Abstract
Rationale
The lack of measurable single cell contractility of human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs) currently limits the utility of hiPSC-CMs for evaluating contractile performance for both basic research and drug discovery.
Objective
To develop a culture method that rapidly generates contracting single hiPSC-CMs and allows quantification of cell shortening with standard equipment used for studying adult cardiac myocytes (CMs).
Methods and Results
Single hiPSC-CMs were cultured for 5 – 7 days on a 0.4 – 0.8 mm thick mattress of undiluted Matrigel (“mattress hiPSC-CM”) and compared to hiPSC-CMs maintained on control substrate (<0.1 mm thick 1:60 diluted matrigel, “control hiPSC-CM”). Compared to control hiPSC-CM, mattress hiPSC-CMs had more rod-shape morphology and significantly increased sarcomere length. Contractile parameters of mattress hiPSC-CMs measured with video-based edge detection was comparable to that of freshly isolated adult rabbit ventricular CMs. Morphological and contractile properties of mattress hiPSC-CM were consistent across cryopreserved hiPSC-CMs generated independently at another institution. Unlike control hiPSC-CM, mattress hiPSC-CMs display robust contractile responses to positive inotropic agents such as myofilament calcium sensitizers. Mattress hiPSC-CMs exhibit molecular changes that include increased expression of the maturation marker cardiac troponin I and significantly increased action potential upstroke velocity due to a 2-fold increase in sodium current (INa).
Conclusions
The Matrigel mattress method enables the rapid generation of robustly contracting hiPSC-CMs and enhances maturation. This new method allows quantification of contractile performance at the single cell level, which should be valuable to disease modeling, drug discovery and preclinical cardiotoxicity testing.
Keywords: Contractility, induced pluripotent stem cells, maturation, cardiac, stem cell, contraction, excitation-contraction coupling, cardiac function
INTRODUCTION
Given species-differences in cardiac myocyte (CM) physiology, human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CMs) are gaining recognition for their potential in human heart disease modeling, preclinical cardiotoxicity evaluation and drug discovery.1 Realization of this potential depends on the ability to assess excitation-contraction (E-C) coupling including contractile properties of individual hiPSC-CMs. To date, most functional evaluations of isolated hiPSC-CMs have focused on electrophysiology2 and calcium (Ca) handling3 measurements, while contractile properties have not been routinely evaluated due to limited cell shortening of hiPSC-CMs cultured under standard conditions. In principle, a variety of biophysical techniques such as atomic force microscopy, traction force microcopy, and micropost deflection are available to assess mechanical properties of isolated hiPSC-CMs; however, because these do not address the issue of limited cell shortening, they have not been widely adopted.4–7 Alternatively, engineered heart tissues (EHTs) can be utilized to assess contractile properties of hiPSC-CMs or mixtures of hiPSC-CMs and support cells8–10; however, compared to hiPSC-CMs, EHTs are difficult and expensive to generate and do not allow simultaneous assessment of contractility and Ca handling at the single cell level. Here, we report a simple and rapid method that generates rod-shaped hiPSC-CMs with aligned myofilaments, which exhibit features of physiological and molecular maturation, and robust cell shortening with each cardiac cycle, thereby enabling straightforward contractility assessment and hence E-C coupling analysis.
METHODS
An expanded Methods section is provided in the Online Data Supplement.
Preparation of Matrigel Mattress substrates
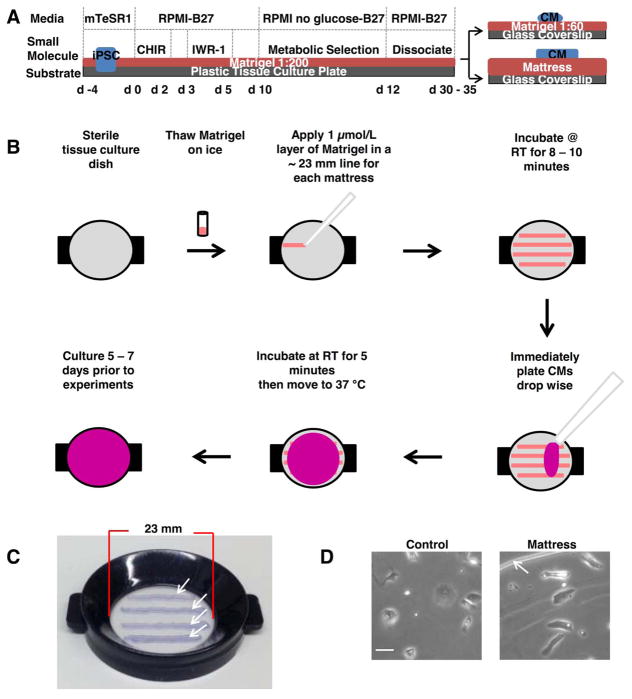
Matrigel mattress platforms were prepared the same day of CM dissociation and seeding. Briefly, mattresses were arrayed on a glass coverslip (Corning) or Delta TPG Dish (Fisher Scientific). Application of 1 μL lines of completely thawed, ice cold, undiluted growth factor-reduced Matrigel [8 – 12 mg/mL] (Corning) were arrayed in parallel. Matrigel was evenly pipetted at a 45 degree angle (Figure 1B) with a P2 pipet and corresponding tip. The mattresses were allowed to incubate for 8 – 10 minutes at room temperature at which point 200 μL of RPMI 1640 medium, 2 % B-27 supplement (Invitrogen), and 1 % Pen-Strep (Life Technologies), with the addition of 40,000 CMs were immediately added, halting mattress polymerization. Each mattress had a width of approximately 0.85 mm. The thickness of each mattress was found to be in the range of 0.4 – 0.8 mm and each line was approximately 23 mm long.
Figure 1. Overview of Matrigel mattress method.
A, Schematic of cardiac induction and Matrigel mattress method plating protocol. B, Matrigel mattress method work flow. C, Matrigel mattresses plated on Delta TPG Dish (Fisher Scientific), white arrows indicate edge of each mattress, blue dye included to visualize mattress. D, hiPSC-CMs plated on control (left panel) and mattress (right panel). Scale bar 50 μm. White arrows indicate edge of mattress platform.
RESULTS
HiPSC-CMs are routinely cultured on substrates such as gelatin or Matrigel, a commercially available extracellular matrix (ECM) preparation11 (Figure 1A). However, under these standard culturing conditions, hiPSC-CMs typically display variable morphology without a dominant axis of myofibril alignment, which is different from freshly-isolated mature adult CMs. As a result, individual hiPSC-CMs cultured under traditional methods exhibit limited cellular shortening (Online Video I). We hypothesized that hiPSC-CMs may be induced to shorten if maintained on a culture substrate with suitable biophysical and biochemical properties12. Of a variety of culture substrates tested,13 we discovered that culturing single hiPSC-CMs on undiluted Matrigel with a thickness of at least 0.4 mm, which we term “Matrigel mattress,” promoted myofibril alignment (Online Figure I), a rod-like cell shape (Online Figure II and Online time-lapse movie) and robust hiPSC-CM shortening (Online Video 2). In this method, hiPSC-CMs are first generated by small molecule-based cardiac differentiation2,14 and maintained on standard cardiac induction substrate. Then on day 30 – 35 of cardiac differentiation, single hiPSC-CMs are dissociated and re-plated on Matrigel mattress, and cultured for an additional 5 – 7 days prior to analysis (Figure 1A,B). Of hiPSC-CMs cultured in the standard manner (e.g., Matrigel 1:60 dilution),11 only a small fraction (3.3 ± 0.5 %) of cells exhibited visible cell contractions. In contrast, essentially all (91.0 ± 5.0 %) hiPSC-CMs maintained on Matrigel mattress, henceforth referred as “mattress hiPSC-CMs,” exhibited visible cellular contractions (P < 0.0001, Figure 2C, lower panel). Mattress hiPSC-CM reached peak contractility after 3 days on the mattress and these properties remained stable for up to 14 days at which point the matrigel loses its integrity. When quantitated with video-based edge detection, spontaneously-contracting mattress hiPSC-CMs exhibited ~ 9.0 ± 1.5 % cell shortening compared to 0.4 ± 0.2 % for control hiPSC-CMs (P < 0.0001, Online Table I). Cell shortening of this magnitude has previously been reported only for late-stage hiPSC-CMs after 80 – 120 days in culture.15
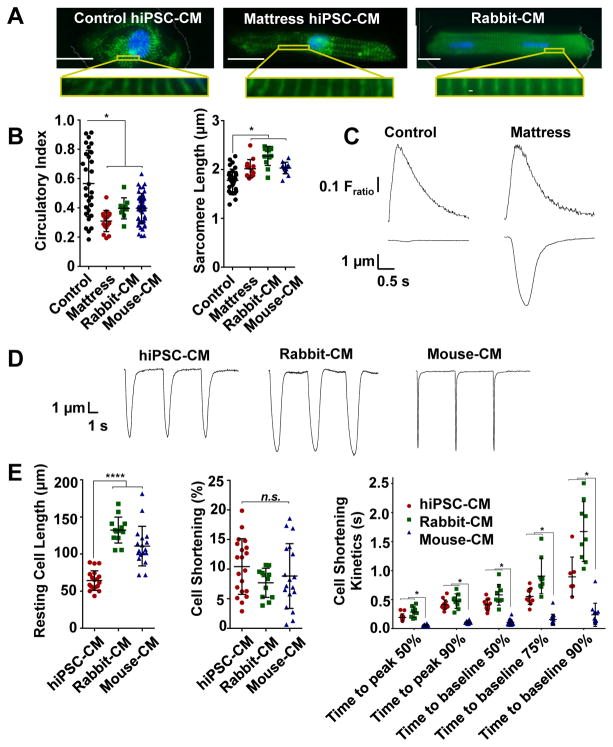
Figure 2. Matrigel mattress morphometry and contractility.
A, Immunofluorescence staining of cardiac myocyte (CM) structural marker α-actinin (green) and nucleus (blue, DAPI) for control human induced pluripotent stem cell-derived cardiac myocytes (hiPSC-CM) (left), mattress hiPSC-CM (center) and rabbit-CM (right). Scale bar 20 μm. 63X. B, Cell morphometry measuring circularity index (left) and sarcomere length (right) are plotted for indicated CMs. Error bars, s.d. (n = 9 – 48 cells per group). C, Representative control and mattress hiPSC-CM Ca transients (upper panel) and contraction traces (lower panel), spontaneously contracting. D, Representative contraction traces for indicated CM. (0.2 Hz). E, Contractility assessment measuring resting cell length (left), cell shortening (center) and cell shortening kinetics (right) are plotted. Error bars, s.d. (n = 6 – 20 cells per group) **** P< 0.0001 vs hiPSC-CM. *P<0.05 vs mouse-CM. n.s., Not significant.
We next compared mattress hiPSC-CMs to acutely-isolated rabbit and mouse CMs. Strikingly, mattress hiPSC displayed comparable cell shortening (Figure 2D,E) despite an overall shorter resting cell length. Contractile kinetics (i.e., time to peak and time to baseline) of mattress hiPSC-CMs were more similar to those of rabbit-CMs, which are thought to be a closer model of human-CMs than mouse-CMs.16 These contractile properties were replicated across multiple independently generated hiPSC-CM lines including post recovery from cryopreservation (Online Table I). Cell shortening of mattress hiPSC-CM was associated with acquisition of morphological features21 comparable to that of freshly isolated adult-CMs including cell elongation, decreased circularity (Figure 2A,B) and increased sarcomere length relative to control hiPSC-CMs (Figure 2B and Online Figure I). The position on the mattress (i.e., center versus edge) had no significant effect on the hiPSC-CMs shape. The contraction force produced by individual hiPSC-CMs on mattress can be calculated from the cell length, cell shortening and the elastic modulus of the matrigel mattress (5.8 kPa, Online Figure III E). Mattress hiPSC-CMs exhibit an average contraction force of 0.3 ± 0.1 mN per mm2 cross-sectional area. The calculated force values based on our cell shortening measurements were closely correlated with values measured independently in the same mattress hiPSC-CMs using traction force microscopy (Online Figure III).
We then examined whether culturing hiPSC-CMs on a Matrigel mattress affected key aspects of E-C coupling such as intracellular Ca handling, electrophysiology, and CM-specific molecular profile. We found Ca handling properties were not significantly different between control and mattress hiPSC-CMs (Figure 2C, upper panel, and Online Table II), and hiPSC-CMs maintained under both conditions displayed robust caffeine-releasable Ca release from sarcoplasmic reticulum (SR) stores. However, compared to control hiPSC-CMs, mattress hiPSC-CMs displayed a modest increase in maximum return velocity of twitch Ca transient and faster caffeine-induced Ca decay. Additionally, electrophysiological parameters of mattress and control hiPSC-CM were similar (Online Figure IV). For instance, the amplitude and mean diastolic potential of both control and mattress hiPSC-CMs were comparable to freshly isolated rabbit-CMs. Of note, however, the upstroke velocity of mattress hiPSC-CMs was markedly greater than that of control hiPSC-CMs (Online Table III), a finding that is consistent with the over two-fold higher sodium current density of mattress compared to control hiPSC-CM (Online Figure V D). Finally, transcriptional profiling of a subset of cardiac genes involved in E-C coupling (Online Figure V A) showed differential expression in mattress hiPSC-CMs. For instance, expression of TNNI3, encoding cardiac troponin I (cTnI), a CM maturation marker which is minimally expressed in hiPSC-CMs maintained under traditional conditions17, was markedly elevated in mattress hiPSC-CMs. Western blot analysis of cTnI and slow skeletal troponin I (ssTnI), the fetal isoform predominant in traditional hiPSC-CMs, confirmed that mattress hiPSC-CMs exhibited higher cTnI levels in conjunction with a stoichiometric reduction in ssTnI levels (Online Figure VI B,C).
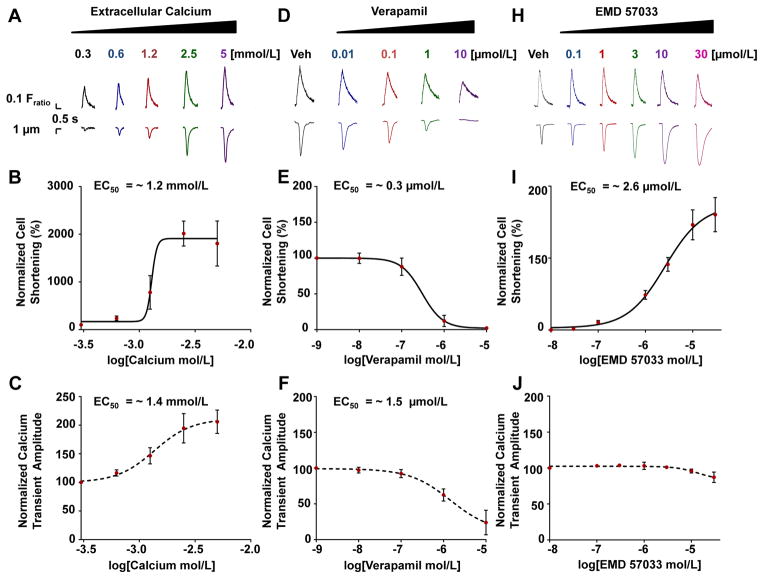
Since culturing hiPSC-CMs on Matrigel mattress permits simultaneous analysis of Ca handling and contractility at the single cell level, we next examined the E-C coupling in response to pharmacological interventions (Figure 3). Mattress hiPSC-CMs increased contractility to inotropic interventions of increasing extracellular Ca, with an EC50 (i.e., effective concentration yielding 50% of maximal response) of ~ 1.2 mmol/L10 (Figure 3 A–C). Treatment with the negative inotrope Verapamil, an L-type Ca channel antagonist, led to a concentration-dependent decrease in cellular contraction with an EC50 of ~ 0.3 μmol/L10 (Figure 3 D–F) Treatment with the myofilament Ca sensitizer EMD57033 led to a concentration-dependent increase in contraction with an EC50 of ~ 2.6 μmol/L18 (Figure 3 H–J) with negligible effects on the intracellular Ca transient amplitude (Figure 3J). To our knowledge, this is the first direct demonstration that individual hiPSC-CMs are capable of altering contractility in response to changes in myofilament Ca sensitivity, a finding that previously could not be observed using hiPSC-CMs maintained on traditional substrates or by evaluating changes in Ca handling alone (Figure 3J).
Figure 3. Pharmacological analysis.
(A, D and H) Representative Ca transients (upper panel) and contraction traces (lower panel) treated with indicated compound. Contraction and Ca concentration-response curves. (B and C) Extracellular Ca (Hill slope = 18.7 and 2.6 respectively). (E and F) Verapamil (Hill slope = −1.8 and −0.9 respectively). (I and J) EMD57033 (Hill slope = 1.1). (n = 5 – 20 cells per group). EC50, effective concentration 50 % of max response. Veh, vehicle.
DISCUSSION
Here, we demonstrate that culturing hiPSC-CMs on Matrigel mattress for 5 – 7 days leads to relative morphological, molecular, and functional maturation, enabling robust cell shortening in a much shorter time frame than traditional methods such as EHT or long-term culture.15,22 It is worth noting that mattress hiPSC-CMs exhibit an average contraction force per cross-sectional area that is comparable to that of EHTs made from hiPSC.22 Since hiPSC-CMs on Matrigel mattress and standard culture substrate showed similar Ca handling (Figure 2 C upper panel and Online Table 2), our results suggest that the enhanced sarcomere alignment along the long axis of the cell (Figure 2 A and online Figure I) is a primary mechanism for the enhanced contractile response of mattress hiPSC-CM. Other factors such as the lower substrate stiffness of the mattress are likely contributory and will need to be explored in future studies. While other techniques such as microcontact printing have been shown to improve sarcomere alignment and direct hiPSC-CMs towards a mature-like phenotype with contractile function,22,23 our Matrigel mattress method enables rapid assessment of contractile properties and Ca handling at the single cell level using standard equipment available in any lab studying adult primary cardiac myocytes. The Matrigel mattress method can also serve as a foundation for development of a high-throughput multiplexed platform to simultaneously monitor Ca handling and contractility of individual patient-derived hiPSC-CMs. Since Matrigel is commercially available with little apparent lot-to-lot variability, easily prepared, and requires no additional functionalization, we anticipate that the present method can be immediately adopted for a wide spectrum of applications including disease modeling, drug discovery and preclinical cardiotoxicity evaluation.
Supplementary Material
Novelty and Significance.
What Is Known?
Single human induced pluripotent stem cell derived cardiac myocyte (hiPSC-CMs) under standard culture conditions display limited changes in cellular geometry (i.e., cell shortening) with each cardiac cycle.
Existing techniques to assess hiPSC-CM contractility are expensive, time consuming and technically challenging. As a result, investigations of hiPSC-CM function have primarily focused on their electrophysiological and Ca handling properties.
What New Information Does This Article Contribute?
Culturing hiPSC-CM on commercially available Matrigel Mattress rapidly (3 days) promotes cell elongation, sarcomere alignment and single cell contractility.
Mattress hiPSC-CM display single cell shortening properties comparable to that of acutely-isolated adult rabbit CM
Mattress hiPSC-CMs display positive inotropic response to myofilament Ca sensitizer, EMD57033 with negligible effect on Ca handling.
Current methods to evaluate contractile properties of single hiPSC-CMs are difficult because single hiPSC-CM cultured on standard conditions largely lack contractile responses. Here, we establish a new culture method “the Matrigel Mattress” that rapidly (3 days) generates elongated hiPSC-CMs that exhibit robust cell shortening. Using this method, we characterized the contractile properties of hiPSC-CMs and their response to positive and negative inotropic agents. We show that hiPSC-CMs from multiple laboratories have comparable contractile properties, even after recovery from cryopreservation. We further demonstrate that these contractile properties are comparable to adult rabbit ventricular cardiac myocytes. The Matrigel method can be readily implemented for basic science studies, drug discovery and preclinical cardiac safety assessment.
Acknowledgments
The CC2 hiPSC line was a generous gift from Drs. Aaron Bowman, Kevin Ess and Dina Neely at Vanderbilt University. This work was supported by the United States National Institutes of Health (NIH) R01HL088635, R01HL071670 and R01HL0108173 (B.C.K.), R01HL0104040 (C.C.H.), R01ES016931 (Aaron Bowman), and R01NS078289 (Kevin C. Ess); by the American Heart Association (AHA) 13IRG13680003 (B.C.K.), 12SDG12050597 (H.S.H.), 12POST12080080 (D.K.), and 14POST19550002 (J.E.H.); and by the Veterans Administration Merit Grant BX000771 (C.C.H.).
SOURCES OF FUNDING
This work was partly supported by the United States National Institutes of Health [HL88635, HL71670 & HL108173 to B.C.K.; NIH HL104040 to C.C.H.]; by the American Heart Association [13IRG13680003 to B.C.K., 12SDG12050597 to H.S.H., 12POST12080080 to D.K.], and by the Veterans Administration Merit Grant BX000771 to C.C.H.
Nonstandard Abbreviations and Acronyms
- CM
Cardiac myocyte
- hiPSC
Human induced pluripotent stem cell
- E-C
Excitation-contraction
- Ca
Calcium
- EHT
Engineered heart tissue
- ECM
Extracellular matrix
- SR
Sarcoplasmic reticulum
- cTnI
Cardiac troponin I
- ssTnI
Slow skeletal troponin I
- EC50
Half maximal effective concentration
Footnotes
DISCLOSURES
J.C.W. is a co-founder of Stem Cell Theranostics. All other authors declare no conflict of interest.
References
- 1.Hayakawa T, Kunihiro T, Ando T, Kobayashi S, Matsui E, Yada H, Kanda Y, Kurokawa J, Furukawa T. Image-based evaluation of contraction–relaxation kinetics of human-induced pluripotent stem cell-derived cardiac myocytes: Correlation and complementarity with extracellular electrophysiology. J Mol Cell Cardiol. 2014;77:178–191. doi: 10.1016/j.yjmcc.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Burridge PW, Matsa E, Shukla P, et al. Chemically defined generation of human cardiac myocytes. Nat Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang HS, Kryshtal DO, Feaster TK, Sánchez-Freire V, Zhang J, Kamp TJ, Hong CC, Wu JC, Knollmann BC. Comparable calcium handling of human iPSC-derived cardiac myocytes generated by multiple laboratories. J Mol Cell Cardiol. 2015;85:79–88. doi: 10.1016/j.yjmcc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez ML, Graham BT, Pabon LM, Han SJ, Murry CE, Sniadecki NJ. Measuring the contractile forces of human induced pluripotent stem cell-derived cardiac myocytes with arrays of microposts. J Biomech Eng. 2014;136:051005. doi: 10.1115/1.4027145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahola A, Kiviaho AL, Larsson K, Honkanen M, Aalto-Setälä K, Hyttinen J. Video image-based analysis of single human induced pluripotent stem cell derived cardiac myocyte beating dynamics using digital image correlation. Biomed Eng Online. 2014;13:39. doi: 10.1186/1475-925X-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Sun N, Bruce MA, Wu JC, Butte MJ. Atomic Force Mechanobiology of Pluripotent Stem Cell-Derived Cardiac myocytes. PLoS One. 2012;7:e37559. doi: 10.1371/journal.pone.0037559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, Delgado SM, Wakatsuki T, Crone WC, Pruitt BL, Palecek SP. Effects of Substrate Mechanics on Contractility of Cardiac myocytes Generated from Human Pluripotent Stem Cells. Int J Cell Biol. 2012;2012:508294. doi: 10.1155/2012/508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13:3599–3608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costa KD, Lee EJ, Holmes JW. Creating Alignment and Anisotropy in Engineered Heart Tissue: Role of Boundary Conditions in a Model Three-Dimensional Culture System. Tissue Eng. 2003;9:567–577. doi: 10.1089/107632703768247278. [DOI] [PubMed] [Google Scholar]
- 10.Stoehr A, Neuber C, Baldauf C, Vollert I, Friedrich FW, Flenner F, Carrier L, Eder A, Schaaf S, Hirt MN, Aksehirlioglu B, Tong CW, Moretti A, Eschenhagen T, Hansen A. Automated analysis of contractile force and Ca2+ transients in engineered heart tissue. Am J Physiol Heart Circ Physiol. 2014;306:H1353–1363. doi: 10.1152/ajpheart.00705.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma A, Marceau C, Hamaguchi R, et al. Human Induced Pluripotent Stem Cell–Derived Cardiac myocytes as an In Vitro Model for Coxsackievirus B3–Induced Myocarditis and Antiviral Drug Screening Platform. Circ Res. 2014;115:556–566. doi: 10.1161/CIRCRESAHA.115.303810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Tsai P, Wieder ED, Kribben A, Van Putten V, Schrier RW, Nemenoff RA. Vascular Smooth Muscle Cells Grown on Matrigel. J Biol Chem. 1994;269:19653–19658. [PubMed] [Google Scholar]
- 13.Chun YW, Balikov DA, Feaster TK, Williams CW, Sheng CC, Lee JB, Boire TC, Neely MD, Bellan LM, Ess KC, Bowman AB, Sung HJ, Hong CC. Combinatorial polymer matrices enhance in vitro maturation of human induced pluripotent stem cell-derived cardiac myocytes. Biomaterials. 2015;67:52–64. doi: 10.1016/j.biomaterials.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sánchez-Freire V, Lee AS, Hu S, Abilez OJ, Liang P, Lan F, Huber BC, Ong S-G, Hong WX, Huang M, Wu JC. Effect of Human Donor Cell Source on Differentiation and Function of Cardiac Induced Pluripotent Stem Cells. J Am Coll Cardiol. 2014;64:436–448. doi: 10.1016/j.jacc.2014.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundy SD, Zhu WZ, Regnier M, Laflamme MA. Structural and functional maturation of cardiac myocytes derived from human pluripotent stem cell. Stem Cells Dev. 2013;22:1991–2002. doi: 10.1089/scd.2012.0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 17.Bedada FB, Chan SS, Metzger SK, Zhang L, Zhang J, Garry DJ, Kamp TJ, Kyba M, Metzger JM. Acquisition of a quantitative, stoichiometrically conserved ratiometric marker of maturation status in stem cell-derived cardiac myocytes. Stem Cell Reports. 2014;3:594–605. doi: 10.1016/j.stemcr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lues I, Beier N, Jonas R, Klockow M, Haeusler G. The Two Mechanisms of Action of Racemic Cardiotonic EMD 53998, Calcium Sensitization and Phosphodiesterase Inhibition, Reside in Different Enantiomers. J Cardiovasc Pharmacol. 1993;21:883–892. doi: 10.1097/00005344-199306000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Knollmann BC, Knollmann-Ritschel BE, Weissman NJ, Jones LR, Morad M. Remodelling of ionic currents in hypertrophied and failing hearts of transgenic mice overexpressing calsequestrin. J Physiol. 2000;525(Pt 2):483–498. doi: 10.1111/j.1469-7793.2000.t01-1-00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harmer AR, Abi-Gerges N, Morton MJ, Pullen GF, Valentin JP, Pollard CE. Validation of an in vitro contractility assay using canine ventricular myocytes. Toxicol Appl Pharmacol. 2012;260:162–172. doi: 10.1016/j.taap.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Po-Ling K, Lee H, Bray M-A, Geisse NA, Huang Y-T, Adams WJ, Sheehy SP, Parker KK. Myocyte Shape Regulates Lateral Registry of Sarcomeres and Contractility. Am J Pathol. 2012;181:2030–2037. doi: 10.1016/j.ajpath.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoehr A, Neuber C, Baldauf C, Vollert I, Friedrich FW, Flenner F, Carrier L, Eder A, Shaaf S, Hirt MN, Aksehirlioglu B, Tong CW, Moretti A, Eschenhagen T, Hansen A. Automated analysis of contractile force and Ca2+ transients in engineered heart tissue. Am J Physiol Heart Circ Physiol. 2014;306:H1353–H1363. doi: 10.1152/ajpheart.00705.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, McCain ML, Yang L, He A, Pasqualini FS, Agarwal A, Yuan H, Jiang D, Zhang D, Zangi L, Geva J, Roberts AE, Ma Q, Ding J, Chen J, Wang D, Li K, Wang J, Wanders RJA, Kulik W, Vaz F, Laflamme MA, Murry CE, Chien KR, Kelly RI, Church GM, Parker KK, Pu WT. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Medicine. 2014;20:616–623. doi: 10.1038/nm.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.