Abstract
Purpose
Metastases to the kidney are a rare entity, historically described in autopsy studies. The primary aim of this study was to describe the presentation, treatment, and outcomes of patients with metastatic tumors to the kidney treated at a tertiary referral center.
Patients and Methods
We retrospectively identified 151 patients diagnosed with a primary non-renal malignancy with renal metastasis. Clinical, radiographic and pathologic characteristics were assessed. Overall survival (OS) was calculated using Kaplan-Meier methods.
Results
Median patient age was 56.7 years. The most common presenting symptoms were flank pain (30%), hematuria (16%) and weight loss (12%). Most primary cancers were carcinomas (80.8%). The most common primary tumor sites were lung (43.7%), colorectal (10.6%), ENT (6%), breast (5.3%), soft tissue (5.3%), and thyroid (5.3%). Renal metastases were typically solitary (77.5%). Concordance between radiologist and clinician imaging assessment was 54.0%. Three ablations and 48 nephrectomies were performed. For non-surgical patients, renal metastasis diagnosis was made with FNA or biopsy. Median OS from primary tumor diagnosis was 3.08 years and median OS from time of metastatic diagnosis was 1.13 years. For patients treated with surgery, median OS from primary tumor diagnosis was 4.81 years, and OS from metastatic diagnosis was 2.24 years.
Conclusions
Metastases to the kidney are a rare entity. Survival appears to be longer in patients who are candidates for, and are treated with surgery. Surgical intervention in carefully selected patients with oligometastatic disease and good performance status should be considered. A multi-disciplinary approach with input from urologists, oncologists, radiologists, and pathologists is needed to achieve the most optimal outcomes for this specific patient population.
Keywords: kidney, metastasis, fine needle aspiration, needle core biopsy, malignancy, nephrectomy
INTRODUCTION
Historically, the kidney was considered to be an organ where tumors rarely metastasized. In a study of 1000 autopsies in 1948, Abrams et al. [1] found that the kidney was only the twelfth most frequently organ involved with metastatic disease, with an incidence of 12.6%. Additional autopsy studies seem to support these findings; Bracken et al. [2] reported a frequency of 7.2% in over ten thousand autopsies, while Klinger [3], who excluded lower gastrointestinal and gynecological tumors, reported a more conservative rate of 2.36%.
With improvements in oncologic care and medical imaging, there has been a shift towards diagnosis of renal metastases in the still-living patient. Improvements in computed tomography (CT) led to incidental discovery of many more renal masses, and a number of case reports in the late twentieth century describe a wide spectrum of differentiating characteristics between primary and secondary renal masses. Although most reports noted that metastases rarely enhanced [4, 5], depending on the primary tumor, the renal mass could be unilateral [6] or bilateral [7], solitary [5] or multifocal [6], and with equally variable shape [8]. Some studies have found exophytic masses to be more indicative of metastasis on CT. [9, 10] Improved accuracy of biopsy procedures has also allowed for a more definitive identification of suspicious renal lesions [11]. However, the natural history of these renal metastases in living patients, as well as their treatment has not been described in detail in the past.
The primary aim of this study was to comprehensively describe the natural history of histologically confirmed metastases to the kidney from initial diagnosis to management and outcomes. By analyzing the radiological, pathological, and clinical data, we have provided a comprehensive profile of this challenging clinical scenario.
PATIENTS AND METHODS
Study Population
This study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center. We performed a retrospective review of the medical records of 189 adult patients who were diagnosed between November 1985 and November 2013 with a metastatic malignancy to the kidney, as confirmed by pathology. These patients were encountered from an institutional pathology database of all consecutive patients with cancer who underwent a renal mass fine needle aspiration, biopsy or nephrectomy (partial or radical) at The University of Texas MD Anderson Cancer Center.
All patients underwent a history and physical examination, and radiologic imaging using CT, MRI, or PET scan, based on the primary treating physician discretion and clinical guidelines. In addition, all patients underwent a percutaneous fine needle aspiration, percutaneous renal biopsy or nephrectomy and therefore had renal mass tissue available for pathologic evaluation. Immunohistochemistry was used if necessary to confirm the diagnosis. Patient charts were used to confirm demographic information, symptoms at time of presentation, imaging results, pathology information, reason for surgery (when performed), and vital status at last follow-up.
Exclusion criteria were concurrent diagnosis of the primary and metastatic tumor to the kidney (N=20), direct extension of the tumor to the kidney instead of true metastases to the kidney (N=13), lack of follow-up after establishing the diagnosis of metastatic disease to the kidney (N=5). As a result, the final study population included 151 patients.
Statistical Analysis
Continuous variables were tabulated and summarized using medians and interquartile ranges. Concordance between initial radiologist and clinician (oncologist or urologist) reads of the renal imaging studies was studied. Overall concordance was calculated as the total number of congruous reads divided by total number of reads. Concordance for primary versus metastasis included only those reads where primary or metastasis was chosen as the evaluation. Overall survival (OS) was defined as a) time from primary diagnosis to date of last follow-up or death and b) time from metastasis to date of last follow-up or death. OS was calculated using the Kaplan and Meier methods for the study population in general as well as a subset of patients who underwent surgery. We then conducted a multivariable Cox proportional hazards model controlling for potential confounding factors including age, gender, tumor size, number of symptoms, histology, primary organ site, and presence of non-renal metastasis. Tests of the proportional hazards assumption were conducted using Schoenfeld residuals.
RESULTS
Demographics, Primary Tumor Site, and Clinical Presentation of Metastasis
The median age of the cohort at kidney metastasis diagnosis was 56.7 years (IQR 48.6–64.9). Forty-eight percent of the patients were female and 84.8% were white. The most common presenting symptom associated with the renal metastasis was abdominal/flank pain (45, 30%; 95% CI: 23 – 38%), followed by hematuria (24, 16%; 95% CI: 11 – 23%), weight loss (18, 12%; 95% CI: 7–18%), night sweats (6, 4%; 95% CI: 1 – 9%) and fever (6, 4%; 95% CI: 1 – 4%). Few patients presented with multiple symptoms, and 85 patients (56.7%) were completely asymptomatic (Table 1).
Table 1.
Demographics and presenting symptoms
| Total | No Renal Surgery | Renal Surgery | |||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | p-value |
| Demographics | |||||||
| Number of patients | 151* | 100 | 48 | ||||
| Age | |||||||
| Median, years (IQR) | 56.7 (48.6–64.9) | 58.1 (49.4 – 64.8) | 54.9 (47.6 – 63.8) | 0.549 | |||
| Gender | 0.726 | ||||||
| Male | 78 | 51.7 | 50 | 50.0 | 26 | 54.2 | |
| Female | 73 | 48.3 | 50 | 50.0 | 22 | 45.8 | |
| Race | 0.798 | ||||||
| White | 128 | 84.8 | 83 | 83.0 | 43 | 89.6 | |
| Black | 10 | 6.6 | 8 | 8.0 | 2 | 4.2 | |
| Asian | 1 | 0.7 | 1 | 1.0 | 0 | 0.0 | |
| Hispanic | 12 | 8 | 8 | 8.0 | 3 | 6.3 | |
| Presenting symptoms | 0.637 | ||||||
| Hematuria | |||||||
| No | 126 | 84 | 84 | 84.8 | 39 | 81.3 | |
| Yes | 24 | 16 | 15 | 15.2 | 9 | 18.8 | |
| Abdominal/flank pain | 0.850 | ||||||
| No | 105 | 70 | 68 | 68.7 | 34 | 70.8 | |
| Yes | 45 | 30 | 31 | 31.3 | 14 | 29.2 | |
| Weight loss | 0.180 | ||||||
| No | 132 | 88 | 84 | 84.8 | 45 | 93.8 | |
| Yes | 18 | 12 | 15 | 15.2 | 3 | 6.3 | |
| Night Sweats | 0.178 | ||||||
| No | 144 | 96 | 93 | 93.9 | 48 | 100.0 | |
| Yes | 6 | 4 | 6 | 6.1 | 0 | 0.0 | |
| Fever | >0.999 | ||||||
| No | 144 | 96 | 95 | 96.0 | 46 | 95.8 | |
| Yes | 6 | 4 | 4 | 4.0 | 2 | 4.2 | |
| Number of symptoms | 0.488 | ||||||
| 0 | 85 | 56.7 | 52 | 52.5 | 30 | 62.5 | |
| 1 | 39 | 26.0 | 29 | 29.3 | 10 | 20.8 | |
| 2+ | 26 | 17.3 | 18 | 18.2 | 8 | 16.8 | |
The 151 patients include 3 patients treated with ablation, and these were not included in the No Surgery or Surgery groups in this table.
Carcinomas constituted the majority of primary cancers (80.8%). The most common primary tumor site was the lung (43.7%), followed by colorectal (10.6%), ENT (6%), breast (5.3%), soft tissue (5.3%), thyroid (5.3%), and unknown primary (5.3%). Most patients (69.5%) did not have any metastatic disease at the time of their original primary tumor diagnosis, although most ultimately developed metastases in other organs in addition to the kidney (80.8% of the total population). Less common primary tumor sites and number of organs with metastasis are listed in Table 2.
Table 2.
Primary tumor characteristics
| Total | No Renal Surgery |
Renal Surgery |
|||||
|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | p-value |
| Diameter of largest renal mass | 0.003 | ||||||
| Median, cm (IQR) | 4.1 (2.3 – 6.3) | 4.0 (2.3 – 5.0) | 6.15 (3.5 – 8.2) | ||||
| Primary Histology | 0.024 | ||||||
| Carcinoma | 122 | 80.8 | 87 | 87.0 | 34 | 70.8 | |
| Sarcoma | 18 | 11.9 | 6 | 6.0 | 10 | 20.8 | |
| Other | 11 | 7.3 | 7 | 7.0 | 4 | 8.3 | |
| Primary organ site | 0.028* | ||||||
| Lung | 66 | 43.7 | 50 | 50.0 | 16 | 33.3 | |
| Colorectal | 16 | 10.6 | 6 | 6.0 | 9 | 18.8 | |
| ENT | 9 | 6.0 | 8 | 8.0 | 1 | 2.1 | |
| Breast | 8 | 5.3 | 5 | 5.0 | 3 | 6.3 | |
| Soft tissue | 8 | 5.3 | 3 | 3.0 | 4 | 8.3 | |
| Thyroid | 8 | 5.3 | 6 | 6.0 | 2 | 4.2 | |
| Unknown | 8 | 5.3 | 5 | 5.0 | 3 | 6.3 | |
| Gynecologic | 7 | 4.6 | 6 | 6.0 | 1 | 2.1 | |
| Skin | 5 | 3.3 | 2 | 2.0 | 3 | 6.3 | |
| Pancreas | 4 | 2.7 | 1 | 1.0 | 3 | 6.3 | |
| Hematologic | 3 | 2.0 | 3 | 3.0 | 0 | 0.0 | |
| Prostate | 3 | 2.0 | 3 | 3.0 | 0 | 0.0 | |
| Bone | 2 | 1.3 | 0 | 0.0 | 2 | 4.2 | |
| Peritoneum | 2 | 1.3 | 1 | 1.0 | 0 | 0.0 | |
| Small bowel | 1 | 0.7 | 1 | 1.0 | 0 | 0.0 | |
| Thymus | 1 | 0.7 | 0 | 0.0 | 1 | 2.1 | |
| Presence of non-renal metastatic disease at time of primary tumor diagnosis | 0.570 | ||||||
| No | 105 | 69.5 | 67 | 67.0 | 35 | 72.9 | |
| Yes | 46 | 30.5 | 33 | 33.0 | 13 | 27.1 | |
| Total number of organs with metastasis | 0.900 | ||||||
| 0 | 29 | 19.3 | 18 | 18.0 | 11 | 23.4 | |
| 1 | 49 | 32.7 | 33 | 33.0 | 14 | 29.8 | |
| 2 | 30 | 20.0 | 22 | 22.0 | 8 | 17.0 | |
| 3 | 23 | 15.3 | 15 | 15.0 | 7 | 14.9 | |
| 4 | 11 | 7.3 | 6 | 6.0 | 5 | 10.6 | |
| 5 | 7 | 4.7 | 5 | 5.0 | 2 | 4.3 | |
| 6 | 1 | 0.7 | 1 | 1.0 | 0 | 0.0 | |
Compares Lung v. Colorectal v. other
Imaging and Concordance
The vast majority of the renal masses in this study were detected on CT imaging (97.7%). Renal ultrasound (18.5%), and MRI images (9.3%) were less frequently used. A total of 177 renal masses were found in our patient cohort, with most renal tumors (77.5%) being solitary, 11.3% with 2 tumors, and 11.3% with more than 2 tumors. In the 131 tumors with available size information, the median tumor diameter was 4.1 cm (IQR 2.3–6.3).
Renal imaging reads (by radiologist and clinician) were categorized as NA (no report available), benign, primary, metastatic, or unsure (Table 3). Fifteen patients for whom radiologist and/or clinician reports were missing were excluded, for a remaining total of 136. Very few images were interpreted as benign by either radiologists (7, 5.1%) or clinicians (4, 2.8%). A kidney metastasis was much less likely to be diagnosed by a radiologist (65, 47.5%) than a clinician (107, 74.3%). On the other hand, a primary renal tumor diagnosis was more favored by radiologists (27, 19.7%) than clinicians (15, 10.4%). A minority of reports offered no definitive diagnosis, with radiologist reads (38, 27.7%) outnumbering clinician ones (18, 12.5%).
Table 3.
Radiologist and Clinician Concordance
| Initial clinician evaluation | |||||
|---|---|---|---|---|---|
| Initial radiologist evaluation | Benign | Metastasis | Primary | Unsure | Total |
| Benign | 4 | 2 | 0 | 1 | 7 |
| Metastasis | 0 | 58 | 2 | 5 | 65 |
| Primary | 0 | 13 | 9 | 4 | 27 |
| Unsure | 0 | 27 | 4 | 7 | 38 |
| Total | 4 | 100 | 15 | 17 | 136 |
Concordance between radiologist and clinician for primary renal tumor versus metastasis specifically was 81.7% (95% CI: 71.6 – 89.4). This was calculated using only those images for which both the radiologist and the clinician offered a diagnosis of either a primary or metastasis. However, if all images are included (for example when the clinician diagnosed primary and the radiologist diagnosed unsure), the concordance on diagnosis becomes 54.0% (95% CI: 44.9% – 63.0%).
Biopsy and Surgery
One hundred eighteen patients underwent FNA (78.2%), 40.4% had needle core biopsy, and 35.8% had both concurrently. Twenty-six patients (17.2%) were not biopsied, and this group typically proceeded straight to surgery.
Overall, 3 ablations (2%) and 48 nephrectomies (31.8%) were performed, including 9 partial (18.8%) and 39 radical nephrectomies (81.2%). Surgery was performed with curative intent in 21 patients (44.7%), for a suspected primary tumor in 11 patients (23.4%), for chemotherapy failure/palliation in 10 patients (21.3%), and for non-functioning symptomatic kidney in 5 patients (10.6%).
Tumor diameter (IQR) was larger in the renal surgery group compared with the non-surgery group [6.15cm (3.5 - 8.2) versus 4.0cm (2.3 – 5.0), p=0.003]. The primary tumor of origin was comparable between the 48 patients who underwent surgery and those who did not. In this surgical group, the most common organ was again lung (16, 33.3%), followed by colorectal (9, 18.8%) and soft tissue (4, 8.3%). Three each of breast, pancreas, skin, and unknown primary (each 6.3%) also underwent surgery. Other surgical details are displayed in Table 4.
Table 4.
Surgical Details
| Characteristic | N | % |
|---|---|---|
| Renal Surgery Done | ||
| No | 100 | 66.2 |
| Ablation | 3 | 2.0 |
| Yes | 48 | 31.8 |
| Type of surgery | ||
| Partial nephrectomy | 9 | 18.8 |
| Radical nephrectomy | 39 | 81.2 |
| Reason for surgery | ||
| Curative intent | 21 | 44.7 |
| Suspected renal primary tumor | 11 | 23.4 |
| Chemotherapy failure/palliation | 10 | 21.3 |
| Poorly functioning kidney | 5 | 10.6 |
Outcomes
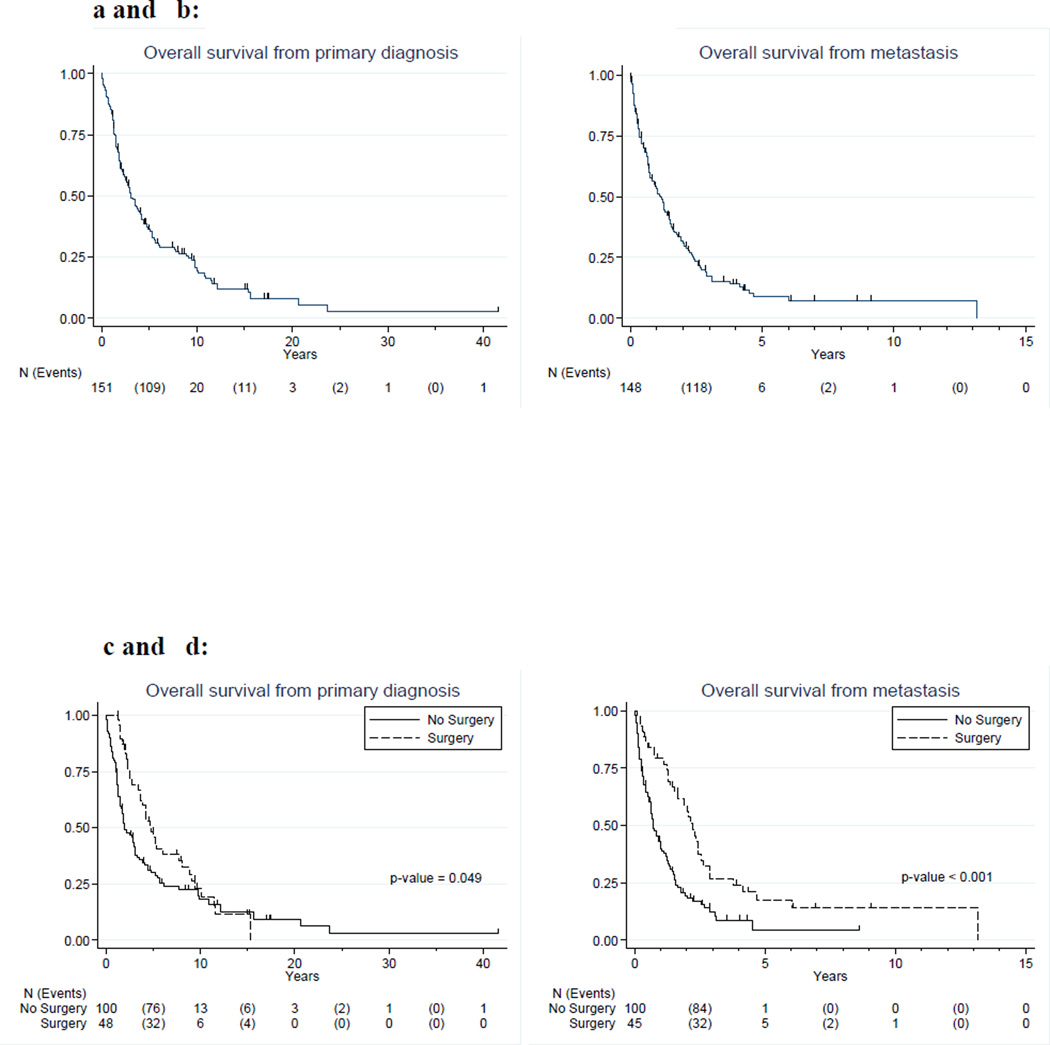
One hundred eleven patients died of disease (73.5%) and 21 (13.9%) were alive with disease. Only 3 patients (2%) who had cancer at last follow-up died of other causes. The median OS from time of renal metastasis diagnosis was 1.13 years (95% CI: 0.76 – 1.46) for the whole study population. OS from time of renal metastasis diagnosis was 2.24 years (95% CI: 1.55 – 2.57) for patients who underwent renal surgery, and 0.72 years (95% CI: 0.55 – 1.02) for patients who did not undergo renal surgery, log-rank p<0.001. Detailed survival plots and estimates are depicted in Figure 1 and shown in Table 5. In the multivariable model (Table 6), survival from time of primary diagnosis was not statistically significant (HR = 0.61; p = 0.069), but the proportional hazards assumption was not met for this model; therefore the true hazards ratio for this model varies over time, making it difficult to interpret both the hazards ratio and the model results in this specific group. On multivariable analysis, overall survival measured from time of renal metastasis was improved for those who had renal surgery (HR = 0.46; p = 0.005).
Figure 1.
a and b: Kaplan Meier curves of OS of all patients
c and d: Kaplan-Meier curves of OS of patients treated with or without surgery
Table 5.
Overall survival estimates
| Probability of Survival | ||||||||
|---|---|---|---|---|---|---|---|---|
| Overall survival | Median | 95% CI | 1-yr | 95% CI | 3-yr | 95% CI | 5-yr | 95% CI |
| Overall | ||||||||
| Time from primary diagnosis | 3.08 | 2.33 – 4.16 | 0.85 | 0.79 – 0.90 | 0.51 | 0.43 – 0.59 | 0.36 | 0.28 – 0.44 |
| Time from metastasis | 1.13 | 0.76 – 1.46 | 0.53 | 0.45 – 0.61 | 0.17 | 0.11 – 0.24 | 0.09 | 0.04 – 0.15 |
| No Surgery | ||||||||
| Time from primary diagnosis | 1.95 | 1.53 – 3.07 | 0.78 | 0.69 – 0.85 | 0.41 | 0.31 – 0.51 | 0.30 | 0.21 – 0.39 |
| Time from metastasis | 0.72 | 0.55 – 1.02 | 0.42 | 0.32 – 0.52 | 0.12 | 0.06 – 0.21 | 0.04 | 0.01 – 0.15 |
| Surgery | ||||||||
| Time from primary diagnosis | 4.81 | 3.67 – 7.72 | 1.00 | -- | 0.69 | 0.53 – 0.80 | 0.48 | 0.33 – 0.62 |
| Time from metastasis | 2.24 | 1.55 – 2.57 | 0.79 | 0.64 – 0.89 | 0.27 | 0.14 – 0.41 | 0.18 | 0.07 – 0.32 |
Table 6.
Multivariable Cox proportional Hazards model
| OS from primary diagnosis | OS from renal metastasis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Age at diagnosis (per year) | 1.02 | 1.00 | 1.04 | 0.034 | 1.01 | 0.99 | 1.03 | 0.349 |
| Gender | ||||||||
| Male (Referent) | ||||||||
| Female | 1.02 | 0.67 | 1.57 | 0.913 | 1.08 | 0.72 | 1.63 | 0.700 |
| Tumor size of largest mass | 1.05 | 0.97 | 1.14 | 0.187 | 1.07 | 0.99 | 1.15 | 0.091 |
| Number of symptoms | ||||||||
| 0 (Referent) | ||||||||
| 1 | 1.68 | 0.96 | 2.94 | 0.072 | 1.28 | 0.73 | 2.26 | 0.388 |
| >=2 | 1.59 | 0.87 | 2.93 | 0.135 | 1.47 | 0.77 | 2.81 | 0.238 |
| Primary Histology | ||||||||
| Carcinoma (Referent) | ||||||||
| Sarcoma | 0.77 | 0.33 | 1.79 | 0.541 | 0.56 | 0.23 | 1.34 | 0.193 |
| Other | 0.81 | 0.34 | 1.94 | 0.630 | 0.95 | 0.38 | 2.35 | 0.909 |
| Primary organ site | ||||||||
| Lung (Referent) | ||||||||
| Colorectal | 0.23 | 0.11 | 0.49 | <0.001 | 0.41 | 0.19 | 0.89 | 0.024 |
| Other | 0.22 | 0.12 | 0.41 | <0.001 | 0.60 | 0.36 | 1.01 | 0.055 |
| Presence of non-renal metastases at time of diagnosis | 1.53 | 0.93 | 2.50 | 0.092 | 0.69 | 0.42 | 1.14 | 0.148 |
| Surgery for renal metastases | 0.61* | 0.36 | 1.04 | 0.069 | 0.46 | 0.27 | 0.80 | 0.005 |
The assumption of non-proportionality was not met for this term in the model that examines time from primary diagnosis.
DISCUSSION
This study of 151 adult patients with metastases to the kidney is, to our knowledge, the largest and most comprehensive profile of this clinical scenario to date, documenting demographic, radiological, and pathological data, along with surgical management outcomes. We found that the most common primary tumors in descending order were lung, colorectal, ENT, and breast/soft tissue/thyroid. This is in line with historical reports noting the lung as the most prevalent source of metastases, followed by a combination of colorectal, stomach, and breast malignancies [1–7, 12–18]. Over half of the patients in our cohort were asymptomatic. Several previous papers have noted changes in laboratory findings, from albuminuria to microscopic hematuria, [6, 17] but clinically patients rarely complain of specific urological symptoms [3, 4]. Interestingly over two-thirds of the patients presented with solitary metastases to the kidney, but in line with other accounts, the majority of them ultimately went on to develop metastases in other organs [4, 15, 17, 19].
Initial diagnosis of metastases to the kidney typically resulted from routine imaging. Siegel et al. [20] found that inter-observer concordance for enhancement of renal masses showed increasing variability with complexity, and other studies have repeatedly demonstrated the lack of pathognomonic characteristics for a renal metastasis. [4–8] In our cohort, radiologists more commonly called the primary renal tumors over metastases, likely because the former is more prevalent than the latter. [4] Clinician call on the radiology read seemed to be better in comparison likely due to knowledge of the patient’s history, the physical examination and specific knowledge about the disease biology and behavior. The fact that almost half of all the patients were symptomatic on presentation is suggestive, although none of the symptoms were specific, and could also be found in association with a renal primary. Certainly, in the patient with a known primary and suspicious renal lesion on imaging, a high degree of suspicion should be employed.
FNA was performed in 78.2% of our patients, and concurrent core biopsy was obtained in almost half of them. Improvements in biopsy technique and consistent results have led to favoring of FNA over core biopsies[16, 21, 22], and indeed, in our dataset only 3 patients had unclear FNA findings. Two of these patients had concurrent core biopsies performed with satisfactory outcomes, and one proceeded straight to surgery for a suspected renal primary. An argument has been made for using larger-gauge needles for enhanced results, especially should the need for tissue preparation arise[23]. This could explain why patients earlier in our series typically only had FNA, but more recent patients often underwent both.
In the past, a renal mass in the presence of a non-renal malignancy used to be considered a clear indication for biopsy, as metastatic disease would typically require systemic treatment[13, 24]. We have previously reported [25] that amongst patients with non-renal malignancies and subsequently discovered renal masses, progression of the primary non-renal malignancy was strongly predictive for metastasis. In fact, none of the 54 patients without signs of progression of the primary non-renal tumor were found to have renal metastases. As such, in patients with simply clinically-localized disease, biopsy is not indicated, as the mass rarely represented a metastasis.
Interesting findings of our study were the outcomes from surgical intervention in this heterogeneous group. Survival appeared to be improved in patients who underwent renal surgical resection. The majority (44.7%) of these surgeries were carried out for curative intent in patients with oligometastatic disease. Surgery was less commonly (21.3%) performed for palliative purposes. While surgery is considered the standard of care in many patients with renal masses thought to be renal cell carcinoma[26–28], there are no clearly established guidelines for tumors metastatic to the kidney. However, it seems that surgery should be considered in carefully selected patients with metastases to the kidney.
Outside of this publication, there is a paucity of more current literature on metastases to the kidney. In a previous report from our institution [25], we looked at 100 patients with renal masses in the setting of other malignancies, and concluded that the only predictive factors for a secondary over a renal primary were progression of the non-renal malignancy and lack of enhancement of the renal mass on imaging (metastatic rate in the study was 19%). In contrast, Patel et al. [19] found that 0.9% of their 2340 patients were diagnosed with renal metastases by histology or regression on imaging in concert with the primary after appropriate treatment. This lower value is possibly related to both the referral patterns of the hospital involved, which prominently featured lymphomas and melanomas, as well as the decidedly less accurate method of diagnosing a metastasis via imaging alone (and not including histology).
Our study has limitations that are worth mentioning. The study period was spread over a relatively long time, but this allowed capturing of many patients with this rare condition for the purposes of this study. We did not capture every single patient with renal metastases, but only those who either had biopsy or surgery on the kidney at our institution. Renal metastases are likely more common in patients with widespread metastases, and therefore the proportions in our study are likely to underestimate the true incidence of metastases to the kidney. The primary tumors were from different organs, with different histology and heterogeneity of the primary tumor biology, which in turn act as a major determinant of outcomes. The primary tumor distribution could differ from other reference centers, based on potentially different referral patterns. The study was retrospective with multiple specialties and different practice patterns involved. Radiographs were not re-reviewed for the purposes of this study, but this could be construed as a strength as it reflects real-time daily clinical practice patterns. Survivor bias might partially explain the improved survival time among surgical patients when measured from primary diagnosis; those who died prior to receiving surgery were automatically included among non-surgical patients. However, given the relatively short time between metastasis and surgery, the effect of survivor bias when examining survival from time of metastasis is minimal. Selection bias for patients undergoing surgery are most like ones with minimal metastatic disease and good performance status, which could explain the better survival outcomes in surgical patients. Another explanation for the better observed outcomes in the surgical group could be related to the difference in systemic therapies used in this cohort.
CONCLUSIONS
Metastases to the kidney are still a rare entity. These are typically bronchogenic in nature, solitary, and asymptomatic. Diagnostic concordance between radiologist and clinician was lower than expected. Thus, a high index of suspicion and a careful review of the scans by all involved physicians is necessary in the patient with a non-renal malignancy who presents with a suspicious renal mass on imaging. Since renal metastases appear early in the metastatic process and survival appears to be relatively longer in patients treated with surgery, surgical intervention in carefully selected patients with oligometastatic disease and good performance status should be considered. A multi-disciplinary approach with input from urologists, oncologists, radiologists, and pathologists is needed to achieve the most optimal outcomes for this specific patient population.
Acknowledgments
Funding
Supported by the NIH/NCI under award number P30CA016672 and used the Biostatistics Resource Group.
Financial Disclosures
Jose A. Karam has served as a one-time consultant to Pfizer in 2013. Christopher G. Wood has received research funding from Pfizer and served as a consultant and on its advisory board.
Footnotes
Conflicts of Interest
None of these disclosures by both authors are related to the current manuscript.
REFERENCES
- 1.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950 Jan;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Bracken RB, Chica G, Johnson DE, Luna M. Secondary renal neoplasms: an autopsy study. Southern medical journal. 1979 Jul;72:806–807. doi: 10.1097/00007611-197907000-00013. [DOI] [PubMed] [Google Scholar]
- 3.Klinger ME. Secondary tumors of the genito-urinary tract. The Journal of urology. 1951 Jan;65:144–153. doi: 10.1016/S0022-5347(17)68470-2. [DOI] [PubMed] [Google Scholar]
- 4.Pagani JJ. Solid renal mass in the cancer patient: second primary renal cell carcinoma versus renal metastasis. Journal of computer assisted tomography. 1983 Jun;7:444–448. doi: 10.1097/00004728-198306000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt GM, Bernardino ME, Graham SD., Jr CT diagnosis of renal metastases. Journal of computer assisted tomography. 1983 Dec;7:1032–1034. doi: 10.1097/00004728-198312000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Choyke PL, White EM, Zeman RK, Jaffe MH, Clark LR. Renal metastases: clinicopathologic and radiologic correlation. Radiology. 1987 Feb;162:359–363. doi: 10.1148/radiology.162.2.3797648. [DOI] [PubMed] [Google Scholar]
- 7.Mitnick JS, Bosniak MA, Rothberg M, Megibow AJ, Raghavendra BN, Subramanyam BR. Metastatic neoplasm to the kidney studied by computed tomography and sonography. Journal of computer assisted tomography. 1985 Jan-Feb;9:43–49. doi: 10.1097/00004728-198501000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Nishitani H, Onitsuka H, Kawahira K, et al. Computed tomography of renal metastases. Journal of computer assisted tomography. 1984 Aug;8:727–730. doi: 10.1097/00004728-198408000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Honda H, Coffman CE, Berbaum KS, Barloon TJ, Masuda K. CT analysis of metastatic neoplasms of the kidney. Comparison with primary renal cell carcinoma. Acta radiologica. 1992 Jan;33:39–44. [PubMed] [Google Scholar]
- 10.Prkačin I, Naumovski-Mihalić S, Dabo N, Palčić I, Vujanić S, Babić Z. Comparison of CT analyses of primary renal cell carcinoma and of metastatic neoplasms of the kidney. Radiology and Oncology. 2001:35. [Google Scholar]
- 11.Diamantis A, Magiorkinis E, Koutselini H. Fine-needle aspiration (FNA) biopsy: historical aspects. Folia histochemica et cytobiologica / Polish Academy of Sciences, Polish Histochemical and Cytochemical Society. 2009;47:191–197. doi: 10.2478/v10042-009-0027-x. [DOI] [PubMed] [Google Scholar]
- 12.Bates AW, Baithun SI. The significance of secondary neoplasms of the urinary and male genital tract. Virchows Archiv : an international journal of pathology. 2002 Jun;440:640–647. doi: 10.1007/s00428-001-0549-x. [DOI] [PubMed] [Google Scholar]
- 13.Gattuso P, Ramzy I, Truong LD, et al. Utilization of fine-needle aspiration in the diagnosis of metastatic tumors to the kidney. Diagnostic cytopathology. 1999 Jul;21:35–38. doi: 10.1002/(sici)1097-0339(199907)21:1<35::aid-dc10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 14.Giashuddin S, Cangiarella J, Elgert P, Levine PH. Metastases to the kidney: eleven cases diagnosed by aspiration biopsy with histological correlation. Diagnostic cytopathology. 2005 Jun;32:325–329. doi: 10.1002/dc.20243. [DOI] [PubMed] [Google Scholar]
- 15.Hietala SO, Wahlqvist L. Metastatic tumors to the kidney. A postmortem, radiologic and clinical investigation. Acta radiologica: diagnosis. 1982;23:585–591. doi: 10.1177/028418518202300610. [DOI] [PubMed] [Google Scholar]
- 16.Leiman G. Audit of fine needle aspiration cytology of 120 renal lesions. Cytopathology : official journal of the British Society for Clinical Cytology. 1990;1:65–72. doi: 10.1111/j.1365-2303.1990.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 17.Wagle DG, Moore RH, Murphy GP. Secondary carcinomas of the kidney. The Journal of urology. 1975 Jul;114:30–32. doi: 10.1016/s0022-5347(17)66935-0. [DOI] [PubMed] [Google Scholar]
- 18.Newsam JE, Tulloch WS. Metastatic tumours in the kidney. British journal of urology. 1966 Feb;38:1–6. doi: 10.1111/j.1464-410x.1966.tb09671.x. [DOI] [PubMed] [Google Scholar]
- 19.Patel U, Ramachandran N, Halls J, Parthipun A, Slide C. Synchronous renal masses in patients with a nonrenal malignancy: incidence of metastasis to the kidney versus primary renal neoplasia and differentiating features on CT. AJR American journal of roentgenology. 2011 Oct;197:W680–W686. doi: 10.2214/AJR.11.6518. [DOI] [PubMed] [Google Scholar]
- 20.Siegel CL, Fisher AJ, Bennett HF. Interobserver variability in determining enhancement of renal masses on helical CT. AJR American journal of roentgenology. 1999 May;172:1207–1212. doi: 10.2214/ajr.172.5.10227490. [DOI] [PubMed] [Google Scholar]
- 21.Pilotti S, Rilke F, Alasio L, Garbagnati F. The role of fine needle aspiration in the assessment of renal masses. Acta cytologica. 1988 Jan-Feb;32:1–10. [PubMed] [Google Scholar]
- 22.Truong LD, Todd TD, Dhurandhar B, Ramzy I. Fine-needle aspiration of renal masses in adults: analysis of results and diagnostic problems in 108 cases. Diagnostic cytopathology. 1999 Jun;20:339–349. doi: 10.1002/(sici)1097-0339(199906)20:6<339::aid-dc4>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 23.Murphy WM, Zambroni BR, Emerson LD, Moinuddin S, Lee LH. Aspiration biopsy of the kidney. Simultaneous collection of cytologic and histologic specimens. Cancer. 1985 Jul 1;56:200–205. doi: 10.1002/1097-0142(19850701)56:1<200::aid-cncr2820560134>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer imaging : the official publication of the International Cancer Imaging Society. 2009;9:44–55. doi: 10.1102/1470-7330.2009.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanchez-Ortiz RF, Madsen LT, Bermejo CE, et al. A renal mass in the setting of a nonrenal malignancy: When is a renal tumor biopsy appropriate? Cancer. 2004 Nov 15;101:2195–2201. doi: 10.1002/cncr.20638. [DOI] [PubMed] [Google Scholar]
- 26.Yagoda A, Abi-Rached B, Petrylak D. Chemotherapy for advanced renal-cell carcinoma: 1983–1993. Seminars in oncology. 1995 Feb;22:42–60. [PubMed] [Google Scholar]
- 27.Onufrey V, Mohiuddin M. Radiation therapy in the treatment of metastatic renal cell carcinoma. International journal of radiation oncology, biology, physics. 1985 Nov;11:2007–2009. doi: 10.1016/0360-3016(85)90285-8. [DOI] [PubMed] [Google Scholar]
- 28.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007 May 1;109:1763–1768. doi: 10.1002/cncr.22600. [DOI] [PubMed] [Google Scholar]