SUMMARY
Mast cells (MCs) contribute to the pathogenesis of obesity and diabetes. This study demonstrates that leptin deficiency slants MCs toward anti-inflammatory functions. MCs in the white adipose tissues (WAT) of lean humans and mice express negligible leptin. Adoptive transfer of leptin-deficient MCs expanded ex vivo mitigates diet-induced and pre-established obesity and diabetes in mice. Mechanistic studies show that leptin-deficient MCs polarize macrophages from M1 to M2 functions because of impaired cell signaling and an altered balance between pro- and anti-inflammatory cytokines, but do not affect T-cell differentiation. Rampant body weight gain in ob/ob mice, a strain that lacks leptin, associates with reduced MC content in WAT. In ob/ob mice, genetic depletion of MCs exacerbates obesity and diabetes, and repopulation of ex vivo expanded ob/ob MCs ameliorates these diseases.
Keywords: Mast cell, leptin signaling, obesity, diabetes, macrophage polarization
INTRODUCTION
Obesity and diabetes involve chronic inflammation (Gregor and Hotamisligil, 2011) mediated by a repertoire of inflammatory cells, including macrophages, B cells, T cells, neutrophils, eosinophils, and mast cells (MCs) (Weisberg et al., 2003; DeFuria et al., 2013; Matarese et al., 2012; Talukdar et al., 2012; Wu et al., 2011; Liu et al., 2011). We previously established that activated MCs operate in diet-induced obesity (DIO) and diabetes in mice by releasing interferon-γ (IFN-γ) and interleukin-6 (IL6), cysteinyl cathepsins, chymase, and tryptase to activate vascular cells or adipocytes and promote adipose tissue angiogenesis, liver glycogenesis, and adipocyte differentiation (Liu et al., 2009). Mice with DIO exhibit increased plasma MC activators substance P and IgE (Moreno et al., 2014). Human plasma concentrations of tryptase, a MC-specific enzyme, increase with rising body mass index (BMI) (Fenger et al., 2012) and correlate with body fat mass, glycated hemoglobin, fasting insulin level, and homeostasis model assessment-estimated insulin resistance (HOMA-IR) index (Ramalho et al., 2012). Although a recent study using anemic MC-deficient KitW/Wv mice and CpaCre/+ mice that affect more than just MCs (Reber et al., 2012) claimed a negligible role of MCs in obesity and diabetes (Gutierrez et al., 2015), MC stabilizer ketotifen reduces BMI, HbA1c, fasting blood glucose, low-density lipoproteins, and triglyceride, and increases adiponectin and high-density lipoproteins among obese and diabetic patients (El-Haggar et al., 2015).
Macrophages (F4/80+CD11b+) drive obesity-associated inflammation and diseases. WAT from obese humans and animals (Weisberg et al., 2003) exhibit increased macrophage numbers. Selective depletion of macrophages or abrogation of the CCR2-MCP-1 (monocyte chemotactic protein-1) cascade that mediates macrophage recruitment reduces WAT inflammation and improves insulin sensitivity in DIO mice (Patsouris et al., 2008; Weisberg et al., 2006). Macrophage polarization also influences obesity. M2 macrophages, also called alternatively activated macrophages, express arginase (Arg)-1 and CD206 (mannose receptor) and associate with metabolic homeostasis in lean WAT (Chawla et al., 2011). M2 cells also produce IL10, an anti-inflammatory cytokine, in response to Th2 cytokines IL4 and IL13 (Lumeng et al., 2007), and contribute to the maintenance of insulin sensitivity (Liao et al., 2011). IFN-γ drives the polarization of M1 macrophages, also called classically activated macrophages, and promotes M1 cell expression of pro-inflammatory cytokines TNF-α, IL6, IL1β, IL12, and MCP-1 (Mathis, 2013). M1 cells express CD11c and high levels of iNOS, TNF-α, and IL6, which impede insulin signaling in adipocytes and therefore promote insulin resistance and obesity-associated inflammation (Weisberg et al., 2003, 2006; Lumeng et al., 2007). Past observations of obese WAT revealed a skewed M1/M2 polarization that enhances WAT inflammation (Shaul et al., 2010). This observation engendered the hypothesis that M1 cells predominate in WAT during obesity. The stimulation of M2 cells using omega-3 fatty acids or thiazolidinediones during obesity improves metabolic function and mutes adipose tissue inflammation (Patsouris et al., 2008; Oh et al., 2010). Several conditions regulate macrophage polarization, including exposure to free fatty acids, fetuin-A, Krüppel-like factor-4, or cold temperatures (Chawla et al., 2011; Liao et al., 2011; Nguyen et al., 2011; Pal et al., 2012). The most abundant immune cells in WAT after macrophages are CD3+ T cells. Compared to lean mice, WAT from obese mice (Morris et al., 2013; Winer et al., 2009) contain increased numbers of CD4+ and CD8+ T cells. Moreover, WAT from obese mice harbors fewer regulatory T cells (Tregs) that maintain self-tolerance and dampen Th1 and Th17 responses.
The peptide hormone and satiety factor leptin regulates body weight by suppressing appetite and stimulating energy expenditure. Its serum concentration and gene expression in adipocytes correlate with body fat storage and remain elevated in obese individuals. Leptin modulates a wide range of immune and inflammatory processes. Inflammatory stimuli (e.g. tumor necrosis factor-α [TNF-α], IL1, or lipopolysaccharide [LPS]) stimulate leptin expression in adipose tissues (Grunfeld et al., 1996), and leptin in turn promotes proliferation, survival, migration/chemotaxis, cytokine/protease/adhesion molecule expression, and oxidative stress of nearly all tested cell types. Leptin-responsive cells include those implicated in cardiometabolic metabolic diseases, such as dendritic cells, basophils, endothelial cells, and neutrophils (Suzukawa et al., 2011; Ottonello et al., 2004; Mattioli et al., 2009; Kato et al., 2011). Leptin also activates monocyte proliferation and cytokine/chemokine expression, oxidative burst (Tsiotra et al., 2013; Santos-Alvarez et al., 1999), macrophage phagocytosis, cytokine expression, oxidative stress, and chemotaxis (Gruen et al., 2007). It also promotes Th1 cell cytokine and adhesion molecule expression and proliferation/survival and constrains Treg expansion (Mattioli et al., 2005; Lord et al., 1998; Martín-Romero et al., 2000; Fernández-Riejos et al., 2008; De Rosa et al., 2007). Leptin drives naïve T-cells towards Th1 polarization by stimulating the synthesis of IL2, IL12, IFN-γ and inhibiting T-cell expression of IL10 and IL4 (Mattioli et al., 2005; Martín-Romero et al., 2000; Batra et al., 2010). In obese humans, plasma leptin levels correlate with inflammatory biomarkers (C-reactive protein, serum amyloid A, and IL6) (Meyers et al., 2008). Compared to controls, patients with metabolic syndrome have 6-fold higher plasma leptin concentrations that correlate positively with WAT MC numbers (Chaldakov et al., 2001). Leptin deficiency associates with reduced circulating CD4+ T-cell counts and impaired T-cell proliferation and cytokine release (Farooqi et al., 2002).
This study revealed that WAT from lean humans and mice expressed higher leptin levels than those from obese subjects, but MCs in WAT from lean subjects expressed negligible leptin. Leptin deficiency of MCs favored acquisition of an anti-inflammatory slant that polarized macrophages towards M2 functions without affecting T-cell differentiation. Leptin-deficient mice lacking MCs showed augmented body weight gain and glucose intolerance. Restoration of leptin-deficient MCs to genetic obese and DIO mice or mice with pre-established diseases limited the progression of these diseases.
RESULTS
MCs in lean adipose tissues show reduced leptin expression
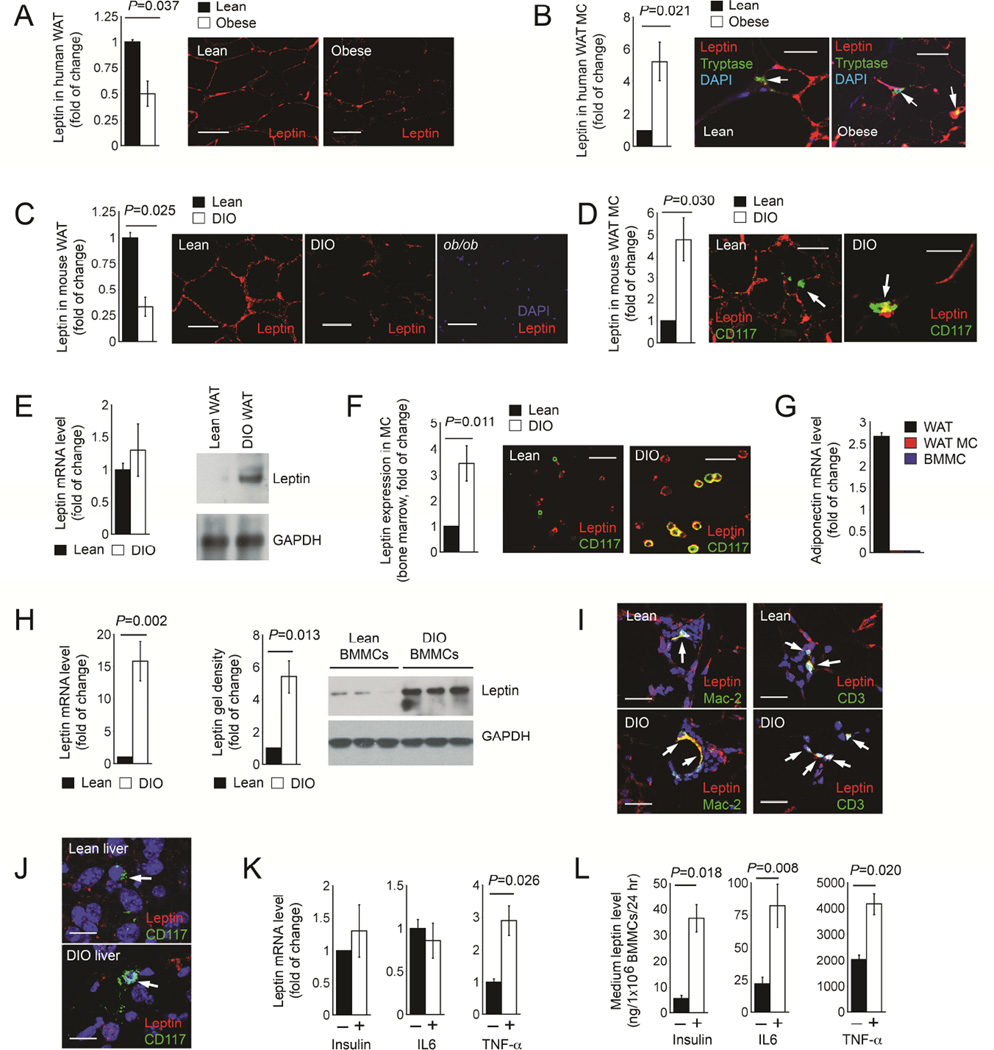
Obese patients (Chaldakov et al., 2001) and DIO mice (El-Haschimi et al., 2000) exhibit increased plasma leptin levels. Yet immunostaining of WAT paraffin sections from obese and lean humans demonstrated that WAT from lean subjects (n=17) contained twice as much leptin as in WAT from obese patients (n=17) (Figure 1A). In contrast, WAT MC in lean subjects contained little or no leptin, while those from obese patients had five-fold higher leptin (Figure 1B). We revealed a similar pattern of leptin expression in WAT from DIO C57BL/6 mice. Leptin levels in WAT from lean mice were about 3-fold higher than those from DIO mice (Figure 1C), but leptin expression in MCs from lean WAT was also five-fold lower than in MCs from DIO WAT (Figure 1D). WAT sections from leptin-deficient ob/ob mice showed no staining, affirming the specificity of the leptin antibody (Figure 1C). To verify these observations, we used cell sorter-purified CD117+FcεR1a+ MCs in WAT from lean and DIO mice. Although leptin mRNA levels were comparable between MCs from lean and DIO mice (Figure 1E, left), immunoblot demonstrated leptin expression in MCs isolated from DIO WAT, but negligibly in MCs isolated from lean WAT (Figure 1E, right). Bone marrow contains mature MCs (Stevens and Rosenthal, 2001). Immunofluorescent double staining also detected three-fold higher leptin expression in MCs from bone marrow preparation from DIO mice than those from lean mice (Figure 1F). RT-PCR detected adiponectin expression in WAT from WT lean mice (positive control), but not in cell sorter-purified MCs from lean WAT or BMMCs (negative control) (Figure 1G), affirming the purity of isolated WAT MCs. Both RT-PCR and immunoblot analysis also revealed much higher leptin expression in BMMCs from DIO mice than in those from lean mice (Figures 1H). To test whether other immune cells in lean WAT also show reduced leptin expression, we performed immunofluorescent double staining for leptin and Mac-2 or CD3. As expected, DIO WAT contained many more Mac-2+ macrophages and CD3+ T cells than lean WAT, but we found comparable leptin expression in macrophages and T cells from lean and DIO WAT (Figure 1I). The MCs from various organs in DIO mice did not all express leptin. Immunofluorescent staining detected negligible leptin expression in MCs from livers from either lean or DIO mice (Figure 1J). Increased insulin and inflammatory cytokines in DIO mice may explain higher leptin expression in DIO MCs than in lean MCs. We tested this hypothesis by stimulating BMMCs with insulin, IL6, and TNF-α. Although only TNF-α increased leptin RNA levels, all three stimuli increased leptin secretion from BMMCs (Figures 1K and 1L).
Figure 1.
Leptin expression in lean and obese humans and mice. A. Leptin immunofluorescent staining of WAT from lean and obese humans. B. Leptin and MC tryptase immunofluorescent double staining of WAT from lean and obese humans. C. Leptin immunofluorescent staining of WAT from lean and DIO mice. D. Leptin and CD117 immunofluorescent double staining of WAT from lean and DIO mice. E. RT-PCR (left) and immunoblot (right) detected leptin expression in MCs isolated from lean and DIO WAT. F. Leptin and CD117 immunofluorescent double staining of total bone marrow cell preparation from lean and DIO mice. G. RT-PCR detected adiponectin expression in WAT, purified MCs from WAT, and BMMCs, all from lean mice. H. RT-PCR (left) and immunoblot (right) detected leptin expression in BMMCs prepared from lean and DIO mice. GAPDH in panels E and H ensured equal protein loading. I. Leptin and Mac-2 or CD3 immunofluorescent double staining of WAT from lean and DIO mice. J. Leptin and CD117 immunofluorescent double staining of liver from lean and DIO mice. RT-PCR (K) and culture medium ELISA (L) detected leptin expression in WT BMMCs with different treatments as indicated. N=17 per group for human WAT samples, n=12 per group for mouse WAT and liver samples, n=3~6 for RT-PCR, and n=6 for leptin ELISA. Representative data for panels A-D, F, and H are shown to the right. Scale: 50 µm.
Leptin deficiency favors acquisition of an anti-inflammatory by MCs
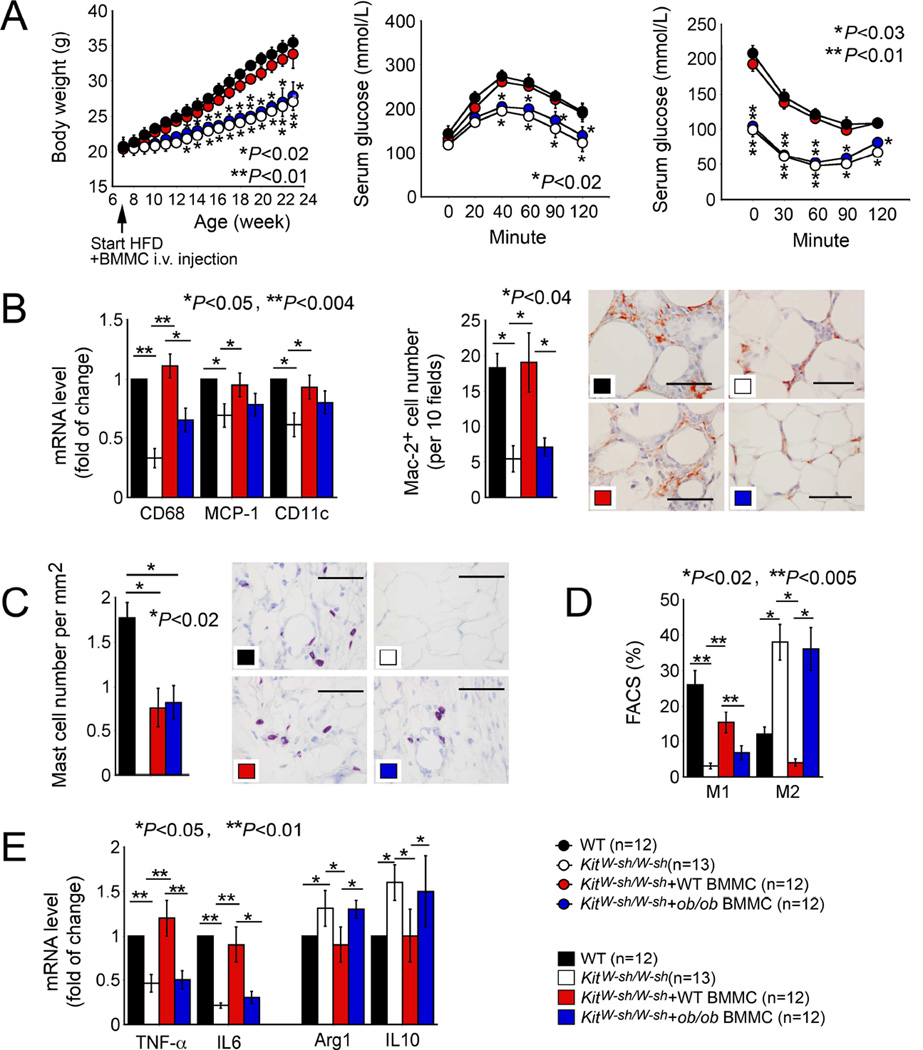
Low expression of leptin in MCs from lean WAT raised the hypotheses that leptin expression affects MC functions that may affect obesity and associated complications. To test this conjecture, we first produced DIO in wild-type (WT) and MC-deficient KitW-sh/W-sh mice by feeding mice a Western diet (Liu et al., 2009). KitW-sh/W-sh mice gained less weight and had better glucose and insulin tolerance than WT mice (Figures 2A). KitW-sh/W-sh mice had significantly lower VAT (visceral adipose tissue) and SAT (subcutaneous adipose tissue) weights, WAT adipocyte sizes, and serum insulin levels than WT mice (Figures S1A–S1C). Adoptive transfer of BMMCs from WT mice, but not those from ob/ob mice that lack leptin, normalized body weight gain, VAT and SAT weight, adipocyte size, glucose and insulin tolerance, and serum insulin concentrations in KitW-sh/W-sh -recipient mice (Figure 2A and Figure S1A–S1C). BMMCs from ob/ob mice also failed to reverse reduced WAT expression of macrophage CD68 or M1 macrophage CD11c, chemokine MCP-1, and Mac2+ macrophage accumulation in WAT in KitW-sh/W-sh recipient mice, unlike those from WT mice, as determined by RT-PCR and immunostaining, respectively (Figure 2B). As we reported (Liu et al., 2009), leptin deficiency did not affect donor BMMC homing to WAT or other organs, such as spleens. Toluidine blue staining revealed partial but equal recovery of donor WT and ob/ob BMMCs in recipient mouse WAT (Figure 2C), although we also detected similarly reduced numbers of CD3+ T cells and increased numbers of NIMP+ neutrophils in WAT from recipient mice receiving WT and ob/ob BMMCs (Figure S1D). In spleens, donor WT and ob/ob BMMCs also had partial and similar recovery, but MC deficiency or reconstitution did not affect significantly spleen contents of macrophages, T cells, or neutrophils (Figure S1E). FACS analysis demonstrated that MC deficiency reduced WAT F4/80+CD11c+ M1 macrophages and increased F4/80+CD206+ M2 macrophages. Repopulation of WT BMMCs, but not ob/ob BMMCs, increased WAT M1 cells and decreased M2 cells in recipient KitW-sh/W-sh mice (Figure 2D). mRNA profiles of M1 markers TNF-α and IL6, and M2 markers Arg-1 and IL10 affirmed the altered M1 and M2 slant in macrophages in WAT from donor WT and ob/ob BMMCs (Figure 2E).
Figure 2.
Obesity and diabetes in WT and MC-deficient KitW-sh/W-sh mice and those receiving BMMCs from WT and ob/ob mice. A. Body weight gain, glucose tolerance, insulin tolerance. B. RT-PCR determined WAT expression of CD68, MCP-1, and CD11c. Immunostaining detected Mac2+ macrophage in WAT. C. Toluidine blue staining detected MC numbers in WAT from different groups of mice as indicated. D. FACS analysis determined WAT F4/80+CD11c+ M1 and F4/80+CD206+ M2 macrophages. E. RT-PCR determined M1 markers (TNF-α and IL6) and M2 markers (arginase-1 and IL10) in WAT from different mouse groups as indicated. The number of mice per group is indicated in the parenthesis. Representative data for panels B (scale: 50 µm) and C (scale: 50 µm) are shown to the right.
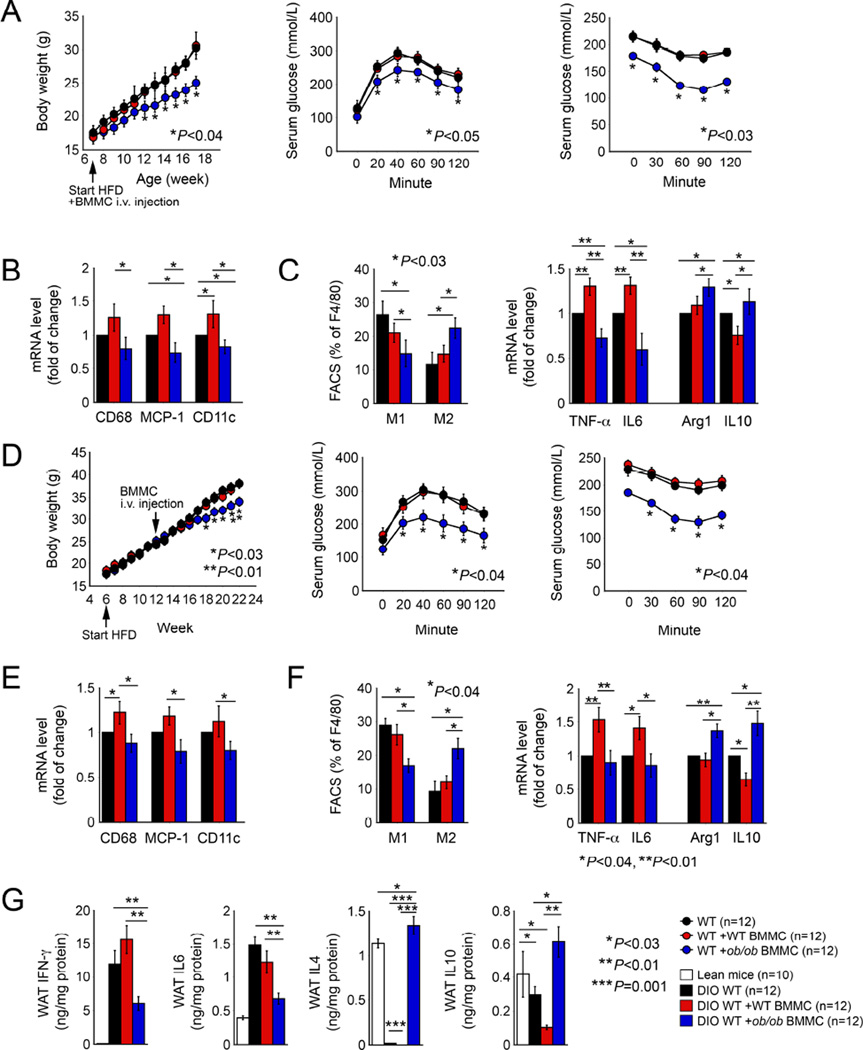
In WT DIO mice, administration of additional WT BMMCs did not affect body weight gain, SAT or VAT weight, adipocyte size, glucose and insulin tolerances, or serum insulin levels. However, WT mice that received ob/ob BMMCs showed significantly reduced obesity (body weight gain, WAT weight, adipocyte size, and serum insulin levels) and improved glucose and insulin tolerance, and reduced serum insulin levels (Figures 3A and Figure S2A). Additional WT BMMCs increased WAT expression of CD68, MCP-1, and CD11c, as determined by RT-PCR, although the total WAT Mac2+ macrophage content did not change. In contrast, additional ob/ob BMMCs reduced CD68, MCP-1, and CD11c expression and macrophage accumulation in WAT from recipient WT mice (Figures 3B and S2B). WT BMMCs did not significantly affect WAT M1 or M2 cells, but ob/ob BMMCs significantly reduced WAT M1 cells and increased M2 cells as determined by FACS analysis. WAT from recipients of WT BMMCs showed significantly increased mRNAs encoding the M1 markers TNF-α and IL6 and decreased mRNA for the M2 marker IL10, while those receiving ob/ob BMMCs had significantly reduced TNF-α and IL6 mRNA and increased Arg-1 and IL10 mRNA (Figure 3C).
Figure 3.
Obesity and diabetes in WT mice and those with pre-established obesity and diabetes receiving WT and ob/ob BMMCs. A. Body weight gain, glucose tolerance, insulin tolerance. B. RT-PCR determined WAT expression of CD68, MCP-1, and CD11c. C. FACS analysis determined WAT F4/80+CD11c+ M1 and F4/80+CD206+ M2 macrophages and RT-PCR determined WAT TNF-α, IL6, arginase-1, and IL10 expression. D. Body weight gain, glucose tolerance, insulin tolerance from mice with pre-established obesity and diabetes followed by MC repopulation. E. RT-PCR determined WAT expression of CD68, MCP-1, and CD11c. F. FACS analysis determined WAT F4/80+CD11c+ M1 and F4/80+CD206+ M2 macrophages and RT-PCR determined TNF-α, IL6, arginase-1, and IL10 in WAT. G. ELISA determined WAT tissue IFN-γ, IL6, IL4, and IL10. Panels A to C used the same mice and panels D to G were from the same mice. Number of mice per group is indicated in each parenthesis.
Leptin-deficient BMMCs also attenuated pre-established diseases. We produced obese and diabetic mice by feeding WT mice a Western diet for six weeks, then gave these mice either WT or ob/ob BMMCs, followed by continued consumption of a Western diet for another 10 weeks. Mice that received ob/ob BMMCs showed significant reduction in body weight gain, VAT and SAT weight, WAT adipocyte sizes, improvement in glucose and insulin tolerances, and a reduction in serum insulin levels, whereas donor WT BMMCs did not affect these variables in recipient mice (Figures 3D and S2C). Again, WT BMMCs increased WAT expression of CD68, MCP-1, CD11c, as determined by RT-PCR. In contrast, ob/ob BMMCs reduced expression of all of these inflammatory markers in WAT and suppressed WAT Mac2+ macrophage accumulation (Figures 3E and S2D). In these mice with pre-established obesity and diabetes, WT BMMCs did not change WAT M1 or M2 cell contents as measured by FACS, but significantly increased WAT mRNA of TNF-α and IL6, and decreased mRNA of IL10. In contrast, ob/ob BMMCs reduced WAT F4/80+CD11c+ M1 cell content and WAT expression of TNF-α and IL6, and increased F4/80+CD206+ M2 macrophages and their expression of Arg-1 and IL10 (Figure 3F). In WT mice, DIO increased WAT pro-inflammatory cytokines IFN-γ and IL6, and reduced WAT anti-inflammatory IL4 and IL10. Donor WT BMMCs further increased WAT IFN-γ and reduced WAT IL4 and IL10. In contrast, donor ob/ob BMMCs significantly reduced recipient mice WAT IFN-γ and IL6, and brought up WAT IL4 and IL10 levels to those in WAT from lean mice (Figure 3G).
Together, in both previously healthy WT mice and those with pre-established obesity and diabetes, ob/ob BMMCs, but not WT BMMCs, attenuated the development of dysmetabolism, corresponding to the less inflammatory profile of MCs lacking leptin expression.
Leptin-deficient MCs polarize macrophages toward M2 functions
Reduced leptin expression in MCs from lean human WAT (Figure 1B) and in MCs from lean mouse WAT and bone marrow (Figures 1D–1F) suggests that these MCs exert correspondingly distinct functions from those in obese WAT. Reduced obesity and diabetes in MC-deficient KitW-sh/W-sh mice reconstituted with ob/ob BMMCs (Figure 2), in WT mice receiving ob/ob BMMC, including those with pre-established obesity and diabetes (Figure 3), supported this hypothesis and suggested a shift in MCs from pro-inflammatory to anti-inflammatory functions in the absence of leptin expression. Leptin production by MCs in WAT may not only influence their own functions, but also those of neighboring cells in WAT, including macrophages and T cells.
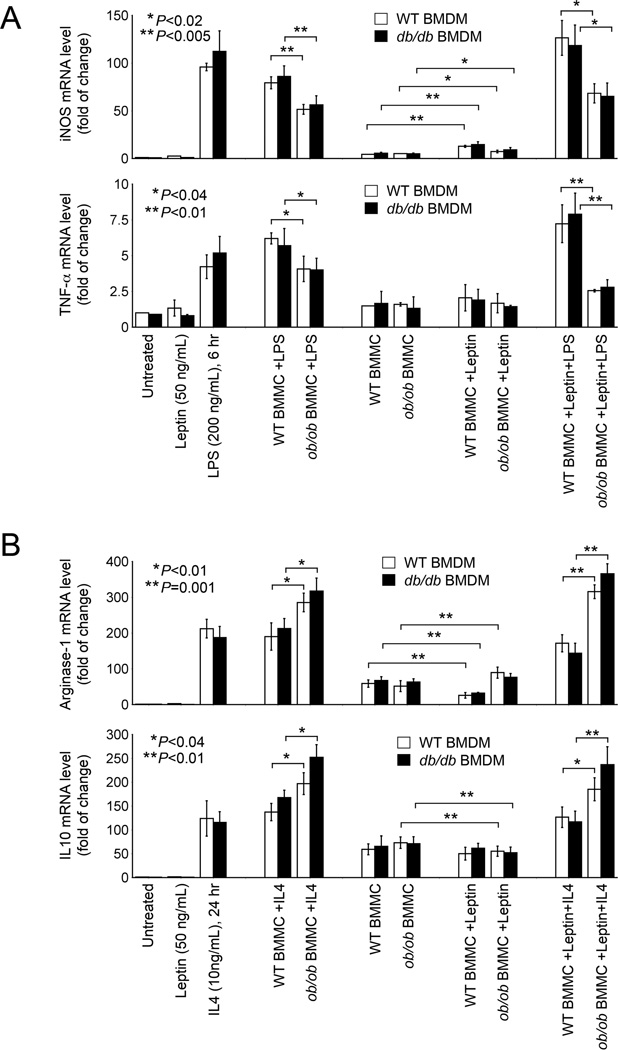
Macrophages are the most abundant inflammatory cells in WAT (Weisberg et al., 2003). Immunofluorescent staining localized leptin receptor ObR expression to Mac-2+ macrophages in WAT from WT DIO mice. WAT from ObR-deficient db/db mice confirmed the anti-mouse ObR antibody specificity. Immunoblot analysis also detected ObR expression in bone marrow-derived macrophages (BMDMs) from WT mice, but not in those from db/db mice (Figures S3A and S3B). BMMCs from ob/ob and WT mice may interact differently with macrophages in WAT or in other peripheral organs. This hypothesis may explain the observations in Figures 2 and 3: One round of repopulation of ob/ob BMMCs reduced WAT M1 cell numbers and M1 markers (TNF-α and IL6), and increased WAT M2 cell numbers and M2 markers (Arg-1 and IL10) in KitW-sh/W-sh mice, WT mice, or WT mice with pre-established obesity and diabetes. Yet the shift from M1 to M2 slant in WAT from these recipient mice (Figures 2 and 3) after receiving ob/ob BMMCs might be a consequence of reduced obesity and diabetes. To test the hypothesis that ob/ob BMMCs influenced macrophage polarization directly, we co-cultured BMDMs from WT mice with and without M1 macrophage stimulus LPS (Zheng et al., 2013), M2 cell stimulus IL4 (Ying et al., 2013), or leptin alone. LPS induced macrophage expression of M1 markers iNOS and TNF-α. Only ob/ob BMMCs, but not WT BMMCs suppressed significantly this activity of LPS. Addition of leptin did not affect this inhibitory activity of ob/ob BMMCs (Figure 4A). In contrast, IL4 induced macrophage expression of M2 markers Arg-1 and IL10. Only ob/ob BMMCs, but not WT BMMCs further increased the expression of these anti-inflammatory molecules. Again, addition of leptin did not affect this activity of ob/ob BMMCs (Figure 4B). At the baseline without LPS or IL4 treatment, leptin acted as a pro-inflammatory molecule to induce BMDM expression of iNOS in the presence of WT or ob/ob BMMCs (Figure 4A), and suppressed BMDM expression of Arg-1 and IL10 in presence of WT or ob/ob BMMCs (Figure 4B). Relative to the expression of M1 and M2 markers in LPS- and IL4-treated macrophages, however, such baseline levels of these markers were rather moderate. Of note, BMDMs from leptin receptor-deficient db/db mice acted identically to WT BMDMs in all treatments (Figures 4A and 4B), suggesting that the presented role of ob/ob BMMCs in inhibiting M1 molecule expression but enhancing M2 molecule expression was not due to the intrinsic leptin of MCs.
Figure 4.
Leptin-deficient MCs polarize macrophages towards M2 functions. RT-PCR determined the expression of M1 markers iNOS and TNF-α (A) or M2 markers Arg-1 and IL10 (B) in WT BMDMs treated with and without leptin, LPS, IL4, WT BMMCs, or ob/ob BMMCs as indicated.
Under the same conditions, however, CD4+ and CD8+ T cells from WT or ob/ob mice did not affect LPS or IL4-induced iNOS, TNF-α, Arg-1, and IL10 expression in BMDMs from WT or db/db mice (Figures S3A and S3B). Our observations suggest a function of leptin-deficient MCs in slanting macrophage polarization from M1 towards M2 phenotypes, and such activity of leptin deficiency may be unique to MCs.
Altered cytokine expression in leptin-deficient BMMCs accounts for their anti-inflammatory activities
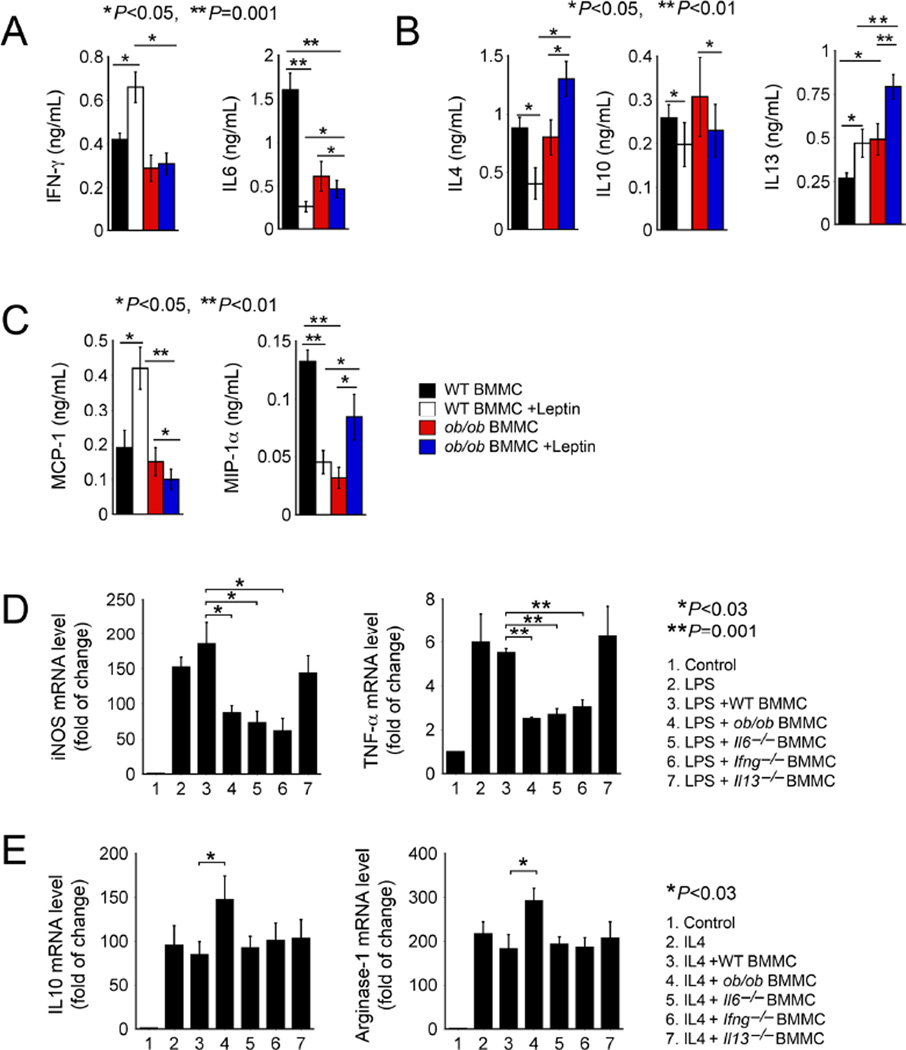
Leptin-deficient BMMCs polarized macrophages towards M2 functions in recipient mice (Figures 2D, 3C and 3F) and in cultured BMDMs (Figure 4). Exogenous leptin or absence of leptin receptor expression on macrophages did not affect such activity of leptin-deficient BMMCs, suggesting an intrinsic leptin-independent role of these BMMCs in M2 macrophage polarization. To test this hypothesis, we performed RayBio® cytokine and chemokine antibody arrays by comparing WT and ob/ob BMMCs in the presence and absence of leptin. Of the 39 cytokines and chemokines represented in this array, eotaxin, IFN-γ, IL1α, IL4, IL6, IL10, IL13, MCP-1, MIP-1α, and MIP-1γ in the culture medium from ob/ob BMMCs showed most robust changes from those in media from WT BMMCs (Figure S4). To verify these observations, we measured by ELISA selected proteins including pro-inflammatory IFN-γ and IL6, anti-inflammatory IL4, IL10, and IL13, and chemokines MCP-1 and MIP-1α. Although leptin induced IFN-γ and reduced IL6 in WT BMMCs, ob/ob BMMCs produced significantly reduced amounts of both of these pro-inflammatory proteins (Figure 5A). In contrast, ob/ob BMMC culture media contained significantly higher concentrations of the anti-inflammatory cytokines IL4 and IL13 either before or after leptin treatment, although IL10 expression showed no significant difference between the two types of cells before and after the treatment (Figure 5B). Similarly, leptin induced MCP-1 but reduced MIP-1α in WT BMMCs. Leptin-deficient ob/ob BMMCs released less of these chemokines before and after leptin treatment (Figure 5C). To test the hypothesis that such differences in production of pro- or anti-inflammatory cytokines accounted for ob/ob BMMC influences on macrophage polarization, we assessed BMDM polarization in the presence of LPS or IL4 using WT and ob/ob BMMCs as experimental controls. In LPS-treated BMDMs, LPS-induced expression of M1 markers iNOS and TNF-α was significantly suppressed by BMMCs from ob/ob, Il6− / − and Ifng− / − mice, but not by those from WT or Il13− / − mice (Figure 5D), suggesting that reduced IFN-γ and IL6 expression in ob/ob BMMCs (Figure 5A) accounted for ob/ob BMMC activity in reducing macrophage expression of M1 markers iNOS and TNF-α (Figure 4A). In contrast, IL4-induced macrophage expression of M2 markers IL10 and Arg-1 was further enhanced by ob/ob BMMCs, but not by those from WT, Il6− / −, Ifng− / −, and Il13− / − mice (Figure 5E), suggesting that molecules other than IFN-γ, IL6, and IL13 accounted for ob/ob BMMC activity in enhancing BMMC expression of Arg-1 and IL10 (Figure 4B).
Figure 5.
Leptin-deficient MCs polarize M2 macrophages by altered cytokine expression. ELISA determined culture medium IFN-γ and IL6 (A), IL4, IL10, and IL13 (B), and MCP-1 and MIP-1α (C) in WT and ob/ob BMMCs treated with and without leptin. RT-PCR determined the expression of M1 markers iNOS and TNF-α (D) and M2 markers IL10 and arginase-1 (E) in BMDMs treated with and without LPS or IL4 and BMMCs from different mice as indicated.
Altered laptin signaling in leptin-deficient BMMCs accounts for their corresponding cytokine expression
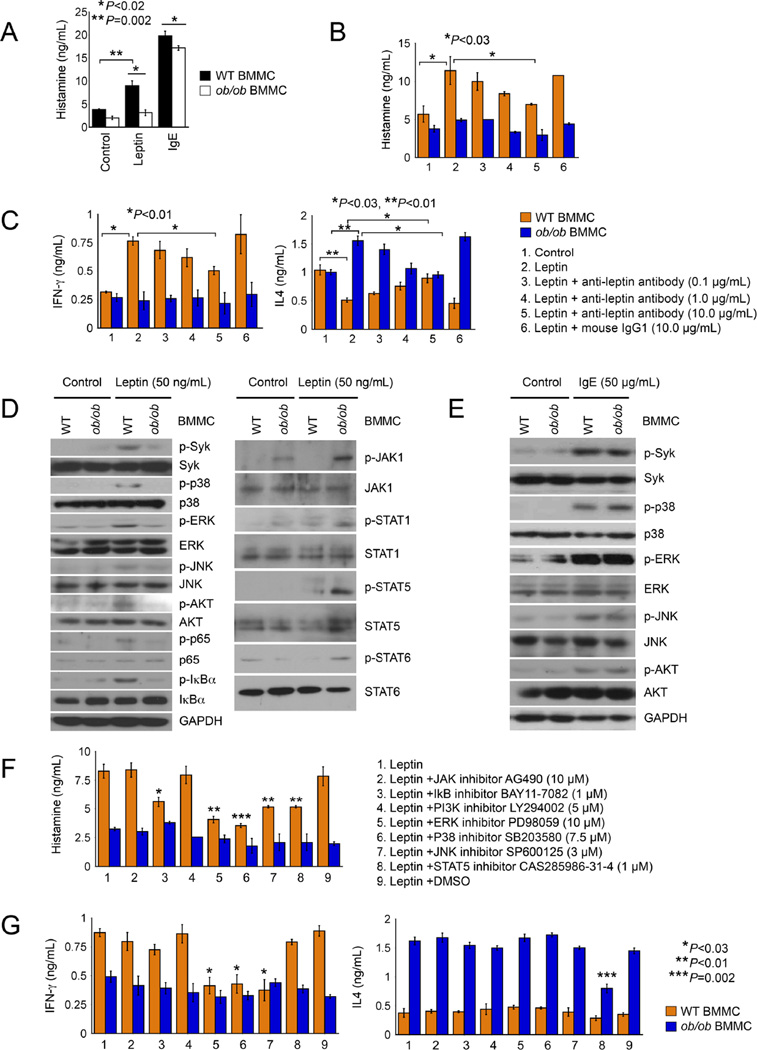
Differences in production of IL6, IFN-γ, IL4 and IL13 between ob/ob and WT BMMCs (Figures 5A and 5B) may reflect differences in BMMC degranulation or activation, or differences in cell signaling that regulates the expression of these cytokines. IgE is one of the most common MC activators (Xu and Shi, 2015). Both leptin and IgE activated WT BMMCs, as determined by histamine release. However, leptin-induced histamine release was fully blunted in ob/ob BMMCs, and we also detected moderate but significant reduction of IgE-induced histamine release from ob/ob BMMCs (Figure 6A). Such differences between WT and ob/ob BMMCs in responding to leptin or IgE stimulation were not due to their differences in expression of leptin receptor ObR or IgE receptor FcεR1. ObR immunoblot analysis did not reveal any differences between WT and ob/ob BMMCs, before or after leptin induction (Figure S5A). FACS analysis also did not find any differences of FcεR1 expression on IgE-activated WT and ob/ob BMMCs (Figure S5B). Leptin neutralizing antibody dose-dependently blocked leptin-induced WT BMMC activation (Figure 6B). The same antibody also dose-dependently inhibited WT BMMC release of IFN-γ, inhibited ob/ob BMMC release of IL4, but increased WT BMMC release of IL4 (Figure 6C). These observations confirmed a direct action of leptin in inducing WT BMMC IFN-γ expression, suppressing WT BMMC IL4 expression, and enhancing ob/ob BMMC expression of IL4.
Figure 6.
Leptin stimulation of MCs and pro- and anti-inflammatory signaling and cytokine expression. A. ELISA determined culture medium histamine levels in WT and ob/ob BMMCs treated with or without leptin or IgE. ELISA determined medium histamine (B) and IFN-γ and IL4 levels (C) in WT and ob/ob BMMCs treated with and without leptin, anti-leptin antibody-treated leptin or control IgG1-treated leptin as indicated. D. Immunoblot of pro-inflammatory (left) and anti-inflammatory (right) signaling molecules in BMMCs from WT and ob/ob mice treated with or without leptin. E. Immunoblot of pro-inflammatory signaling molecules in BMMCs from WT and ob/ob mice treated with or without IgE. ELISA determined culture medium histamine (F) and IFN-γ and IL4 (G) in WT and ob/ob BMMCs treated with or without leptin, different signaling molecule inhibitors, or vehicle DMSO.
Consistent to above discussed activities of leptin on WT and ob/ob BMMC cytokine expression, we demonstrated corresponding changes in leptin signaling in ob/ob BMMCs vs. WT BMMCs. Leptin-induced phosphorylations of Syk, p38, ERK, JNK, AKT, IκB α, and p65 that are responsible for pro-inflammatory cytokine expression were all blunted in ob/ob BMMCs (Figure 6D, left). In contrast, leptin-induced phosphorylations of JAK1, STAT1, STAT5, and STAT6 that are responsible for anti-inflammatory cytokines were all enhanced in ob/ob BMMCs (Figure 6D, right). Such activities of ob/ob BMMCs were selective to leptin. IgE-induced phosphorylations of Syk, p38, ERK, JNK, and AKT in ob/ob BMMCs did not differ from those of WT BMMCs (Figure 6E). Consistent with these cell signaling changes, synthetic inhibitors for IκBα, ERK, p38, JNK, and STAT5 all inhibited WT BMMC degranulation (Figure 6F), but only inhibitors for ERK, p38, and JNK blocked WT BMMC expression of IFN-γ, and only STAT5 inhibitor blocked WT BMME IL4 production (Figure 6G). Under baselines without leptin stimulation, none of the tested cell signaling inhibitors affected WT and ob/ob BMMC release of IFN-γ, IL4, or histamine (Figure S5C).
Mast cell leptin deficiency does not affect CD4+ T-cell differentiation
T cells are the second most abundant inflammatory cells in WAT (Wu et al., 2007). Leptin deficiency did not affect T-cell influences on macrophage polarization (Figures S3C and S3D), but ob/ob BMMCs might affect T-cell differentiation in addition to macrophage polarization. To test this hypothesis, we cultured naïve CD4+ T cells with WT and ob/ob BMMCs using leptin as a positive control. Leptin increased Th1 differentiation (Mattioli et al., 2005; Lord et al., 1998; Martín-Romero et al., 2000; Fernández-Riejos et al., 2008; De Rosa et al., 2007) and reduced Th2 differentiation, but neither WT nor ob/ob BMMCs affected CD4+ T-cell differentiation (Figure S5D), excluding a direct role of ob/ob BMMCs on CD4+ T cells.
Low numbers of MCs in ob/ob mice contribute to obesity and diabetes in these mice
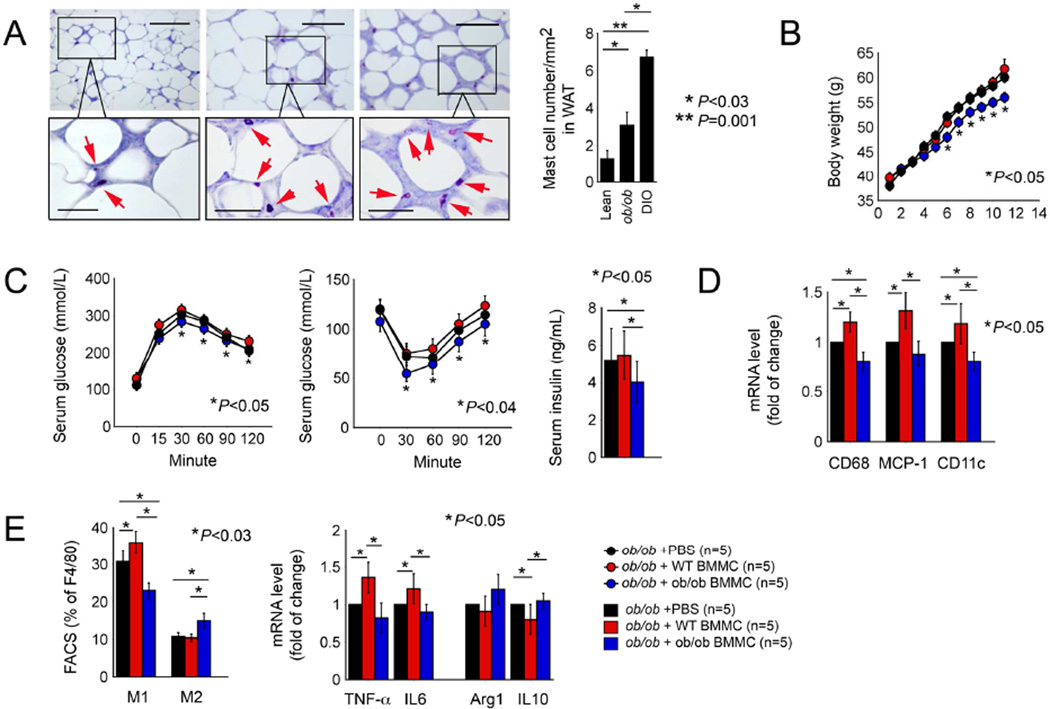
We previously demonstrated higher numbers of MCs in WAT from DIO mice than those in WAT from lean mice (Liu et al., 2009), whereas MCs in WAT, mesenteric and perinephric fat, skeletal muscle, and the liver, spleen, and thymus from ob/ob mice resembled those in lean mice (Altintas et al., 2012), suggesting MC deficiency in ob/ob mice. Using toluidine blue staining, we detected higher numbers of MCs in WAT from ob/ob mice than those in WAT from lean mice, but fewer than half of those in WAT from DIO mice (Figure 7A). Low numbers of these anti-inflammatory MCs may contribute to increased body weight gain in ob/ob mice. To test this hypothesis, we adoptively transferred 1×107ob/ob BMMCs or WT BMMCs to 5-week-old recipient ob/ob mice. Compared to those that received PBS, recipient ob/ob mice showed no differences in body weight gain (Figure 7B), glucose and insulin tolerances and serum insulin levels (Figure 7C) after receiving WT BMMCs. In contrast, one round of transfer of ob/ob BMMCs significantly reduced body weight gain, increased glucose and insulin tolerances, and reduced serum insulin levels (Figures 7B and 7C), demonstrating the ability of a few more ob/ob MCs to ameliorate obesity and diabetes in ob/ob mice. While adoptive transfer of WT BMMCs increased WAT expression of CD68, MCP-1, and CD11c, indicating increased WAT inflammation as expected, one round of administration of donor ob/ob BMMCs significantly reduced the expression of these inflammatory markers (Figure 7D). Consistent with our hypothesis, adoptive transfer of WT BMMCs increased WAT M1 cells as determined by FACS analysis, enhanced WAT expression of TNF-α and IL6, and decreased IL10 expression (Figure 7E). In contrast, adoptive transfer of ob/ob BMMCs significantly reduced WAT expression of all three inflammatory markers (CD68, MCP-1, and CD11c), reduced WAT M1 cell numbers and increased M2 numbers (Figure 7E). These data suggest that MC deficiency affects body weight gain in ob/ob mice. To probe this hypothesis further, we generated ob/ob KitW-sh/W-sh mice that lack MCs. Compared with male ob/ob mice that showed low numbers of MCs in WAT, genetic depletion of MCs in male ob/ob KitW-sh/W-sh mice significantly increased body weight gain, decreased glucose and insulin tolerance, increased VAT weight, and induced splenomegaly, likely because of increased inflammation, enlarged WAT adipocyte size, and increased WAT macrophage content (Figures S6A–S6E). Female mice yielded the same results (Figures S6F–S6H).
Figure 7.
Additional leptin-deficient MCs improve obesity and diabetes in ob/ob mice. A. Toluidine blue staining of MCs in WAT from lean, ob/ob, and DIO mice. Representative data are shown to the left. Scale: 200 µm. Inset scale: 50 µm. Body weight gain (B) and glucose tolerance, insulin tolerance, and serum insulin levels (C) in ob/ob mice received PBS, WT BMMCs, and ob/ob BMMCs. D. RT-PCR determined WAT expression of CD68, MCP-1, and CD11c. E. FACS analysis determined F4/80+CD11c+ M1 and F4/80+CD206+ M2 macrophages and RT-PCR determined M1 markers (TNF-α and IL6) and M2 markers (arginase-1 and IL10) in WAT from different mouse groups as indicated. Number of mice per group is indicated in the parenthesis.
DISCUSSION
This study revealed a previously untested function of leptin expression on MCs, originated from our initial observation that WAT from lean humans and mice expressed abundant leptin, but MCs from these WAT were leptin-deficient. Leptin deficiency blunted MC expression of pro-inflammatory cytokines and associated signaling pathways, but enhanced MC expression of anti-inflammatory cytokines and associated cell signaling. We demonstrated that it was the reduced pro-inflammatory cytokines and increased anti-inflammatory cytokines, but not leptin itself that converted leptin-deficient MCs into anti-inflammatory cells that carried unique activity in polarizing M2 macrophages, which predominate in lean WAT (Chawla et al., 2011; Lumeng et al., 2007; Liao et al., 2011). These activities of leptin-deficient MCs may contribute to the improved obesity and diabetes in DIO mice, genetic obese mice, and those with pre-established diseases, although additional mechanisms may also exist.
Leptin-deficient ob/ob BMMCs affected macrophage polarization not because these cells lack leptin, but rather because they have altered pro-inflammatory and anti-inflammatory cytokine expression profile due to the leptin lack. In macrophage polarization assays, with or without leptin did not affect the activity of ob/ob BMMCs in changing the macrophage expression of iNOS, TNF-α, Arg-1, and IL10 (Figure 4). The observations that Ifng− / − and Il6− / − BMMCs acted similarly to ob/ob BMMCs in macrophage polarization (Figures 5D and 5E) and that ob/ob BMMCs showed muted cell signaling for pro-inflammatory cytokine expressions but enhanced cell signaling for anti-inflammatory cytokine expression (Figures 6D and 6G) support this hypothesis. In IL4-treated macrophages, only ob/ob BMMCs, but not those from Ifng− / −, Il6− / −, and Il13− / −mice increased the expression of IL10 and Arg-1 (Figure 5E). Therefore, MC-derived IFN-γ and IL6 may explain reduced M1 macrophages by ob/ob BMMCs. Untested mediators other than IFN-γ, IL6, and IL13 from ob/ob BMMCs may contribute to enhanced macrophage IL4 expression and M2 macrophage polarization, a hypothesis that was not studied in this study.
Increased inflammation in WAT from obese humans and mice contributed to the elevated leptin expression of MCs in these tissues. MCs in obese WAT from humans and mice showed elevated leptin expression. MCs in bone marrow and BMMCs from DIO mice also expressed more leptin than those from lean mice. These observations may be explained by increased inflammation in DIO mice. In WAT from DIO mice, we detected much higher levels of pro-inflammatory IFN-γ and IL6 but lower anti-inflammatory IL4 and IL10 than those in WAT from lean mice (Figure 3G). High levels of WAT insulin, IL6, and TNF-α may all stimulate BMMC leptin expression (Figures 1K and 1L), consistent with prior studies that TNF-α stimulates adipose tissue leptin expression (Grunfeld et al., 1996) and circulates at lower concentrations in lean vs. obese subjects (Kern et al., 2001). It remains to be explained that obese humans and DIO mice have higher plasma leptin concentrations than do lean subjects (Meyers et al., 2008; Chaldakov et al., 2001), but we detected 2 to 3 fold more leptin in WAT from lean humans and mice than in WAT from obese subjects (Figures 1A and 1C). Leptin may exert different activities depending on the environment or target cell types. Indeed, we demonstrated that leptin induced MC expression of pro-inflammatory IFN-γ as we expected (Mattioli et al., 2005; Lord et al., 1998; Martín-Romero et al., 2000; Fernández-Riejos et al., 2008; De Rosa et al., 2007), but reduced MC expression of both pro-inflammatory IL6 and anti-inflammatory IL4 (Figures 5A and 5B). Therefore, detailed activities of leptin on MCs under different conditions merit in depth investigation.
Leptin deficiency did not affect IgE signaling. Leptin deficiency in MCs mutated the pro-inflammatory signaling pathway and cytokine production with concurrent increase of the anti-inflammatory signaling transduction and cytokine expression (Figures 6C, 6D and 6G). Such activity of leptin-deficient MCs did not necessarily associate with IgE-induced MC activation. Compared with those of WT BMMCs, IgE-induced histamine release in leptin-deficient MCs remained high (Figure 6A) and IgE signaling in ob/ob BMMCs was unaffected (Figure 6E).
Inflammation in WAT from DIO mice did not affect leptin expression in macrophages and T cells. Different from MCs, macrophages and T cells in lean and DIO WAT expressed similar level of leptin, suggesting that reduced leptin expression in lean WAT is MC-specific and does not apply to other immune cells. In CD4+ and CD8+ T cells, leptin enhances Th1 cytokine expression (IL2 and IFN-γ) and reduces Th2 cytokine expression (IL4 and IL10) (Lord et al., 1998; Martín-Romero et al., 2000). In WT BMMCs, leptin stimulated the signaling pathways that also enhance pro-inflammatory cytokine expression but reduced anti-inflammatory cytokine expression (Figures 6C, 6D and 6G). At this point, leptin acted similarly on T cells and MCs in controlling Th1 and Th2 cytokine expression. Leptin regulates Th1 immunity by promoting Th1 differentiation and proliferation and constraining Treg expansion (Mattioli et al., 2005; 2008; De Rosa et al., 2007), consistent with altered Th1 and Th2 cytokines (Lord et al., 1998; Martín-Romero et al., 2000). However, only ob/ob BMMCs, but not ob/ob CD4+ T cells or CD8+ T cells reduced the expression of BMDM M1 markers iNOS and TNF-α and increased the expression of BMDM M2 markers Arg-1 and IL10 (Figure 4, S3C, and S3D). Therefore, leptin deficiency in T cells may have different role from that in MCs. Further, MCs in bone marrow or differentiated from bone marrow from DIO mice expressed more leptin than those from lean mice, but liver MCs from lean or DIO mice had hardly detectable leptin (Figure 1J). Therefore, lower leptin expression in MCs from lean subjects than those from obese subjects may not apply to MCs in all organs, a phenomenon that remains to be further explained.
Despite these as yet unanswered questions, the present data provide mechanistic insight into heretofore unsuspected functions of MCs in obesity and diabetes, and point to approaches to modulate these prevalent diseases. Therefore, MCs may act differently in obesity and diabetes depending on their expression of leptin. A recent study, however, argues against this hypothesis (Gutierrez et al., 2015). Although MC-deficient KitW/ Wvmice had protection from DIO and glucose tolerance, MC-specific depletion by inserting Cre to the MC carboxypeptidase A3 gene in Cpa3cre/+ mice did not affect body weight gain and glucose tolerance in either DIO or genetic ob/ob obese mice. In recipient KitW/Wvmice, adoptive transfer of bone marrow cells from Cpa3cre/+ and Cpa3+/+ mice reversed equally reduced body weight and improved glucose tolerance. In WT mice on a C57BL/6 background, MC stabilization with disodium cromoglycate (DSCG) also did not affect body weight gain (Gutierrez et al., 2015). Yet this study may not exclude a role of MCs in obesity and diabetes. The Cre insertion to the carboxypeptidase A3 gene in Cpa3Cre/+mice possibly depleted other cells in addition to MCs that express the carboxypeptidase A3 gene, including basophils (Voehringer et al., 2004), some T cell progenitors and thymic T cells (Feyerabend et al., 2009; Taghon et al., 2007), and some hematopoietic progenitor cells (Franco et al., 2010). For example, obesity inversely correlates with the production of thymic T cells (Yoshida et al., 2014). Depletion of thymic T cells in Cpa3Cre/+ mice will likely enhance obesity. Although anemic KitW/W-v mice (Reber et al., 2012) also showed reduced obesity and glucose tolerance (Gutierrez et al., 2015), these mice also have reduced numbers of neutrophils by 50~80% (Chervenick & Boggs, 1969) and basophils (Lantz et al., 1998), besides a dearth of MCs. Reduction of these cells may all contribute to the reduced obesity and diabetes in KitW/W-v mice. For example, neutrophils can play an important role in obesity and diabetes (Talukdar et al., 2012). Despite this fact, donor bone marrow cells from Cpa3+/+ and Cpa3Cre/+ mice might correct MC deficiency in KitW/W-v mice. This bone marrow may concurrently correct the neutrophil reduction in these mice. Therefore, donor bone marrow from both Cpa3+/+ and Cpa3Cre/+ mice will likely increase obesity, if neutrophils remain essential in KitW/W-v mice. In contrast, KitW-sh/W-sh mice may have increased numbers of neutrophils and basophils (Grimbaldeston et al., 2005), and reduced obesity and diabetes can be fully reversed by WT BMMCs, but not those lacking MC inflammatory cytokines, excluding the possible role of cells other than MCs (Liu et al., 2009). Indeed, a recent randomized controlled study among obese patients with type 2 diabetes showed that MC inhibition with ketotifen reduced BMI, HbA1c, fasting blood glucose, blood total cholesterol, and low-density lipoprotein and triglycerides, but increased blood high-density lipoprotein and adiponectin (El-Haggar et al., 2015). These clinical data support a role of MCs in these metabolic diseases, although confirmation of these preliminary results requires more comprehensive clinical studies.
EXERIMENTAL PROCEDURES
Mice
WT (C57BL/6), Il6− / − (C57BL/6, N11), Ifng− / − (C57BL/6, N10), ob/ob (C57BL/6, N10), and db/db (C57BL/6, N22) mice were obtained from the Jackson Laboratories (Bar Harbor, ME). MC-deficient KitW-sh/W-sh (C57BL/6, N>15) and Il13− / − (C57BL/6, N11) have been described previously (Liu et al., 2009; Stanya et al., 2013). BMMCs were prepared by differentiating bone marrow cells in IL3 and stem cell factor as described previously (Liu et al., 2009). BMMC adoptive transfer was performed using 5- or 12 week-old male KitW-sh/W-sh, WT, or ob/ob mice by tail-vein injection (1×107 cells per mouse). Two weeks post-reconstitution, mice started consuming a Western diet (22.6% protein, 45.2% carbohydrate, 20.1% fat, and 1.25% cholesterol; Research Diets, New Brunswick, NJ) for 10 to 16 weeks. Body weight was monitored weekly. By the end of each course of Western diet consumption, we performed glucose tolerance and insulin tolerance assays as described (Liu et al., 2009). Mice were euthanized, blood samples were collected after intracardiac puncture on heparinized syringes, and subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), spleen, kidney, and liver were removed, weighed, and used for subsequent gene expression studies and histological and fluorescence-activated cell sorting (FACS) analyses. Mouse fasting plasma insulin was measured in pre-fasted mice (16 hours) using the Ultra Sensitive Insulin ELISA kit (Crystal Chem, Downers Grove, IL). Plasma leptin levels were measured using Bio-Plex Pros Assays (Bio-Rad, Hercules, CA). Mice were bred and maintained according to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health. Harvard Medical School Standing Committee on Animals approved all animal protocols.
BMMC cell signaling
BMMCs from WT and ob/ob mice were stimulated with leptin (50 ng/ml) or IgE (50 µg/ml) for 30 minutes, followed by immunoblot analysis to detect p-Syk (1:500, Abcam), p-JAK2 (1:500), p-STAT3 (1:500), p-STAT5 (1:500), p-STAT6 (1:500), p-AKT (1:500), p-ERK (1:500), p-JNK (1:500), p-IκBα (1:500), p-p38 (1:500), p-p65 (1:500), each non-phosphorylated proteins (1:500), and GAPDH (1:2000), all of which were purchased from Cell Signaling Technology (Danvers, MA).
BMMCs from WT and ob/ob mice were also used to detect histamine, IFN-γ, and IL4 productions with or without cell signaling inhibitors, including JAK inhibitor AG490 (10 µM), IκB inhibitorBAY11–7082 (1 µM), PI3K inhibitor LY294002 (5 µM), ERK inhibitor PD98059 (10 µM), p38 inhibitor SB203580 (7.5 µM), JNK inhibitor SP600125 (3 µM), and STAT5 inhibitor CAS285986-31-4 (1 µM). BMMC (106/well) were pretreated with these inhibitors for 1 h, followed by incubation with or without recombinant mouse leptin (50 ng/ml) for another 24 h. Cell culture medium were collected for ELISA to determine histamine (Eagle bisciences, Inc. Nashua, NH), IFN-γ (eBioscience), and IL4 (eBioscience), according to manufacturers’ instructions.
For in vitro leptin blocking experiments, leptin (50 ng/ml) was pre-incubated with or without neutralization antibody (0.1–10 µg/ml) for 15 minutes and then incubated with BMMCs from WT and ob/ob mice for 48 h. Cell culture medium were collected for ELISA to determine histamine, IFN-γ, and IL4.
Statistical analyses
All data from humans, mice, or cell cultures were expressed as means±SEM. A nonparametric Mann-Whitney test was used for statistical analysis, followed by a post-hoc Bonferroni correction. P < 0.05 was considered statistically significant.
Supplementary Material
Highlights.
Mast cells in WAT from lean humans and mice are leptin-deficient
Reconstitution of leptin-deficient mast cells mitigates obesity and diabetes in mice
Leptin-deficient mast cells promote M2 macrophage polarization
Leptin-deficient mast cells slant anti-inflammatory signaling in response to leptin
In Brief.
Pro-inflammatory mast cells play detrimental roles in obesity and diabetes. Zhou et al. show that mast cells in white adipose tissues from lean humans and mice are leptin-deficient and polarize macrophages towards anti-inflammatory M2 phenotype. Adoptive transfer of leptin-deficient mast cells mitigates obesity and diabetes in obese mice.
ACKNOWLEDGEMENTS
The authors thank Ms. Chelsea Swallom for her editorial assistance. This study is supported by National Institutes of Health grants HL60942, HL81090, HL123568 (GPS), HL48743, and HL080472 (PL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplementary information includes Supplementary Methods and ten Supplementary Figures.
AUTHOR CONTRIBUTIONS
Y.Z., X.Y., H.C., S.S., J.R., L.Z., A.H.I., F.B., C.L., J.L., J.T., K.C., C.H.L., and G.S.H. performed the experiments, provided materials, and analyzed the data. P.L. and G.P.S. design the experiments and wrote the manuscript.
REFERENCES
- Altintas MM, Nayer B, Walford EC, Johnson KB, Gaidosh G, Reiser J, De La Cruz-Munoz N, Ortega LM, Nayer A. Leptin deficiency-induced obesity affects the density of mast cells in abdominal fat depots and lymph nodes in mice. Lipids Health Dis. 2012;11:21. doi: 10.1186/1476-511X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra A, Okur B, Glauben R, Erben U, Ihbe J, Stroh T, Fedke I, Chang HD, Zeitz M, Siegmund B. Leptin: a critical regulator of CD4+ T-cell polarization in vitro and in vivo. Endocrinology. 2010;151:56–62. doi: 10.1210/en.2009-0565. [DOI] [PubMed] [Google Scholar]
- Chaldakov GN, Fiore M, Stankulov IS, Hristova M, Antonelli A, Manni L, Ghenev PI, Angelucci F, Aloe L. NGF, BDNF, leptin, and mast cells in human coronary atherosclerosis and metabolic syndrome. Arch. Physiol. Biochem. 2001;109:357–360. doi: 10.1076/apab.109.4.357.4249. [DOI] [PubMed] [Google Scholar]
- Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervenick PA, Boggs DR. Decreased neutrophils and megakaryocytes in anemic mice of genotype W/W. J Cell Physiol. 1969;73:25–30. doi: 10.1002/jcp.1040730104. [DOI] [PubMed] [Google Scholar]
- DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc. Natl. Acad. Sci. U. S. A. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- El-Haggar SM, Farrag WF, Kotkata FA. Effect of ketotifen in obese patients with type 2 diabetes mellitus. J Diabetes Complications. 2015;29:427–432. doi: 10.1016/j.jdiacomp.2015.01.013. [DOI] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Matarese G, Lord GM, Keogh JM, Lawrence E, Agwu C, Sanna V, Jebb SA, Perna F, Fontana S, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Invest. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenger RV, Linneberg A, Vidal C, Vizcaino L, Husemoen LL, Aadahl M, Gonzalez-Quintela A. Determinants of serum tryptase in a general population: the relationship of serum tryptase to obesity and asthma. Int. Arch. Allergy Immunol. 2012;157:151–158. doi: 10.1159/000327535. [DOI] [PubMed] [Google Scholar]
- Fernandez-Riejos P, Goberna R, Sanchez-Margalet V. Leptin promotes cell survival and activates Jurkat T lymphocytes by stimulation of mitogen-activated protein kinase. Clin. Exp. Immunol. 2008;151:505–518. doi: 10.1111/j.1365-2249.2007.03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyerabend TB, Terszowski G, Tietz A, Blum C, Luche H, Gossler A, Gale NW, Radtke F, Fehling HJ, Rodewald HR. Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity. 2009;30:67–79. doi: 10.1016/j.immuni.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Franco CB, Chen CC, Drukker M, Weissman IL, Galli SJ. Distinguishing mast cell and granulocyte differentiation at the single-cell level. Cell Stem Cell. 2010;6:361–368. doi: 10.1016/j.stem.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. Am J Pathol. 2005;167:835–848. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen ML, Hao M, Piston DW, Hasty AH. Leptin requires canonical migratory signaling pathways for induction of monocyte and macrophage chemotaxis. Am. J. Physiol. Cell Physiol. 2007;293:C1481–C1488. doi: 10.1152/ajpcell.00062.2007. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J. Clin. Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez DA, Muralidhar S, Feyerabend TB, Herzig S, Rodewald HR. Hematopoietic Kit Deficiency, rather than Lack of Mast Cells, Protects Mice from Obesity and Insulin Resistance. Cell Metab. 2015;21:678–691. doi: 10.1016/j.cmet.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Kato H, Ueki S, Kamada R, Kihara J, Yamauchi Y, Suzuki T, Takeda M, Itoga M, Chihara M, Ito W, et al. Leptin has a priming effect on eotaxin-induced human eosinophil chemotaxis. Int. Arch. Allergy Immunol. 2011;155:335–344. doi: 10.1159/000321195. [DOI] [PubMed] [Google Scholar]
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2001;280:E745–E751. doi: 10.1152/ajpendo.2001.280.5.E745. [DOI] [PubMed] [Google Scholar]
- Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- Liao X, Sharma N, Kapadia F, Zhou G, Lu Y, Hong H, Paruchuri K, Mahabeleshwar GH, Dalmas E, Venteclef N, et al. Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 2011;121:2736–2749. doi: 10.1172/JCI45444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Romero C, Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell. Immunol. 2000;199:15–24. doi: 10.1006/cimm.1999.1594. [DOI] [PubMed] [Google Scholar]
- Matarese G, Procaccini C, De Rosa V. At the crossroad of T cells, adipose tissue, and diabetes. Immunol. Rev. 2012;249:116–134. doi: 10.1111/j.1600-065X.2012.01154.x. [DOI] [PubMed] [Google Scholar]
- Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17:851–859. doi: 10.1016/j.cmet.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli B, Giordani L, Quaranta MG, Viora M. Leptin exerts an anti-apoptotic effect on human dendritic cells via the PI3K–Akt signaling pathway. FEBS Lett. 2009;583:1102–1106. doi: 10.1016/j.febslet.2009.02.029. [DOI] [PubMed] [Google Scholar]
- Mattioli B, Straface E, Quaranta MG, Giordani L, Viora M. Leptin promotes differentiation and survival of human dendritic cells and licenses them for Th1 priming. J Immunol. 2005;174:6820–6828. doi: 10.4049/jimmunol.174.11.6820. [DOI] [PubMed] [Google Scholar]
- Meyers JA, Liu AY, McTiernan A, Wener MH, Wood B, Weigle DS, Sorensen B, Chen-Levy Z, Yasui Y, Boynton A, et al. Serum leptin concentrations and markers of immune function in overweight or obese postmenopausal women. J. Endocrinol. 2008;199:51–60. doi: 10.1677/JOE-07-0569. [DOI] [PubMed] [Google Scholar]
- Moreno M, Puig J, Serrano M, Moreno-Navarrete JM, Ortega F, Ricart W, Fernandez-Real JM. Circulating tryptase as a marker for subclinical atherosclerosis in obese subjects. PloS one. 2014;9:97014. doi: 10.1371/journal.pone.0097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris DL, Cho KW, Delproposto JL, Oatmen KE, Geletka LM, Martinez-Santibanez G, Singer K, Lumeng CN. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottonello L, Gnerre P, Bertolotto M, Mancini M, Dapino P, Russo R, Garibotto G, Barreca T, Dallegri F. Leptin as a uremic toxin interferes with neutrophil chemotaxis. J. Am. Soc. Nephrol. 2004;15:2366–2372. doi: 10.1097/01.ASN.0000139321.98029.40. [DOI] [PubMed] [Google Scholar]
- Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat. Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of CD11c–positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalho R, Almeida J, Beltrao M, Pirraco A, Costa R, Sokhatska O, Guardao L, Palmares C, Guimaraes JT, Delgado L, et al. Neurogenic inflammation in allergen-challenged obese mice: A missing link in the obesity-asthma association? Exp. Lung Res. 2012;38:316–324. doi: 10.3109/01902148.2012.699589. [DOI] [PubMed] [Google Scholar]
- Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–625. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell. Immunol. 1999;194:6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes. 2010;59:1171–1181. doi: 10.2337/db09-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanya KJ, Jacobi D, Liu S, Bhargava P, Dai L, Gangl MR, Inouye K, Barlow JL, Ji Y, Mizgerd JP, et al. Direct control of hepatic glucose production by interleukin-13 in mice. J. Clin. Invest. 2013;123:261–271. doi: 10.1172/JCI64941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens EC, Rosenthal NS. Bone marrow mast cell morphologic features and hematopoietic dyspoiesis in systemic mast cell disease. Am J Clin Pathol. 2001;116:177–182. doi: 10.1309/Q2WJ-46CL-YRFT-M5JF. [DOI] [PubMed] [Google Scholar]
- Suzukawa M, Nagase H, Ogahara I, Han K, Tashimo H, Shibui A, Koketsu R, Nakae S, Yamaguchi M, Ohta K. Leptin enhances survival and induces migration, degranulation, and cytokine synthesis of human basophils. J. Immunol. 2011;186:5254–5260. doi: 10.4049/jimmunol.1004054. [DOI] [PubMed] [Google Scholar]
- Taghon T, Yui MA, Rothenberg EV. Mast cell lineage diversion of T lineage precursors by the essential T cell transcription factor GATA-3. Nat Immunol. 2007;8:845–855. doi: 10.1038/ni1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiotra PC, Boutati E, Dimitriadis G, Raptis SA. High insulin and leptin increase resistin and inflammatory cytokine production from human mononuclear cells. Biomed Res. Int. 2013;2013:487081. doi: 10.1155/2013/487081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D, Shinkai K, Locksley RM. Type 2 immunity reflects orchestrated recruitment of cells committed to IL-4 production. Immunity. 2004;20:267–277. doi: 10.1016/s1074-7613(04)00026-3. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW., Jr CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J. Clin. Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Ghosh S, Perrard XD, Feng L, Garcia GE, Perrard JL, Sweeney JF, Peterson LE, Chan L, Smith CW, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JM, Shi GP. Emerging role of mast cells and macrophages in cardiovascular and metabolic diseases. Endocr Rev. 2012;33:71–108. doi: 10.1210/er.2011-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying W, Cheruku PS, Bazer FW, Safe SH, Zhou B. Investigation of macrophage polarization using bone marrow derived macrophages. J. pVis. Exp. 2013:76. doi: 10.3791/50323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Nakashima E, Kubo Y, Yamaoka M, Kajimura J, Kyoizumi S, Hayashi T, Ohishi W, Kusunoki Y. Inverse associations between obesity indicators and thymic T-cell production levels in aging atomic-bomb survivors. PLoS One. 2014;9:91985. doi: 10.1371/journal.pone.0091985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XF, Hong YX, Feng GJ, Zhang GF, Rogers H, Lewis MA, Williams DW, Xia ZF, Song B, Wei XQ. Lipopolysaccharide-induced M2 to M1 macrophage transformation for IL-12p70 production is blocked by Candida albicans mediated up-regulation of EBI3 expression. PloS one. 2013;8:63967. doi: 10.1371/journal.pone.0063967. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.