Abstract
A method for detection of fucosylated glycans from haptoglobin in patient serum has been developed that provides enhanced sensitivity. The workflow involves isolation of the haptoglobin using an HPLC-based affinity column followed by glycan removal, extraction and desialylation. The fucosylated glycans are then derivatized by Meladrazine which significantly enhances the detection of the glycans in electrospray ionization. The separation of the derivatized glycans in a HILIC column shows 8 glycans from haptoglobin can be detected using less than 1 μL serum sample, with excellent reproducibility and quantitation, where without derivatization the glycans could not be detected. The ratio of the fucosylated peaks to their corresponding non-fucosylated forms shows that the fucosylated glycans are upregulated in the case of hepatocellular carcinoma (HCC) samples versus cirrhosis samples, where the relatively low abundance bifucosylated tetra-antennary form can be detected and may be a particularly good marker for HCC.
Keywords: liquid chromatography-mass spectrometry, electrospray ionization, hepatocellular cancer, cirrhosis, fucosylation, Meladrazine
Introduction
Fucosylation has been used as a potential marker for various cancers, including pancreatic, ovarian, lung and liver cancers among others1-4. In the case of hepatocellular carcinoma (HCC) in particular there have been several recent core-fucosylated markers that could distinguish between HCC and liver cirrhosis5, 6. For example, AFP-L3, which depends on a core-fucosylated (CF) glycopeptide of AFP, has been used as an alternative marker for HCC7, 8. Other work has shown that one of the 4 core-fucosylation sites in ceruloplasmin provides an improved detection specifically between ALC-related cirrhosis and ALC-related HCC9. Other investigations have shown that core-fucosylation in haptoglobin is a potential marker that can outperform AFP for detection of HCC versus cirrhosis10.
In mass spectrometry (MS) analysis of glycans enzymatically cleaved from proteins, the poor ionization efficiency of glycans is an important issue that needs to be improved, especially for detection of minor peaks that might be important markers of diseases. Derivatization plays a significant role in detection intensity enhancement. Permethylation is one strategy used to improve glycan response in MS11, however, the reaction which covers all hydroxyl groups may result in complex mass spectra due to the disparate labeling sites. Compared with permethylation, the reductive amination and hydrazine based labeling procedure provides only one possibility to react with an aldehyde group in the glycan, thus forming a single derivative product12. A N2,N2,N4,N4-Tetraethyl-6-hydrazinyl-1,3,5-triazine-2,4-diamine (Meladrazine) reagent has been recently developed as an alternative agent which can provide superior sensitivity for glycan analysis in mass spectrometry13.
In this work, we have developed a quantitative method to detect fucosylated glycan structures at low levels in haptoglobin with a minimal amount of sample by utilizing the Meladrazine derivatization reagent. The method involves haptoglobin extraction by an HPLC based affinity column14 with subsequent glycan extraction and sialic acid removal. The glycans are then derivatized with the agent that allows one to detect glycans from 1 μL of patient serum by HILIC separation and electrospray mass spectrometry. This is demonstrated for samples from HCC patients versus those with cirrhosis. It is shown that a relatively small but distinctive peak of a bifucosylated tetra-antennary glycan can distinguish HCC from cirrhosis. The method is shown to be quantitative and reproducible and allows sensitive detection of glycans from serum samples.
Materials and Methods
Materials
Peptide-N-glycosidase (PNGase F, 500 U/μL) was purchased from New England Biolabs (Ipswich, MA, USA). Neuraminidase, β-mercaptoethanol, acetic acid, HPLC-grade acetonitrile (ACN), HPLC-grade methanol, water, and porous graphitized carbon tips (PGC tips) were purchased from Sigma-Aldrich (St. Louis, MO). Anti-human haptoglobin antibody and haptoglobin standard were obtained from Abcam (Cambridge, MA).
Serum Samples
A total of 14 serum samples from patients were provided by the University Hospital, Ann Arbor, Michigan, according to IRB approval, which include 8 HCC cases (3 HBV-, 2 HCV-, and 3 ALC-related) and 6 cirrhosis cases (1 HBV-, 3 HCV-, and 2 ALC-related). The clinical features of the patient samples are summarized in Table S1. The cancer group consisted of 8 primary HCCs at various clinical stages (BCLC classification): stage A (n = 4), stage B (n = 3), stage C (n = 1), and stage D (n = 0). Among the early stage HCC patients (BCLC stages A and B), there were 3 HBV-, 1 HCV- and 3 ALC-related HCCs. The only advanced HCC patient was an HCV-related HCC sample. Samples were aliquoted and stored at −80 °C before use.
Isolation of Haptoglobin from Serum
Haptoglobin was purified from up to 20 μL patient serum using an anti-haptoglobin antibody immobilized HPLC column developed in-house as reported previously14. The purification was performed on a Beckman Coulter Proteome™ Lab PPS system (Fullerton, CA) with a flow rate of 0.5 mL/min for 40 min. A custom-made antibody conjugated resin column (4.6 mm × 50 mm) was used for serum haptoglobin enrichment. Briefly, the serum sample was diluted with 200 μL of dilution buffer (10 mM TBS, 150 mM NaCl, pH 7.4) and then loaded onto the HPLC column. The bound fraction was eluted with stripping buffer (0.1 M Glycine, pH 2.5) and neutralized with the neutralization buffer (0.1 M Tris-HCl, pH 8.0). The eluted haptoglobin fraction (~ 3.5 mL) was desalted using a 4 mL YM-3 centrifugal device (Millipore, Billerica, MA) by buffer exchange three times with HPLC water and then dried down in a SpeedVac concentrator. One-tenth of the eluted haptoglobin was checked by SDS-PAGE followed by silver staining using ProteoSilver™ Plus Silver Stain Kit (Sigma) to confirm the purity of haptoglobin.
Deglycosylation and Desialylation of Haptoglobin
Half of the eluted haptoglobin was dissolved in water and denatured by adding 1 μL of fresh denaturing buffer (0.2% SDS, 100 mM β-mercaptoethanol) with incubation at 60 °C for 30 min. After the solution was cooled to room temperature, 50 mM ammonium bicarbonate solution was added to make a final concentration of 15 mM. One unit of PNGase F was added to release N-glycans. The reaction mixture was incubated at 37 °C for 18 hours and quenched by heating at 95 °C for 10 min. Subsequently, the mixture was dried and reconstituted in 30 μL 20 mM ammonium acetate followed by desialylation with 40 mU neuraminidase (Sigma-Aldrich, St. Louis, MO) at 37 °C for 20 h. The desialylated N-glycans were dried in a SpeedVac and redissolved in 10 μL of water (with 0.1% TFA), then purified using porous graphitized carbon tips (PGC tips) as described previously10. The resulting glycan samples were dried in a SpeedVac and stored at −20 C for further use.
Labeling of Haptoglobin glycans
The labeling procedure used a new labeling reagent Meladrazine according to a previously reported method15, with some modifications. A 50 mM solution of the labeling reagent was made in 3:1 MeOH : acetic acid (v/v). 20 μL reagent solution was added to each tube containing purified glycans, which greatly exceeded the glycan molarity. The reaction mixture was run at 60 °C for 3 hours. It was then dried under vacuum and dissolved in 10 μL of solution which was used as the initial LC-MS condition (80% ACN, 20% 10mM ammonium formate in water, pH 6.5) for LC-MS analysis.
LC-MS Analysis
LC-MS analysis was performed using a Paradigm MG4 micropump system (Michrom Biosciences, Auburn, CA) and ESI-LTQ mass spectrometer (Thermo Fisher Scientific, San Jose, CA). Separation of derivatized glycans was performed on hydrophilic interaction chromatography (HILIC). A HALO Penta-HILIC column (2.1 mm × 150 mm, 5 μm particle size) was used. Mobile phases A and B were 10 mM ammonium formate in water (pH 6.5) and 98% ACN contained 0.1% formic acid, respectively. 10 μL of sample was injected at the initial gradient condition (80% mobile phase). The gradient was then ramped from 80% to 50% solvent B over 25 min with a total run time of 40 min. Glycans were eluted at 0.2 mL/min. The positive ion mode was used for analysis. The MS detection range was set at 800 to 2000 m/z. The ESI spray voltage and capillary voltage were set at 5 kV and 35 V, respectively, with the stainless steel capillary heating to 275 °C.
Data Analysis
Structures and m/z values of derivatized glycans were calculated and identified by GlycoWorkbench Software. GlycoWorkbench is a software tool developed by the EUROCarbDB initiative to assist the manual interpretation of MS data.16 Often used residues of glycans are included in the software. The list of structural constituents comprises an exhaustive collection of saccharides, substituents, reducing-end markers and saccharide modifications. All the stereo-chemical information about a glycan, including the linkage position and ring configuration, can be specified. The display of a glycan is dependent only on its structure and the chosen notation. In our case, by specifying the molecular weight of labeling reagent, we applied the tool to calculate the theoretical exact mass of each derived glycan first and then input the experimented m/z value to search for structures to confirm the results. The identified glycans were quantified automatically with Xcalibur Qual Browser 2.1 (Thermo Fisher Scientific, San Jose, CA) using the peak area from the extracted ion chromatogram (XIC) with the following settings: (1) peaks were extracted with a 1 Da (±0.5 Da) mass window, (2) scan filter was set as full MS, and (3) Genesis peak detection algorithm was used.
Results and Discussion
Fucosylation degree changes are closely related to HCC and other cancers17. Researchers have found that bifucosylated N-glycans with both core and antennary fucosylation are significantly elevated in patients with HCC compared to that in liver cirrhosis10, 18. ESI-LC-MS in particular has been demonstrated to have great advantages for detection and analysis of glycans. This generally requires separation of the glycans and derivatization to enhance detection. In this study we have developed a sensitive ESI-LC-MS method for haptoglobin fucosylation analysis in HCC and liver cirrhosis based on a new reagent13 which can enhance the sensitivity for detection.
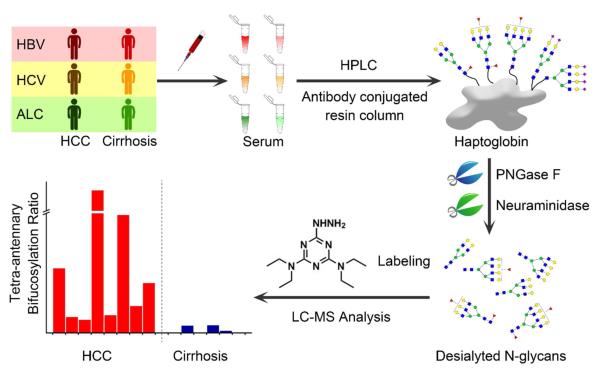
Figure 1 outlines the workflow for the method used herein. HCC and cirrhosis patients with three major etiologies, HCV, HBC and ALC, were selected to test this glycomic detection method. Initially haptoglogin was isolated using an anti-haptoglobin antibody immobilized HPLC column developed in-house14 and then the N-glycans were enzymatically released by PNGase F from the haptoglobin and desialylated by neuraminidase. Sialic acids were removed so as to avoid interference for glycan profiling attributed to loss of sialic acids during the ionization process and to reduce the glycan heterogeneity with various amounts of sialic acids. Also glycans with similar structures but different sialic acid content collapsed to one peak after removing sialic acids, which increased the MS detection sensitivity.
Figure 1.
Workflow of LC-MS analysis of haptoglobin and fucosylation changes in hepatocellular carcinoma (HCC) and liver cirrhosis of the three most common etiologies, infection with hepatitis B virus (HBV), infection with hepatitis C virus (HCV), and heavy alcohol consumption (ALC).
The desialylated N-glycans were then labeled by an s-triazine based labeling reagent Meladrazine developed previously13 before LC-MS analysis. Compared with the permethylation and reductive amination approaches, the hydrazine labeling procedure has been applied with distinct advantages. In comparison, permethylation often results in mass spectra with several peaks for each glycan, corresponding to different labeling sites. This increases the complexity of analysis and makes MS data identification more difficult, especially for complex samples. Also, some sensitivity is sacrificed due to this multiple peaks per glycan. Compared to the reductive amination approach, there is efficient reactivity with the aldehyde group where the reaction product hydrazone is much more stable than imine, and thus achieves direct analysis in ESI-MS without any further desalting or other purification. The elimination of these procedures greatly reduced the sample pre-treatment time and increased the recovery rate and the reproducibility of the method. The reagent provided enhanced signal intensity and sensitivity so that the fucosylation changes in serum haptoglobin from HCC and cirrhosis patients could be readily analyzed.
Isolation of Haptoglobin from Serum
Haptoglobin was isolated from serum of patients with HCC (n = 8) and liver cirrhosis (n = 6), respectively. The antibody conjugated HPLC column enriched haptoglobin from serum was further evaluated by gel electrophoresis in combination with silver staining. Figure S1 shows the SDS-PAGE image of one of the enriched haptoglobin samples, where only haptoglobin is observed without impurities. By applying the Kaleidoscope protein marker (Bio-Rad, Hercules, CA), haptoglobin β chain with ~40 kDa, α-2 chain with ~20 kDa and α-1 chain with ~ 13 kDa were observed.
Limit of Detection
To evaluate the limit of detection (LOD) of the ESI-LC-MS method, haptoglobin standard from normal serum was used. N-glycans were first enzymatically released from haptoglobin standards then desialylated using neuraminidase, followed by PGC purification and Meladrazine labeling for ESI-LC-MS analysis. Since the standard haptoglobin was purified from normal samples, fucosylated glycans were always present at low abundance. The three most intense non-fucosylated but desialylated N-glycans with bi-antennary structure (Hex5HexNAc4), tri-antennary structure (Hex6HexNAc5) and tetra-antennary structure (Hex7HexNAc6), respectively, were chosen as models to make calibration curves, which are shown in Figure S2 with correlation coefficients (R2) between 0.989 to 0.997. Calibration curves for peak area (A) versus the initial amount of haptoglobin (m) were fitted using a linear equation (A) = a (m) + b. Their linearity and LODs (S/N=3) are summarized in Table 1. Acceptable linearity was obtained with absolute LODs as low as 0.1, 0.1, and 0.5 μg haptoglobins for the three glycans at a S/N ratio of 3, respectively. The labeling by s-triazine based labeling reagent Meladrazine played an important role in N-glycan detection. The multi-nitrogen atoms in the reagent structure generate positive charges during the ionization process, thus increasing the ionization efficiency of the N-glycans derivatized by the reagent in the positive mode. A comparison between Meladrazine and the commercial labeling reagent 2-aminobenzamide (2-AB), which was developed based on reductive amination reaction, was performed. 2-AB labeled DP7 was analyzed and compared with the reagent Meladrazine under the same separation and detection conditions. As shown in Figure S3, an approximately six-fold increase is obtained for the 2-AB labeled sample while the Meladrazine labeled glycan shows around a forty-fold increase. These results demonstrate the superior performance of Meladrazine in signal enhancement over commercial 2-AB. According to a previous report, the glycan analysis method with Meladrazine labeling prior to LC-MS did enhance the signal intensity by a factor of 3915, which made it applicable for glycan profiling from haptoglobin isolated from small amounts of serum.
Table 1.
The calibration curve fittings and LODs (S/N = 3) for three non-fucosylated glycans in haptoglobin standard
| Glycan | Calibration curve | Absolute LOD of initial amount of Haptoglobin (μg) |
|---|---|---|
| Bi-antennary | A = 8.40×105m + 2.07×104 | 0.1 |
| Tri-antennary | A = 6.15×105m − 4.82×104 | 0.1 |
| Tetra-antennary | A = 8.78×104m + 7.15×104 | 0.5 |
Desialylated N-Glycan Profiling of Serum Haptoglobin
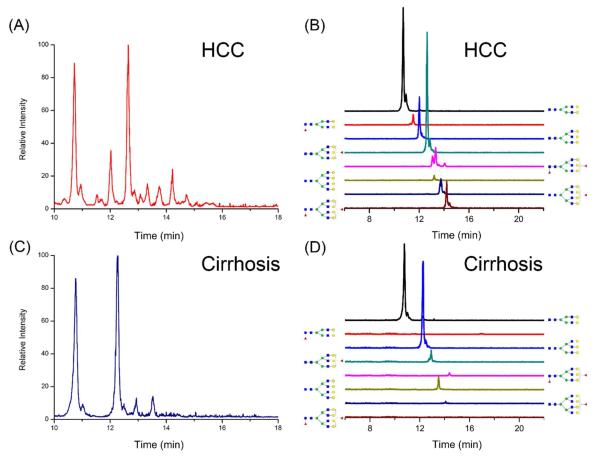
Before glycan profiling analysis, an intact glycan sample enzymatically cut from haptoglobin from a HCC patient without labeling was tested under the same conditions, where almost no signal of any glycans could be observed. This is shown in Figure S4, where (A) is the LC-MS base peak chromatogram of the intact glycan sample without any notable peaks while (B) is that of the glycan labeled by Meladrazine with the resulting glycan detection. Based on the enhancement effect due to labeling, a typical profile of desialylated N-glycans of human haptoglobin from HCC and cirrhosis patients is illustrated in Figure 2. Eight glycans were extracted with excellent S/N ratio and identified in the HCC sample, as shown in Figures 2(A), (B) and Table S2, which includes the nonfucosylated bi-, tri, and tetra-antennary glycans (m/z 1876.94, 1130.51 and 1312.91), monofucosylated bi-, tri, and tetra-antennary glycans (m/z 1020.51, 1203.12 and 1385.50), and bifucosylated tri- and tetra-antennary glycans (m/z 1276. 52 and 1458.61). However, only six types of N-glycans could be detected in the cirrhosis sample, as shown in Figures 2(C) and (D). Table S2 also lists the retention time data for eight glycans in HCC sample, which correspond to Figure 2. Glycans with higher molecular weight are generally more hydrophilic and have longer retention times on the HILIC column. The retention times of glycans shows a corresponding trend with the hydrophilicities of their structures. The retention time trend further provides confirmation for each structures determination.
Figure 2.
The base peak chromatograms of desialylated N-glycans in haptoglobin from patient sera with hepatocellular carcinoma (A) and liver cirrhosis (C) and the extracted ion chromatograms of identified glycans corresponding to hepatocellular carcinoma (B) and liver cirrhosis (D).
It should be noted that we used the same HCC and cirrhosis serum samples as those in our previous study10, where the oligosaccharide compositions of the eight typical glycans of serum haptoglobin were confirmed by MS/MS analysis at low energy CID using MALDI-QIT-TOF mass spectrometry. Herein, we also performed MS/MS analysis of the glycans on an LTQ in a data-dependent acquisition manner where the top three most abundant precursors in each full scan spectrum were selected for MS/MS using CID. Figure S5 shows a representative ESI-MS/MS spectrum of glycans at m/z 1876.94, which is the biantennary nonfucosylated glycan in haptoglobin. The result showed that the biantennary glycan at m/z 1876.94 was well fragmented while other glycan ions with a 2+ charge were rarely fragmented. Therefore, we referred to our previous work10 for glycan structure determination. GlycoWorkbench software was also employed in glycan identification. The glycan profiling results and abundance differences between HCC and cirrhosis are in good accordance with previous reports by MALDI-MS analysis10.
Fucosylation Degree of Haptoglobin N-Glycan in HCC and Cirrhosis Samples
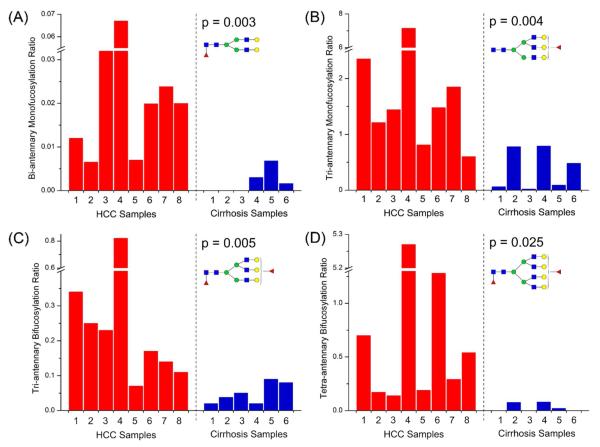
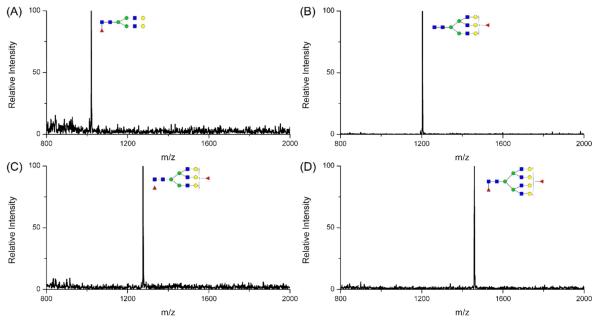
The fucosylation ratio was calculated by the peak area of the fucosylated glycan divided by that of glycan in the same antennary structures but without fucosylation. All the fucosylation ratios are listed in Table S3, with accompanying p-values between the HCC and cirrhosis groups. Four fucosylation types presented significant discrimination between cancer and cirrhosis with p values all below 0.05, which include the monofucosylated bi-antennary, monofucosylated tri-antennary, bifucosylated tri-antennary and bifucosylated tetra-antennary (Figure 3). Their corresponding mass spectra are presented in Figure 4 where there is a clear and distinct single peak for each type of fucosylated glycan. Even for the lowest bifucosylation ratio observed in tri-antennary and tetra-antennary structures, a mass spectrum with S/N over 3 could be obtained as shown in Figure S6. Since most HCC samples were BCLC early stages, the difference in fucosylation ratio between HCC and cirrhosis glycans provide a potential means to distinguish early HCC from cirrhosis. All four fucosylation types of glycans show a p value below 0.05, which demonstrates significant discrimination between cancer and cirrhosis. However, the bifucosylated tetra-antennary glycan is the best potential performer among them. The monofucosylated bi-antennary glycan is always in extremely low abundance compared with other types of fucosylated glycans even in HCC samples. As shown in Figure 3(A), the highest fucosylation ratio is lower than 0.07 while the lowest fucosylation ratio of other three types is still greater than 0.1. Figure 3(B) and Figure 3(C) illustrate that the monofucosylated tri-antennary and bifucosylated tri-antennary glycans presented relative higher abundance in cirrhosis samples compared to the bifucosylated form. The bifucosylated tetra-antennary glycan with relatively low abundance but large differences between HCC and cirrhosis is thus the most suitable glycan to discriminate these two states.
Figure 3.
The ratio of the fucosylated peaks to their corresponding non-fucosylated forms of (A) monofucosylated bi-antennary, (B) monofucosylated tri-antennary, (C) bifucosylated tri-antennary and (D) bifucosylated tetra-antennary desialylated N-glycans in haptoglobin from patient sera with hepatocellular carcinoma (red column) and liver cirrhosis (blue column).
Figure 4.
Mass spectra of (A) monofucosylated bi-antennary, (B) monofucosylated tri-antennary, (C) bifucosylated tri-antennary and (D) bifucosylated tetra-antennary desialylated N-glycans in haptoglobin from patient sera with hepatocellular carcinoma
Reproducibility and sensitivity of the method
Repeated trials were carried out to evaluate the reproducibility of the method. Three copies of haptoglobin were first enriched in parallel from the same serum sample. After PNGase F deglycosylation and neuraminidase desialylation, desalting and purification, glycans were derivatizated by the labeling reagent, followed by LC-MS analysis. All of the sample pretreatment procedures, separation and detection conditions were the same for the three samples. The relative standard deviation (RSD) of retention time and peak area of eight primary N-glycans are listed in Table 2, which are all below 0.3% and 8%, respectively. Even those glycans with low abundance could be detected via the method, where the method appears to be robust and stable for real sample applications.
Table 2.
RSD of peak area of glycans from HCC haptoglobin (n = 3)
| Structure | RSD of Retention Time | RSD of Peak Area |
|---|---|---|
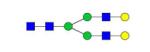
|
0.10% | 0.68% |
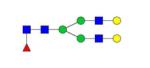
|
0.09% | 4.71% |
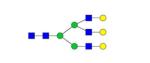
|
0.05% | 6.73% |
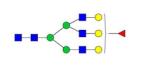
|
0.14% | 3.89% |
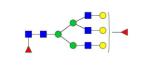
|
0.19% | 7.46% |
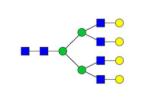
|
0.14% | 5.00% |
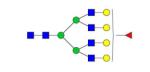
|
0.04% | 3.40% |
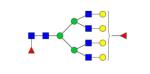
|
0.23% | 2.29% |

|
N-acetylglucosamine |

|
Mannose |
|
| |||

|
Galactose |

|
Fucose |
Different initial volumes of serum (10 μL, 5μL and 1μL, respectively) were utilized to investigate the sensitivity of the method. The result demonstrates that the four critical fucosylated glycans could all be identified in only 1 μL serum with S/N of 3 with good linearity. As shown in Figure S7, the correlation coefficients (R2) were 0.980, 0.995, 0.993 and 0.999 for each type of glycan. The slightly poor linearity of monofucosylated bi-antennary glycan was attributed to the low abundance of this glycan compared to others.
Conclusions
In this work, we have developed a work-flow for analysis of fucosylated glycans of haptoglobin from patient serum. Using this method we were able to extract haptoglobin from serum and isolate the glycans for further analysis. We separated the glycans with HILIC HPLC and with the use of the derivatizing agent Meladrazine we were able to detect 8 glycans for analysis by ESI-MS down to 1 μL of serum. We could not detect the glycans at the amounts of serum used without the Meladrazine agent. Also we found that desialylation simplified the signal and also increased the sensitivity in detection. Several of the glycans were fucosylated and when compared to their non-fucosylated forms were generally found to be upregulated in HCC versus cirrhosis for a limited sample set used in this work. The method was found to be particularly effective for detection of the bifucosylated tetra-antennary peak which is relatively low abundance and may be a good marker for HCC. The method was also shown to be quantitative and reproducible.
Supplementary Material
Acknowledgement
This work was funded by the National Cancer Institute under grant 1R01 CA160254 (DML) and received partial support from the National Institutes of Health through grant R01 GM 49500 (DML). The work was also supported by the Graduate School of Peking University.
Abbreviations
- HCC
hepatocellular carcinoma
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- ALC
alcohol consumption
- LC-LTQ-MS
liquid chromatography with linear ion trap mass spectrometer
- Meladrazine
N2,N2,N4,N4-Tetraethyl-6-hydrazinyl-1,3,5 triazine-2,4-diamine
- PGC
porous graphite carbon
Footnotes
Supporting Information Available:
This material is available free of charge via the Internet at http://pubs.acs.org.
Supplemental Figure S1. SDS-PAGE electropherogram of isolated haptoglobin from serum.
Supplemental Figure S2. Calibration curves with correlation coefficients (R2) for the three non-fucosylated glycan types in haptoglobin standard.
Supplemental Figure S3. Extracted ion chromatograms of Meladrazine labeled DP7, 2-AB labeled DP7 and intact DP7.
Supplemental Figure S4. Base peak chromatograms of (A) intact desialylated N-glycans and (B) Meladrazine labeled desialylated N-glycans from human haptoglobin with HCC.
Supplemental Figure S5. ESI-MS/MS spectrum of the glycan at m/z 1876.94. Potential structures of fragment ions are labeled.
Supplemental Figure S6. Mass spectra of tri-antennary bifucose and tetra-antennary bifucose at the lowest bifucosylation ratio using 1 uL of serum sample.
Supplemental Figure S7. Calibration curves of four critical fucosylated N-glycans enzymatically released from patient sera with desialylation.
Supplemental Table S1. Summary of Sample Population Characteristics
Supplemental Table S2. The identified desialylated N-glycans in haptoglobin from patient sera with hepatocellular carcinoma and liver cirrhosis
Supplemental Table S3. Summary of Fucosylation Ratio
References
- 1.Tan ZJ, Yin HD, Nie S, Lin ZX, Zhu JH, Ruffin MT, Anderson MA, Simone DM, Lubman DM. Large-Scale Identification of Core-Fucosylated Glycopeptide Sites in Pancreatic Cancer Serum Using Mass Spectrometry. J. Proteome Res. 2015;14:1968–1978. doi: 10.1021/acs.jproteome.5b00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu J, Xie XL, Liu YS, He JT, Benitez R, Buckanovich RJ, Lubman DM. Identification and Confirmation of Differentially Expressed Fucosylated Glycoproteins in the Serum of Ovarian Cancer Patients Using a Lectin Array and LC-MS/MS. J. Proteome Res. 2012;11:4541–4552. doi: 10.1021/pr300330z. [DOI] [PubMed] [Google Scholar]
- 3.Li QK, Gabrielson E, Zhang H. Application of glycoproteomics for the discovery of biomarkers in lung cancer. Proteomics Clin. Appl. 2012;6:244–256. doi: 10.1002/prca.201100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrero JA, Feng ZD, Wang YH, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D, Dalhgren J, Chia D, Lok AS, Wagner PD, Srivastava S, Schwartz M. alpha-Fetoprotein, Des-gamma Carboxyprothrombin, and Lectin-Bound alpha-Fetoprotein in Early Hepatocellular Carcinoma. Gastroenterology. 2009;137:110–118. doi: 10.1053/j.gastro.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee HJ, Cha HJ, Lim JS, Lee SH, Song SY, Kim H, Hancock WS, Yoo JS, Paik YK. Abundance-Ratio-Based Semiquantitative Analysis of Site-Specific N-Linked Glycopeptides Present in the Plasma of Hepatocellular Carcinoma Patients. J. Proteome Res. 2014;13(5):2328–2338. doi: 10.1021/pr4011519. [DOI] [PubMed] [Google Scholar]
- 6.Fanayan S, Hincapie M, Hancock WS. Using lectins to harvest the plasma/serum glycoproteome. Electrophoresis. 2012;33:1746–1754. doi: 10.1002/elps.201100567. [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, Nakata K, Kato Y, Shima M, Ishii N, Koji T, Taketa K, Endo Y, Nagataki S. Early Recognition of Hepatocellular-Carcinoma Based on Altered Profiles of Alpha-Fetoprotein. N. Engl. J. Med. 1993;328:1802–1806. doi: 10.1056/NEJM199306243282502. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa T, Miyoshi E, Yakushijin T, Hiramatsu N, Igura T, Hayashi N, Taniguchi N, Kondo A. Glycomic analysis of alpha-fetoprotein L3 in hepatoma cell lines and hepatocellular carcinoma patients. J. Proteome Res. 2008;7:2222–2233. doi: 10.1021/pr700841q. [DOI] [PubMed] [Google Scholar]
- 9.Yin HD, Lin ZX, Nie S, Wu J, Tan ZJ, Zhu JH, Dai JL, Feng ZD, Marrero J, Lubman DM. Mass-Selected Site-Specific Core-Fucosylation of Ceruloplasmin in Alcohol-Related Hepatocellular Carcinoma. J. Proteome Res. 2014;13:2887–2896. doi: 10.1021/pr500043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu JH, Lin ZX, Wu J, Yin HD, Dai JL, Feng ZD, Marrero J, Lubman DM. Analysis of Serum Haptoglobin Fucosylation in Hepatocellular Carcinoma and Liver Cirrhosis of Different Etiologies. J. Proteome Res. 2014;13:2986–2997. doi: 10.1021/pr500128t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciucanu I, Kerek F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984;131:209–217. [Google Scholar]
- 12.Harvey DJ. Electrospray mass spectrometry and fragmentation of N-linked carbohydrates derivatized at the reducing terminus. J. Am. Soc. Mass Spectrom. 2000;11:900–915. doi: 10.1016/S1044-0305(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 13.Tie C, Zhang XX. A new labelling reagent for glycans analysis by capillary electrophoresis-mass spectrometry. Anal. Methods. 2012;4:357–359. [Google Scholar]
- 14.Zhu JH, Wu J, Yin HD, Marrero J, Lubman DM. Mass spectrometric N-glycan analysis of haptoglobin from patient serum samples using a 96-well plate format. J. Proteome Res. doi: 10.1021/acs.jproteome.5b00662. DOI: 10.1021/acs.jproteome.5b00662 [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao M-Z, Zhang Y-W, Yuan F, Deng Y, Liu J-X, Zhou Y-L, Zhang X-X. Hydrazino-s-triazine based labelling reagents for highly sensitive glycan analysis via liquid chromatography–electrospray mass spectrometry. Talanta. 2015;144:992–997. doi: 10.1016/j.talanta.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 16.Ceroni A, Maass K, Geyer H, Geyer R, Dell A, Haslam SM. GlycoWorkbench: A Tool for the Computer-Assisted Annotation of Mass Spectra of Glycans. J. Proteome Res. 2008;7:1650–1659. doi: 10.1021/pr7008252. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy C, Saldova R, O’Brien ME, Bergin DA, Carroll TP, Keenan J, Meleady P, Henry M, Clynes M, Rudd PM, Reeves EP, McElvaney NG. Increased Outer Arm and Core Fucose Residues on the N-Glycans of Mutated Alpha-1 Antitrypsin Protein from Alpha-1 Antitrypsin Deficient Individuals. J. Proteome Res. 2014;13:596–605. doi: 10.1021/pr400752t. [DOI] [PubMed] [Google Scholar]
- 18.Lin ZX, Yin HD, Lo A, Ruffin MT, Anderson MA, Simeone DM, Lubman DM. Label-free relative quantification of alpha-2-macroglobulin site-specific core-fucosylation in pancreatic cancer by LC-MS/MS. Electrophoresis. 2014;35:2108–2115. doi: 10.1002/elps.201300376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.