Abstract
Objective
Empirical studies of cognitive ability and personality have tended to operate in isolation of one another. We suggest that returning to a unified approach to considering the development of individual differences in both cognition and personality can enrich our understanding of human development.
Method
We draw on previous meta-analyses of longitudinal, behavior genetic studies of cognition and personality across the lifespan, focusing particular attention on age trends in heritability and differential stability.
Results
Both cognition and personality are moderately heritable and exhibit large increases in stability with age; however, marked differences are evident. First, the heritability of cognition increases substantially with child age, while the heritability of personality decreases modestly with age. Second, increasing stability of cognition with age is overwhelmingly mediated by genetic factors, whereas increasing stability of personality with age is entirely mediated by environmental factors. Third, the maturational time-course of stability differs: Stability of cognition nears its asymptote by the end of the first decade of life, whereas stability of personality takes three decades to near its asymptote.
Conclusions
We discuss how proximal gene-environment dynamics, developmental processes, broad social contexts, and evolutionary pressures may intersect to give rise to these divergent patterns.
Keywords: Developmental behavior genetics, personality, cognitive ability, gene-environment interplay, differential stability
Individual differences in cognitive ability and personality fundamentally affect how people interact with the world around them, impacting outcomes as diverse as interpersonal relationships, educational achievement, occupational success, income, happiness, health, and longevity (Deary, Weiss, & Batty, 2010; Moffitt et al., 2011; Ozer & Benet-Martínez, 2006; Roberts, Kuncel, Shiner, Caspi, & Goldberg, 2007; Schmidt & Hunter, 1988). Both cognition and personality are well known for their high differential stabilities over time (Conley, 1984)–individuals tend to maintain their relative standing compared to others across the lifespan.1 In fact, the impressive associations between these psychological differences and important life outcomes may be owed, in part, to their relatively high differential stabilities over time and context: small, seemingly inconsequential, instances of thinking, feeling, and behaving when repeatedly and systematically experienced over time may aggregate to profoundly shape individuals’ life paths. How, then, do these consequential psychological differences emerge over development, and what accounts for the differential stability of these characteristics over tremendous spans of time?
In the current article, we describe our recent meta-analytic work on how genetic and environmental influences on cognition and personality change across nearly the entire lifespan (Briley & Tucker-Drob, 2013; 2014; Tucker-Drob & Briley, 2014; Tucker-Drob, Briley, & Harden, 2013). We suggest that similarities and differences in the empirical patterns of genetic and environmental contributions to stability between cognition and personality may offer insights into how processes of gene-environment correlation and interaction intersect with the developmental, social, and evolutionary pressures that underlie cognition and personality. Thus, on the one hand, gene-environment correlation and interaction may serve as a common set of mechanisms guiding developmental stability of both personality and cognition. On the other hand, differences in the directionality and systematicity of developmental, social, and evolutionary pressures can be used as a framework by which to understand unique trends across traits. We use this dual backdrop to compare and contrast the lifespan trends in genetic and environmental sources of variation and stability for personality and cognitive ability.
Gene-Environment Interplay as a Guiding Framework for Studying Development
Gene-environment interplay is a general term that refers to the multiple ways in which genetic and environmental influences on development depend on one another. The necessity of considering gene-environment interplay is widely recognized in the literature on cognitive ability (Dickens, Turkheimer, & Beam, 2011; Collins, Macoby, Steinberg, Hetherington, & Bornstein, 2000; Tucker-Drob et al., 2013), personality (e.g., Bleidorn, Kanlder, & Caspi, 2014; Roberts & Jackson, 2008), and psychological development more generally (e.g., Bronfenbrenner & Ceci, 1994; Johnson, Penke, & Spinath, 2011; Scarr & McCartney, 1983; Tabery, 2007). Genetic and environmental effects may depend on one another by way of gene-environment correlation, in which individuals come to be nonrandomly exposed to different environments as a function of genetically influenced individual differences, and gene × environment interaction, in which environmental experiences affect individuals differently as a function of genetically-influenced individual differences (Plomin, DeFries, & Loehlin, 1977).
Gene-environment correlations can emerge through passive, evocative, and active mechanisms. Passive gene-environment correlation occurs when the genes that parents pass on to their children also influence the type of rearing environment that they provide to those children. Evocative gene-environment correlation occurs when individuals evoke different experiences from their surroundings on the basis of their genetically-influenced characteristics. Active gene-environment correlation occurs when people actively seek out or create environmental experiences on the basis of their genetically-influenced characteristics (e.g., abilities, preferences, or proclivities). When these environments, in turn, have causal effects on psychological development, dynamic bidirectional processes of gene-environment transactions result (Bronfenbrenner & Ceci, 1994; Scarr & McCartney, 1983; Tucker-Drob et al., 2013; Tucker-Drob & Harden, 2012a). In other words, gene-environment transactions occur when certain individuals are more likely to experience specific environments based on their genotype, and those environments have causal impacts on their development. When these same processes occur systematically over prolonged periods of development, the effects of gene-environment transactions aggregate, such that genetic effects become reinforced and amplified (Dickens & Flynn, 2001; Beam & Turkheimer, 2013). This amplification may be further accelerated as children are given increasing opportunities for active and evocative gene-environment correlation with age (Scarr & McCartney, 1983; Tucker-Drob et al., 2013).
Uneven opportunities for gene-environment correlations within a society may be mechanisms of social inequalities in life outcomes (Tucker-Drob et al., 2013). Socioeconomic advantage is tightly linked with access to enriching curricular and extracurricular educational experiences (Duncan & Murnane, 2011) that children may seek out and evoke on the basis of genetically influenced motivational factors (Tucker-Drob & Harden, 2012a). Under conditions of socioeconomic disadvantage, even children with high natural levels of intellectual interest and achievement motivation are afforded only minimal opportunities to seek out and evoke such enriching experiences. Moreover, parents with greater socioeconomic and interpersonal resources are better able to actively monitor and regulate their adolescent children’s activities and whereabouts (McLoyd, 1998), which diminishes opportunities for gene-environment correlations with respect to interactions with delinquent peers (Dick, Viken, Purcell, Kaprio, Pulkkinen, & Rose, 2007; Mann, Kretsch, Tackett, Harden, & Tucker-Drob, 2015). Thus, social policies which promote opportunities to engage in positive experiences may help to reduce social inequality by promoting gene-environment correlations, and those which restrict opportunities to engage in risky or deleterious experience may help to reduce social inequality by restricting gene-environment correlations. Indeed, key components of efforts to increase academic development involve expanding early educational opportunities and disseminating to parents information about the educational system (Arnold & Doctoroff, 2003; Lareau, 2002; Magnuson & Waldfogel, 2005; Payne & Knowles, 2009). Such policies may enrich gene-environment correlations by arming disadvantaged children with the skills and their parents with the social capital necessary to navigate the complex educational institution.
The second primary mechanism of gene-environment interplay, gene × environment interaction, can also occur through a variety of pathways. Individuals may respond differentially to shared, family-level environments (e.g., parenting, socioeconomic status) based on their genotype. For example, genetic influences on cognitive development may be especially strong in family-level environments that support cognitive development (Harden, Turkheimer, & Loehlin, 2007; Rhemtulla & Tucker-Drob, 2012; Rowe, Jacobson, & Van den Oord, 1999; Scarr-Salapatek, 1971; Taylor, Roehrig, Soden-Hensler, Connor, & Schatschneider, 2010; Tucker-Drob, Rhemtulla, Harden, Turkheimer, & Fask, 2011; Turkheimer, Haley, Waldron, D’Onofrio, & Gottesman, 2003). Additionally, individuals may differ in their potential responses to unique environments and experiences (e.g., peer groups, stressful life events, any experience not necessarily shared with other members of their family) on the basis of their genotypes. For example, the experience of antisocial peer groups, parent-child relationship problems, and low academic achievement in adolescence may accentuate genetic influences on externalizing behavior (Hicks, South, DiRago, Iacono, & McGue, 2009). If environmental disadvantage prevents the expression of genetic potentials for positive psychological outcomes and/or magnifies the expression of genetic risks for maladaptive psychological outcomes, social inequality may be exacerbated.
Gene × environment interaction has important implications for behavior genetic models. When genotypes respond differently to family-level environments, then this effect will lead more genetically similar individuals reared together to resemble one another to a greater extent. Put differently, this effect will differentiate individuals that do not share identical genotypes, resulting in phenotypic variation becoming linked to additive genetic effects. When genotypes respond differentially to unique life experiences, this effect differentiates even genetically identical individuals because they do not share their unique life experiences. This process links phenotypic variation with nonshared environmental effects.
Typically, the effects of gene-environment interplay are investigated at single points in time. Yet these mechanisms may play a role in establishing the stability of psychological characteristics. Gene-environment correlation may build across development. Early genetically influenced differences between individuals, be they small, may influence the manner in which they move through the environment and thus amplify over time. Gene × environment interactions may generate individual differences and lead individuals toward different life trajectories. These processes may also combine. For example, some individuals may respond differentially to the same educational environment. Teachers may pick up on these individual differences and provide student-specific feedback. This combined process of gene-environment interplay may lead to the stability of individual differences. Depending on whether the environmental effects are shared across members of the same family, or unique of each family member, the differential stability of personality and cognition may become linked to genetic or environmental mechanisms. Mechanisms of gene-environment interplay may connect patterns of behavior with consequential outcomes by guiding the development of individual differences. Next, we consider pressures on behavior and how these pressures might explain the role that cognition and personality play in life opportunity.
Developmental, Social, and Evolutionary Pressures on Trait Development
High levels of cognitive ability are adaptive for individuals and for societies, both with respect to modern life (Deary, Weiss, & Batty, 2010) and with respect to human evolutionary history (Penke, Denissen, & Miller, 2007). Reasoning and knowledge build cumulatively over development, with more complex capacities building upon more basic foundational skills (Duncan et al., 2007; Knudsen, Heckman, Cameron, & Shonkoff, 2006; Siegler, 1998), a process facilitated by both person-driven and context-driven learning processes (Bouchard, 1997; Tucker-Drob, Cheung, & Briley, 2014). Modern societies have institutionalized strong, directional social scaffolds, e.g. formal education, for continuous and sustained learning over nearly the entirety of infant, child, and adolescent development. One possible result of these strong directional pressures for learning and cognitive development, when combined with active and evocative selection processes, is the rapid stratification (i.e., inequality) of children’s experiences based on early genetically-influenced variation, and thus the early canalization of their trajectories of cognitive development.
The developmental, social, and evolutionary pressures that have been hypothesized to act on personality are quite different. Although higher levels of cognitive ability are considered universally adaptive, adaptive levels of specific personality traits might depend on context and might vary across the lifespan (Caspi, Roberts, & Shiner, 2006; Nettle, 2007). For example, being less agreeable in childhood and adolescence may encourage individuals to invest in themselves in order to ensure a stable and prosperous future, whereas being more agreeable may be increasingly adaptive in adulthood as social contexts become more stable (increasing the likelihood that kind acts are later reciprocated) and pairbonds and families form. Moreover, the progression of personality development over the lifespan does not follow the same sort of increasingly directional path as cognitive development. Average levels of personality change over the lifespan (Roberts, Walton, & Viechtbauer, 2006), but these changes are not mutually interdependent (Mõttus et al., 2012) as is the case for cognitive abilities (Rhemtulla & Tucker-Drob, 2011; Tucker-Drob, 2011). That is, while reading mastery may promote more general reasoning development (Ritchie, Bates, & Plomin, 2014), becoming more neurotic does not necessarily entail a linked change in agreeableness. Nor it is clear that personality changes are cumulative, in the sense, that one must master average openness before graduating to high openness, in contrast to cognitive development. Indeed, the shifting life roles that individuals adopt over development likely shift the optimal profile of personality, with some periods of the lifespan necessitating risk-taking and others security (Roberts et al., 2006). Thus, social forces on personality are strong, but mutate across childhood and early adulthood, and are less institutionalized, allowing individuals to explore identity development over a protracted period of time. Because adaptive profiles of personality shift with fluctuating social roles, evolutionary pressures may have equipped humans with protracted developmental periods of personality plasticity. A plausible effect of these developmental, social, and evolutionary pressures, when combined with active and evocative processes, may be that trajectories of personality development canalize relatively late in development and respond to idiosyncratically and arbitrarily experienced environments, superimposed on a backdrop of genetically-influenced tendencies.
Similarities and Differences between Personality and Cognition
Following from Cronbach’s (1949) distinction between “maximal” performance and “typical” behavior, the field of differential psychology has largely explored cognitive and personality development in isolation. Many findings from these parallel lines of research are, however, remarkably similar. Both cognitive ability and personality are hierarchically organized (Deary, 2001; John, Naumann, & Soto, 2008; McCrae & Costa, 2008; Spearman, 1904), follow consistent lifespan patterns of mean-level development (McArdle, Ferrer-Caja, Hamagami, & Woodcock, 2002; Roberts et al., 2006), and become increasingly stable across the lifespan (Bayley, 1949; Roberts & DelVecchio, 2000). Many of these empirical results hold across cultures (Georgas, Weiss, van de Vijver, & Saklofske, 2003; McCrae et al., 1999) and in nonhuman populations (Gosling, 2001; Herrmann, Hernández-Lloreda, Call, Hare, & Tomasello, 2010; Weiss & King, 2014). In modern societies, both cognitive ability and personality have implications for social disparities in life opportunity (Damian, Su, Shanahan, Trautwein, & Roberts, 2014; Shanahan, Bauldry, Roberts, Macmillan, & Russo, 2014).
At the same time, behavior genetic research on the factor structure of cognition and personality hint at potential differences between the two domains. Variation shared by diverse measures of cognitive ability result largely from pleiotropic genetic effects (Alarcón, Plomin, Fulker, Corley, & DeFries, 1999; Casto, DeFries, & Fulker, 1995; Rijsdijk, Vernon, & Boomsma, 2002). Based on this evidence, the “generalist genes” hypothesis predicts that the same genes affect a wide variety of cognitive abilities and disabilities, resulting in the positive manifold (Kovas & Plomin, 2006; Plomin & Kovas, 2005). From a developmental perspective, these results are consistent with models in which the emergence of the general factor of ability results from reciprocal gene-environment transactions: Initial genetic effects are narrow and specific to individual abilities; these abilities lead to exposure to higher quality environments; these environments improve all abilities in concert (Dickens, 2007; van der Maas et al., 2006).
In contrast, facet-level analyses of personality indicate that multiple, distinguishable sets of genetic and environmental influences are necessary to explain personality dimensions (Briley & Tucker-Drob, 2012; Franić, Borsboom, Dolan, & Boomsma, 2014; Jang, McCrae, Angleitner, Riemann, & Livesley, 1998; Jang et al., 2002; Johnson & Krueger, 2004; McCrae et al., 2001). This is no strong evidence for “generalist” genes for personality: the Big Five tap heterogeneous and relatively independent sets of etiological influences. The developmental pressures on personality traits do not appear to follow the same pattern of mutually reinforcing cumulative growth at a highly general level that is seen with cognitive abilities. In summary, genes have a widespread effect on broad cognitive measures but more limited effects on narrow and distinct personality facets. It is possible that these features of personality and cognition emerge as individuals mature, gene-environment interplay accumulates, and patterns of behavior solidify (Cheung, Harden, & Tucker-Drob, in press; van der Maas et al., 2006).
Lifespan Trends in Genetic and Environmental Influences
Cognitive ability and personality are well-known for being among the most (differentially) stable psychological characteristics (Conley, 1984). However, the stability of these traits is not uniform over development. Rather, cognition and personality increase in their differential stabilities with age. Measures of cognitive ability in infancy and very early childhood are only weakly predictive of later ability (Lewis & McGurk, 1972). Yet, across the first decade of life, the predictive validity of measures of cognition for later assessments dramatically increases (Bayley, 1949). The level of stability found in adolescence persists across the majority of the lifespan. Gow and colleagues (2011) found that measures of cognition at age 11 substantially predict ability at age 87. Similarly, Larsen, Hartmann, and Nyborg (2008) found minimal evidence of ability reordering across nearly 20 years in midlife. In the personality domain, measures of infant and young child temperament are predictive of later personality (Caspi et al., 2003). Despite this slightly more stable starting point, the stability of personality increases from a stability coefficient of approximately .3 to .7 by middle adulthood (Roberts & DelVecchio, 2000). Correcting stability estimates for the effect of measurement error, there is minimal evidence of reordering over relatively long periods of time in adulthood (Fergusson, 2010). Thus the stability of individual differences in personality follows a similar lifespan trend as cognitive ability, but the increase in stability occurs over the first three decades of life rather than only the first. In this section, we provide a review of our meta-analytic work on the genetic and environmental mechanisms underlying these dramatic developmental increases in stability. We first describe the meta-analytic dataset and discuss empirical work on age trends in the genetic and environmental contributions to cognition and personality variation at single points in time.
Meta-Analytic Dataset
Complete details concerning the source of the meta-analytic data are extensively discussed in previous publications (Briley & Tucker-Drob, 2014; Tucker-Drob & Briley, 2014). To summarize, our dataset contains parameters from longitudinal, behavior genetic studies of personality and cognition. For cognition, this included 21 articles or chapters based on 15 independent samples composed of 12,721 sibling pairs of various types (e.g., monozygotic twins, dizygotic twins, adoptive siblings, etc.). Behavior genetic parameters were estimated for 150 pairs of time points and measures, with baseline ages ranging from .5 years to 72.7 years. The average test-retest interval was 5.9 years (SD = 5.5). The majority of data points were clustered over the first 20 years of the lifespan. Considerable data was also available for late adulthood, with relatively few studies focusing on midlife. Effect sizes were coded based on whether general intelligence (62.3% of effect sizes), fluid intelligence (37.7%), or crystallized intelligence (16.2%) was under investigation. Results differed minimally across measures of ability, and we therefore focus on trends for all measures of cognition (see Tucker-Drob & Briley, 2014 for additional details).
For personality, the meta-analytic data was drawn from 24 articles based on 21 independent samples composed of 21,057 sibling pairs. Behavior genetic parameters were estimated for 251 combinations of time points and measures, with baseline ages ranging from 1.0 year to 84.3 years. The average test-retest interval was 5.4 years (SD = 2.9). In contrast to cognition, data density for personality was high across the lifespan. Effect sizes were coded based on Big Five trait, report format (self- vs. informant-report), and trait generality (broad vs. narrow). Extraversion and neuroticism were the most studied traits with over 90 associated effect sizes each. Openness, the least studied trait, was represented by 30 effect sizes. Results were remarkably similar across each of the Big Five. Informant-reports made up 23.3% of the total dataset. Results also did not differ substantially by report format, and sensitivity analyses focusing only on self-reports or informant-reports uncovered largely similar trends (see Briley & Tucker-Drob, 2014 for additional details). Lifespan trends in genetic and environmental stability for personality are not obscured by differences in report format and the associated potential influence of method bias. Broad and narrow measures of personality were represented nearly equally, and again, results were very consistent across trait generality. For these reasons, we focus on trends for all measures of personality.
Heritability and Environmentality
Univariate behavior genetic methodology typically decomposes variance into additive genetic effects (which serve to make more genetically related individuals more similar on the phenotype), shared environmental effects (which serve to make individuals raised together more similar on the phenotype, regardless of genetic relatedness), and nonshared environmental effects (which serve to differentiate individuals raised together, even genetically identical individuals, i.e. monozygotic twins, and may include measurement error). This decomposition is accomplished by comparing the phenotypic resemblance of pairs of individuals who vary in their degree of genetic relatedness (e.g., monozygotic twins share 100% of their genes, whereas dizygotic twins share approximately 50% of their segregating genes, on average) and/or shared rearing experience (e.g. genetically unrelated adopted siblings raised together, monozygotic twins raised apart). (see Johnson et al., 2011 for a more complete description of the nuances of behavior genetic models).2
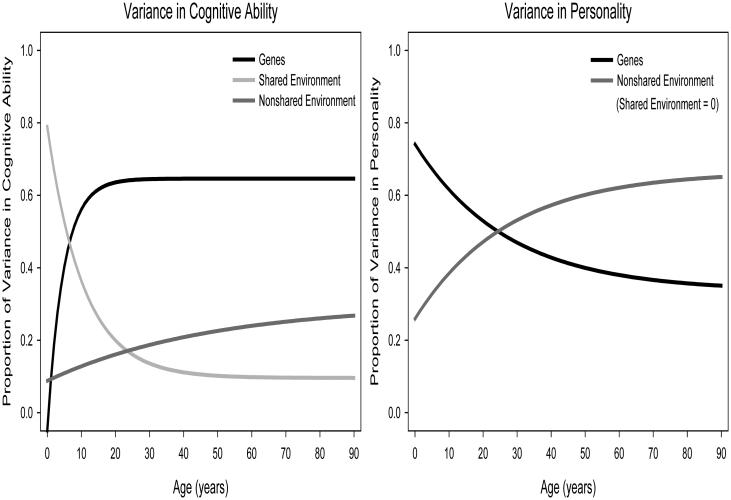
Figure 1 displays lifespan trends in the proportion of variation in cognitive ability and personality associated with genetic and environmental effects. Genetic influences on cognitive ability increase dramatically over the first two decades of life (Briley & Tucker-Drob, 2013). In early childhood, approximately 20% of the variation in cognitive ability is associated with genetic variation, but this increases to nearly 70% by late adolescence. In parallel, shared environmental effects on cognition decrease from explaining the majority of variation in ability to having minimal or no effect. Nonshared environmental influences explain approximately 20% of the variation in ability regardless of developmental period. The decline in shared environmental influences primarily results from decay processes, meaning that early shared environmental effects (e.g., effects of family-of-origin socioeconomic status and parenting that serve to make children raised together more similar to one another in their cognitive abilities), do not persist at full strength across time. The increase in genetic influences, on the other hand, is primarily due to amplification effects, in that early genetic effects explain greater amounts of variability at later ages.
Figure 1.
Proportion of variation in cognitive ability and personality attributable to genetic, shared environmental, and nonshared environmental effects based on data from Briley and Tucker-Drob (2013; 2014) and Tucker-Drob and Briley (2014). Shared environmental effects on personality are infrequently encountered, and for the current purposes have been fixed to zero, rather than estimated at zero.
Variation in personality is attributable to genetic and nonshared environmental effects in relatively equal proportion (Briley & Tucker-Drob, 2014). Shared environmental effects were not consistently observed at any point in the lifespan based on the studies included in the meta-analysis (cf., Buchanan, McGue, Keyes, & Iacono, 2009). Genetic influences may be slightly larger in infancy and very early childhood, but it is not entirely clear whether this trend depends on method effects (i.e., contrast effects due to reliance on parent reports). Even if not due to method effects, the decline in genetic effects on personality is modest, particularly compared to the increase in heritability for cognition. The least conservative estimate of the decline in genetic effects on personality implies a decrease of only approximately 20%, from explaining 75% of the variation to 55% over the first 20 years of life.
Taken together, the lifespan trends in heritability and environmentality imply drastically different developmental patterns. Genetic influences on cognitive ability increase substantially over early life, and in contrast, genetic influences on personality decrease modestly over early life, adolescence, and into adulthood.
Genetic and Environmental Stability
Longitudinal behavior genetic models decompose the stability of phenotypes into additive genetic, shared environmental, and nonshared environmental pathways. Conceptually, these models ask whether the genetic or environmental factors that influence a phenotype at one point in time also influence the phenotype at a later point in time. For example, a longitudinal correlated factors model (Neale & Maes, 2005) decomposes variation in a phenotype at multiple points in time into genetic and environmental effects and estimates the correlation between these factors. In this context, genetic stability refers to the correlation between genetic effects at an initial time point and effects at a later time point. Do the genetic effects that make some individuals extraverted or bright in adolescence also make them extraverted and bright in adulthood? Similarly, do the same family-level or individual-level environmental experiences impact personality and cognition at different points in time?
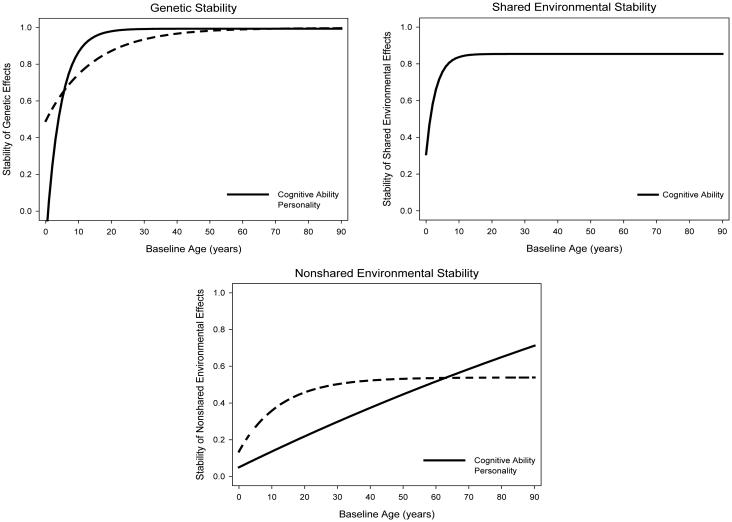
Figure 2 displays lifespan trends in the stability of genetic, shared environmental, and nonshared environmental effects on cognition and personality. Genetic effects on cognition are not at all stable in very early life, but genetic stability increases dramatically over the first decade of life (Tucker-Drob & Briley, 2014). Beginning around age 10, perfect stability of genetic effects is observed. Put differently, the genes that matter for cognition in late childhood are the same genes that matter in adolescence, adulthood, and old age. The shared environmental influences that lead children to perform better on cognitive tasks in early childhood also lead to better performance in adolescence. The stability of shared environmental effects increases from a stability coefficient of approximately .4 to .8 over the first decade of life. Nonshared environmental effects on cognition do not persist across time in childhood, but nonshared environment stability increases linearly across the lifespan. In adulthood and old age, the unique life experiences that influence ability exhibit stability coefficients of approximately .4 and .6, respectively.
Figure 2.
Stability of genetic, shared environmental, and nonshared environmental effects on cognitive ability and personality assuming approximately a six year test-retest interval based on data from Briley and Tucker-Drob (2013; 2014) and Tucker-Drob and Briley (2014). Shared environmental stability of personality is undefined because shared environmental effects account for no variation in personality.
The genetic and nonshared environmental influences on personality also increase in stability across the lifespan, but the details differ from cognition. Genetic influences on personality are already fairly stable in infancy (stability coefficient of .5), but perfect stability is not reached until mid-adulthood, much slower than the trend observed for cognition. Because personality is not affected by the shared environment, shared environmental stability is undefined. The increase in stability of nonshared environmental effects is compressed compared to cognition. Whereas the stability of nonshared environmental effects on cognition increase linearly across the lifespan, increasing even from adulthood to old age, nonshared environmental effects increase only across the first 30 years of life for personality followed by a plateau near a stability coefficient of .5.
Genetic and Environmental Contributions to Stability
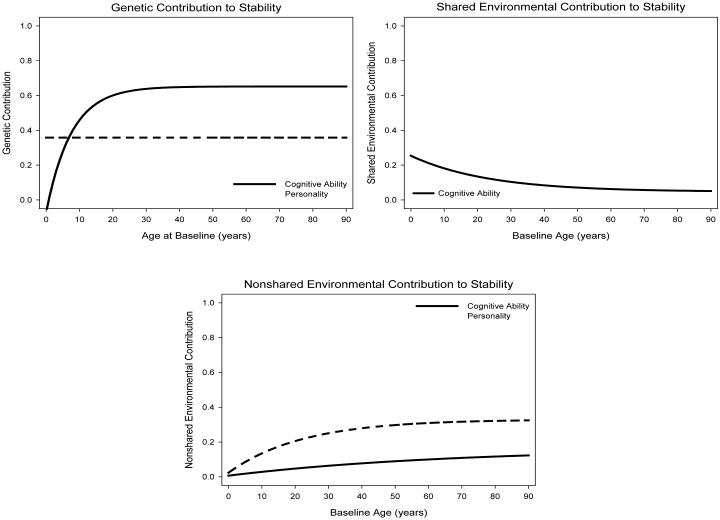
The magnitude of genetic and environmental effects is an empirically distinct question from the stability of those effects. Effects can be highly stable, but not matter much for the phenotype, or effects can be relatively unstable across time, but explain the majority of variation in the phenotype at any given age. The contribution to stability combines these sources of information. How much of the observed stability coefficient in raw correlation units is attributable to genetic and environmental sources? Figure 3 highlights lifespan trends in the genetic and environmental contributions to stability. For cognitive ability, genetic influences increase in both magnitude and stability; as a result, genetic effects increasingly contribute to phenotypic stability with child development (Tucker-Drob & Briley, 2014). By early adulthood, genes contribute approximately .6 correlation units to the observed stability. Further, the increase in phenotypic stability is almost entirely due to genetic effects, as shared environmental contributions fade out and nonshared environmental contributions are relatively flat across the lifespan. Therefore, although both environmental sources of variation do increase in stability, neither shared nor unshared environmental influences contribute much to the increasing phenotypic stability of cognition, because the shared environment accounts for little variance in cognition after children leave the home environment, and the nonshared environment accounts only modest portions variation and is only modestly stable.
Figure 3.
Genetic, shared environmental, and nonshared environmental contributions to stability in raw correlation units assuming approximately a six year test-retest interval based on data from Briley and Tucker-Drob (2014) and Tucker-Drob and Briley (2014). Shared environmental contributions for personality are undefined because shared environmental effects account for no variation in personality.
Despite the phenotypic similarities between cognition and personality, the results for genetic and environmental contributions to stability are entirely different between the two domains. Genes contribute about .35 correlation units to the stability of personality at all ages of lifespan (Briley & Tucker-Drob, 2014). As a result of the countervailing trends of decreasing heritability and increasing genetic stability, no age-trends in the genetic contribution emerge across the lifespan. This means that genes are a crucial stabilizing force for personality and are the primary stabilizing force at all ages, but genetic effects are unable to explain the increasing phenotypic stability of personality across the lifespan. Nonshared environmental contributions to stability are essentially zero in early childhood, but increasing contributions from the nonshared environment entirely explain the increase in phenotypic stability. The nonshared environmental contribution to stability plateaus in midlife at a level of approximately .33 correlation units.
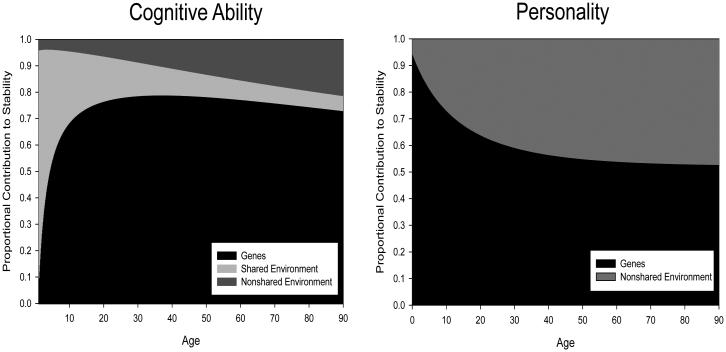
The results presented in Figure 3 demonstrate the absolute genetic and environmental contributions to stability with age. Because phenotypic stability itself changes with age, it is also useful to consider the proportional contributions to stability attributable to genetic and environmental sources. Figure 4 presents this information and highlights the striking differences between cognition and personality. In early life, shared environmental effects are the major stabilizing force for cognition. Over the first decade of life, the proportional contribution of genetic effects increases from approximately 10% to approximately 70% in the first decade of life, and largely remains at that level for the remainder of the life span. The proportional contribution of the nonshared environmental gradually increases from nearly 0% to about 20% by late life. In sharp contrast, genetic effects explain nearly 100% of the stability of personality in early life, but this proportion is slowly shaved away as the nonshared environmental contribution increases across the lifespan. By late adulthood, just over 50% of the stability of personality is attributable to genetic factors, with the remaining proportion attributable to nonshared environmental factors. Whereas genetically-linked processes guide the stabilization of cognition, unique life experiences guide the stabilization of personality across the lifespan.
Figure 4.
Proportional contribution to observed stability from genetic, shared environmental, and nonshared environmental sources on cognitive ability and personality assuming approximately a six year test-retest interval based on data from Briley and Tucker-Drob (2014) and Tucker-Drob and Briley (2014).
Implications: Are All Phenotypes the Same?
In genetics research, it is common to discuss “model phenotypes,” such as height (e.g., Lettre et al., 2008). Model phenotypes are easy to measure with reliable, valid, and quick methods allowing for massive consortia on a given topic. This empirical step is necessary to explore and test novel genetic theories and rapidly advancing technologies. An implicit or explicit assumption that sometimes follows from this work is that complex psychological phenotypes will display the same pattern of results, albeit linked to different genetic polymorphisms. From this point of view, insights into the structure of genetic effects, such as infinitesimal models of pleiotropic genetic influences, should hold as general biological truths. However, the biological and social mechanisms guiding psychological development may be more complicated than with height. Indeed, as we have highlighted in this article, complex psychological phenotypes even differ from one another in the empirical patterns of genetic and environmental mechanisms of development (cf. Harden et al., in press).
Our meta-analytic work indicates that genetic influences on cognition and its differential stability increase dramatically over child development, whereas genetic influences on personality and its stability are relatively uniform over development, with the increasing stability of personality over childhood through middle adulthood primarily attributable to nonshared environmental mechanisms. This occurs in spite of relatively similar age trends in the observed stabilities of cognition and personality. How can we understand these differing trends in light of how gene-environment interaction and correlation intersect with the differing developmental, social, and evolutionary pressures on cognition and personality?
A key point of contrast lies in the fact that cognitive development occurs via a cumulative directional process (e.g., Siegler, 1998), whereas personality development occurs over a gradually stabilizing fluctuating process (e.g., Nettle, 2007; Caspi et al., 2005). Through universal education, cognitive development is promoted continuously over child and adolescent development (Knudsen et al., 2006). If early genetically-influenced traits affect how children progress through directed schooling, however subtly, then variation in cognitive ability will become increasingly tied to genotypic variation (Bouchard, 1997). In other words, gene-environment transactions with the educational environment may result in a genetic multiplier effect for cognition (Dickens & Flynn, 2001). Potentially small initial differences in ability may be identified and reinforced by teachers, parents, and peers, which amplify genetic influences on cognition. Objectively shared environmental supports for cognitive development (e.g., stimulating family-level resources or parenting practices) may also give children a head start for academic growth. These supports, however, may not have uniform effects across individuals. Rather, high resource environments may promote genetic potentials for cognitive development and at the same time reduce effective estimates of shared environmenttality (Rhemtulla & Tucker-Drob, 2012; Tucker-Drob et al., 2011; Tucker-Drob, 2012). This may occur because higher opportunity macroenvironments facilitate the processes by which children select and evoke learning experiences on the basis of their genetically influenced “noncognitive” traits. For example, we have found that genetic variance in motivational factors, such as intellectual interest, is more strongly predictive of knowledge and academic achievement among children and adolescents raised in higher socioeconomic status circumstances, where opportunities for learning are more plentiful (Tucker-Drob & Harden, 2012a,b; Tucker-Drob & Briley, 2012; Tucker-Drob, Cheung, & Briley, 2014).
Individuals are not “trained” toward certain personalities to the same extent or with the same consistency as with cognition. Rather, they can freely explore a variety of different identities and relationship patterns (Caspi et al., 2005; McAdams & Olson, 2010).3 Early genetic influences on personality may not have a large, propulsive effect on reinforcing and amplifying growth. Rather, genetic influecnes on personality are moderately maintained over development. Our results indicate that, against a backdrop of relatively constant heritability and relatively constant genetic contributions to overall stability, personality stability increases because individuals encounter novel social experiences, and these unique environments become more stable as individuals progress through infancy, childhood, adolescence, and young adulthood. That is, variation in personality becomes increasingly linked to unique life experiences as dispositions are molded to meet the demands of slowly building social responsibilities (e.g., Specht et al., 2014). This might, in part, reflect the slow increase in the repercussions of people’s personality-relevant life decisions: For example, childhood peer groups may only last a year, a university experience is typically four years, but the choice of a career or spouse (or a mortgage) may last decades. By the time people reach age 30, when the nonshared environmental stability plateaus, many of the major life decisions regarding adult social roles – whom to marry (or whether to marry at all), whether to have children and how many, where to live, what to do for work – have been or are nearly cemented
While the types of life experiences that have a lasting impact on personality slowly come to be realized over the first approximately thirty years of life (e.g., family formation; Hutteman et al., 2014), the social and institutional structures that guide cognitive development crystallize extremely early in development (Duncan et al., 2007). Cognitive development depends on enriching and demanding experiences, and children are tracked based on their ability by schools and also based on socioeconomic resources (i.e., neighborhood-level resources affect school quality; Duncan & Murnane, 2011). Taken together, the development, social, and institutional pressures on cognition identify, magnify, and stratify levels of ability in the population such that genetically-linked mechanisms primarily explain the life course trajectories of cognition. By way of gene-environment transactions and gene × environment interactions, these sources of genetic variation are dependent on the existence of social scaffolds guiding development. Indeed, it has been suggested that identifying and adjusting these scaffolds to enrich positive person-environment transactions across socioeconomic strata may be important for addressing social inequality (Bronfrenbrenner & Ceci, 1994).
Personality development may be sensitive to social, occupational, and romantic roles (Roberts, Wood, & Smith, 2005) or major life events (e.g., “snares,” such as teen pregnancy or incarceration; Moffitt et al., 2011), and individuals form these roles over an extended period of time. But, in contrast to cognition, the developmental, social, and institutional pressures on personality unfold slowly over development. Individuals with specific constellations of traits follow diverse pathways to adulthood, and individuals may differentially respond to such unique life experiences on the basis of genetically influenced characteristics. For example, people may navigate the demands of highly competitive occupations (e.g., lawyer) based on their genetically influenced characteristics and proceed along pathways that align with their personality (i.e., choosing corporate law vs. community law). These mechanisms of gene-environment interplay may guide initially random or fluctuating environmental inputs toward increasingly sustained environmental experiences in which developmental trajectories are stabilized.
Finally, many of these same types of processes may have resulted in differing evolutionary pressures on cognition and personality in ancestral populations, resulting in different genetic architectures and lifespan trends in psychological development (see Buss & Penke, 2014; Penke, Denissen, & Miller, 2007). Cognition, as a dimension with clear adaptive and maladaptive ends, may have faced selection pressures promoting early plasticity and rapid canalization. This observation is consistent with the rapid increase in genetic stability of cognition over the first decade of life. Personality, as several dimensions with adaptive effects that may vary across social or physical context (Nettle, 2007), may have faced selection pressures promoting extended plasticity as organisms explore and conform to uncertain environmental pressures. This may help to explain why genetic contributions to personality stability are constant, rather than increasing, over development.
Conclusion: Toward A More Unified Differential Psychology
As laid out by Cronbach (1949), a number of features unify the study maximal performance and typical behavior. Psychometric methods are used to assess individual differences reliably and to discern the mechanisms guiding those differences. However, these mechanisms appear very different for two of the pillars of differential psychology, cognitive ability and personality. Broadening the study of personality and cognitive ability to consider how research in each domain can inform the other can stimulate progress in understanding both domains. An important goal of such cross-domain research is to understand more fully the mechanisms of gene-environment interplay that guide psychological development and link individual differences with important life outcomes.
Acknowledgement
Paige Harden provided valuable comments on previous versions of this article.
Funding statement
Elliot M. Tucker-Drob was supported by National Institutes of Health (NIH) Research Grant R21HD069772. Daniel A. Briley was supported by NIH Training Grant T32HD007081. The Population Research Center at the University of Texas at Austin is supported by NIH Center Grant R24HD042849.
Footnotes
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Differential stability (i.e., the stability of individual differences in reference to a population) is also often referred to as rank-order stability (i.e., the stability of one’s ranking relative to others) and is empirically assessed with a test-retest correlation (i.e., how well do scores at an initial time point predict scores in a longitudinal follow-up?).
Behavior genetic decompositions can be corrected for the effects of measurement error (unreliability) when information about test reliability is available. Correcting for unreliability reduces the nonshared environmentality estimate and increase nonshared environmental stability. Importantly, our previous analyses indicated that, while corrections for measurement error affected the overall magnitudes of nonshared environementality and nonshared environmental stability, such corrections did not dramatically alter the age trends (see Briley & Tucker-Drob, 2014; Tucker-Drob & Briley, 2014).
Some have suggested that, because personality is predictive of important life outcomes, interventions targeting personality training “are promising avenues for addressing poverty and disadvantage” (Mathilde, Duckworth, Heckman, & Kautz, 2011, p. 3). In fact, one might expect that if such interventions are effective and universally implemented over the entirety of childhood and adolescent development, then the developmental genetics of the targeted personality traits may come to more closely resemble those observed for cognitive ability and academic achievement.
References
- Alarcón M, Plomin R, Fulker DW, Corley R, DeFries JC. Molarity not modularity: Multivariate genetic analysis of specific cognitive abilities in parents and their 16-year-old children in the Colorado adoption project. Cognitive Development. 1999;14:175–193. [Google Scholar]
- Almlund M, Duckworth AL, Heckman JJ, Kautz TD. Personality psychology and economics. National Bureau of Economic Research; 2011. (No. w16822) [Google Scholar]
- Arnold DH, Doctoroff GL. The early education of socioeconomically disadvantaged children. Annual Review of Psychology. 2003;54:517–545. doi: 10.1146/annurev.psych.54.111301.145442. doi: 10.1146/annurev.psych.54.111301.145442. [DOI] [PubMed] [Google Scholar]
- Bayley N. Consistency and variability in the growth of intelligence from birth to eighteen years. The Pedagogical Seminary and Journal of Genetic Psychology. 1949;75:165–196. doi: 10.1080/08856559.1949.10533516. doi: 10.1080/08856559.1949.10533516. [DOI] [PubMed] [Google Scholar]
- Beam CR, Turkheimer E. Phenotype–environment correlations in longitudinal twin models. Development and Psychopathology. 2013;25:7–16. doi: 10.1017/S0954579412000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleidorn W, Kandler C, Caspi A. The behavioral genetics of personality development in adulthood – Classic, contemporary, and future trends. European Journal of Personality. 2014;28:244–255. [Google Scholar]
- Bouchard TJ., Jr. Experience producing drive theory: How genes drive experience and shape personality. Acta Paediatrica Supplement. 1997;422:60–64. doi: 10.1111/j.1651-2227.1997.tb18347.x. [DOI] [PubMed] [Google Scholar]
- Briley DA, Tucker-Drob EM. Broad bandwidth or high fidelity? Evidence from the structure of genetic and environmental effects on the facets of the five factor model. Behavior Genetics. 2012;42:743–763. doi: 10.1007/s10519-012-9548-8. doi: 10.1007/s10519-012-9548-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley DA, Tucker-Drob EM. Explaining the increasing heritability of cognitive ability across development: A meta-analysis of longitudinal twin and adoption studies. Psychological Science. 2013;24:1704–1713. doi: 10.1177/0956797613478618. doi: 10.1177/0956797613478618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley DA, Tucker-Drob EM. Genetic and environmental continuity in personality development: A meta-analysis. Psychological Bulletin. 2014;140:1303–1331. doi: 10.1037/a0037091. doi: 10.1037/a0037091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfenbrenner U, Ceci SJ. Nature-nurture reconceptualized in developmental perspective: A bio-ecological model. Psychological Review. 1994;101:568–586. doi: 10.1037/0033-295x.101.4.568. [DOI] [PubMed] [Google Scholar]
- Buchanan JP, McGue M, Keyes M, Iacono WG. Are there shared environmental influences on adolescent behavior? Evidence from a study of adoptive siblings. Behavior Genetics. 2009;39:532–540. doi: 10.1007/s10519-009-9283-y. doi: 10.1007/s10519-009-9283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss DM, Penke L. Evolutionary personality psychology. In: Cooper L, Larsen R, editors. Handbook of personality processes and individual differences. American Psychological Association; Washington, DC: 2014. [Google Scholar]
- Caspi A, Harrington H, Milne B, Amell JW, Theodore RF, Moffitt TE. Children’s behavioral styles at age 3 are linked to their adult personality traits at age 26. Journal of Personality. 2003;71:495–514. doi: 10.1111/1467-6494.7104001. doi: 10.1111/1467-6494.7104001. [DOI] [PubMed] [Google Scholar]
- Caspi A, Roberts BW, Shiner RL. Personality development. Annual Review of Psychology. 2005;56:453–484. doi: 10.1146/annurev.psych.55.090902.141913. [DOI] [PubMed] [Google Scholar]
- Casto SD, DeFries JC, Fulker DW. Multivariate genetic analysis of Wechsler Intelligence Scale for Children-Revised (WISC-R) factors. Behavior Genetics. 1995;25:25–32. doi: 10.1007/BF02197239. [DOI] [PubMed] [Google Scholar]
- Cheung AK, Harden KP, Tucker-Drob EM. From specialist to generalist: Developmental changes in the genetic structure of early child abilities. Developmental Psychobiology. doi: 10.1002/dev.21309. in press. [DOI] [PubMed] [Google Scholar]
- Collins WA, Maccoby EE, Steinberg L, Hetherington EM, Bornstein MH. Contemporary research on parenting: The case for nature and nurture. American Psychologist. 2000;55:218–232. doi: 10.1037/0003-066X.55.2.218. [PubMed] [Google Scholar]
- Conley JJ. The hierarchy of consistency: A review and model of longitudinal findings on adult individual differences in intelligence, personality, and self-opinion. Personality and Individual Differences. 1984;5:11–25. doi: 10.1016/0191-8869(84)90133-8. [Google Scholar]
- Cronbach LJ. Essentials of psychological testing. Harper; New York: 1949. [Google Scholar]
- Damian RI, Su R, Shanahan M, Trautwein U, Roberts BW. Can personality traits and intelligence compensate for background disadvantage? Predicting status attainment in adulthood. Journal of Personality and Social Psychology. 2014 Nov 17; doi: 10.1037/pspp0000024. Advanced online publication. http://dx.doi.org/10.1037/pspp0000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deary IJ. Intelligence: A very short introduction. Oxford University Press; New York, NY: 2001. [Google Scholar]
- Deary IJ, Weiss A., & Batty, G. D. Intelligence and personality as predictors of illness and death: How researchers in differential psychology and chronic disease epidemiology are collaborating to understand and address health inequalities. Psychological Science in the Public Interest. 2010;11:53–79. doi: 10.1177/1529100610387081. [DOI] [PubMed] [Google Scholar]
- Dick DM, Viken R, Purcell S, Kaprio J, Pulkkinen L, Rose RJ. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. Journal of Abnormal Psychology. 2007;116:213–218. doi: 10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickens WT. What is g? Brookings Institution; Washington, DC: 2007. [Google Scholar]
- Dickens WT, Flynn JR. Heritability estimates versus large environmental effects: The IQ paradox resolved. Psychological Review. 2001;108:346–369. doi: 10.1037/0033-295x.108.2.346. [DOI] [PubMed] [Google Scholar]
- Dickens WT, Turkheimer E, Beam C. The social dynamics of the expression of genes for cognitive ability. In: Kendler KS, Jaffee S, Romer D, editors. The dynamic genome and mental health: The role of genes and environments in youth development. Oxford University Press; New York, NY: 2011. pp. 103–127. [Google Scholar]
- Duncan GJ, Dowsett CJ, Claessens A, Magnuson K, Huston AC, Klebanov P, Japel C. School readiness and later achievement. Developmental Psychology. 2007;43:1428. doi: 10.1037/0012-1649.43.6.1428. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Murnane RJ. Whither opportunity? Rising inequality, schools, and children’s life chances. Russell Sage Foundation; 2011. [Google Scholar]
- Franić S, Borsboom D, Dolan CV, Boomsma DI. The Big Five personality traits: Psychological entities or statistical constructs. Behavior Genetics. 2014;44:591–604. doi: 10.1007/s10519-013-9625-7. [DOI] [PubMed] [Google Scholar]
- Georgas J, Weiss LG, van de Vijver FJ, Saklofske DH. Culture and children’s intelligence: Cross-cultural analysis of the WISC-III. Academic Press; San Diego, CA: 2003. [Google Scholar]
- Gosling SD. From mice to men: What can we learn about personality from animal research? Psychological Bulletin. 2001;127:45–86. doi: 10.1037/0033-2909.127.1.45. doi: 10.1037/0033-2909.127.1.45. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Johnson W, Pattie A, Brett CE, Roberts B, Starr JM, Deary IJ. Stability and change in intelligence from age 11 to ages 70, 79, and 87: The Lothian birth cohorts of 1921 and 1936. Psychology and Aging. 2011;26:232–240. doi: 10.1037/a0021072. doi: 10.1037/a0021072. [DOI] [PubMed] [Google Scholar]
- Harden KP, Patterson M, Briley DA, Engelhardt LE, Kretsch N, Mann FD, Tackett J, Tucker-Drob EM. Developmental changes in genetic and environmental influences on rule-breaking and aggression: Age and pubertal development. Journal of Child Psychology and Psychiatry. doi: 10.1111/jcpp.12419. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden KP, Turkheimer E, Loehlin JC. Genotype-by-environment interaction in adolescents’ cognitive aptitude. Behavior Genetics. 2007;37:273–283. doi: 10.1007/s10519-006-9113-4. doi: 10.1007/s10519-006-9113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E, Hernández-Lloreda MV, Call J, Hare B, Tomasello M. The structure of individual differences in the cognitive abilities of children and chimpanzees. Psychological Science. 2010;21:102–110. doi: 10.1177/0956797609356511. doi: 10.1177/0956797609356511. [DOI] [PubMed] [Google Scholar]
- Hicks BM, South SC, DiRago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry. 2009;66:640–648. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutteman R, Bleidorn W, Keresteš, Brković I, Butković A, Denissen JJA. Reciprocal associations between parenting challenges and parents’ personality development in young and middle adulthood. European Journal of Personality. 2014;28:168–179. doi: 10.1002/per.1932. [Google Scholar]
- Jang KL, Livesley WJ, Angleitner A, Riemann R, Vernon PA. Genetic and environmental influences on the covariance of facets defining the domains of the five-factor model of personality. Personality and Individual Differences. 2002;33:83–101. doi: 10.1016/S0191-8869(01)00137-4. [Google Scholar]
- Jang KL, McCrae RR, Angleitner A, Riemann R, Livesley WJ. Heriatbility of facet-level traits in a cross-cultural twin sample: Support for a hierarchical model of personality. Journal of Personality and Social Psychology. 1998;74:1556–1565. doi: 10.1037//0022-3514.74.6.1556. doi: 10.1037/0022-3514.74.6.1556. [DOI] [PubMed] [Google Scholar]
- John OP, Naumann LP, Soto CJ. Paradigm shift to the integrative Big Five trait taxonomy: History, measurement, and conceptual issues. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality. Guilford Press; New York, NY: 2008. pp. 114–158. [Google Scholar]
- Johnson W, Krueger RF. Genetic and environmental structure of adjectives describing the domains of the Big Five Model of personality: A nationwide US twin study. Journal of Research in Personality. 2004;38:448–472. doi: 10.1016/j.jrp.2003.11.001. [Google Scholar]
- Johnson W, Penke L, Spinath FM. Heritability in the era of molecular genetics: Some thoughts for understanding genetic influences on behavioural traits. European Journal of Personality. 2011;25:254–266. doi: 10.1002/per.836. [Google Scholar]
- Knudsen EI, Heckman JJ, Cameron JL, Shonkoff JP. Economic, neurobiological, and behavioral perspectives on building America’s future workforce. Proceedings of the National Academy of Sciences. 2006;103:10155–10162. doi: 10.1073/pnas.0600888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovas Y, Plomin R. Generalist genes: Implications for cognitive sciences. TRENDS in Cognitive Sciences. 2006;10:198–203. doi: 10.1016/j.tics.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Krueger RF. Phenotypic, genetic, and nonshared environmental parallels in the structure of personality: A view from the Multidimensional Personality Questionnaire. Journal of Personality and Social Psychology. 2000;79:1057–1067. doi: 10.1037//0022-3514.79.6.1057. doi: 10.1037/0022-3514.79.6.1057. [DOI] [PubMed] [Google Scholar]
- Larsen L, Hartmann P, Nyborg H. The stability of general intelligence from early adulthood to middle-age. Intelligence. 2008;36:29–34. doi: 10.1016/j.intell.2007.01.001. [Google Scholar]
- Lareau A. Invisible inequality: Social class and childrearing in Black and White families. American Sociological Review. 2002;67:747–776. [Google Scholar]
- Lettre G, Jackson AU, Gieger C, Schumacher FR, Berndt SI, Samma S, Hirschhorn JN. Identification of ten loci associated with height highlights new biological pathways in human growth. Nature Genetics. 2008;40:584–591. doi: 10.1038/ng.125. doi: 10.1038/ng.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis GJ, Bates TC. How genes influence personality: Evidence from multi-facet twin analyses of the HEXACO dimensions. Journal of Research in Personality. 2014;51:9–17. doi: 10.1016/j.jrp.2014.04.004. [Google Scholar]
- Lewis M, McGurk H. Evaluation of infant intelligence: Infant intelligence test scores-true or false? Science. 1972;178:1174–1177. doi: 10.1126/science.178.4066.1174. doi: 10.1126/science.178.4066.1174. [DOI] [PubMed] [Google Scholar]
- Magnuson KA, Waldfogel J. Early childhood care and education: Effects on ethnic and racial gaps in school readiness. The Future of Children. 2005;15:169–196. doi: 10.1353/foc.2005.0005. [DOI] [PubMed] [Google Scholar]
- Mann FD, Kretsch N, Tackett J, Harden KP, Tucker-Drob EM. Person × environment interactions in adolescent delinquency: Sensation seeking, peer deviance, and parental monitoring. Personality and Individual Differences. 2015;76:129–134. doi: 10.1016/j.paid.2014.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams DP, Olson BD. Personality development: Continuity and change over the life course. Annual Review of Psychology. 2010;61:517–542. doi: 10.1146/annurev.psych.093008.100507. doi: 10.1146/annurev.psych.093008.100507. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Ferrer-Caja E, Hamagami F, Woodcock RW. Comparative longitudinal structural analyses of the growth and decline of multiple intellectual abilities over the life span. Developmental Psychology. 2002;38:115–142. doi: 10.1037/0012-1649.38.1.115. [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr. The five-factor theory of personality. In: John OP, Robins RW, Pervin LA, editors. Handbook of personality. Guilford Press; New York, NY: 2008. pp. 159–181. [Google Scholar]
- McCrae RR, Costa PT, Jr., de Lima M, Simões A, Ostendorf F, Angleitner A, Piedmont RL. Age differences in personality across the adult life span: Parallels in five cultures. Developmental Psychology. 1999;35:466–477. doi: 10.1037//0012-1649.35.2.466. doi: 10.1037/0012-1649.35.2.466. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Jang KL, Livesley WJ, Riemann R, Angleitner A. Sources of structure: Genetic, environmental, and artifactual influences on the covariation of personality traits. Journal of Personality. 2001;69:511–535. doi: 10.1111/1467-6494.694154. doi: 10.1111/1467-6494.694154. [DOI] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53:185. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Arseneault L, Belskey D, Dickson N, Hancox RJ, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proceedings of the National Academy of Sciences. 2011;108:2693–2698. doi: 10.1073/pnas.1010076108. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mõttus R, Johnson W, Starr JM, Deary IJ. Correlates of personality trait levels and their changes in very old age: The lothian birth cohort 1921. Journal of Research in Personality. 2012;46:271–278. [Google Scholar]
- Neale MC, Maes HHM. Methodology for genetic studies of twins and families. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2005. [Google Scholar]
- Nettle D. Personality: What makes you the way you are. Oxford University Press; 2007. [Google Scholar]
- Ozer DJ, Benet-Martínez V. Personality and the prediction of consequential outcomes. Annual Review of Psychology. 2006;57:401–421. doi: 10.1146/annurev.psych.57.102904.190127. [DOI] [PubMed] [Google Scholar]
- Payne C, Knowles T. Promise and peril: Charter schools, urban school reform, and the Obama administration. Harvard Educational Review. 2009;79:227–239. [Google Scholar]
- Penke L, Denissen JJA, Miller GF. The evolutionary genetics of personality. European Journal of Personality. 2007;21:549–587. doi: 10.1002/per.629. [Google Scholar]
- Plomin R, Kovas Y. Generalist genes and learning disabilities. Psychological Bulletin. 2005;131:592–617. doi: 10.1037/0033-2909.131.4.592. doi: 10.1037/0033-2909.131.4.592. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. doi: 10.1037/0033-2909.84.2.309. [PubMed] [Google Scholar]
- Rhemtulla M, Tucker-Drob EM. Gene-by-socioeconomic status interaction on school readiness. Behavior Genetics. 2012;42:549–558. doi: 10.1007/s10519-012-9527-0. doi: 10.1007/s10519-012-9527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijsdijk FV, Vernon PA, Boomsma DI. Application of hierarchical genetic models to Raven and WAIS subtests: A Dutch twin study. Behavior Genetics. 2002;32:199–210. doi: 10.1023/a:1016021128949. [DOI] [PubMed] [Google Scholar]
- Rhemtulla M, Tucker-Drob EM. Correlated longitudinal changes across linguistic, achievement, and psychomotor domains in early childhood: Evidence for a global dimension of development. Developmental Science. 2011;14:1245–1254. doi: 10.1111/j.1467-7687.2011.01071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SJ, Bates TC, Plomin R. Does Learning to Read Improve Intelligence? A Longitudinal Multivariate Analysis in Identical Twins From Age 7 to 16. Child development. 2014;86:23–36. doi: 10.1111/cdev.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: A quantitative review of longitudinal studies. Psychological Bulletin. 2000;126:3–25. doi: 10.1037/0033-2909.126.1.3. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Jackson JJ. Sociogenomic personality psychology. Journal of Personality. 2008;76:1523–1544. doi: 10.1111/j.1467-6494.2008.00530.x. doi: 10.1111/j.1467-6494.2008.00530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, Kuncel NR, Shiner R, Caspi A, Goldberg LR. The power of personality: The comparative validity of personality traits, socioeconomic status, and cognitive ability for predicting important life outcomes. Perspectives on Psychological Science. 2007;2:313–345. doi: 10.1111/j.1745-6916.2007.00047.x. doi: 10.1111/j.1745-6916.2007.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts BW, Walton KE, Viechtbauer W. Patterns of mean-level change in personality traits across the life course: A meta-analysis of longitudinal studies. Psychological Bulletin. 2006;132:1–25. doi: 10.1037/0033-2909.132.1.1. doi: 10.1037/0033-2909.132.1.1. [DOI] [PubMed] [Google Scholar]
- Roberts BW, Wood D, Smith JL. Evaluating Five Factor Theory and social investment perspectives on personality trait development. Journal of Research in Personality. 2005;39:166–184. doi: 10.1016/j.jrp.2004.08.002. [Google Scholar]
- Rowe DC, Jacobson KE, van den Oord E. Genetic and environmental influences on vocabulary IQ: Parental education level as a moderator. Child Development. 1999;70:1151–1162. doi: 10.1111/1467-8624.00084. doi: 10.1111/1467-8624.00084. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype → environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Scarr-Salapatek S. Race, social class, and IQ. Science. 1971;174:1285–1295. doi: 10.1126/science.174.4016.1285. doi: 10.1126/science.174.4016.1285. [DOI] [PubMed] [Google Scholar]
- Schmidt FL, Hunter JE. The validity and utility of selection methods in personnel psychology: Practical and theoretical implications of 85 years of research findings. Psychological Bulletin. 1988;124:262–274. [Google Scholar]
- Siegler RS. Emerging minds: The process of change in children's thinking. Oxford University Press; 1998. [Google Scholar]
- Shanahan MJ, Bauldry S, Roberts BW, Macmillan R, Russo R. Personality and the reproduction of social class. Social Forces. 2014;93:209–240. [Google Scholar]
- Spearman C. “General intelligence” objectively determined and measured. American Journal of Psychology. 1904;15:201–293. [Google Scholar]
- Specht J, Bleidorn W, Denissen JJA, Hennecke M, Hutteman R, Kandler C, Zimmermann J. What drives adult personality development? A comparison of theoretical perspectives and empirical evidence. European Journal of Personality. 2014;28:216–230. doi: 10.1002/per.1966. [Google Scholar]
- Tabery J. Biometric and developmental gene-environment interactions: Looking back, moving forward. Development and Psychopathology. 2007;19:961–976. doi: 10.1017/S0954579407000478. doi: 10.1017/S0954579407000478. [DOI] [PubMed] [Google Scholar]
- Taylor J, Roehrig AD, Soden-Hensler B, Connor CM, Schatschneider C. Teacher quality moderates the genetic effects on early reading. Science. 2010;328:512–514. doi: 10.1126/science.1186149. doi: 10.1126/science.1186149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM. Global and domain-specific changes in cognition throughout adulthood. Developmental Psychology. 2011;47:331–343. doi: 10.1037/a0021361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM. Preschools reduce early academic achievement gaps: A longitudinal twin approach. Psychological Science. 2012;23:310–319. doi: 10.1177/0956797611426728. Doi: 10.1177/0956797611426728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Briley DA. Socioeconomic status modifies interest-knowledge associations among adolescents. Personality and Individual Differences. 2012;53:9–15. doi: 10.1016/j.paid.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Briley DA. Continuity of genetic and environmental influences on cognition across the life span: A meta-analysis of longitudinal twin and adoption studies. Psychological Bulletin. 2014;140:949–979. doi: 10.1037/a0035893. doi: 10.1037/a0035893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Harden KP. Intellectual interest mediates gene × socioeconomic status interaction on adolescent academic achievement. Child Development. 2012a;83:743–757. doi: 10.1111/j.1467-8624.2011.01721.x. doi: 10.1111/j.1467-8624.2011.01721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Harden KP. Learning motivation mediates gene-by-socioeconomic status interaction on early mathematics achievement. Learning and Individual Differences. 2012b;22:37–45. doi: 10.1016/j.lindif.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Briley DA, Harden KP. Genetic and environmental influences on cognition across development and context. Current Directions in Psychological Science. 2013;22:349–355. doi: 10.1177/0963721413485087. doi: 10.1177/0963721413485087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Cheung AK, Briley DA. Gross domestic product, science Interest, and science achievement: A person × nation interaction. Psychological Science. 2014;25:2047–2057. doi: 10.1177/0956797614548726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker-Drob EM, Rhemtulla M, Harden KP, Turkheimer E, Fask D. Emergence of a Gene × Socioeconomic Status interaction on infant mental ability between 10 months and 2 years. Psychological Science. 2011;22:125–133. doi: 10.1177/0956797610392926. doi: 10.1177/095679610392926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, nofrio B. M., & Gottesman, I. I. Socioeconomic status modifies heritability of IQ in young children. Psychological Science. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- van der Maas HLJ, Dolan CV, Grasman RPPP, Wicherts JM, Huizenga HM, Raijmakers MEJ. A dynamical model of general intelligence: The positive manifold of intelligence by mutualism. Psychological Review. 2006;113:842–861. doi: 10.1037/0033-295X.113.4.842. [DOI] [PubMed] [Google Scholar]
- Weiss A, King JE. Great ape origins of personality maturation and sex differences: A study of orangutans and chimpanzees. Journal of Personality and Social Psychology. 2014 Nov 17; doi: 10.1037/pspp0000022. Advanced online publication. http://dx.doi.org/10.1037/pspp0000022. [DOI] [PubMed] [Google Scholar]
- Yamagata S, Atsunobu S, Ando J, Ono Y, Kijima N, Yoshimura K, Jang KL. Is the genetic structure of human personality universal? A cross-cultural twin study from North America, Europe, and Asia. Journal of Personality and Social Psychology. 2006;90:987–998. doi: 10.1037/0022-3514.90.6.987. doi: 10.1037/0022-3514.90.6.987. [DOI] [PubMed] [Google Scholar]