital discharge. After study completion, each caregiver received a U.S. $20 check by mail.
Intervention
Enhanced caregiver training (Enhanced-CT)
A registered nurse interventionist delivered the training that had two components: management of patient symptoms and caregiver stress management (Table 1). Symptom areas included prevention of infection, management of fatigue, pain control, and maintenance of nutrition and proper elimination. In the first component, the nurse provided training in symptom management strategies. For example, for shortness of breath, discussions focused on positioning and pursed-lip breathing for symptom alleviation. If the patient had no active symptoms, the discussion focused on symptom prevention. The nurse also provided technical care skills if warranted (e.g., care of a central catheter). The nurse encouraged caregivers to identify and discuss areas of home care that worried them and included education and training to those specific areas.
Table 1. Teaching Areas for the Enhanced Caregiver Training Program.
| Prevention of Infection: signs and symptoms of infection; skills for patient care as applicable (examples are central line care, dressing change, Foley catheter care, wound care, etc.) |
| Management of Fatigue: activity-rest cycle, (example is napping in mid-morning and mid-afternoon, with each nap no longer than 15-20 minutes). Pleasant activity scheduling (examples are timing busiest activities or enjoyable activities in the morning when energy is at a high level). |
| Pain Control: assessment; pain medication management; nonpharmacologic interventions |
| Maintenance of Nutrition and Proper Elimination: maintaining or increasing caloric intake; management for constipation or diarrhea |
| Home Care Issues: assessment of issues that caregiver perceives to be stressful and in need of assistance; discussion of medication regimen for patient |
| Strategies for Managing Psychological Distress: deep breathing, progressive muscle relaxation; pleasant imagery |
For caregiver stress management, the skills training focused on deep breathing, progressive muscle relaxation, and pleasant imagery, which are strategies previously used by the research team. These strategies have demonstrated efficacy in helping cancer patients and caregivers manage stress and reduce negative emotions [20]. For this training, a three-step behavioral rehearsal procedure was used: a) the nurse modeled how one could apply the skill to a particular problem (e.g., using relaxation to manage anxiety), b) the caregiver practiced the skill and was given positive and corrective feedback on performance, and c) the caregiver practiced the sill until mastery was obtained.
Prior to the training, the nurse reviewed the patient's problem list and clinical progress notes to tailor the training to ongoing concerns and to ensure that discharge is anticipated within at least 5 days or sooner. The manualized training followed a structured topical outline with standard activities for each teaching area. Each teaching area began with needs assessment so that discussions were individualized to caregivers. Caregivers also received a one-page handout on each symptom containing information that would be discussed. This was to minimize note-taking distractions among caregivers. Discussions were interactive; caregivers were given ample opportunity to ask questions. Training usually was conducted at the patient's bedside and patient participation was encouraged to simulate the dyadic nature of home caregiving. Training lasted one to two hours and, if desired, caregivers could spread this out over two sessions.
Comparison education (EDUC)
Caregivers received standardized, manualized training about local community resources, home health, respite care, hospice, palliative care, living will, and medical power of attorney. Similar to the Enhanced-CT group, the training was interactive and conducted at the patient's bedside. A social worker or a nurse delivered the training, which lasted for approximately one hour.
Intervention fidelity
The study interventionists were provided with a manual outlining the treatment protocol and trained in delivering the intervention through modeling, role-play, and feedback. We recorded the first five training sessions and the study Principal Investigator (PI) listened to the recordings to monitor for fidelity to the study protocol. The PI and interventionists had monthly debriefing in the first six months of the study. Thereafter, training sessions were recorded quarterly, and the PI and interventionists met quarterly as well.
Measures
Patients completed baseline demographics, functional status, and well-being questionnaires. Caregivers completed measures on self-efficacy for managing patients' cancer symptoms and caregiver stress; preparedness for caregiving, anxiety, depression, and burden at baseline and at 2 and 4 weeks of discharge. Measures of self-efficacy and preparedness were also completed immediately after the training. Additionally, caregivers completed demographics and health literacy questionnaires at baseline.
Caregiver measures: Primary outcomes
Caregiver self-efficacy for managing cancer symptoms and stress was measured using a modified version of a Self-efficacy Scale for Cancer Caregivers [21]. Thirteen items assessed confidence in caregivers' abilities to manage cancer symptoms (12 items) and their stress (1 item). Item ratings ranged from 10 (very uncertain) to 100 (very certain). Self-efficacy for symptom management was the mean of the 12 items, and the one item assessing self-efficacy for stress management was treated as a stand-alone item.
Preparedness for caregiving was measured using The Preparedness for Caregiving scale, a subscale of the Family Caregiving Inventory [22]. This 8-item scale measured physical, emotional, social, and general preparation for caregiving. Caregivers rated their preparedness from “Not at all prepared” (0 points) to “Very well prepared” (4 points). This scale is widely used in informal caregiving research [23,24].
Caregiver measures: Secondary outcomes
Anxiety was measured using the 5-item Profile of Mood States (POMS) anxiety sub-scale. The items are rated based on the strength of emotion, where 0 = “not at all,” and 4 = “extremely.” It has demonstrated adequate psychometric properties [25,26].
Depression was measured using the Center for Epidemiology Studies - Depression Scale (CES-D), a 10-item measure with excellent validity and reliability [27,28].
Caregiver burden was measured using the 24-item Caregiver Reaction Assessment (CRA). The CRA measures reactions to caregiving for family members with a variety of chronic illnesses. It includes items on caregiver esteem, family support, impact on finances, impact on schedule, and impact on health [29].
Another measure included The Rapid Estimate of Adult Literacy in Medicine (REALM-R) [30]. The REALM-R uses recognition and pronunciation of 8 health-related words. A score ≤ 6 indicates poor health literacy.
Patient measures
Patient Functional status was assessed using the 7-item instrumental (IADL) and the 6-item Physical (PADL) subscales of the OARS Multidimensional Functional Assessment Questionnaire (OMFAQ) [31]. Scores were scaled as 1 (need no help), 2 (need some help), or 3 (unable to do).
Well-being was measured using the Quality of Life in Chronic Illness: FACT-G, a 27-item questionnaire with four domains: physical, functional, social/family, and emotional well-being [32-34].
Dyads completed a standardized demographics questionnaire. Caregivers were also asked two additional questions: years of caregiving and relationship to the patient. Duke's medical record for each patient within 30 days of index hospital discharge was also reviewed to determine ED visits and hospital readmissions.
Data Analysis
We estimated sample size based on the primary hypothesis that caregivers in the Enhanced-CT group would have increased self-efficacy for symptom management, as measured by the self-efficacy scale, at post-training compared with the EDUC group using methods in Borm, Fransen, and Lemmens [35]. Based on our previous pilot study, the estimated baseline to post-training correlation was 0.52 [16]. To detect a difference of 0.5 standard deviations at post-training with 85% power, and a type I error rate of 5%, 110 caregivers were needed; however, to account for dropout, we enrolled 138 caregivers.
For all outcomes, general linear models (PROC MIXED in SAS, version 9.2, SAS Institute, Inc., Cary, North Carolina) were used to test for differences in the Enhanced-CT group relative to the EDUC group. Final models included a common intercept, oncology unit (the stratification variable), dummy coded time (baseline, post-training for primary outcomes only, 2, and 4 weeks post-discharge), and intervention arm interactions with each follow-up time point. An unstructured covariance matrix was fit to account for the correlation of caregivers' repeated measures over time. Mean differences between the Enhanced-CT and EDUC at post-training (primary outcomes only), 2, and 4 weeks post-discharge were calculated, along with corresponding 95% confidence intervals (CIs), using SAS ESTIMATE statements.
Measurements from all randomized caregivers, including those who subsequently discontinued the study, were used for the longitudinal analyses (n = 138 caregivers). Caregivers who discontinued the study differed on baseline characteristics, as compared with those who completed the study, so a multiple imputation procedure [36] to estimate missing values was employed. The imputation model included baseline variables that were predictors of dropout in addition to treatment group, the oncology unit used for stratification in the randomization process, and the caregiver outcomes at baseline, post-training, 2, and 4 week post-discharge. The macro IVEware in SAS (version 0.2) was used to generate 10 imputed datasets via a sequential regression method. General linear models for each outcome were fit to each of these data sets, and the 10-sets of parameter estimates and standard errors were combined using the Rubin rules for multiple imputation (using PROC MIANALYZE in SAS). More information on this general analytic approach can be found elsewhere [37,38].
To account for censoring due to death, 30-day rates of ED visits and readmissions were compared between the Enhanced-CT and EDUC groups using Kaplan-Meier estimators and log-rank test statistic. Primary and secondary outcomes were identified a priori and no adjustments for multiple comparisons were made. A p-value < 0.05 was statistically significant.
Results
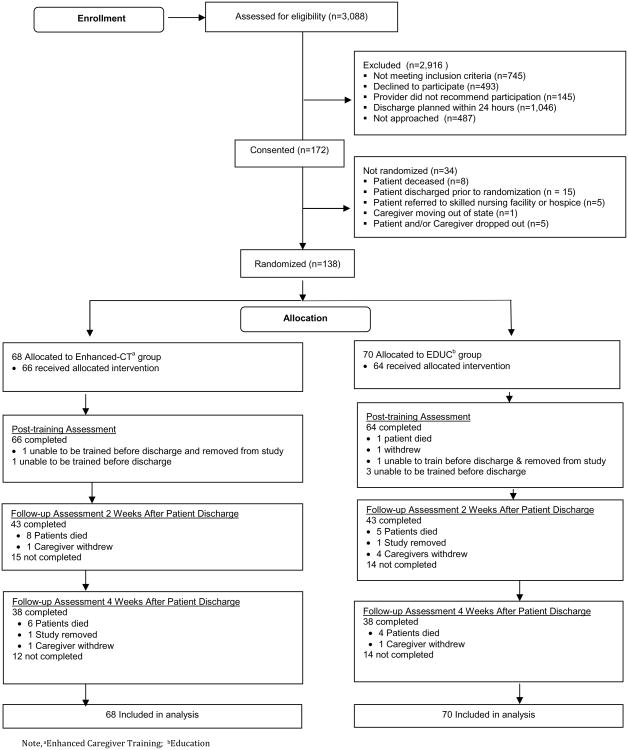
We screened 3,088 electronic records for eligibility (Figure 1). Of the 665 we approached for recruitment, we consented 172 dyads. Of these 172 dyads, we randomized 138 (68 dyads were assigned to the Enhanced-CT group and 70 to the EDUC group). Sixty-six caregivers (97%) in the Enhanced-CT group and 64 caregivers (91%) in the EDUC group completed training and the post-training assessment. Thirty-eight caregivers in the Enhanced-CT group (57% of those assigned) and 38 in the EDUC group (59%) completed the 4-week post-discharge follow-up. Caregivers who completed the study were older, had been a caregiver for less time, were less likely to be working, had lower anxiety (POMS) and depressive (CES-D) symptoms (CES-D), and had higher baseline confidence to manage stress than caregivers who did not complete the study. Patients of caregivers who completed the study were older, more likely to be female, more likely to have a hematological malignancy, needed less help (ADL/IADL) and had lower functional well-being (FACT-G) than patients of caregivers who did not complete the study. We included all of these baseline characteristics in the multiple imputation model.
Fig. 1. Study Consort Diagram.
Characteristics of dyads are described in Tables 2 and 3. The mean age of caregivers was 55 and majority were female, White, married, had at least some college years, and spouses of care recipients. Of those caregivers who completed the REALM (n = 122), only four (3%) had a high risk for poor literacy. On average, caregiving duration was approximately 19 months. The baseline mean self-efficacy among participating caregivers was high at 70.4 (72.0 for Enhanced-CT vs. 69.0 for EDUC). Caregivers in the Enhanced-CT group spent longer time on training compared to those in the EDUC group (mean of 110.7 minutes vs. 56.5 minutes) and were more likely to have their loved ones with cancer participate in the training (66.7% vs. 48.4%). Patients who participated were afforded opportunities to actively engage in the training by asking questions or clarifying information received. Only one caregiver in the Enhanced-CT group received a booster to the training. Patient participants had a mean age of 57. They shared the same demographic characteristics as caregivers except that the majority were male.
Table 2. Caregiver baseline characteristics and primary outcomes assessed at baseline.
| Overall N = 138 | Enhanced-CTa Group N = 68 | EDUCb Group N = 70 | |
|---|---|---|---|
|
| |||
| Age, mean in years (SD) | 55.3 (13.2) | 56.2 (12.7) | 54.4 (13.7) |
|
| |||
| Female gender | 115 (83.3) | 56 (82.4) | 59 (84.3) |
|
| |||
| Race | |||
| White | 106 (76.8) | 51 (75.0) | 55 (78.6) |
| Black | 25 (18.1) | 13 (19.1) | 12 (17.1) |
| Other | 7 (5.1) | 4 (5.9) | 3 (4.3) |
|
| |||
| Hispanic/Latino ethnicity | 7 (5.1) | 2 (2.9) | 5 (7.1) |
|
| |||
| Marital Status: currently married | 115 (83.3) | 58 (85.3) | 57 (81.4) |
|
| |||
| Currently working | 68 (49.3) | 32 (47.1) | 36 (51.4) |
|
| |||
| Household finances: have difficulty or have to make adjustments to pay the bills1 | 33 (25.2) | 18 (28.6) | 15 (22.1) |
|
| |||
| Highest level of education | |||
| High school graduate or less | 41 (29.7) | 24 (35.3) | 17 (24.3) |
| Some college, associate's degree, trade school | 53 (38.4) | 25 (36.8) | 28 (40.0) |
| College degree or higher | 44 (31.9) | 19 (27.9) | 25 (35.7) |
|
| |||
| Number of months caring for loved one1, mean (SD) | 19.4 (34.9) | 18.0 (33.2) | 20.7 (36.6) |
|
| |||
| Caregiver relationship to patient | |||
| Spouse | 93 (67.4) | 45 (66.2) | 48 (68.6) |
| Child | 20 (14.5) | 11 (16.2) | 9 (12.9) |
| Parent | 14 (10.1) | 9 (13.2) | 5 (7.1) |
| Other2 | 11 (8.0) | 3 (4.4) | 8 (11.4) |
|
| |||
| Primary outcomes | |||
|
| |||
| Self-efficacy, mean (SD) | 70.4 (17.8) | 72.0 (17.4) | 69.0 (18.3) |
|
| |||
| Stress management, mean (SD) | 74.6 (23.8) | 76.1 (22.3) | 73.2 (25.4) |
|
| |||
| Preparation for caregiving, mean (SD) | 2.9 (0.7) | 2.9 (0.7) | 2.8 (0.6) |
Note, n (%) unless otherwise indicated, SD = standard deviation,
Enhanced Caregiver Training;
Education
Missing data: 7 caregivers have missing data for household finances, 6 caregivers randomized to ECT have missing data for months caring for loved one,
Other relationships include sibling (n = 2), fiancé (n=2), partner (n=1), boyfriend or girlfriend (n=3), cousin (n=1), friend (n=1), daughter-in-law (n=1)
Table 3. Patient baseline characteristics.
| Overall N = 138 | Enhanced-CTa Group N = 68 | EDUCb Group N = 70 | |
|---|---|---|---|
|
| |||
| Age, mean in years (SD) | 57.0 (15.1) | 57.0 (14.2) | 57.0 (16.1) |
|
| |||
| Female gender | 50 (36.2) | 23 (33.8) | 27 (38.6) |
|
| |||
| Race | |||
| White | 107 (77.5) | 52 (76.5) | 55 (78.6) |
| Black | 27 (19.6) | 15 (22.1) | 12 (17.1) |
| Other | 4 (2.9) | 1 (1.5) | 3 (4.3) |
|
| |||
| Hispanic/Latino ethnicity | 3 (2.2) | 2 (2.9) | 1 (1.4) |
|
| |||
| Marital Status: currently married | 99 (71.7) | 49 (72.1) | 50 (71.4) |
|
| |||
| Highest level of education | |||
| High school graduate or less | 42 (30.4) | 19 (27.9) | 23 (32.9) |
| Some college, associate's degree, trade school | 53 (38.4) | 28 (41.2) | 25 (35.7) |
| College degree or higher | 43 (31.2) | 21 (30.9) | 22 (31.4) |
Note, n (%) unless otherwise indicated, SD = standard deviation,
Enhanced Caregiver Training;
Education
Primary Outcomes
After the intervention, caregivers randomized to Enhanced-CT group had a significantly higher level of self-efficacy for managing patients' cancer symptoms (6.1, 95% CI: 3.0-9.3, p<0.001), and their stress (4.8, 95% CI: 0.5-9.1, p=0.03) than those in the EDUC Group (see Table 4). Similarly, after the training, caregivers randomized to the Enhanced-CT group reported significantly higher levels of preparedness for caregiving than those in the EDUC group (0.2, 95% CI: 0.1-0.4, p=0.01). However, these differences were not sustained at 2-week and 4-week post-discharge assessments (see Table 4).
Table 4. Model Results for Survey Measure Outcomes.
| Outcome and Study Time Point | Estimated Mean for Enhanced-CT grp. | Estimated Mean for EDUC grp. | Mean Difference Between Groups (95% CI) | P-value |
|---|---|---|---|---|
| Self-efficacy | ||||
| Baseline | 70.4 | 70.4 | ||
| Post-training | 82.2 | 76.1 | 6.1 (3.0, 9.3) | <0.001 |
| 2-week follow up* | 78.9 | 77.4 | 1.5 (-3.4, 6.3) | 0.56 |
| 4-week follow up** | 77.9 | 76.4 | 1.5 (-3.7, 6.7) | 0.57 |
| Certainty to cope with own stress | ||||
| Baseline | 74.7 | 74.7 | ||
| Post-training | 82.7 | 77.9 | 4.8 (0.5, 9.1) | 0.03 |
| 2-week follow up | 76.8 | 77.6 | -0.9 (-8.1, 6.3) | 0.81 |
| 4-week follow up | 78.5 | 79.6 | -1.1 (-7.9, 5.7) | 0.75 |
| Preparation for caregiving | ||||
| Baseline | 2.9 | 2.9 | ||
| Post-training | 3.2 | 3.0 | 0.2 (0.1, 0.4) | 0.01 |
| 2-week follow up | 3.2 | 3.1 | 0.1 (-0.1, 0.3) | 0.30 |
| 4-week follow up | 3.2 | 3.0 | 0.2 (-0.02, 0.4) | 0.08 |
| CESD-10 | ||||
| Baseline | 9.3 | 9.3 | ||
| 2-week follow up | 7.5 | 7.9 | -0.4 (-2.2, 1.3) | 0.63 |
| 4-week follow up | 7.4 | 8.4 | -1.0 (-2.6, 0.5) | 0.19 |
| POMS Anxiety Subscale | ||||
| Baseline | 7.1 | 7.1 | ||
| 2-week follow up | 5.1 | 5.2 | -0.1 (-1.7, 1.5) | 0.90 |
| 4-week follow up | 4.5 | 5.6 | -1.1 (-2.6, 0.3) | 0.13 |
| CRA Impact on Schedule | ||||
| Baseline | 3.2 | 3.2 | ||
| 2-week follow up | 3.2 | 3.2 | -0.001 (-0.2, 0.2) | 0.99 |
| 4-week follow up | 3.2 | 3.3 | -0.1 (-0.3, 0.2) | 0.52 |
| CRA Caregiver Esteem | ||||
| Baseline | 4.4 | 4.4 | ||
| 2-week follow up | 4.4 | 4.3 | 0.04 (-0.1, 0.2) | 0.60 |
| 4-week follow up | 4.4 | 4.3 | 0.1 (-0.1, 0.2) | 0.40 |
| CRA Lack of Family Support | ||||
| Baseline | 1.9 | 1.9 | ||
| 2-week follow up | 2.0 | 2.2 | -0.2 (-0.5, 0.1) | 0.15 |
| 4-week follow up | 2.0 | 2.2 | -0.2 (-0.5, 0.1) | 0.14 |
| CRA Impact on Health | ||||
| Baseline | 2.2 | 2.2 | ||
| 2-week follow up | 2.1 | 2.2 | -0.05 (-0.3, 0.2) | 0.67 |
| 4-week follow up | 2.2 | 2.2 | -0.1 (-0.4, 0.2) | 0.71 |
| CRA Impact on Finances | ||||
| Baseline | 2.6 | 2.6 | ||
| 2-week follow up | 2.7 | 2.6 | 0.1 (-0.2, 0.4) | 0.56 |
| 4-week follow up | 2.7 | 2.7 | 0.1 (-0.3, 0.4) | 0.75 |
Two weeks post-hospital discharge;
Four weeks post-hospital discharge
Enhanced-CT = Enhanced Caregiver Intervention, EDUC = Comparison, CESD-10 = Center for Epidemiology Studies – Depression Scale, POMS = Profile of Mood States, CRA = Caregiver Reaction Assessment Range of model-estimated correlations between repeated-measures for primary outcomes: self-efficacy (0.39 –0.88); certainty to cope with stress (0.29 – 0.86); preparation for caregiving (0.34 – 0.74)
Secondary Outcomes
In general, caregivers' depressive symptoms and anxiety improved from the baseline to two-week follow-up. However, there was no evidence of intervention group differences in these improvements (all p > 0.05; Table 4). There was little change over time in caregiver burden for Enhanced-CT and EDUC groups. There was little change over time in caregiver burden for Enhanced-CT and EDUC groups. The Kaplan-Meier estimated 30-day ED treat-and-release, direct hospitalization, and ED visits leading to hospitalization rates were 7%, 42% and 22% for Enhanced-CT patients, respectively, and 10%, 46% and 26% for EDUC patients (all p > 0.05).
Discussion
Compared to the EDUC group, caregivers in the Enhanced-CT group had significant increases in self-efficacy for managing patients' cancer symptoms and stress, and preparedness for caregiving immediately after training. Many caregivers stated the importance of having a dedicated time with a nurse to discuss their needs and practice caregiving skills. A caveat for implementation, however, is the additional time required for this training that consequently will require re-examination of nurse workload.
We did not observe sustained intervention effects on self-efficacy and preparedness following hospital discharge. These results are similar to our previous trial of older hospitalized cancer patients [17]. Our training's single-dose approach may have undermined the changing nature of post-discharge caregiver needs [39]. Additional, pre-discharge training is anticipatory at best and may not adequately capture the complex nature of home caregiving [7].
The Enhanced-CT protocol did not improve caregivers' depressive symptoms, anxiety, or burden. Although our strategies of deep breathing, progressive muscle relaxation, and pleasant imagery are associated with stress reduction, [20] their effects may have been curtailed by high, continuous, post-discharge demands on informal caregivers [10,11]. In addition to concerns about the care of their loved ones, caregivers experience substantial increases in time demands, physical exhaustion, financial costs, and personal heath risks [40]. Future caregiving trials should consider supplemental ways of support after hospital discharge such as phone calls or using interactive technologies. Beginning in October 2012, the Centers for Medicaid and Medicare Services began reducing payments to hospitals for patients with selected diagnoses and who are readmitted to the hospital within 30 days of discharge [41]. This new mandate should stimulate hospitals to invest in resources for home transitions. One important resource is a caregiver support program that transcends the physical boundaries of hospitals.
Our study has several limitations. We conducted our study at one of the nation's leading centers for cancer services [42] that caters to medically complex patients with complicated treatment regimens. Thus, our participants have many caregiving needs that are outside the purview of the study's caregiver training protocol. The high medical acuity of our patients also contributed to the attrition rate of our enrolled participants. We only collected data on formal services utilized by dyads. The collection of additional caregiving support, including from family and friends, could have helped in understanding our study outcomes. Second, interventionist fidelity was not assessed by someone not directly involved in the study, raising the possibility of bias in assessments. Data on therapist adherence to protocol would have addressed this fidelity concern. Thus, a more intensive fidelity assessment and ongoing monitoring and supervision might have enhanced treatment effects. The characteristics caregivers and/or patients who dropped out in the study should be accounted for to determine if there are common dyadic features contributing to this attrition. Lastly, caregiver utilization of coping strategies taught during the intervention was not assessed in the home setting. Thus, it is unclear whether the coping strategies were insufficient or if their use was inadequate.
In summary, we found that our Enhanced-CT protocol resulted in short-term improvements in self-efficacy for managing cancer symptoms and stress, and preparedness among caregivers but not in their psychological well-being. The lack of sustained intervention effects may be related to the single-dose nature of our intervention and the changing needs of caregivers after hospital discharge.
Acknowledgments
The authors thank Iris Pounds, Margaret Falkovic, Melanie Paige, Sarah Garrigues, Sophia Duong and Terry Ervin for their assistance. We also extend our gratitude to Duke oncology unit staff and to all study participants for their time and effort.
Funding Support: This study was funded by the National Institute of Nursing Research (P01 NR010948; Clinical Trials identifier NCT00938769). Dr. Abby J. Schwartz receives support from National Institutes of Health grant number T32 AG000029.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest. The authors have full control of all primary data and agree to allow the journal to review the data if requested.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Authors report no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of Duke University Health System Institutional Review Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
Cristina C. Hendrix, Email: cristina.hendrix@dm.duke.edu.
Donald E. Bailey, Jr, Email: Chip.Bailey@dm.duke.edu.
Karen E. Steinhauser, Email: karen.steinhauser@dm.duke.edu.
Maren K. Olsen, Email: maren.olsen@dm.duke.edu.
Karen M. Stechuchak, Email: karen.stechuchak@dm.duke.edu.
Sarah G. Lowman, Email: lowmanster@gmail.com.
Abby J. Schwartz, Email: abby.schwartz@dm.duke.edu.
Richard F. Riedel, Email: richard.riedel@dm.duke.edu.
Francis J. Keefe, Email: francis.keefe@dm.duke.edu.
Laura S. Porter, Email: Laura.Porter@dm.duke.edu.
James A. Tulsky, Email: james.tulsky@dm.duke.edu.
References
- 1.Adler NE, Page AEK. Cancer care for the whole patient: Meeting psychosocial health needs. National Academies Press; Washington, DC: 2008. [PubMed] [Google Scholar]
- 2.Smith BD, Smith GL, Hurria A, et al. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–65. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 3.American Cancer Society. Cancer facts & figures 2014. Atlanta: American Cancer Society; 2014. http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. [Google Scholar]
- 4.Kim Y, Schulz R. Family caregivers' strains: comparative analysis of cancer caregiving with dementia, diabetes, and frail elderly caregiving. J Aging Health. 2008;20:483–503. doi: 10.1177/0898264308317533. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher KL, Koresawa S, West C, et al. Putting cancer pain management regimens into practice at home. J Pain Symptom Manage. 2002;23:369–82. doi: 10.1016/s0885-3924(02)00385-8. [DOI] [PubMed] [Google Scholar]
- 6.Sutton LM, Clipp EC, Winer EP. Mangement of the terminally ill patient. In: Hunter CP, Johnson KA, Muss HB, editors. Cancer in the elderly. Dekker; New York: 2000. pp. 543–572. [Google Scholar]
- 7.Given B, Sherwood PR. Family care for the older person with cancer. Semin Oncol Nurs. 2006;22:43–50. doi: 10.1016/j.soncn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 8.Scherbring M. Effect of caregiver perception of preparedness on burden in an oncology population. Oncol Nurs Forum. 2002;29:E70–E76. doi: 10.1188/02.ONF.E70-E76. [DOI] [PubMed] [Google Scholar]
- 9.Hodges LJ, Humphris GM, Macfarlane G. A meta-analytic investigation of the relationship between the psychological distress of cancer patients and their carers. Soc Sci Med. 2005;60:1–12. doi: 10.1016/j.socscimed.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Cornwell P, Dicks B, Fleming J, et al. Care and support needs of patients and carers early post-discharge following treatment for non-malignant brain tumour: establishing a new reality. Support Care Cancer. 2012;20:2595–2610. doi: 10.1007/s00520-012-1383-1. [DOI] [PubMed] [Google Scholar]
- 11.Shaw J, Harrison J, Young J, et al. Coping with newly diagnosed upper gastrointestinal cancer: a longitudinal qualitative study of family caregivers' role perception and supportive care needs. Support Care Cancer. 2013;21:749–756. doi: 10.1007/s00520-012-1575-8. [DOI] [PubMed] [Google Scholar]
- 12.Foust JB, Vuckovic N, Henriquez E. Hospital to home health care transition: patient, caregiver, and clinician perspectives. West J Nurs Res. 2012;34:194–212. doi: 10.1177/0193945911400448. [DOI] [PubMed] [Google Scholar]
- 13.Nosbusch JM, Weiss ME, Bobay KL. An integrated review of the literature on challenges confronting the acute care staff nurse in discharge planning. J Clin Nurs. 2011;20:754–774. doi: 10.1111/j.1365-2702.2010.03257.x. [DOI] [PubMed] [Google Scholar]
- 14.Plank A, Mazzoni V, Cavada L. Becoming a caregiver: new family carers' experience during the transition from hospital to home. J Clin Nurs. 2012;21:2072–2082. doi: 10.1111/j.1365-2702.2011.04025.x. [DOI] [PubMed] [Google Scholar]
- 15.Hendrix C, Ray C. Informal caregiver training on home care and cancer symptom management prior to hospital discharge: a feasibility study. Oncology nursing forum. 2006;33:793–798. doi: 10.1188/06.ONF.793-798. [DOI] [PubMed] [Google Scholar]
- 16.Hendrix CC, Abernethy A, Sloane R, et al. A pilot study on the influence of an individualized and experiential training on cancer caregiver's self-efficacy in home care and symptom management. Home Healthc Nurse. 2009;27:271–278. doi: 10.1097/01.nhh.0000356777.70503.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hendrix CC, Landerman R, Abernethy AP. Effects of an individualized caregiver training intervention on self-efficacy of cancer caregivers. West J Nurs Res. 2013;35:590–610. doi: 10.1177/0193945911420742. [DOI] [PubMed] [Google Scholar]
- 18.Bandura A. Self-efficacy: The exercise of control. Freeman; New York: 1997. [Google Scholar]
- 19.Bailey DE, Jr, Steinhauser K, Hendrix C, et al. Pairing self-management with palliative care: Intervening in life-limiting illness. J Nurs Healthc Chronic Illn. 2011;3:1–3. doi: 10.1111/j.1752-9824.2011.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varvogli L, Darviri C. Stress Management Techniques: evidence-based procedures that reduce stress and promote health. Health Science Journal. 2011;5:74–89. [Google Scholar]
- 21.Porter LS, Keefe FJ, Garst J, et al. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: associations with symptoms and distress. Pain. 2008;137:306–315. doi: 10.1016/j.pain.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Archbold PG, Stewart BJ, Greenlick MR, et al. Mutuality and preparedness as predictors of caregiver role strain. Res Nurs Health. 1990;13:375–384. doi: 10.1002/nur.4770130605. [DOI] [PubMed] [Google Scholar]
- 23.Grant JS, Elliott TR, Weaver M, et al. Telephone intervention with family caregivers of stroke survivors after rehabilitation. Stroke. 2002;33:2060–2065. doi: 10.1161/01.str.0000020711.38824.e3. [DOI] [PubMed] [Google Scholar]
- 24.Hudson PL, Hayman-White K. Measuring the psychosocial characteristics of family caregivers of palliative care patients: psychometric properties of nine self-report instruments. J Pain Symptom Manage. 2006;31:215–228. doi: 10.1016/j.jpainsymman.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Curran SL. Short Form of the Profile of Mood States (POMS-SF): Psychometric Information. Psychological Assessment. 1995;7:80–83. [Google Scholar]
- 26.Shacham S. A shortened version of the Profile of Mood States. J Pers Assess. 1983;47:305–306. doi: 10.1207/s15327752jpa4703_14. [DOI] [PubMed] [Google Scholar]
- 27.Hall LA, Gurley DN, Sachs B, et al. Psychosocial predictors of maternal depressive symptoms, parenting attitudes, and child behavior in single-parent families. Nurs Res. 1991;40:214–220. [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale a self-report depression scale for research in the general population. Applied psychological measurement. 1977;1:385–401. [Google Scholar]
- 29.Given CW, Given B, Stommel M, et al. The caregiver reaction assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Res Nurs Health. 1992;15:271–283. doi: 10.1002/nur.4770150406. [DOI] [PubMed] [Google Scholar]
- 30.Bass PF, Wilson JF, Griffith CH. A shortened instrument for literacy screening. J Gen Intern Med. 2003;18:1036–1038. doi: 10.1111/j.1525-1497.2003.10651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 32.Cella DF, Tulsky DS. Quality of life in cancer: definition, purpose, and method of measurement. Cancer Invest. 1993;11:327–336. doi: 10.3109/07357909309024860. [DOI] [PubMed] [Google Scholar]
- 33.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 34.Lent L, Hahn E, Eremenco S, et al. Using cross-cultural input to adapt the Functional Assessment of Chronic Illness Therapy (FACIT) scales. Acta Oncol. 1999;38:695–702. doi: 10.1080/028418699432842. [DOI] [PubMed] [Google Scholar]
- 35.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–1238. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Raghunathan TE, Lepkowski JM, Van Hoewyk J, et al. A Multivariate Technique for Multiply Imputing Missing Values Using a Sequence of Regression Models. Survey Methodology. 2001;27:85–96. [Google Scholar]
- 37.Olsen MK, Stechuchak KM, Edinger JD, et al. Move over LOCF: principled methods for handling missing data in sleep disorder trials. Sleep Med. 2012;13:123–132. doi: 10.1016/j.sleep.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 38.van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–242. doi: 10.1177/0962280206074463. [DOI] [PubMed] [Google Scholar]
- 39.Grant JS, Glandon GL, Elliott TR, et al. Caregiving problems and feelings experienced by family caregivers of stroke survivors the first month after discharge. International Journal of Rehabilitation Research. 2004;27:105–111. doi: 10.1097/01.mrr.0000127639.47494.e3. [DOI] [PubMed] [Google Scholar]
- 40.Zarit SH. Caregiver assessment: Voices and views from the field. II. San Francisco, CA: National Center on Caregiving at Family Caregiver Alliance; 2006. Assessment of family caregivers: A research perspective. https://caregiver.org/sites/caregiver.org/files/pdfs/v2_consensus.pdf. [Google Scholar]
- 41.Centers for Medicare & Medicaid Services. Readmissions Reduction Program. 2014 http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html.
- 42.U.S. News and World Report, Health. Duke University Hospital; 2014. http://health.usnews.com/best-hospitals/area/nc/duke-university-medical-center-6360355/cancer. [Google Scholar]