Abstract
Vascular resistance networks control tissue blood flow in concert with regulating arterial perfusion pressure. In response to increased metabolic demand, vasodilation arising in arteriolar networks ascends to encompass proximal feed arteries. By reducing resistance upstream, ascending vasodilation (AVD) increases blood flow into the microcirculation. Once initiated [e.g., through local activation of K+ channels in endothelial cells (ECs)], hyperpolarization is conducted through gap junctions along the endothelium. Via EC projections through the internal elastic lamina, hyperpolarization spreads into the surrounding smooth muscle cells (SMCs) through myoendothelial gap junctions (MEGJs) to promote their relaxation. Intercellular signaling through electrical signal transmission (i.e., cell-to-cell conduction) can thereby coordinate vasodilation along and among the branches of microvascular resistance networks. Perivascular sympathetic nerve fibers course through the adventitia and release norepinephrine to stimulate SMCs via α-adrenoreceptors to produce contraction. In turn, SMCs can signal ECs through MEGJs to activate K+ channels and attenuate sympathetic vasoconstriction. Activation of K+ channels along the endothelium will dissipate electrical signal transmission and inhibit AVD, thereby restricting blood flow into the microcirculation while maintaining peripheral resistance and perfusion pressure. This review explores the origins and nature of intercellular signaling governing blood flow control in skeletal muscle with respect to the interplay between AVD and sympathetic innervation. Whereas these interactions are integral to physical daily activity and athletic performance, resolving the interplay between respective signaling events provides insight into how selective interventions can improve tissue perfusion and oxygen delivery during vascular disease.
Keywords: arterioles, calcium-activated potassium channel, conducted dilation, endothelium, exercise, gap junctions, microcirculation, resistance arteries, sympathetic nerves, vascular smooth muscle, ascending vasodilation
Introduction
It is both humbling and a tremendous honor to receive the Malpighi Award from the European Society for Microcirculation. My studies of the microcirculation began formally in 1984 at the University of Virginia as a postdoctoral fellow under the mentorship of Brian Duling and to whom this article is dedicated in memorium. With now over three decades of sharing the search, such recognition by the ESM truly reflects the efforts and achievements of the outstanding individuals with whom I have the privilege of sharing this passion in my laboratory. The relationships presented here are developed with respect to how the phenomenon of “propagated vasodilation” in the microcirculation has evolved into a multifaceted field of investigation. In accord with the ability of vasomotor responses to spread along and among branches of the resistance vasculature, such coordinated regulation among arterioles and their proximal feed arteries has become recognized as “conducted”, “spreading” and “ascending” vasodilation with respect to the context in which it has been studied. Given my focus on understanding the control of muscle blood flow during exercise, concepts are presented with controversies addressed. Given the nature of the Malpighi Award, the perspective conveyed herein reflects the development and evolution of my laboratory and research program, which I respectfully submit as an example of how studying the microcirculation can open many doors.
The underlying theme of this article is career development thus I address primarily young investigators studying the microcirculation as it is they (you!) who represent the future of our discipline. Indeed, my decision to pursue advanced training stemmed from the desire to be able to have trainees of my own one day. Through my own extraordinary mentors at UC Berkeley, The University of Michigan and The University of Virginia, I developed a sense of how this could be done. Since its inception (1987) in the Noll Laboratory for Human Performance Research at Penn State, my laboratory has focused on studies of microvascular signaling and exercise hyperemia. However this article is not intended as a review of either topic. Rather, my goal is convey key personal experiences as a new field of investigation was defined and has matured. I shall refer to particular experiences as “Career-Defining Moments” (CDM) in hope that others may reflect on their own. I also wish to convey a bit of philosophical perspective garnered along the way.
Even while our publications serve as scientific “currency,” the impact of individual papers typically wanes over time with but a select few earning recognition as “classics” (e.g., [1–3]). In the current environment, young investigators (and those who judge them) are often more concerned with the quantity rather than quality and originality of their work. One of the greatest rewards as a Principal Investigator and mentor is to facilitate the development of eager trainees into new investigators who are prepared to identify and implement original inquiry. Given the pressures to remain abreast of developments in one’s own area of investigation, typically less attention is given to papers published more than a few years ago. Further, when compared to many of the articles I read as a graduate student, the writing style in current publications tends to be much more focused on specific relationships under investigation. To harken back to earlier days, when research articles encompassed more of the “story” of how particular questions evolved and why findings were interpreted in a particular way, I have attempted to present this article in such fashion. Though I may appear to digress occasionally, such is the case when one deviates from their intended path to gain perspective. My hope is that rather than detracting from the overall presentation, these complementary bits of information help to convey relationships that are integral to the story being told.
Background and perspective
The decision to focus on studying the microcirculation for a career evolved from my undergraduate and early graduate work in exercise physiology. Inspiration came from the desire to understand how the human body is able to support extreme levels of energy turnover during intense physical activity. My first research project (Master’s thesis at Berkeley) involved human subjects (fellow graduate students and willing friends!) to address a classic problem in human energetics: What governs oxygen consumption, particularly during the recovery period when it remains elevated despite the cessation of external work? We found that lactic acid, produced in proportion to exercise intensity and long purported to regulate recovery oxygen consumption, actually had no discernable effect! [4]. Perusal of such correlations in the ‘classic’ literature used to substantiate these relationships revealed them to be a matter of when samples were collected and the availability of a suitable precursor (e.g., muscle glycogen)… So much for cause-and-effect! As a doctoral student at Michigan, my research focused on skeletal muscle performance and how it was affected by injury and physical training [5, 6]. As with athletic performance, each of these relationships involves key roles for the microcirculation. Thus, my fate was sealed to focus on blood flow regulation in light of the coupling between metabolic demand and oxygen delivery during exercise. These interactions underscore a most impressive response of the vascular supply.
In accord with the span of its energetic requirements, the dynamic range of skeletal muscle blood flow when transitioning from rest to maximal perfusion during exercise (i.e., functional hyperemia) is greater than any other tissue in the body. Expressed relative to tissue mass, muscle blood flow can range from less than 5 ml/min/100g at rest to well over 250 (and perhaps more than 400) ml/min/100g during intense running and cycling [7, 8]. Thus, blood flow to skeletal muscle can vary 50- to 100-fold and it is the resistance vasculature (i.e., arterioles and their feed arteries) that governs this incredible range of tissue perfusion [9]. In turn, with skeletal muscle typically comprising 30–50% of total body mass, the ability of its vascular supply to dilate and reduce total peripheral resistance is so great that vasodilation must be restricted to prevent a fall in arterial perfusion pressure [10]. As developed later in this article, this regulatory role is served primarily by the sympathetic nervous system [10, 11].
Classic studies of functional vasodilation in response to contractile activity had focused on the regulation of tissue oxygen pressure (pO2) [2, 3] and the ability of “vasodilator substances” produced by active skeletal muscle fibers to relax vascular smooth muscle cells (SMCs) [12, 13]. In practice, functional vasodilation entails the ability to override (i.e., “escape”) sympathetic vasoconstriction [10, 11, 14] through mechanisms that – remarkably – are still being resolved. A year after beginning my doctoral studies, the role of the endothelium in governing the relaxation of vascular smooth muscle was identified in the rabbit aorta [1], the principal systemic artery. Ensuing research confirmed and extended endothelium-dependent dilation throughout the resistance vasculature [15–18]. In light of integrating vasodilation (i.e., SMC relaxation) via the endothelium and vasoconstriction (i.e., SMC contraction) through sympathetic nerves, an underlying goal of this review is to identify and integrate respective roles in blood flow control.
When learning to study the microcirculation – rather than the consequences of what may have been done to it while preparing it for study – my strongest impression was the absolute requirement for punctiliousness. Indeed, as I try to explain to prospective trainees, one must be prepared for dedicating the time and effort requisite to collecting meaningful data. As manifested in works of Marcello Malpighi (1628–1694), it is revolutionary to reveal secrets of nature by studying things that are too small to be seen by the naked eye. For example, Malpighi was the first (in 1661) to observe red blood cells coursing through pulmonary capillaries of the frog [19], thereby establishing the functional link between arteries and veins that had been inferred by William Harvey (1578–1657) in his treatise “De Motu Cordis” (“On the Motion of the Heart and Blood”) [20]. Such insightful, definitive observations were integral to Malpighi’s founding the science of microscopic anatomy while pioneering the use of histology towards understanding the structural organization of tissues and organs in plants and animals.
Professor Duling advised (and experience has confirmed) the need for caution when viewing through the microscope, to remain objective and to be attentive to what nature is trying to tell you. August Krogh was a master when studying the microcirculation of animals [2, 3] to gain insight into his less invasive studies of human performance [21]. A take-home message from all of this is that one should not be afraid to make mistakes during the process of discovery! Thus, a favorite adage of mine is that any experiment worth doing well is worth doing half-assed first. In other words, if you have an idea, try it out as best you can with what you’ve got… See if something is there that warrants further study! The hard part then lies in designing and executing the definitive experiment(s) to test your hypothesis rigorously, while recognizing that one’s findings are only as good as their controls. As exemplified by Malpighi’s discoveries, a most rewarding challenge for an investigator is the implementation of a novel scientific approach that enables new and definitive insight into the workings of nature, especially when it involves microcirculation!
Ascension of propagated vasodilation
When beginning to study the microcirculation in 1984, I was directed to the phenomenon of “propagated vasodilation” and was told (by Professor Duling) that it was “an idea whose time may have come.” The sole reprint request from his 1970 article [22] had been mounted over his desk for years… This paper described the spread of vasodilation along arterioles of the hamster cheek pouch in response to stimulating a discrete site with an appropriate stimulus. Thus, vasoactive agents were delivered as a brief (< 1 s) pulse from a micropipette positioned with its tip adjacent to the wall of an arteriole (diameter, ~ 30 μm), thereby confining the stimulus to within 20–40 μm [22]. In response to acetylcholine (ACh), dilation spread (termed “propagated vasodilation”) bi-directionally along the arteriole over distances encompassing several hundred μm. In contrast, other vasodilators (e.g., papaverine and eledoisin) produced dilation only within the vicinity of their direct effect. The ability to inhibit propagated vasodilation using local anesthetics (i.e., lidocaine or procaine) was interpreted to suggest neural activation as the underlying mechanism [22]. However, transmission along vascular smooth muscle was also considered in accord with Hilton’s conclusions regarding AVD in the cat hindlimb [23]. A few years later, experiments performed on sheep carotid arteries implicated the activation of K+ channels as a mechanism by which procaine (as well as lignocaine) affected electrical activity in the vessel wall [24]. Nevertheless, definitive studies had resolved neural control of arterioles in tissues of frogs and hamsters [25]. Indeed, the original observation of spreading vasodilatation in the web of the frog hindlimb was attributed to a local axon reflex [26]. While defining regional variation in the nature of innervation among vascular beds and animal species, ensuing publications have confirmed cholinergic, nitroxidergic, purinergic and sensory as well as adrenergic (i.e., sympathetic) innervation of the microvasculature (reviewed in [27]).
In the late 1970’s using preparations dissected from the small intestine of guinea pigs, Hirst and Neild employed dual intracellular microelectrodes to demonstrate that current injected into a submucosal arteriole (diameter, 30–75 μm) at one site evoked changes in membrane potential (Vm) at remote sites along the vessel and its contiguous branches [28]. These studies thereby demonstrated that an electrical signal could spread for at least 2–3 mm along the arteriolar wall. In light of studies that advanced electrical conduction in smooth muscle [29, 30], signaling over such distances was attributed to electrical transmission between neighboring SMCs. While Hirst and Neild were the first to define the cable properties of electrical conduction along arterioles, their preparations were neither pressurized nor perfused and electrical signal transmission was evaluated in the absence of vasomotor responses [28]. Though the endothelium was not disrupted, its role in governing vascular smooth muscle function had not yet been considered. Reflecting on the idea whose time had come, the role of propagated vasodilation in blood flow control was ripe for investigation - particularly in light of AVD during exercise [23, 31].
As illustrated in Figure 1, the functional significance of AVD is that events originating in distal microvessels spread upstream into the proximal arterial supply in order to attain maximal blood flow [32]. In the absence of AVD, any constriction of feed arteries upstream from arteriolar networks effectively restricts tissue blood flow into the microcirculation, even when arterioles within the tissue dilate maximally [32, 33]. Such differences in vasomotor control within resistance networks [12, 31, 34] provide an effective mechanism for maximizing the capillary surface area for oxygen extraction, particularly when total blood flowing into the microcirculation is limited by proximal vasoconstriction [31]. Regional differences in the nature of vasomotor control within vascular networks also points to opportunities for developing therapeutic interventions targeted to increase total blood flow into a tissue or tissue region (e.g., feed arteries and proximal arterioles) vs. expanding the surface area for gas and solute exchange (e.g., distal arterioles and capillary networks) with the tissue parenchyma.
Figure 1. Ascending vasodilation in a microvascular resistance network.
A. Illustration depicts a feed artery (FA) arising from a large conduit artery. The FA carries blood into the tissue (e.g., skeletal muscle; dotted line) and gives rise to primary (1A) arterioles, a and b. These 1A’s each give rise to second order (2A) arterioles, which branch into third-order (3A) and then terminal (TA) arterioles, with each TA giving rise to groups of capillaries (postcapillary microvessels omitted for clarity). B. Vasodilation originating in 3A and 2A (for branches c and d) “ascends” into the parent 1A (branch a) but not into the proximal FA. At the same time, branch b may receive little or no dilator signal from its daughter 2A and 3A (not shown) supplying an inactive region of the muscle. Under these conditions, lack of ascending vasodilation into FA limits tissue blood flow. C. With greater dilation of its daughter arterioles, vasodilation ascends into branch b which, together with signals along branch a are summed in the FA. Dilation of the FA reduces the proximal resistance restricting tissue blood flow. Total blood flow into the microcirculation increases dramatically.
My initial experiments as a postdoctoral fellow focused on the feed arteries that supply arteriolar networks of the cremaster muscle in anesthetized hamsters. As this model enabled imaging of resistance networks during blood flow control, we resolved AVD in response to the contraction of striated muscle fibers [32]. Determining that feed arteries external to the cremaster muscle (and confirmed using the gracilis muscle) dilate in concert with their arteriolar networks during muscle contraction substantiated earlier observations of AVD in cats [23, 31]. Our focus then turned to resolving the mechanism underlying how vasodilation actually traveled along the vessel wall. Several possibilities were apparent. First was a role for vasodilator nerves (above). Second, increased flow through the lumen of arteries had been proposed as a mechanism of vasodilation [35, 36]. Thus, when vessels dilate downstream, the resulting flow increase through upstream vessels could promote vasodilation [37]. A third mechanism entailed myogenic relaxation, whereby the increased flow velocity through the feed artery reduced transmural pressure (in accord with Bernoulli’s principle), resulting in vasodilation. To perform a definitive experiment, we needed a way to eliminate respective variables!
The hamster cheek pouch preparation enables access to relatively long unbranched arterioles while they remain perfused by the systemic circulation [38]. We developed a protocol whereby a single arteriole (diameter, ~25 μm) was occluded simultaneously at 2 sites using micropipettes separated from each other by several hundred microns. This maneuver created a “sealed segment” (Figure 2) of constant volume (and diameter) and devoid of blood flow while occluded at each end. With tetrodotoxin added to block neural propagation, the arteriole was stimulated with ACh from a micropipette positioned proximal to the first occluder while diameter was observed at a remote site distal to the second occluder. Using this paradigm, the first CDM for me in studying the microcirculation came late one afternoon. With respective micropipettes positioned as in Figure 2, the ACh stimulus was delivered at the proximal site and – to my absolute amazement – vasodilation occurred along the arteriole beyond the distal occluder despite no vasomotor response within the occluded segment! It should be recognized that even if SMCs relaxed within the sealed segment, it could not change in volume (or diameter) while occluded at each end. This paradigm served as a direct test (and confirmation) of the hypothesis that a vasodilator signal could travel along the arteriolar wall independent of nerves or mechanical signaling [39].
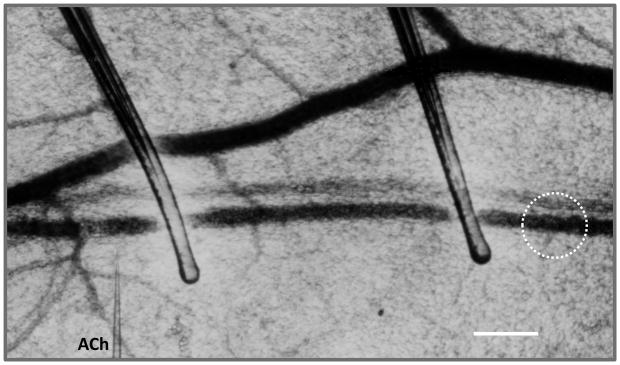
Figure 2. Conducted vasodilation in vivo.
A sealed arteriolar segment (~ 500 μm long) was created in the hamster cheek pouch using a pair of occlusion micropipettes. The stimulus micropipette containing acetylcholine (ACh) was positioned to the left of the proximal occluder with its tip (1 μm) adjacent to the arteriolar wall. In response to a pulse of ACh (200 ms, 1000 nA), vasodilation was measured to the right of the distal occluder (i.e., within broken circle) with no change in diameter or volume (i.e., in either pressure or flow) within the occluded segment. Scale bar = 100 μm. Original (unpublished) image from experiments performed in Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science 1986;234:868–870.
My initial (half-assed) experiment to determine whether vasodilation could spread along an occluded arteriole (where blood flow could not change) involved a single occluder. When presenting these preliminary findings at a conference, I was challenged by an astute observer who recognized that even though the signal traveled through the site of occlusion (where neither transmural pressure nor shear stress could change), the arteriole beyond the occluder filled with blood as it dilated. Thus neither changes in shear stress nor in transmural wall stress could be excluded as mechanical signals for vasodilation. Sealing a segment between dual occluders solved this limitation by eliminating changes in blood flow (or diameter) within the sealed segment. Remarkably, the signal conducted through both sites of occlusion and the sealed segment. As a positive control for the effects of damaging cells, if a pipette was rubbed across the arteriole to damage its cells, the propagated response was lost. Thus, nearly 2 years after beginning my postdoctoral training, a definitive experiment set our thinking (and ensuing research efforts) on a fundamentally new course: Instead of responding to nerves, or to changes in transmural pressure or blood flow (i.e., shear stress), we proposed that intercellular signaling (i.e., cell-to-cell conduction) through gap junctions was the mechanism underlying propagated vasodilation in arterioles [39, 40]. Ensuing experiments demonstrated that simultaneous stimulation of paired daughter arterioles downstream evoked vasomotor responses that summed in their parent arterioles upstream [41]. The latter findings illustrated that information arising at discrete locations on distal branches of a resistance network was integrated to determine the response of proximal branches. In accord with earlier findings [28], electrical signaling was implicated as a mechanism for conducting vasomotor responses along the arteriolar wall [40]. Complementary studies from independent laboratories indicated that signals initiated from capillaries could govern arterioles that controlled their perfusion [42, 43]. Nevertheless, early (half assed?) experiments were unable to resolve a definitive role for gap junctions or to discern whether the endothelium (vs. smooth muscle) was integral to signal transmission. Nor did these studies preclude a role for perivascular nerves!
New beginnings
As a young investigator attempting to establish independence, one of the greatest (of many) difficulties entails determining which avenue of investigation to develop as “your own.” I began by exploring a role for conducted vasodilation (CVD) in the microcirculation of striated muscle using the hamster cremaster muscle [44, 45]. A key experiment focused on terminal arterioles having high levels of resting vasomotor tone, such that no red blood cells were flowing through their lumen. As none of the capillaries supplied by these arterioles were perfused, I tested the hypothesis that CVD underlies microvascular recruitment and capillary perfusion with red blood cells. The key finding was that, in response to an ACh stimulus delivered at the distal end of a terminal arteriole, dilation traveled upstream (i.e., ascended) into the parent arteriole, resulting in perfusion of the terminal arteriole and the capillaries it supplied [46]. As the response was unaffected by tetrodotoxin (i.e., was not mediated by nerves), AVD within the microcirculation was shown to promote arteriolar recruitment and capillary perfusion along striated muscle fibers [46]. Complementary experiments using the cremaster preparation in the Sarelius laboratory illustrated that contracting bundles of striated muscle fibers could evoke a similar response [47, 48], confirming a functional role for arteriolar conduction in matching local perfusion with metabolic demand. It should be recognized that although used widely as an intravital preparation to study the microcirculation [44, 49, 50] and as a source of isolated microvessels for in vitro studies [51], the cremaster muscle does not attach to the skeleton nor does its contraction promote locomotion. Moreover, the cremaster muscle serves a functional role only in males. Thus alternative approaches were required to study the microcirculation of limb muscles that are recruited during physical exercise irrespective of gender.
In accord with classic studies of the soleus and extensor digitorum longus (EDL) hindlimb muscles of rats as representative of “slow-twitch” and “fast-twitch” muscle fiber types, respectively [52, 53], we developed the rat hindlimb as a preparation for intravital imaging [54]. Perfusion pressure measured at the distal end of feed arteries (e.g., just above the dotted line in Figure 1) was 20–30% lower than systemic arterial pressure, thereby indicating that feed arteries indeed functioned as proximal sites of resistance to skeletal muscle blood flow. Remarkably, feed arteries of both soleus and EDL dilated in response to muscle contraction, confirming AVD as a physiological response in true skeletal muscles [54]. Such findings helped to lay the foundation for questions that have since driven the research in several laboratories including my own: What is the cellular pathway for signal transmission along the vessel wall? What are the vasoactive signals and how do they travel? Are these signals under physiological regulation and if so, how? As developed below, addressing these relationships has given rise to more focused questions as “conduction” has evolved and matured into a field of investigation in its own right.
Cellular pathways for signal transmission
The two principal cellular elements of the microvessel wall are SMCs and endothelial cells (ECs) [55]. In light of classical studies of the “nexus” and electrical conduction in smooth muscle [29, 30, 56] and of electrical conduction along strips of guinea pig taenia coli [57] and rabbit aorta [58], the first studies of electrical signaling along arterioles were interpreted to reflect intercellular coupling between SMCs [28]. These latter experiments were performed on isolated preparations that enabled access to the arteriolar wall with microelectrodes. As vessels were approached from the abluminal surface, it was assumed that recordings were from SMCs. However one of the limitations of intracellular recording from multicellular preparations is the uncertainty of which cell was actually recorded from. Inclusion of a fluorescent dye in the recording microelectrode had been used by neurophysiologists to identify projections of individual neurons [59]. Further, as gap junctions enable passage of molecules ≤ ~1 kDa, dye transfer from the injected cell to neighboring cells was taken to indicate effective coupling through gap junctions [60, 61]. For example, Lucifer yellow (MW=457 Da; [62]) and propidium iodide (MW= 668 Da) are dye tracers that we have used for such purpose [63, 64] (Figure 3). Nevertheless, it should be recognized that the absence of dye coupling does not preclude the existence of electrical coupling [59], as the size and charge characteristics of dye molecules may impede their diffusion through gap junction channels [65] to a far greater extent than the diffusion of ions (e.g., K+ and Cl−) during electrical charge transfer.
Figure 3. Dye labeling during intracellular recording.

In an isolated pressurized feed artery of the hamster cheek pouch retractor muscle, Lucifer yellow dye (green; MW = 457 Da) was retained within a SMC and did not spread to adjacent cells. Note cytoplasmic distribution as SMC encircles the vessel. In contrast, propidium iodide (red; MW = 668 Da) microinjected into the central EC spread readily to surrounding ECs, thereby labeling respective nuclei Scale bar = 10 μm. Image from: Emerson, G.G. and S.S. Segal. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res 2000;87:474–479, 2000. Used with permission from Wolters Kluwer Health.
We had advanced the hypothesis that cell-to-cell signaling was the basis of propagated vasodilation in the hamster cheek pouch [39, 40]. A key advantage of the cheek pouch preparation is that microvessels supplying this thin epithelial tissue can be readily accessed by micropipettes (Figure 2). In 1991, a travel award from the Microcirculatory Society, Inc. supported my visit to the University of Geneva to collaborate with Jean-Louis Bény, who had begun using dye labeling during intracellular recording from cells in isolated strips of pig coronary arteries [61]. In his laboratory, we performed the first intracellular recordings from cheek pouch arterioles in vivo with Lucifer yellow dye in the microelectrode [66]. Following recording, preparations had to be transferred from the electrophysiology rig to a fluorescence microscope to confirm electrode placement. Anxiously fiddling with knobs in the dark, another CDM occurred when we observed robust dye transfer along the endothelium following recording from an EC. Conversely, we found no evidence of dye transfer when recording from (circumferential) SMCs nor was there dye transfer between ECs and SMCs. Thus the endothelium appeared to be the most robust cellular pathway for signal transmission along the arteriolar wall. Ensuing studies of EC morphology in arterioles from several tissues, including the hamster cheek pouch, supported this conclusion [67]. Being oriented along the vessel axis in the direction of blood flow, individual ECs lining arterioles are able to contact numerous SMCs while each SMC may contact nearly as many ECs as they encircle the intima (Figure 4). Indeed, this functional morphology suggested a key role for the endothelium in coordinating SMC relaxation along branches of resistance networks.
Figure 4. Structural and functional arrangements of microvascular smooth muscle cells and endothelial cells.
A. Schematic two-dimensional depiction of how endothelial cells (EC) and smooth muscle cells (SMC) are oriented to each other in arterioles and resistance arteries. EC align along the vessel axis in the direction of blood flow. In contrast, SMC are oriented perpendicular to the flow stream and encircle the intima within the intact vessel (see Figure 8A). Note corresponding orientations of EC vs. SMC nuclei. Internal elastic lamina lies between respective cell layers with holes for endothelial projections (see Figure 5). B. Schematic longitudinal section along top and bottom edge of vessel wall depicting adjacent SMC (in cross-section) in contact with the abluminal surface of consecutive EC. Along downstream segment, hyperpolarization (shaded) has conducted longitudinally and radially into surrounding SMC (arrows) to coordinate vasodilation. The signal is shown spreading into the upstream segment; note longitudinal gradients in hyperpolarization and SMC relaxation. Neighboring cells are coupled to each other via gap junctions (not shown), which are robust between neighboring EC (heavier arrows depicting current flow), sparse between adjacent SMC and restricted to discrete myoendothelial junctions between EC and SMC (small arrows depicting current flow; see inset).
Several years elapsed before intracellular recording with dye labeling was integrated with intravital microscopy to observe vasomotor responses concomitant with electrical signaling in vivo [68]. During this time, a controversy developed as to whether arteriolar ECs and SMCs are electrically coupled to each other, particularly in the intact system. Experiments performed on cheek pouch arterioles isolated for in vitro studies found that Vm and depolarization to phenylephrine (PE), an agonist selective for the α1-adrenoreceptor (α1AR), evoked depolarization of ECs in addition to SMCs [69]. Because α1ARs are expressed on SMCs (but not ECs) of arterioles [70], these findings implied that respective cell layers were coupled electrically to each other through myoendothelial gap junctions (MEGJs). In striking contrast, when cheek pouch arterioles were studied in vivo, PE was found to depolarize SMCs and depolarization (with ensuing vasoconstriction) conducted along the smooth muscle layer without affecting Vm in the endothelium [68]. Further, although hyperpolarization was conducted along either the endothelium or smooth muscle, respective cells had a distinct response waveform [68]. Thus, while either ECs or SMCs could provide a pathway for conduction and did so according to the stimulus, respective cell layers did not appear to be electrically coupled to each other when arterioles were controlling blood flow in vivo. To find my laboratory at odds with that of my mentor regarding such a fundamental question was one of the most difficult periods of my career. However, controversy in science is often the incentive for developing new experimental paradigms!
In the preceding experiments, respective cell layers of the arteriolar wall remained intact. Thus an alternative approach was needed to resolve roles for smooth muscle vs. endothelium as well as to test for myoendothelial coupling. Those familiar with fluorescence imaging know well that the excitation of a fluorochrome can damage cells through the production of reactive oxygen species; fluorescein is especially phototoxic in this regard [71]. Early studies investigated the role of the endothelium in governing reactivity of pial arterioles on the surface of the mouse brain [72]. Thus, sodium fluorescein was injected into the tail vein during illumination with intense green light. The interaction of light with dye (“light-dye treatment,” LDT) damaged the endothelium as confirmed by loss of vasodilation to ACh or bradykinin, while vasodilation to papaverine (a smooth muscle relaxant) was preserved [72]. A similar approach in arterioles of the rat cremaster muscle was found to eliminate the dilator response to an acute increase in blood flow velocity, thereby implicating a role for shear stress exerted on the endothelium as a mediator of vasodilation [73]. In accord with these findings, we attempted to use fluorescein dye in the hamster cheek pouch to resolve the role(s) of endothelium vs. smooth muscle in the conduction of vasodilation and vasoconstriction. However the sodium salt of fluorescein (MW=332) is hydrophilic and readily escapes from the vascular compartment. Accordingly, following dye injection into the femoral vein, our initial (half-assed?) experiments in the cheek pouch found inconsistent disruption of both ECs and SMCs (assessed by loss of dilation to ACh and of constriction to PE delivered from micropipettes) in the region of LDT [74]. A better experimental approach was needed!
We reasoned that conjugating the fluorochrome to albumin (MW ~65 kDa) should promote its retention within the vascular compartment when injected intravenously. In practice, this modification to the LDT protocol enabled selective damage to the endothelium [74]. Further, using a micropipette to apply albumin-conjugated fluorescein to the abluminal surface of an arteriole selectively disrupted SMCs while the endothelium remained intact [74]. Damaging SMCs eliminated the conduction of vasoconstriction through the treated segment without affecting the conduction of vasodilation [74]. Although damaging ECs prevented ACh from initiating vasodilation at the site of EC damage, ACh delivered onto the arteriole beyond the site of EC damage resulted in conduction past the site of EC injury. Thus, as implied by our earlier findings, once initiated from an intact vessel region, vasodilation could travel along either the SMC or the EC layer of these arterioles in vivo. Ensuing experiments in which SMCs and ECs were damaged selectively at different sites along the same arteriole illustrated that, depending upon the nature of the stimulus, either SMCs or ECs could serve as the cellular conduction pathway. However, the loss of conducted responses when SMCs and ECs were damaged at separate sites along the same arteriole indicated that respective cell layers were not directly coupled to each other in vivo [75] – a conclusion that was consistent with our earlier findings [68]. Attenuation of CVD during the inhibition of nitric oxide (NO) synthase further suggested that a “wave” of NO was being released along the arteriole during the conducted response however it would be several years before the underlying Ca2+ signaling was resolved. Ensuing experiments from an independent laboratory using intracellular recordings from arterioles in the mouse cremaster muscle also indicated that arteriolar ECs and SMCs are not electrically coupled to each other in vivo [76].
Given the presence of MEGJs in cheek pouch arterioles as well as feed arteries [77], one explanation that may resolve the controversy surrounding myoendothelial coupling in vitro vs. in vivo is that these discrete sites of heterocellular coupling through MEGJs can be modulated; e.g., through phosphorylation and/or nitrosylation of Cx subunits [78–80]. Thus, one hypothesis that remains to be resolved is that MEGJs may be functionally “closed” under physiological conditions in vivo and “open” during the isolation of vessels for in vitro studies. Once the ability to isolate, cannulate and pressurize individual arterioles was established [51], this experimental approach (i.e., pressure myography) became central to research efforts in many laboratories. In studying such preparations, it should be recognized that preparing isolated vessels involves loss of blood flow and transmural pressure, chilling the excised tissue to near-freezing temperatures (typically 4 °C), cutting the vessel free from surrounding tissue and the network in which it has interacted with other vessel branches [12, 81] - all during immersion and perfusion with artificial solutions while being chilled, rewarmed and pressurized… before performing the actual experiment! That these preparations remain viable and provide valuable information is not the concern. Rather, it should be recognized that all reports of myoendothelial coupling are based upon isolated vessel preparations studied in vitro [69, 82–91] or their constitutive ECs and SMCs when grown in culture [92]. A controversy that remains to be resolved is whether MEGJs in a given vessel are effectively closed under physiological “homeostatic” conditions and then open during “stress” from insult or injury.
Functional morphology: myoendothelial coupling
While lining the entire cardiovascular system, the endothelium exhibits regional variability in structure and function [93, 94]. Throughout the resistance vasculature, ECs are coupled functionally to each other through gap junction channels that are comprised primarily of Connexin (Cx)37, Cx40 and Cx43 albeit with regional variation [95, 96]. While present in SMCs, the expression of gap junctions between ECs is far greater [77, 95, 97]. An important feature of resistance vessels is the intimate association of SMCs with their underlying ECs (Figure 5). Nearly 50 years ago, classic studies of the cellular ultrastructure along arteriolar networks in the fascia of rabbit skeletal muscle revealed discrete sites at which ECs extended projections through the internal elastic lamina (IEL). At that time, the presence of “myoendothelial junctions” in electron micrographs was interpreted to suggest that “humoral transmitter substances” (e.g., catecholamines) taken up from the blood stream by luminal ECs were made more readily available for diffusion to the surrounding SMCs [55]. As functional studies became more sophisticated, the role of the endothelium in mediating smooth muscle relaxation via the release of autacoids (e.g., NO) was identified [1]. However, in rat mesenteric arteries, endothelium-dependent vasodilation was found to prevail when NO production was inhibited and was associated with smooth muscle hyperpolarization, leading to the recognition of endothelium-dependent hyperpolarizing factor (EDHF; see [98] for review). While EDHF gained recognition as a cytochrome p450 metabolite of arachidonic acid [99] and was found to be effective in the hamster microcirculation [100, 101], complementary studies invoked a role for K+ ion as an EDHF [102]. Moreover, findings that hyperpolarization initiated via ECs can lead to hyperpolarization and relaxation of SMCs along arterioles in the absence of electrical coupling between respective cell layers in vivo [68, 76] implicated a physiological role for EDHF in the microcirculation.
Figure 5. Immunolabeling and fluorescence imaging of vessel wall morphology.
Optical sections (thickness, 1 μm) progressing though mouse mesenteric artery. A. SMCs (red, labelled for smooth muscle α-actin) with slender, transversely oriented nuclei (blue, stained with TOPRO-3) and collagen fibers (green autofluorescence). B. Internal elastic lamina (IEL, green autofluorescence) containing small holes (i.e., “fenestrae”) enabling EC projections to contact SMC for myoendothelial coupling. EC nuclei (blue, TOPRO-3) are oval and oriented along the vessel axis. Note nuclei from EC and SMC within the same 1 μm optical section as IEL holes, documenting the intimate association of respective cell layers. Scale bars = 50 μm. Immunolabeling and imaging performed by Dr. Erika Boerman.
Electrophysiological studies of arterioles had typically relied upon a single microelectrode for intracellular recording. Approaching vessels from the abluminal surface ensured that the first cell penetrated was a SMC. Opening the vessel to expose the lumen (“en face”) [61, 85] or manipulating a microelectrode through the hole remaining from a severed branch [69] are strategies that have been used to record from the endothelium while it remains associated with the vessel wall. However such preparations no longer experience transmural pressure, which otherwise evokes depolarization and constriction via the myogenic response [103, 104]. When vessels are unpressurized, Vm is typically more negative (−60 to −75mV) [28, 69] than obtained in pressurized vessels (−30 to −40 mV) [68, 84]. Thus the baseline and operating range for electrical signaling varies under respective conditions. With this difference in mind, and in deference to the earlier studies using paired microelectrodes [28], we performed dual simultaneous intracellular recordings in isolated pressurized feed arteries (diameter, 50–70 μm)of the hamster retractor muscle. Another CDM occurred when we found ECs and SMCs to be electrically coupled to each other during conducted vasomotor responses [84]. A brief pulse of ACh from a micropipette (as described earlier) or the intracellular injection of negative current evoked the conduction of hyperpolarization and vasodilation [84]. Simultaneous recording from an EC (confirmed by axial orientation and robust dye transfer to neighboring ECs) and a SMC (circumferential orientation with little or no dye transfer; Figure 3) demonstrated superimposable electrical responses of both cell layers. Further, current injection into an EC produced a corresponding electrical response in SMC and vice versa along with the conduction of vasodilation during hyperpolarization or vasoconstriction during depolarization [84]. Electron micrographs confirmed the localization of gap junctions at myoendothelial contacts [77, 84]. Thus, functional MEGJs were identified in resistance microvessels and their role in intercellular signaling between endothelial and smooth muscle layers has evolved into a vigorous area of investigation [69, 82–91, 105–107]. Whether, and if so, how, the role of EDHF vs. direct electrical coupling through MEGJs may change under experimental conditions (i.e., in vivo vs. isolated vessels) remains poorly understood [108] and requires further investigation.
In isolated feed arteries of the retractor muscle, selective LDT of the endothelium abolished conducted responses (initiated by ACh) beyond the region of damage, whereas LDT of SMCs did not affect conduction through the region of SMC damage [18]. These findings illustrated that the endothelium served as the principal cellular pathway for signal transmission (Figures 3 and 4). As an “exercise guy”, it was essential for me to determine whether such a role for the endothelium prevailed in the intact system. Thus we performed experiments using rhythmic contractions of the retractor muscle in anesthetized hamsters. Remarkably, feed arteries dilated upstream from the contracting muscle and blood flow increased > 4 fold [33]. Performing LDT to disrupt the endothelium midway along the feed artery prevented AVD in response to muscle contraction and abolished CVD in response to local stimulation with ACh (as observed for the same vessels in vitro). Associated with the loss of AVD was a ~50% attenuation of the blood flow response to muscle contraction, thereby confirming the critical role of feed artery dilation in attaining maximal perfusion during metabolic demand [33]. Thus, inhibiting conduction along the endothelium (and thereby AVD) effectively restricts blood flowing into the microcirculation. In a recent study, the endothelium of the vascular supply to the rat brain was found to perform a similar role in response to neuronal activity; i.e., AVD along the endothelium was integral to the hyperemic response during neurovascular coupling [109]. In light of endothelial dysfunction associated with vascular aging and disease [110, 111], such findings imply that impaired AVD in skeletal muscle and brain may contribute, respectively, to diminished physical activity and impaired cognitive function.
Coming from the other direction
The sympathetic nervous system is activated in accord with the intensity of physical exertion [112]. A key outcome is the redistribution of cardiac output away from inactive regions (e.g., internal organs) to supply oxygen and nutrients to active skeletal muscles [113]. In skeletal muscle, sympathetic innervation extends along resistance arteries (Figure 6) to encompass arteriolar networks [114]. A classic relationship in exercise physiology centers on the interaction between sympathetic nerve activity (SNA) and dilation of the vascular supply in response to muscle contraction [113, 115, 116]. The overriding of sympathetic vasoconstriction by the metabolic demands of exercising skeletal muscle came to be recognized as “functional sympatholysis” [11, 14]. However if vasodilation were to attain maximal levels throughout the resistance vasculature of skeletal muscle (recall that it comprises 30–50% of total body mass), total peripheral resistance would no longer be sufficient to maintain arterial perfusion pressure - even during maximal cardiac output [10]. This dynamic interaction was well-demonstrated in chronically-instrumented dogs running on treadmills, where intra-arterial infusion of prazosin (to inhibit αARs) increased hindlimb blood flow in proportion to running intensity as well as the level of αAR inhibition [117, 118]. The take-home message here is that vasoconstriction and blood flow restriction by the sympathetic nervous system increase with the intensity of exercise and the mass of muscle engaged in the activity.
Figure 6. Perivascular sympathetic innervation.
Whole-mount preparation of superior epigastric artery from mouse abdominal skeletal muscle. A. Immunolabeling for tyrosine hydroxylase (green), a limiting enzyme in catecholamine synthesis. Dotted lines indicate vessel edges. B. Immunolabeling for protein gene product 9.5 (PGP9.5; red), a neuronal marker. C. Overlay (yellow) of preceding panels confirming sympathetic origin for perivascular innervation of skeletal muscle. Scale bars = 50 μm. Immunolabeling and imaging performed by Dr. Erika Boerman.
In addressing where SNA was exerting its effect on the vascular supply, Folkow’s classic studies of perfusion pressure and blood flow in the hindlimb of anesthetized cats determined that vasoconstriction was maintained in proximal arteries while vasodilation was manifest downstream within the muscle [31]. More recent intravital studies of the hamster retractor muscle revealed that dilation of feed arteries increased with the level of motor unit recruitment and decreased with the intensity of SNA, even when arterioles within the contracting muscle remained fully dilated [116]. These graded relationships along the resistance network (i.e., regional differences in vasomotor control; alluded to earlier) ensure that feed arteries remain sufficiently constricted to maintain arterial perfusion pressure while dilation of arterioles within the active muscle maximizes capillary perfusion and the surface area for oxygen extraction - particularly as blood flow is limited by proximal resistance (Figure 1). Experiments performed in the hamster cremaster muscle had shown that stimulating perivascular sympathetic nerves suppressed CVD along arterioles [119]. Ensuing studies of retractor muscle feed arteries in vivo [120] and in vitro [121] demonstrated that increasing the level of SNA progressively inhibited CVD, with additive roles for α1AR and α2AR activation. Thus SNA can modulate AVD in a graded manner and do so by more than one signaling pathway. Whether sympathetic modulation of CVD was manifested constitutively (i.e., without being induced by exercise, perivascular nerve stimulation or pharmacological activation of αARs) remained to be ascertained.
In human subjects, responses of leg blood flow to graded stationary cycling reduced by nearly 1/3 in older (~63 years) compared to younger (~26 years) males [122] however the mechanism was unknown. Shortly thereafter it was reported that, under resting conditions, blood flow through the femoral artery was attenuated by 25–30% in older vs. younger males in conjunction with elevated SNA and αAR-mediated vasoconstriction [123, 124]. To investigate these relationships in the microcirculation of a skeletal muscle integral to locomotion, we developed the gluteus maximus muscle as a preparation for intravital microscopy in the C57BL/6 mouse [125]. In males, our first clue that age was having an effect was a ~50% attenuation of CVD in old (~20 months) vs. young (~4 months) mice. Ensuing studies found that rapid onset vasodilation (ROV) in response to single tetanic contractions was impaired in old male mice but not in their female counterparts [126]. Inhibiting αARs with phentolamine restored ROV in the old males, while subtle treatment of young mice of either sex with norepinephrine (1 nM) had no effect on resting diameter yet suppressed ROV to resemble that of old males [126]. Further, while there was no difference in steady-state dilation between age groups during rhythmic muscle contractions, arteriolar red blood cell velocity and blood flow were depressed by > 50% in old vs. young males [126]. This outcome is consistent with elevated SNA impairing AVD to restrict muscle blood flow with advanced age. Moreover, these relationships we first defined in the microcirculation of mouse skeletal muscle were found to prevail in human subjects [127], thereby validating the use of rodents for more invasive studies of mechanisms underlying the effects of aging in humans.
The gluteus maximus muscle is comprised of two distinct regions each governed by its respective motor nerve and vascular supply [128]. Microvascular networks of respective regions are often joined by anastomotic arterioles and venules. Taking advantage of these anatomical relationships in male mice [129], we tested whether the contraction of one muscle region could initiate vasodilation that spread into the inactive muscle region. Under control conditions, dilation of the anastomotic arteriole was constrained to the active muscle region. However, following inhibition of αARs with phentolamine, vasodilation spread from the active muscle region into the inactive muscle region [129]. These findings were the first to suggest that a constitutive level of αAR activation effectively restricts the spread of vasodilation. Thus, sympathetic neuromodulation can direct blood flow and oxygen delivery to where it is needed most: active muscle fibers during exercise.
Complementary and bidirectional signaling pathways
In the late 1980s, studies of CVD initiated by ACh in hamster cheek pouch arterioles suggested that these dilations consisted of two components: an initial “fast” response, whereby the vessel appeared to “pop” open within the first couple of seconds, and a slower increase in diameter that progressed over the next several seconds [39–41]. Thus, multiple signaling pathways mediating smooth muscle relaxation was apparent. During the same time frame, the activation of K+ channels concomitant with a rise in [Ca2+]i was identified as a mechanism of EC hyperpolarization in response to stimulating muscarinic receptors with ACh [130]. Ensuing studies identified essential roles for small- (SKCa) and intermediate- (IKCa) conductance Ca2+-activated K+ channels as the basis of EC hyperpolarization to ACh (see [131] for an historical perspective on the definitive studies defining these relationships). We therefore reasoned that SKCa and IKCa activation may be involved in the rapid component of CVD. This hypothesis was tested in pressurized feed arteries of the hamster retractor muscle. Treating the site of ACh stimulation with apamin + charybdotoxin (to block the initiation of hyperpolarization via KCa channel activation) eliminated the “fast” dilator response and thereby revealed the secondary “slow” component of conduction [132]. Ensuing experiments in which ECs were loaded with a Ca2+-sensitive dye (Fura-2) revealed a Ca2+ “wave” traveling along the endothelium that corresponded with the “slow” component [132]. This was a CDM in resolving distinct yet complementary signaling pathways that contributed to CVD. Although a Ca2+ wave had been implicated during intravital studies of cheek pouch arterioles [75], actually recording this component of the conducted response was a most rewarding breakthrough. Ensuing experiments employed confocal imaging to resolve the rise in endothelial [Ca2+]i that resulted in the lowering of SMC [Ca2+]i [133]. The latter effect was explained by the closure of voltage-gated (L-type) Ca2+ channels as hyperpolarization spread through MEGJs into the surrounding SMCs. To investigate these relationships in vivo, collaborative efforts enabled intravital imaging of the microcirculation in transgenic mice expressing a Ca2+-sensitive protein (GCaMP2) selectively within arteriolar ECs [134], thereby establishing the functional significance of this signaling pathway in vivo (Figure 7). Collectively, these experiments illustrated that rapid (i.e., electrical) signaling coordinates arteriolar dilation over several mm (e.g., across daughter and parent branches; Figure 1) while the ensuing endothelial Ca2+ wave can further “tune” the magnitude and duration of dilation along several hundred microns; i.e., along vessel segments closer to the stimulus origin.
Figure 7. Endothelial calcium wave contributes to ascending vasodilation in vivo.
Sequential images of an arteriole within the cremaster muscle of transgenic mice expressing GCaMP2 (a Ca2+ sensitive fluorescent protein) in ECs to illustrate the initiation and spread of a Ca2+ wave and vasodilation in response to local stimulation with a brief pulse of ACh (in accord with Figure 2). A. Initiation: Note local rise of intracellular Ca2+ in ECs across the intima. Arrowhead corresponds to ACh micropipette; arrow indicates direction of blood flow. B. Transmission: The Ca2+ wave has spread bi-directionally along arteriolar endothelium. C. Ensuing dilation along upstream segments. Scale bars = 50 μm. Complementary images from experiments performed in Tallini YN, Brekke JF, Shui B, Doran R, Hwang S, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC-GCaMP2 transgenic mice. Circ Res 2007;101:1300–1309.
A functional role for Ca2+ signaling between SMCs and ECs had been implicated in arterioles isolated from the hamster cheek pouch [83]. Using the Ca2+ indicator dye Fluo-3, stimulating the arteriole with PE was found to increase Ca2+ within SMCs that, in turn, gave rise to elevated Ca2+ within underlying ECs [83]. The rise in EC Ca2+ was reported to stimulate NO production through activating endothelial NO synthase (Figure 8), thereby providing negative feedback to attenuate vasoconstriction [83]. Ensuing experiments expanded the role of endothelial feedback in modulating SMC contraction to include the activation of endothelial KCa channels [89]. These respective findings inferred that [Ca2+]i increased globally, throughout the cytoplasm of affected cells. However, new insight into the molecular organization of MEJs (Figure 8) complemented by refined experimental design and confocal imaging of Ca2+ signaling microdomains have provided alternative interpretations [90, 91, 135].
Figure 8. Bidirectional myoendothelial calcium and electrical signaling.
Schematic illustration of myoendothelial feedback; key steps in signaling events are enumerated. Acetylcholine binds to Gq protein-coupled muscarinic (M3) receptor (1) resulting in the liberation of inositol 1,4,5-trisphosphate (IP3), which binds to its receptor (IP3R) in the endoplasmic reticulum (ER), releasing Ca2+ to activate KCa channels (2); the efflux of K+ through the plasma membrane (3) hyperpolarizes the cell interior. With SMC, norepinephrine (NE) released from perivascular sympathetic nerve terminals (PVSN) binds to the Gq protein-coupled α1 adrenergic receptor (4), also liberating IP3, which activates IP3R in the sarcoplasmic reticulum (SR, 5) to release Ca2+ into the cytosol. Elevation of IP3 and Ca2+ within SMC promotes their diffusion through myoendothelial gap junctions (MEGJ; 6 & 7) at endothelial projections through holes in the IEL (see Figure 5). In turn, local elevation of second messengers may stimulate Ca2+ release from ER within the projection (8). Elevation of K+ within the endothelial projection can activate IKCa (9), with K+ efflux and hyperpolarization providing negative feedback through the MEGJ (10) to attenuate Ca2+ entry into SMC (11) through voltage-gated Ca2+ channels (VGCC). At the same time, the local rise in extracellular K+ can stimulate the electrogenic Na+/K+ ATPase (12) and inward-rectifying K+ channels (not shown), further contributing to SMC hyperpolarization. Influx of Ca2+ through transient receptor potential (TRP) channels (13) can augment IKCa activation in endothelial projections, further promoting SMC relaxation via myoendothelial conduction of hyperpolarization. Concomitant activation of NO production by endothelial nitric oxide synthase (eNOS, 14) further promotes vasodilation.
With EDH explained by the activation of SKCa and IKCa subsequent to the rise in EC [Ca2+]i, electrophysiological studies of isolated rat mesenteric arteries implicated distinct roles for respective ion channels. Under resting conditions (Vm, ~−50 mV), ACh induced EDH (to ~−75 mV) and relaxation of SMCs, with both responses attributed to the activation of SKCa. However, when SMCs were first depolarized (to ~−40 mV) with PE, their repolarization to ~−50 mV upon stimulation with ACh was attributed to the activation of IKCa, with SKa contributing the remaining ~−25 mV hyperpolarization [136]. These findings led to the hypothesis that respective KCa channels, which appeared to have distinct roles in EDH, may be differentially localized in the endothelium [137]. Confocal microscopy and ultrastructural immunohistochemistry illustrated that IKCa were localized within punctate holes of the IEL in association with EC projections; localization of Cx37 and Cx40 to these same sites implicated association of IKCa with MEGJs (Figure 8). In contrast, SKCa channels were found in association with Cx37, Cx40 and Cx43 that formed gap junctions at EC borders [137]. Thus, differentially activated components of EDH appear related to the topological separation of SKCa and IKCa. Novel experiments using mouse ECs and SMCs grown in co-culture addressed the directionality and contribution of Ca2+ vs. inositol 1,4,5-trisphosphate (IP3) diffusion and the role of respective Cx isoforms in myoendothelial signaling [92, 138, 139]. While providing critical new insight into the directionality and effects of Ca2+ vs. IP3 flux through MEGJs under highly controlled conditions, it should be recognized that co-cultures lack the presence of an IEL, exhibit a far greater number of EC-SMC contacts than occur in actual resistance vessels, and have never experienced blood flow or transmural pressure. Nevertheless, and as supported by more recent studies, conclusions based upon co-culture experiments have been strengthened by complementary in vivo paradigms [140].
To observe Ca2+ signaling of native endothelium while avoiding optical interference from SMCs, mesenteric arteries from transgenic mice expressing GCaMP2 in ECs were opened for en face fluorescence imaging. Thereby, Ca2+ signaling microdomains were resolved that corresponded to EC projections through the IEL [85]. Spatially fixed Ca2+ release events characterized as “pulsars” were mediated by IP3 receptors (IP3Rs) localized to the endoplasmic reticulum (ER) within EC projections. While spontaneously active under basal conditions, stimulation with ACh (and the ensuing IP3 production) increased pulsar activity several-fold. Consistent with earlier findings [137], immunostaining for IKCa was localized to holes in the IEL, as was staining for IP3Rs [85]. Thus, local release of Ca2+ from IP3Rs was associated with activation of IKCa within EC projections located at holes the IEL. When activated locally, these channels produce hyperpolarization and when coupled with MEGJ localization within these microdomains [137], a mechanism was identified for Ca2+-dependent signaling of hyperpolarization from ECs to SMCs. Further insight into these spatial and functional relationships was gained through recent studies of feed arteries of the hamster retractor muscle [88]. Ultrastructural tomography resolved EC projections through the IEL that directly contacted overlying SMCs. These projections contained ER membranes expressing IP3Rs in conjunction with IKCa. Following loading with a Ca2+ - sensitive dye (Fluo-4), confocal imaging revealed that stimulating SMCs with PE evoked discrete Ca2+ events in ECs (termed “Ca2+ wavelets”) that were attributed to the activation of IP3Rs within the EC projections. As inhibiting IKCa or endothelial IP3Rs enhanced vasoconstriction to the α1AR agonist PE, the bidirectional signaling pathway entailed activation of SMCs with generation of IP3 and Ca2+ as second messengers, which may then diffuse through MEGJs to activate IP3Rs of the ER within EC projections (Figure 8). The ensuing activation of IKCa provided negative feedback to attenuate SMC depolarization and vasoconstriction [88]. Thus, rather than ECs responding to global changes in [Ca2+]i as initially proposed [89, 141], more detailed studies have revealed endothelial projections as discrete microdomains of heterocellular signaling at MEJs that modulate the contractile status of SMCs [82, 86, 88, 90, 91, 135, 142]. The take-home message for bidirectional signaling through MEGJs is that, inresponse to activation of αARs on SMCs, negative fe dback from ECs modulates vasoconstriction whereas activation of muscarinic receptors on the endothelium drives SMC relaxation. In both scenarios, myoendothelial signalling is integral to vasomotor (and thereby blood flow) control in the resistance vasculature. It should also be recognized that the activation of αARs and muscarinic receptors with physiological agonists in isolated preparations enables the resolution (and thereby the study) of functional relationships that may otherwise be difficult to ascertain in the intact system.
Simplifying the model and putting things together
As experimental approaches to investigate the nature of signaling between SMCs and ECs continued to evolve, we turned our attention towards understanding the biophysical properties of native intact microvascular endothelium. Professor Jackson’s laboratory had developed methods for isolating arteriolar smooth muscle from its endothelium to study ion channel signaling events intrinsic to microvascular SMCs and ECs of the rat and hamster [143, 144]. We refined these procedures to isolate intact endothelial “tubes” from microvessels of the mouse [145], thereby eliminating MEJs and the influence of SMCs on endothelial function. The superior epigastric artery of the mouse was selected as a feed artery from abdominal skeletal muscle as it enables the isolation of long (several mm) intact segments. This experimental model is well-suited for studying the intrinsic properties of electrical conduction and Ca2+ signaling along native intact microvascular endothelium in the absence of transmural pressure or blood flow. Control experiments evaluated Ca2+ and Vm in freshly isolated endothelial tubes during exposure to graded levels of stimulation with ACh. Remarkably, the response curves for respective signaling events corresponded to the response curve for vasodilation [146], confirming that endothelial tubes retained their integrity for functional signaling. In light of earlier studies that had implicated the endothelium as the cellular pathway for conducting vasodilation, pairs of intracellular microelectrodes were used to inject current into one EC while simultaneously recording Vm in a remote EC located at defined separation distances (typically 500–2000 μm, corresponding to ~15–60 ECs placed end-to-end). A key finding was that the amplitude of the Vm response in the remote EC increased or decreased in a linear manner with the amplitude and polarity of the injected current. [63, 146]. This behavior confirmed the lack of voltage-gated ion channels in the endothelium. Further, the Vm response decayed exponentially with the distance between electrodes, indicating that gap junctions remained intact following isolation of the endothelium and that the electrical signal conducted from cell to cell in a manner consistent with what had been observed for the endothelium of intact feed arteries [18].
Intercellular conduction of electrical signals requires effective coupling among neighboring cells as enabled through gap junctions. Indeed, disruption of gap junctions (e.g., by deleting the Connexin40 subunit) impaired conducted responses along arterioles of the mouse cremaster muscle [147, 148]. Though perhaps less apparent, the high electrical resistance of plasma membranes is integral to signal transmission from cell to cell. Thus, we questioned whether opening ion channels in the plasma membrane would make ECs electrically “leaky”. The endothelial tube preparation proved to be an ideal model for these experiments, as SKCa/IKa are highly expressed in these cells [111] and the pharmacological tools for manipulating these channels are well defined [131, 149]. Moreover, as there are no other cell types or hemodynamic stimuli that could intervene, intrinsic biophysical properties of native microvascular endothelium could be defined. A key finding from these experiments was that the activation of SKCa/IKa resulted in the loss of the electrical signal at remote recording sites. Thus, opening ion channels in the plasma membrane inhibited electrical signal transmission while gap junctions remained fully patent, as confirmed by robust dye transfer from an injected EC to its surrounding neighbors [150]. Thus, using a novel approach, these experiments revealed that intercellular signal transmission along the endothelium could be modulated independent of gap junctions by manipulating ion channels in the plasma membrane [63].
In light of earlier discussion, these findings from endothelial tubes provide a foundation from which to reconsider myoendothelial signaling. In particular, how stimulation of αARs can attenuate the conduction of electrical signals and suppress AVD during exercise. As shown in rat mesenteric arteries, SNA increased the activity of Ca2+ “pulsars” observed within ECs, indicating that signals initiated on SMCs (where the αARs are activated during SNA) enhanced Ca2+ signaling in the endothelium [85]. In turn, vasoconstriction during SNA was shown to be enhanced by inhibiting IKCa [86]. These findings are consistent with the activation of αARs on SMCs giving rise to localized Ca2+ signaling (pulsars) and hyperpolarization (via IKCa activation) in endothelial projections to provide negative feedback that attenuates sympathetic vasoconstriction. During SNA, norepinephrine is released from perivascular nerves that course along feed arteries and arteriolar networks. Thus secondary to αAR stimulation, IKCa (along with nearby SKCa) would also be activated along the endothelium (via MEGJs; above). In turn, dissipating the electrical signal through plasma membranes reduces the effective distance of signal transmission along the endothelium [150]. This interpretation explains how SNA inhibits CVD (via αAR activation) along feed arteries studied in vitro [121] and of AVD into feed arteries supplying skeletal muscle in vivo [116]. In a reciprocal manner, the increase in muscle blood flow during exercise can be explained by inhibiting sympathetic vasoconstriction, exemplified in running dogs by the increase in hindlimb blood flow upon pharmacological blockade of αARs [117, 118]. It should also be recognized that the activation of ion channels in SMCs can inhibit intercellular signal transmission and the conduction vasomotor responses [121, 151, 152]. Thus, in light of direct coupling between ECs and SMCs through MEGJs, increasing the loss of electrical charge through membranes in either cell layer can effectively dissipate conducted (i.e., ascending) vasomotor responses.
Summary and translation
Aging and vascular disease are accompanied by enhanced SNA, endothelial dysfunction in association with oxidative stress and impaired tissue perfusion [110, 153–155]. Hydrogen peroxide is a common reactive oxygen species and was recently found to activate KCa channels in the endothelium of feed arteries [111]. This effect of oxidative stress inhibits intercellular conduction in a manner complementary to the actions of SNA described above. Taken together, these findings help to explain why blood flow is restricted with aging. Thus, combining enhanced SNA with oxidative stress can lead to the opening of ion channels in cell membranes, thereby dissipating electrical signals and impairing conduction. With AVD thereby compromised, blood flow into the microcirculation is restricted [33, 126]. Attributing such effects of aging and SNA to specific membrane proteins points to targeting selective therapeutic interventions to restore tissue perfusion. Nevertheless, whereas the activation of KCa channels can be an effective strategy to promote vasodilation and improve tissue blood flow [156, 157], potentially adverse effects on the ability to conduct electrical signals should also be considered. For example, the coordination of capillary perfusion within and between respective branches of resistance networks [129] (Figure 1) may be compromised with “global”dilation of arterioles secondary to the activation of KCa channels and loss of vasomotor tone.
In presenting the evolution and application of research on “vascular conduction” from the perspective of this author’s laboratory, it should be recognized that complementary findings and lines of investigation have been and continue to be developed by a growing body of investigators contributing to this field; many of my colleagues are cited herein. In addition to the moment-to-moment coordination of vasomotor control emphasized here, vascular conduction appears to be integral to intercellular communication that underlies remodeling of vascular networks [158]. Beyond our interest in skeletal muscle, cell-to-cell conduction in vascular resistance networks has been implicated as being integral to tubuloglomerular feedback among nephrons in the kidney [159], to neurovascular coupling in the brain [109, 160] and to the matching of ventilation and perfusion in the lung [161]. Nearly every cell, tissue and organ in the body relies upon the microcirculation to maintain homeostasis. In turn, with aging, disease and organ dysfunction, it is implicit that the microcirculation is involved. Elucidating the role of intercellular signaling within and between the endothelium and smooth muscle, how these events are modulated by nerves and how they may be influenced by surrounding tissue, blood flow and environmental factors represent unlimited opportunities for microvascular research in the future, particularly in translating what we observe and discover through the microscope towards the treatment of patients and optimizing their quality of life.
Acknowledgments
This article was written from the author’s perspective of how this area of research has developed over the last 30+ years. The contributions of all who have contributed to this development are gratefully acknowledged. There is no intention to exclude any work not cited; references are selected as being representative of particular aspects of this story while recognizing that there is much more to endothelium-dependent vasodilation and blood flow control than addressed herein.
Grant Support
This article reflects research supported by grants R37-HL041026, R01-HL086483 and R01-HL056786 to S. S. Segal from the National Heart, Lung and Blood Institute of the National Institutes of Health, United States Public Health Service. The content of this article is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations used in text
- ACh
acetylcholine
- AR
adrenoreceptor
- AVD
ascending vasodilation
- [Ca2+]i
intracellular calcium concentration
- CDM
career defining moment
- Cx
connexin
- CVD
conducted vasodilation
- EC
endothelial cell
- ER
endoplasmic reticulum
- EDH
endothelium dependent hyperpolarization
- EDHF
endothelium dependent hyperpolarizing factor
- ESM
European Society for Microcirculation
- EDL
extensor digitorum longus
- ER
endoplasmic reticulum
- IEL
internal elastic lamina
- IKCa
intermediate conductance Ca2+-activated K+ channel (KCa 3.1)
- IP3
inositol 1,4,5-trisphosphate
- IP3R
inositol 1,4,5-trisphosphate receptor
- LDT
light-dye treatment
- MEGJ
myoendothelial gap junction
- MEJ
myoendothelial junction
- NO
nitric oxide
- pO2
partial pressure of oxygen
- PE
phenylephrine
- ROV
Rapid onset vasodilation
- SKCa
small conductance Ca2+-activated K+ channel (KCa 2.3)
- SMC
smooth muscle cell
- SNA
sympathetic nerve activity
- Vm
membrane potential
Footnotes
Conflict of Interest
None
References
- 1.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1918;52:409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krogh A. The supply of oxygen to the tissues and the regulation of the capillary circulation. J Physiol. 1919;52:457–474. doi: 10.1113/jphysiol.1919.sp001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segal SS, Brooks GA. Effects of glycogen depletion and work load on postexercise O2 consumption and blood lactate. J Appl Physiol Respir Environ Exerc Physiol. 1979;47:514–521. doi: 10.1152/jappl.1979.47.3.514. [DOI] [PubMed] [Google Scholar]
- 5.Segal SS, Faulkner JA. Temperature-dependent physiological stability of rat skeletal muscle in vitro. Am J Physiol Cell Physiol. 1985;248:C265–270. doi: 10.1152/ajpcell.1985.248.3.C265. [DOI] [PubMed] [Google Scholar]
- 6.Segal SS, White TP, Faulkner JA. Architecture, composition, and contractile properties of rat soleus muscle grafts. Am J Physiol Cell Physiol. 1986;250:C474–479. doi: 10.1152/ajpcell.1986.250.3.C474. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong RB, Laughlin MH. Blood flows within and among rat muscles as a function of time during high speed treadmill exercise. J Physiol. 1983;344:189–208. doi: 10.1113/jphysiol.1983.sp014933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Segal SS. Regulation of blood flow in the microcirculation. Microcirculation. 2005;12:33–45. doi: 10.1080/10739680590895028. [DOI] [PubMed] [Google Scholar]
- 10.Rowell LB. Neural control of muscle blood flow: Importance during dynamic exercise. Clin Exp Pharmacol Physiol. 1997;24:117–125. doi: 10.1111/j.1440-1681.1997.tb01793.x. [DOI] [PubMed] [Google Scholar]
- 11.Thomas GD, Segal SS. Neural control of muscle blood flow during exercise. J Appl Physiol. 2004;97:731–738. doi: 10.1152/japplphysiol.00076.2004. [DOI] [PubMed] [Google Scholar]
- 12.Granger HJ, Goodman AH, Granger DN. Role of resistance and exchange vessels in local microvascular control of skeletal muscle oxygenation in the dog. Circ Res. 1976;38:379–385. doi: 10.1161/01.res.38.5.379. [DOI] [PubMed] [Google Scholar]
- 13.Skinner NS, Jr, Costin JC. Interactions of vasoactive substances in exercise hyperemia: O2, K+, and osmolality. Am J Physiol. 1970;219:1386–1392. doi: 10.1152/ajplegacy.1970.219.5.1386. [DOI] [PubMed] [Google Scholar]
- 14.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- 15.Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- 16.Kaley G, Rodenburg JM, Messina EJ, Wolin MS. Endothelium-associated vasodilators in rat skeletal muscle microcirculation. Am J Physiol Heart Circ Physiol. 1989;256:H720–725. doi: 10.1152/ajpheart.1989.256.3.H720. [DOI] [PubMed] [Google Scholar]
- 17.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol Heart Circ Physiol. 1990;259:H1063–1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- 18.Emerson GG, Segal SS. Endothelial cell pathway for conduction of hyperpolarization and vasodilation along hamster feed artery. Circ Res. 2000;86:94–100. doi: 10.1161/01.res.86.1.94. [DOI] [PubMed] [Google Scholar]
- 19.Malpighi M. http://www.britannica.com/EBchecked/topic/360486/Marcello-Malpighi http://en.wikipedia.org/wiki/Marcello_Malpighi http://www.nndb.com/people/033/000095745/ http://micro.magnet.fsu.edu/optics/timeline/people/malpighi.html.
- 20.Harvey W. http://en.wikipedia.org/wiki/William_Harvey http://www.fordham.edu/halsall/mod/1628harvey-blood.asp.
- 21.Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duling BR, Berne RM. Propagated vasodilation in the microcirculation of the hamster cheek pouch. Circ Res. 1970;26:163–170. doi: 10.1161/01.res.26.2.163. [DOI] [PubMed] [Google Scholar]
- 23.Hilton SM. A peripheral arterial conducting mechanism underlying dilatation of the femoral artery and concerned in functional vasodilatation in skeletal muscle. J Physiol. 1959;149:93–111. doi: 10.1113/jphysiol.1959.sp006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacobs A, Keatinge WR. Effects of procaine and lignocaine on electrical and mechanical activity of smooth muscle of sheep carotid arteries. Br J Pharmacol. 1974;51:405–411. doi: 10.1111/j.1476-5381.1974.tb10676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lutz BR, Fulton GP, Akers RP. The neuromotor mechanism of the small blood vessels in membranes of the frog (rana pipiens) and the hamster (mesocricetus auratus) with reference to the normal and pathological conditions of blood flow. Exp Med Surg. 1950;8:258–287. [PubMed] [Google Scholar]
- 26.Krogh A, Harrop GA, Rehberg PB. Studies on the physiology of capillaries. III. The innervation of the blood vessels in the hind legs of the frog. J Physiol. 1922;56:179–189. doi: 10.1113/jphysiol.1922.sp002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westcott EB, Segal SS. Perivascular innervation: A multiplicity of roles in vasomotor control and myoendothelial signaling. Microcirculation. 2013;20:217–238. doi: 10.1111/micc.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirst GD, Neild TO. An analysis of excitatory junctional potentials recorded from arterioles. J Physiol. 1978;280:87–104. doi: 10.1113/jphysiol.1978.sp012374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burnstock G, Prosser CL. Conduction in smooth muscles: Comparative electrical properties. Am J Physiol. 1960;199:553–559. doi: 10.1152/ajplegacy.1960.199.3.553. [DOI] [PubMed] [Google Scholar]
- 30.Prosser CL, Burnstock G, Kahn J. Conduction in smooth muscle: Comparative structural properties. Am J Physiol. 1960;199:545–552. doi: 10.1152/ajplegacy.1960.199.3.545. [DOI] [PubMed] [Google Scholar]
- 31.Folkow B, Sonnenschein RR, Wright DL. Loci of neurogenic and metabolic effects on precapillary vessels of skeletal muscle. Acta Physiol Scand. 1971;81:459–471. doi: 10.1111/j.1748-1716.1971.tb04924.x. [DOI] [PubMed] [Google Scholar]
- 32.Segal SS, Duling BR. Communication between feed arteries and microvessels in hamster striated muscle: Segmental vascular responses are functionally coordinated. Circ Res. 1986;59:283–290. doi: 10.1161/01.res.59.3.283. [DOI] [PubMed] [Google Scholar]
- 33.Segal SS, Jacobs TL. Role for endothelial cell conduction in ascending vasodilatation and exercise hyperaemia in hamster skeletal muscle. J Physiol. 2001;536:937–946. doi: 10.1111/j.1469-7793.2001.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Westcott EB, Goodwin EL, Segal SS, Jackson WF. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol. 2012;590:1849–1869. doi: 10.1113/jphysiol.2011.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingebrigtsen R, Leraand S. Dilatation of a medium-sized artery immediately after local changes of blood pressure and flow as measured by ultrasonic technique. Acta Physiol Scand. 1970;79:552–558. doi: 10.1111/j.1748-1716.1970.tb04757.x. [DOI] [PubMed] [Google Scholar]
- 36.Lie M, Sejersted OM, Kiil F. Local regulation of vascular cross section during changes in femoral arterial blood flow in dogs. Circ Res. 1970;27:727–737. doi: 10.1161/01.res.27.5.727. [DOI] [PubMed] [Google Scholar]
- 37.Davies PF. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duling BR. The preparation and use of the hamster cheek pouch for studies of the microcirculation. Microvasc Res. 1973;5:423–429. doi: 10.1016/0026-2862(73)90059-9. [DOI] [PubMed] [Google Scholar]
- 39.Segal SS, Duling BR. Flow control among microvessels coordinated by intercellular conduction. Science. 1986;234:868–870. doi: 10.1126/science.3775368. [DOI] [PubMed] [Google Scholar]
- 40.Segal SS, Duling BR. Conduction of vasomotor responses in arterioles: A role for cell-to-cell coupling? Am J Physiol Heart Circ Physiol. 1989;256:H838–845. doi: 10.1152/ajpheart.1989.256.3.H838. [DOI] [PubMed] [Google Scholar]
- 41.Segal SS, Damon DN, Duling BR. Propagation of vasomotor responses coordinates arteriolar resistances. Am J Physiol Heart Circ Physiol. 1989;256:H832–837. doi: 10.1152/ajpheart.1989.256.3.H832. [DOI] [PubMed] [Google Scholar]
- 42.Dietrich HH, Tyml K. Capillary as a communicating medium in the microvasculature. Microvasc Res. 1992;43:87–99. doi: 10.1016/0026-2862(92)90008-d. [DOI] [PubMed] [Google Scholar]
- 43.Song H, Tyml K. Evidence for sensing and integration of biological signals by the capillary network. Am J Physiol Heart Circ Physiol. 1993;265:H1235–1242. doi: 10.1152/ajpheart.1993.265.4.H1235. [DOI] [PubMed] [Google Scholar]
- 44.Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc Res. 1973;5:384–394. doi: 10.1016/0026-2862(73)90054-x. [DOI] [PubMed] [Google Scholar]
- 45.Grant RT. Direct observation of skeletal muscle blood vessels (rat cremaster) J Physiol. 1964;172:123–137. doi: 10.1113/jphysiol.1964.sp007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segal SS. Microvascular recruitment in hamster striated muscle: Role for conducted vasodilation. Am J Physiol Heart Circ Physiol. 1991;261:H181–189. doi: 10.1152/ajpheart.1991.261.1.H181. [DOI] [PubMed] [Google Scholar]
- 47.Berg BR, Cohen KD, Sarelius IH. Direct coupling between blood flow and metabolism at the capillary level in striated muscle. Am J Physiol Heart Circ Physiol. 1997;272:H2693–2700. doi: 10.1152/ajpheart.1997.272.6.H2693. [DOI] [PubMed] [Google Scholar]
- 48.Cohen KD, Berg BR, Sarelius IH. Remote arteriolar dilations in response to muscle contraction under capillaries. Am J Physiol Heart Circ Physiol. 2000;278:H1916–1923. doi: 10.1152/ajpheart.2000.278.6.H1916. [DOI] [PubMed] [Google Scholar]
- 49.Hill MA, Simpson BE, Meininger GA. Altered cremaster muscle hemodynamics due to disruption of the deferential feed vessels. Microvasc Res. 1990;39:349–363. doi: 10.1016/0026-2862(90)90048-v. [DOI] [PubMed] [Google Scholar]
- 50.Bagher P, Segal SS. The mouse cremaster muscle preparation for intravital imaging of the microcirculation. J Vis Exp. 2011:e2874. doi: 10.3791/2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duling BR, Gore RW, Dacey RG, Jr, Damon DN. Methods for isolation, cannulation, and in vitro study of single microvessels. Am J Physiol Heart Circ Physiol. 1981;241:H108–116. doi: 10.1152/ajpheart.1981.241.1.H108. [DOI] [PubMed] [Google Scholar]
- 52.Close R, Hoh JF. Influence of temperature on isometric contractions of rat skeletal muscles. Nature. 1968;217:1179–1180. doi: 10.1038/2171179a0. [DOI] [PubMed] [Google Scholar]
- 53.Segal SS, Faulkner JA, White TP. Skeletal muscle fatigue in vitro is temperature dependent. J Appl Physiol. 1986;61:660–665. doi: 10.1152/jappl.1986.61.2.660. [DOI] [PubMed] [Google Scholar]
- 54.Williams DA, Segal SS. Feed artery role in blood flow control to rat hindlimb skeletal muscles. J Physiol. 1993;463:631–646. doi: 10.1113/jphysiol.1993.sp019614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rhodin JA. The ultrastructure of mammalian arterioles and precapillary sphincters. J Ultrastruct Res. 1967;18:181–223. doi: 10.1016/s0022-5320(67)80239-9. [DOI] [PubMed] [Google Scholar]
- 56.Dewey MM, Barr L. Intercellular connection between smooth muscle cells: The nexus. Science. 1962;137:670–672. doi: 10.1126/science.137.3531.670-a. [DOI] [PubMed] [Google Scholar]
- 57.Barr L, Berger W, Dewey MM. Electrical transmission at the nexus between smooth muscle cells. J Gen Physiol. 1968;51:347–368. doi: 10.1085/jgp.51.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mekata F. Current spread in the smooth muscle of the rabbit aorta. J Physiol. 1974;242:143–155. doi: 10.1113/jphysiol.1974.sp010698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ransom BR, Kettenmann H. Electrical coupling, without dye coupling, between mammalian astrocytes and oligodendrocytes in cell culture. Glia. 1990;3:258–266. doi: 10.1002/glia.440030405. [DOI] [PubMed] [Google Scholar]
- 60.Larson DM, Sheridan JD. Junctional transfer in cultured vascular endothelium: II. Dye and nucleotide transfer. J Membr Biol. 1985;83:157–167. doi: 10.1007/BF01868747. [DOI] [PubMed] [Google Scholar]
- 61.Bény JL, Gribi F. Dye and electrical coupling of endothelial cells in situ. Tissue Cell. 1989;21:797–802. doi: 10.1016/0040-8166(89)90030-x. [DOI] [PubMed] [Google Scholar]
- 62.Stewart WW. Lucifer dyes--highly fluorescent dyes for biological tracing. Nature. 1981;292:17–21. doi: 10.1038/292017a0. [DOI] [PubMed] [Google Scholar]
- 63.Behringer EJ, Segal SS. Tuning electrical conduction along endothelial tubes of resistance arteries through Ca2+-activated K+ channels. Circ Res. 2012;110:1311–1321. doi: 10.1161/CIRCRESAHA.111.262592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Emerson GG, Segal SS. Electrical activation of endothelium evokes vasodilation and hyperpolarization along hamster feed arteries. Am J Physiol Heart Circ Physiol. 2001;280:H160–167. doi: 10.1152/ajpheart.2001.280.1.H160. [DOI] [PubMed] [Google Scholar]
- 65.Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- 66.Segal SS, Bény JL. Intracellular recording and dye transfer in arterioles during blood flow control. Am J Physiol Heart Circ Physiol. 1992;263:H1–7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- 67.Haas TL, Duling BR. Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc Res. 1997;53:113–120. doi: 10.1006/mvre.1996.1999. [DOI] [PubMed] [Google Scholar]
- 68.Welsh DG, Segal SS. Endothelial and smooth muscle cell conduction in arterioles controlling blood flow. Am J Physiol Heart Circ Physiol. 1998;274:H178–186. doi: 10.1152/ajpheart.1998.274.1.H178. [DOI] [PubMed] [Google Scholar]
- 69.Xia J, Little TL, Duling BR. Cellular pathways of the conducted electrical response in arterioles of hamster cheek pouch in vitro. Am J Physiol Heart Circ Physiol. 1995;269:H2031–2038. doi: 10.1152/ajpheart.1995.269.6.H2031. [DOI] [PubMed] [Google Scholar]
- 70.Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle α1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol. 2008;155:514–524. doi: 10.1038/bjp.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rumbaut RE, Sial AJ. Differential phototoxicity of fluorescent dye-labeled albumin conjugates. Microcirculation. 1999;6:205–213. [PubMed] [Google Scholar]
- 72.Rosenblum WI. Endothelial dependent relaxation demonstrated in vivo in cerebral arterioles. Stroke. 1986;17:494–497. doi: 10.1161/01.str.17.3.494. [DOI] [PubMed] [Google Scholar]
- 73.Koller A, Kaley G. Endothelium regulates skeletal muscle microcirculation by a blood flow velocity-sensing mechanism. Am J Physiol Heart Circ Physiol. 1990;258:H916–920. doi: 10.1152/ajpheart.1990.258.3.H916. [DOI] [PubMed] [Google Scholar]
- 74.Bartlett IS, Segal SS. Resolution of smooth muscle and endothelial pathways for conduction along hamster cheek pouch arterioles. Am J Physiol Heart Circ Physiol. 2000;278:H604–612. doi: 10.1152/ajpheart.2000.278.2.H604. [DOI] [PubMed] [Google Scholar]
- 75.Budel S, Bartlett IS, Segal SS. Homocellular conduction along endothelium and smooth muscle of arterioles in hamster cheek pouch: Unmasking an NO wave. Circ Res. 2003;93:61–68. doi: 10.1161/01.RES.0000080318.81205.FD. [DOI] [PubMed] [Google Scholar]
- 76.Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- 77.Sandow SL, Looft-Wilson R, Doran B, Grayson TH, Segal SS, Hill CE. Expression of homocellular and heterocellular gap junctions in hamster arterioles and feed arteries. Cardiovasc Res. 2003;60:643–653. doi: 10.1016/j.cardiores.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 78.Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase c regulates gap junctional communication. J Cell Biol. 2000;149:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Straub AC, Johnstone SR, Heberlein KR, Rizzo MJ, Best AK, Boitano S, Isakson BE. Site-specific connexin phosphorylation is associated with reduced heterocellular communication between smooth muscle and endothelium. J Vasc Res. 2009;47:277–286. doi: 10.1159/000265562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 s-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vac Biol. 2011;31:399–407. doi: 10.1161/ATVBAHA.110.215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson PC. The myogenic response in the microcirculation and its interaction with other control systems. J Hypertens Suppl. 1989;7:S33–39. [PubMed] [Google Scholar]
- 82.Bagher P, Beleznai T, Kansui Y, Mitchell R, Garland CJ, Dora KA. Low intravascular pressure activates endothelial cell TRPV4 channels, local Ca2+ events, and IKCa channels, reducing arteriolar tone. Proc Natl Acad Sci U S A. 2012;109:18174–18179. doi: 10.1073/pnas.1211946109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci U S A. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: Role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- 85.Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci U S A. 2008;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nausch LW, Bonev AD, Heppner TJ, Tallini Y, Kotlikoff MI, Nelson MT. Sympathetic nerve stimulation induces local endothelial Ca2+ signals to oppose vasoconstriction of mouse mesenteric arteries. Am J Physiol Heart Circ Physiol. 2012;302:H594–602. doi: 10.1152/ajpheart.00773.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- 88.Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brigdan M, Welsh DG. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol. 2012;302:C1226–1242. doi: 10.1152/ajpcell.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yashiro Y, Duling BR. Integrated Ca2+ signaling between smooth muscle and endothelium of resistance vessels. Circ Res. 2000;87:1048–1054. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]
- 90.Sonkusare SK, Bonev AD, Ledoux J, Liedtke W, Kotlikoff MI, Heppner TJ, Hill-Eubanks DC, Nelson MT. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science. 2012;336:597–601. doi: 10.1126/science.1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sonkusare SK, Dalsgaard T, Bonev AD, Hill-Eubanks DC, Kotlikoff MI, Scott JD, Santana LF, Nelson MT. AKAP150-dependent cooperative TRPV4 channel gating is central to endothelium-dependent vasodilation and is disrupted in hypertension. Sci Signal. 2014;7:ra66. doi: 10.1126/scisignal.2005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- 93.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975;67:863–885. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. Arteries and veins. J Cell Biol. 1976;68:705–723. doi: 10.1083/jcb.68.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hill CE, Rummery N, Hickey H, Sandow SL. Heterogeneity in the distribution of vascular gap junctions and connexins: Implications for function. Clin Exp Pharmacol Physiol. 2002;29:620–625. doi: 10.1046/j.1440-1681.2002.03699.x. [DOI] [PubMed] [Google Scholar]
- 96.Looft-Wilson RC, Payne GW, Segal SS. Connexin expression and conducted vasodilation along arteriolar endothelium in mouse skeletal muscle. J Appl Physiol. 2004;97:1152–1158. doi: 10.1152/japplphysiol.00133.2004. [DOI] [PubMed] [Google Scholar]
- 97.Hakim CH, Jackson WF, Segal SS. Connexin isoform expression in smooth muscle cells and endothelial cells of hamster cheek pouch arterioles and retractor feed arteries. Microcirculation. 2008;15:503–514. doi: 10.1080/10739680801982808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garland CJ, Hiley CR, Dora KA. EDHF: Spreading the influence of the endothelium. Br J Pharmacol. 2011;164:839–852. doi: 10.1111/j.1476-5381.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 100.de Wit C, Esser N, Lehr HA, Bolz SS, Pohl U. Pentobarbital-sensitive edhf comediates ach-induced arteriolar dilation in the hamster microcirculation. Am J Physiol Heart Circ Physiol. 1999;276:H1527–1534. doi: 10.1152/ajpheart.1999.276.5.H1527. [DOI] [PubMed] [Google Scholar]
- 101.Welsh DG, Segal SS. Role of EDHF in conduction of vasodilation along hamster cheek pouch arterioles in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1832–1839. doi: 10.1152/ajpheart.2000.278.6.H1832. [DOI] [PubMed] [Google Scholar]
- 102.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 103.Harder DR. Pressure-dependent membrane depolarization in cat middle cerebral artery. Circ Res. 1984;55:197–202. doi: 10.1161/01.res.55.2.197. [DOI] [PubMed] [Google Scholar]
- 104.Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508 ( Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3. 1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- 107.Straub AC, Lohman AW, Billaud M, Johnstone SR, Dwyer ST, Lee MY, Bortz PS, Best AK, Columbus L, Gaston B, Isakson BE. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature. 2012;491:473–477. doi: 10.1038/nature11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459:897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- 109.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci. 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Behringer EJ, Shaw RL, Westcott EB, Socha MJ, Segal SS. Aging impairs electrical conduction along endothelium of resistance arteries through enhanced Ca2+-activated K+ channel activation. Arterioscler Thromb Vac Biol. 2013;33:1892–1901. doi: 10.1161/ATVBAHA.113.301514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rowell LB, O’Leary DS, Kellog DL., Jr . Integration of cardiovascular control systems in dynamic exercise; Handbook of Physiology, Section 12: Exercise: Regulation and integration of multiple systems. New York: Oxford University Press; 1996. pp. 770–838. [Google Scholar]
- 113.Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- 114.Marshall JM. The influence of the sympathetic nervous system on individual vessels of the microcirculation of skeletal muscle of the rat. J Physiol. 1982;332:169–186. doi: 10.1113/jphysiol.1982.sp014408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strandell T, Shepherd JT. The effect in humans of increased sympathetic activity on the blood flow to active muscles. Acta Med Scand Suppl. 1967;472:146–167. doi: 10.1111/j.0954-6820.1967.tb12622.x. [DOI] [PubMed] [Google Scholar]
- 116.VanTeeffelen JW, Segal SS. Interaction between sympathetic nerve activation and muscle fibre contraction in resistance vessels of hamster retractor muscle. J Physiol. 2003;550:563–574. doi: 10.1113/jphysiol.2003.038984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.O’Leary DS, Robinson ED, Butler JL. Is active skeletal muscle functionally vasoconstricted during dynamic exercise in conscious dogs? Am J Physiol Regulatory Integrative Comp Physiol. 1997;272:R386–391. doi: 10.1152/ajpregu.1997.272.1.R386. [DOI] [PubMed] [Google Scholar]
- 118.Buckwalter JB, Mueller PJ, Clifford PS. Sympathetic vasoconstriction in active skeletal muscles during dynamic exercise. J Appl Physiol. 1997;83:1575–1580. doi: 10.1152/jappl.1997.83.5.1575. [DOI] [PubMed] [Google Scholar]
- 119.Kurjiaka DT, Segal SS. Interaction between conducted vasodilation and sympathetic nerve activation in arterioles of hamster striated muscle. Circ Res. 1995;76:885–891. doi: 10.1161/01.res.76.5.885. [DOI] [PubMed] [Google Scholar]
- 120.Haug SJ, Welsh DG, Segal SS. Sympathetic nerves inhibit conducted vasodilatation along feed arteries during passive stretch of hamster skeletal muscle. J Physiol. 2003;552:273–282. doi: 10.1113/jphysiol.2003.046284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haug SJ, Segal SS. Sympathetic neural inhibition of conducted vasodilatation along hamster feed arteries: Complementary effects of α1- and α2-adrenoreceptor activation. J Physiol. 2005;563:541–555. doi: 10.1113/jphysiol.2004.072900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- 123.Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: Relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- 124.Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: Role for augmented α-adrenergic vasoconstriction. J Physiol. 2001;536:977–983. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bearden SE, Payne GW, Chisty A, Segal SS. Arteriolar network architecture and vasomotor function with ageing in mouse gluteus maximus muscle. J Physiol. 2004;561:535–545. doi: 10.1113/jphysiol.2004.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jackson DN, Moore AW, Segal SS. Blunting of rapid onset vasodilatation and blood flow restriction in arterioles of exercising skeletal muscle with ageing in male mice. J Physiol. 2010;588:2269–2282. doi: 10.1113/jphysiol.2010.189811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Casey DP, Joyner MJ. Influence of α-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol. 2012;113:1201–1212. doi: 10.1152/japplphysiol.00734.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bearden SE, Segal SS. Neurovascular alignment in adult mouse skeletal muscles. Microcirculation. 2005;12:161–167. doi: 10.1080/10739680590904964. [DOI] [PubMed] [Google Scholar]
- 129.Moore AW, Bearden SE, Segal SS. Regional activation of rapid onset vasodilatation in mouse skeletal muscle: Regulation through α-adrenoreceptors. J Physiol. 2010;588:3321–3331. doi: 10.1113/jphysiol.2010.193672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Busse R, Fichtner H, Luckhoff A, Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol Heart Circ Physiol. 1988;255:H965–969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- 131.Feletou M, Vanhoutte PM, Weston AH, Edwards G. EDHF and endothelial potassiun channels: IKCa and SKCa. Br J Pharmacol. 2003;140:225. doi: 10.1038/sj.bjp.0705425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Domeier TL, Segal SS. Electromechanical and pharmacomechanical signalling pathways for conducted vasodilatation along endothelium of hamster feed arteries. J Physiol. 2007;579:175–186. doi: 10.1113/jphysiol.2006.124529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Uhrenholt TR, Domeier TL, Segal SS. Propagation of calcium waves along endothelium of hamster feed arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1634–1640. doi: 10.1152/ajpheart.00605.2006. [DOI] [PubMed] [Google Scholar]
- 134.Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: Measurements in Cx40BAC GCaMP transgenic mice. Circ Res. 2007;101:1300–1309. doi: 10.1161/CIRCRESAHA.107.149484. [DOI] [PubMed] [Google Scholar]
- 135.Billaud M, Lohman AW, Johnstone SR, Biwer LA, Mutchler S, Isakson BE. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacol Rev. 2014;66:513–569. doi: 10.1124/pr.112.007351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: Possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Isakson BE. Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J Cell Sci. 2008;121:3664–3673. doi: 10.1242/jcs.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Isakson BE, Duling BR. Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res. 2005;97:44–51. doi: 10.1161/01.RES.0000173461.36221.2e. [DOI] [PubMed] [Google Scholar]
- 140.Heberlein K, Straub AC, Best AK, Greyson MA, Looft-Wilson RC, Sharma PR, Meher A, Leitinger N, Isakson BE. Plasminogen activator inhibitor-1 regulates myoendothelial junction formation. Circ Res. 2010;106:1092–1102. doi: 10.1161/CIRCRESAHA.109.215723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dora KA, Martin PE, Chaytor AT, Evans WH, Garland CJ, Griffith TM. Role of heterocellular gap junctional communication in endothelium-dependent smooth muscle hyperpolarization: Inhibition by a connexin-mimetic peptide. Biochem Biophys Res Commun. 1999;254:27–31. doi: 10.1006/bbrc.1998.9877. [DOI] [PubMed] [Google Scholar]
- 142.Nagaraja S, Kapela A, Tran CH, Welsh DG, Tsoukias NM. Role of microprojections in myoendothelial feedback--a theoretical study. J Physiol. 2013;591:2795–2812. doi: 10.1113/jphysiol.2012.248948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1997;4:35–50. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 144.Cohen KD, Jackson WF. Membrane hyperpolarization is not required for sustained muscarinic agonist-induced increases in intracellular Ca2+ in arteriolar endothelial cells. Microcirculation. 2005;12:169–182. doi: 10.1080/10739680590904973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Socha MJ, Hakim CH, Jackson WF, Segal SS. Temperature effects on morphological integrity and Ca2+ signaling in freshly isolated murine feed artery endothelial cell tubes. Am J Physiol Heart Circ Physiol. 2011;301:H773–783. doi: 10.1152/ajpheart.00214.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Behringer EJ, Socha MJ, Polo-Parada L, Segal SS. Electrical conduction along endothelial cell tubes from mouse feed arteries: Confounding actions of glycyrrhetinic acid derivatives. Br J Pharmacol. 2012;166:774–787. doi: 10.1111/j.1476-5381.2011.01814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.de Wit C, Roos F, Bolz SS, Kirchhoff S, Kruger O, Willecke K, Pohl U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ Res. 2000;86:649–655. doi: 10.1161/01.res.86.6.649. [DOI] [PubMed] [Google Scholar]
- 148.Figueroa XF, Paul DL, Simon AM, Goodenough DA, Day KH, Damon DN, Duling BR. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ Res. 2003;92:793–800. doi: 10.1161/01.RES.0000065918.90271.9A. [DOI] [PubMed] [Google Scholar]
- 149.Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte P, Weston A. EDHF: Bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- 150.Behringer EJ, Segal SS. Spreading the signal for vasodilatation: Implications for skeletal muscle blood flow control and the effects of ageing. J Physiol. 2012;590:6277–6284. doi: 10.1113/jphysiol.2012.239673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Beleznai TZ, Yarova PL, Yuill KH, Dora KA. Smooth muscle Ca2+ -activated and voltage-gated K+ channels modulate conducted dilation in rat isolated small mesenteric arteries. Microcirculation. 2011;18:487–500. doi: 10.1111/j.1549-8719.2011.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hald BO, Jacobsen JC, Braunstein TH, Inoue R, Ito Y, Sorensen PG, Holstein-Rathlou NH, Jensen LJ. BKCa and KV channels limit conducted vasomotor responses in rat mesenteric terminal arterioles. Pflugers Arch. 2012;463:279–295. doi: 10.1007/s00424-011-1049-8. [DOI] [PubMed] [Google Scholar]
- 153.Muller-Delp JM, Gurovich AN, Christou DD, Leeuwenburgh C. Redox balance in the aging microcirculation: New friends, new foes, and new clinical directions. Microcirculation. 2012;19:19–28. doi: 10.1111/j.1549-8719.2011.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Seals DR, Esler MD. Human ageing and the sympathoadrenal system. J Physiol. 2000;528:407–417. doi: 10.1111/j.1469-7793.2000.00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ungvari Z, Kaley G, de Cabo R, Sonntag WE, Csiszar A. Mechanisms of vascular aging: New perspectives. J Gerontol A Biol Sci Med Sci. 2010;65:1028–1041. doi: 10.1093/gerona/glq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kohler R, Kaistha BP, Wulff H. Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease. Expert Opin Ther Targets. 2010;14:143–155. doi: 10.1517/14728220903540257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Feletou M. Calcium-activated potassium channels and endothelial dysfunction: Therapeutic options? Br J Pharmacol. 2009;156:545–562. doi: 10.1111/j.1476-5381.2009.00052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Secomb TW, Pries AR. Information transfer in microvascular networks. Microcirculation. 2002;9:377–387. doi: 10.1038/sj.mn.7800146. [DOI] [PubMed] [Google Scholar]
- 159.Chen YM, Yip KP, Marsh DJ, Holstein-Rathlou NH. Magnitude of TGF-initiated nephron-nephron interactions is increased in SHR. Am J Physiol Renal Fluid Electrolyte Physiol. 1995;269:F198–204. doi: 10.1152/ajprenal.1995.269.2.F198. [DOI] [PubMed] [Google Scholar]
- 160.Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–659. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- 161.Wang L, Yin J, Nickles HT, Ranke H, Tabuchi A, Hoffmann J, Tabeling C, Barbosa-Sicard E, Chanson M, Kwak BR, Shin HS, Wu S, Isakson BE, Witzenrath M, de Wit C, Fleming I, Kuppe H, Kuebler WM. Hypoxic pulmonary vasoconstriction requires connexin 40-mediated endothelial signal conduction. J Clin Invest. 2012;122:4218–4230. doi: 10.1172/JCI59176. [DOI] [PMC free article] [PubMed] [Google Scholar]