Abstract
Background
Women with a history of false-positive mammogram result may be at increased risk of developing subsequent breast cancer.
Methods
Using 1994 to 2009 Breast Cancer Surveillance Consortium data we included women ages 40–74 years with a screening mammogram that resulted in a false-positive with recommendation for additional imaging, false-positive with recommendation for biopsy, or true-negative with no cancer within one year following the examination. We used partly conditional Cox proportional hazards survival models to assess the association between a false-positive mammogram result and subsequent breast cancer, adjusting for potential confounders. Adjusted survival curves stratified by breast density and false-positive result were used to evaluate changes in risk over time.
Results
During 12,022,560 person-years of follow-up, 48,735 cancers were diagnosed. Compared to women with a true-negative examination, women with a false-positive with additional imaging recommendation had increased risk of developing breast cancer (adjusted hazard ratio (aHR)=1.39, 95%CI:1.35–1.44) as did women with a false-positive with a biopsy recommendation (aHR=1.76, 95%CI:1.65–1.88). Results stratifying by breast density were similar to overall results except among women with almost entirely fatty breasts in which aHRs were similar for both false-positive groups. Women with a false-positive result had persistently increased risk of developing breast cancer 10-years after the false-positive examination.
Conclusion/Impact
Women with a history of a false-positive screening mammogram or biopsy recommendation were at increased risk of developing breast cancer for at least a decade, suggesting that prior false-positive screening may be useful in risk prediction models.
Keywords: Screening mammogram, breast cancer, false positive result, breast density, risk prediction models
Introduction
In the United States an estimated 67% of women ages 40 and older undergo screening mammography every 1–2 years (1). Among these screening examinations, approximately 16% of first and 10% of subsequent mammograms will generate a false-positive result (2). Over the course of ten screening mammograms, the estimated cumulative probability of at least one false-positive result is 61% for women screened annually and 42% for women screening biennially (2).
Prior studies examining the risk of breast cancer among women with a history of a false-positive screening mammogram report conflicting findings (3–8). The majority of these studies were conducted in Europe where false-positive rates are substantially lower than in the United States (9–10). The one study conducted in the United States found that a false-positive result was predictive of future breast cancer risk among postmenopausal but not premenopausal women (6). Although breast density is a strong risk factor for breast cancer and is also associated with elevated false-positive rates (11), no previous study has examined whether a false-positive result influences the risk of developing breast cancer differently for those with non-dense vs. dense breasts. Given that false-positives are common in the U.S., the attributable risk associated with false-positive examinations could be substantial if the association with breast cancer is strong.
Our study sought to extend current knowledge by examining whether the relationship between history of a false-positive screening mammogram result and the risk of developing breast cancer varies according to the type of recommendation associated with false-positive results or by mammographic breast density. Using data from the Breast Cancer Surveillance Consortium (BCSC) from 1994 to 2009 from seven registries, we evaluated the association between false-positive mammograms with differing follow-up recommendations (either workup with imaging alone or workup involving biopsy) and breast cancer risk overall and stratified by breast density. We hypothesized that there would be a greater increase in breast cancer risk among women with a history of false-positive biopsy recommendation results compared to those with a false-positive additional imaging recommendation and that this association would be independent of breast density.
Materials and Methods
Data Sources
We utilized data from the BCSC, which are representative of the US population (12). Specifically, we included data from seven registries located in New Hampshire, North Carolina, San Francisco Bay Area, Western Washington state, New Mexico, Colorado and Vermont. Details on the BCSC have been described previously (13). Briefly, at each registry prospective data collection includes self-reported demographic characteristics, indication for mammogram, breast cancer risk factors, Breast Imaging Reporting and Data Systems (BI-RADS) mammography assessment and breast density (14), and the radiologists’ management recommendations. The patient and mammography data are linked with pathology databases and state cancer registry data to obtain information on subsequent cancer diagnoses. Data from each registry are sent to a central Statistical Coordinating Center for quality control and pooled analyses. Each registry and the Statistical Coordinating Center received institutional review board approval for either active or passive consent or a waiver of consent to enroll participants, link study data, and perform analytic studies. All procedures are Health Insurance Portability and Accountability Act compliant and all registries as well as the Statistical Coordinating Center have received a Federal Certificate of Confidentiality.
Study Population
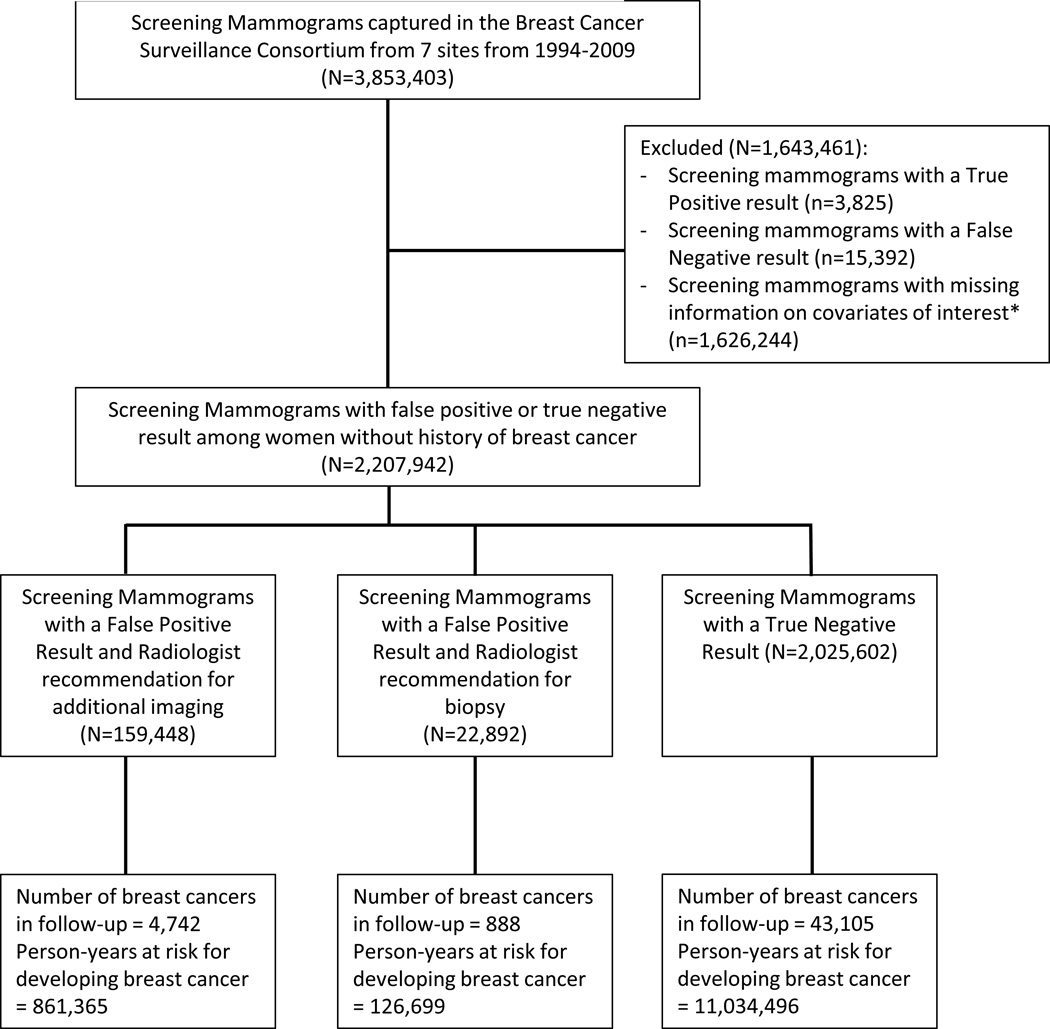
We included screening mammograms received by women ages 40 to 74 years performed between 1994 and 2009. We excluded women with a prior breast cancer diagnosis and women who lacked complete information on age, race/ethnicity, family history of breast cancer, history of breast biopsy, or BI-RADS breast density. From pathology and cancer registry data we classified women as having incident breast cancer if a diagnosis of invasive carcinoma or ductal carcinoma in situ occurred (Figure 1).
Figure 1. Flowchart of Study Population.
Measures and Definitions
At each mammography examination, women completed a questionnaire on demographic and breast health history. Women self-reported race and ethnicity and were categorized as non-Hispanic white, non-Hispanic black, Hispanic, Asian/Native Hawaiian/Pacific Islander, Native American/Native Alaskan, or other. Women were categorized as having a family history of breast cancer if they had at least one first-degree relative with the disease, irrespective of the age of the relative at the time of diagnosis. The time since prior mammography was determined using information from the radiology practice or self-reported information from the woman. History of a breast biopsy was ascertained from both pathology records and self-report. Mammographic breast density was assessed by the radiologist interpreting the screening mammogram using the BI-RADS breast density categories of almost entirely fat, scattered fibroglandular densities, heterogeneously dense, and extremely dense (14).
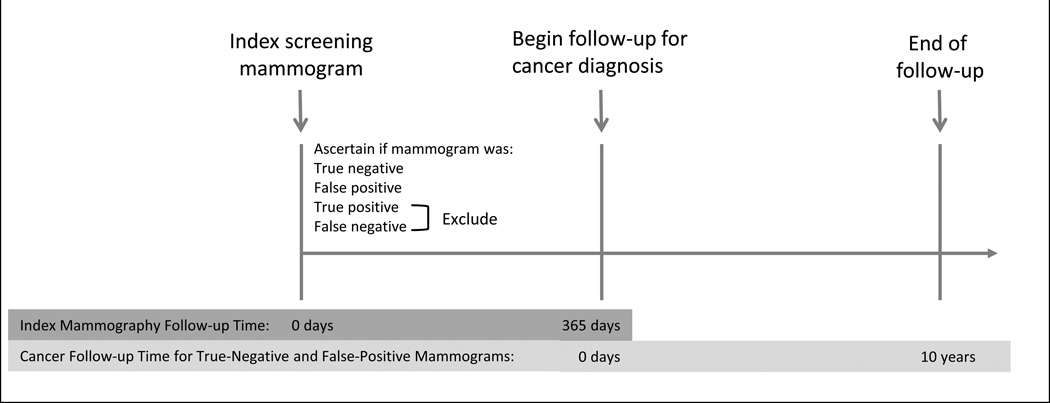
Each index screening mammogram was classified as false-positive, true-negative, false-negative, or true-positive based on (1) the radiologists’ interpretation using the BI-RADS lexicon and (2) if breast cancer was diagnosed within one year of the screening mammogram (Figure 2). Positive assessments were defined as those with an initial BI-RADS assessment of 0, 4, 5, or 3 when accompanied by a recommendation for immediate evaluation. Screening mammograms with a positive assessment and no cancer diagnosis in the following one-year were classified as false-positive examinations. Negative assessments were defined as those with a BI-RADS assessment of 1, 2, or 3 if not accompanied by a recommendation for immediate evaluation. Mammograms with a negative assessment and no cancer diagnosis within one year were classified as true-negative examinations. Screening mammograms with positive assessment and cancer diagnosis within one-year were classified as true-positive. Screening mammograms with a negative assessment and a cancer diagnosis within one-year were classified as false-negative examinations. We excluded true-positive or false-negative mammograms, as cancers associated with these examinations were assumed to have been present at the time of the screening mammogram and our study focused on cancers diagnosed subsequent to the index screening examination.
Figure 2. Study Design.
Follow-up for breast cancer diagnosis began one year after each index screening mammogram and continued for ten years or through the end of 2011, whichever occurred first (Figure 2). Since we were interested in comparing the risk of breast cancer among women with and without a false positive result, we included screening examinations performed through 2009 to allow for a minimum of one year to determine if the screening exam resulted in a false-positive and another year to follow women for breast cancer diagnosis.
Among screening examinations with a false-positive result, we subdivided mammograms, based on the radiologists’ BI-RADS assessment and recommendations following the BI-RADS 4th edition atlas, as those with a recommendation for additional imaging only and those with a recommendation for biopsy. Mammograms with a final BI-RADS assessment at the end of all imaging evaluation of 4, 5, or 0 or 3 when accompanied by a recommendation for biopsy or surgical consultation were classified as false-positive biopsy recommendations. All other false-positives were classified as false-positive imaging recommendations. Hence our interest was in comparing three groups of women: those with a false-positive result who were recommended for additional imaging only (false-positive with imaging recommendation), those with a false-positive result who were recommended for biopsy (false-positive with biopsy recommendation), and those with true-negative examinations.
Statistical Analysis
We compared demographics and risk factors of the 3 groups: false-positive with imaging recommendation, false-positive with biopsy recommendation, and true-negatives. We examined the number of breast cancer cases, the person-years (P-Y) at risk for developing breast cancer and the breast cancer rate overall and stratified by the four BI-RADS breast density categories. Exact Poisson confidence intervals are provided for breast cancer rates.
To determine if women with a history of a false-positive versus a true-negative screening result were more likely to continue to be screened and thus may appear to have higher cancer rates, we computed the proportion of women in each group (true-negative and false-positive) who received subsequent screening mammograms.
We used a partly conditional Cox proportional hazard survival model to assess the association between a false-positive mammography result (with additional imaging or with biopsy recommendation separately) and breast cancer (15). By using the partly conditional Cox model, we were able to include all mammograms received by an individual woman while accounting for within-woman correlation. In these analyses, the mammogram was the unit of analysis. Each false-positive or true-negative mammogram initiated a new follow-up period, which continued until the first of breast cancer diagnosis or censoring by death, end of health plan enrollment (for women in the Western Washington state registry), ten years of follow-up, or the end of the study period. All models were adjusted for age, race/ethnicity, menopausal status, breast density, history of breast biopsy, time since prior mammogram, and family history of breast cancer. We investigated models with three levels of interaction between density and false-positive mammography results. First, we included only main effects of density and false-positive results without interaction terms. This model assumed that the association between false-positive results and survival was the same for all densities. Next we added the interaction between false-positive results and breast density to investigate possible differential effects of false-positive results for women with different breast densities. Finally, we fit a model with baseline hazards stratified by breast density and false-positive results to investigate possible time-varying relationships between false-positive groups for each breast density group. Hence, adjusted hazard ratios and 95% confidence intervals (CI) for false-positive results are reported (1) overall and (2) separately by breast density based on the interaction model. Results of models with stratified baseline hazards based on density and false-positive results are presented graphically, with follow-up beginning at 12 months after the screening mammogram, in the form of adjusted survival curves since no single hazard ratio represents the effect of false-positive results for these models. The proportional hazards assumption was assessed by testing for a statistically significant interaction between the covariate and log time. This test found no evidence of violation of the proportional hazards assumption.
All analyses were conducted using R version 2.15.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
The study population included 2,207,942 screening mammograms performed in 1,297,906 women. The highest proportion of false-positive examinations with recommendations for additional imaging or biopsy occurred among women ages 40–49 years (34.8% and 33.1%, respectively) (Table 1). False-positive results were also common among pre-menopausal women. A higher proportion of false-positive examinations were present among women with heterogeneously or extremely dense breasts compared to women with almost entirely fat breasts or scattered fibroglandular densities.
Table 1.
Characteristics of the Study Population by Screening Mammogram Result, Breast Cancer Surveillance Consortium
| Mammogram Classification | ||||||
|---|---|---|---|---|---|---|
| Characteristic | FP, Additional Imaging N=159,448 |
FP, Biopsy N=22,892 |
True-Negative N=2,025,602 |
|||
| N | % | N | % | N | % | |
| Age, years | ||||||
| 40–49 | 55,505 | 34.8 | 7,577 | 33.1 | 531,206 | 26.2 |
| 50–59 | 51,227 | 32.1 | 7,417 | 32.4 | 661,656 | 32.7 |
| 60–69 | 39,496 | 24.8 | 5,909 | 25.8 | 605,282 | 29.9 |
| 70–74 | 13,220 | 8.3 | 1,989 | 8.7 | 227,458 | 11.2 |
| Race/Ethnicity | ||||||
| White | 137,051 | 86.0 | 19,063 | 83.3 | 1,680,964 | 83.0 |
| Black | 6,646 | 4.2 | 1,413 | 6.2 | 108,256 | 5.3 |
| Asian, Native Hawaiian, Pacific Islander | 5,005 | 3.1 | 876 | 3.8 | 86,173 | 4.3 |
| Hispanic | 9,946 | 6.2 | 1,405 | 6.1 | 138,578 | 6.8 |
| American Indian/Alaska Native | 8,00 | 0.5 | 135 | 0.6 | 11,631 | 0.6 |
| Menopausal Status | ||||||
| Pre/Peri- | 63,111 | 39.6 | 8,749 | 38.2 | 605266 | 29.9 |
| Post- | 96,337 | 60.4 | 14,143 | 61.8 | 1420336 | 70.1 |
| Family History of Breast Cancer | ||||||
| Yes | 25,021 | 15.7 | 3,678 | 16.1 | 311,419 | 15.4 |
| No | 134,427 | 84.3 | 19,214 | 83.9 | 1,714,183 | 84.6 |
| History of breast biopsy | ||||||
| Yes | 38,970 | 24.2 | 6,675 | 29.2 | 425,906 | 21.0 |
| No | 120,478 | 75.6 | 16,217 | 70.8 | 1,599,696 | 79.0 |
| BI-RADS Breast Density | ||||||
| Almost entirely fat | 7,004 | 4.4 | 1,193 | 5.2 | 197,148 | 9.7 |
| Scattered fibroglandular densities | 65,367 | 41.0 | 9,331 | 40.8 | 919,377 | 45.4 |
| Heterogeneously dense | 73,470 | 46.1 | 10,303 | 45.0 | 754,879 | 37.3 |
| Extremely dense | 13,607 | 8.5 | 2,065 | 9.0 | 154,198 | 7.6 |
FP = false-positive; BI-RADS = Breast Imaging-Reporting and Data System
For those with a true-negative result, the total P-Y at risk for developing a breast cancer after the screening examination was 11,034,496 P-Y with a total of 43,105 breast cancers occurring during this time period (Table 2), giving a breast cancer rate per 1000 P-Y of 3.91 (95%CI: 3.87, 3.94). Among those false-positives with imaging recommendation, the breast cancer rate per 1000 P-Y was 5.51 (95%CI: 5.35, 5.66). In false-positives with a biopsy recommendation, the breast cancer rate per 1,000 P-Y was 7.01 (95%CI: 6.56, 7.48). Breast cancer rates stratified by BI-RADS breast density showed similar patterns with cancer rates highest for false-positives with a biopsy recommendation and lowest for those with true-negative results for women with scattered fibroglandular densities, heterogeneously dense, or extremely dense breasts. In the almost entirely fat group, the breast cancer rates were similar regardless of false-positive recommendation type (3.69 with 95%CI: 3.07, 4.41 for false-positive with an imaging recommendation versus 3.98 with 95%CI: 2.53, 5.97, for false-positives with a biopsy recommendation).
Table 2.
Breast Cancer Rate per 1000 person-years by Screening Mammogram Result and Breast Density, Breast Cancer Surveillance Consortium
| Prior Mammogram Result / Recommendation |
Number breast cancer cases |
Person years at risk |
Breast cancer rate (95%CI) per 1000 P-Y |
|---|---|---|---|
| ALL WOMEN: | |||
| True-negative | 43,105 | 11,034,496 | 3.91 (3.87, 3.94) |
| False-positive, additional imaging | 4,742 | 861,365 | 5.51 (5.35, 5.66) |
| False-positive, biopsy | 888 | 126,699 | 7.01 (6.56, 7.48) |
| BY BI-RADS BREAST DENSITY: | |||
| ALMOST ENTIRELY FAT | |||
| True-negative | 2,206 | 993,559 | 2.22 (2.13, 2.31) |
| False-positive, additional imaging | 123 | 33,291 | 3.69 (3.07, 4.41) |
| False-positive, biopsy | 23 | 5,774 | 3.98 (2.53, 5.97) |
| SCATTERED FIBROGLANDULAR DENSITY | |||
| True-negative | 17,884 | 5,070,911 | 3.53 (3.37, 3.47) |
| False-positive, additional imaging | 1,737 | 355,192 | 4.89 (4.51, 4.95) |
| False-positive, biopsy | 315 | 52,612 | 5.99 (5.09, 6.36) |
| HETEROGENEOUSLY DENSE | |||
| True-negative | 19,014 | 4,143,830 | 4.59 (4.52, 4.65) |
| False-positive, additional imaging | 2,372 | 398,280 | 5.96 (5.72, 6.20) |
| False-positive, biopsy | 448 | 56,984 | 7.86 (7.15, 8.62) |
| EXTREMELY DENSE | |||
| True-negative | 4,001 | 826,197 | 4.84 (4.69, 4.99) |
| False-positive, additional imaging | 510 | 74,602 | 6.84 (6.26, 7.45) |
| False-positive, biopsy | 102 | 11,329 | 9.00 (7.35, 10.92) |
BI-RADS = Breast Imaging-Reporting and Data System; CI = confidence interval; P-Y = person years
During one year of follow-up, 19.2% of women with a false-positive result and 21.0% of women with a true negative result received a subsequent screening mammogram. The proportion with a screening mammogram in the two groups are similar at 2, 3, 4 and 5 years of follow-up indicating that women with a false-positive are not more likely to be screened in the future compared to women with a true-negative.
In Cox proportional hazards models adjusted for age, race/ethnicity, history of benign breast biopsy, time since prior mammogram, and family history of breast cancer, the comparison of those with a false-positive result to those with a true-negative result are shown in Table 3, overall and allowing for an interaction between breast density and false-positive results. The adjusted hazard ratio for women with a screening mammogram with a false-positive result with an imaging recommendation was 1.39 relative to women with a true-negative result. Among women with a screening mammogram with a false-positive result with a biopsy recommendation, the adjusted hazard ratio was 1.76 relative to women with a true-negative result. In those women with almost entirely fatty breasts, the adjusted hazard ratio comparing those with a false-positive imaging recommendation to those with a true-negative result is 1.70 and comparing those with a false-positive biopsy recommendation to those with a true-negative result is 1.77. Among those with scattered fibroglandular densities, heterogeneously dense, and extremely dense breasts, having a false-positive imaging recommendation had an increased hazard of 34–44% and having a false-positive biopsy recommendation had 74–87% higher hazard (all p-values<0.01).
Table 3.
Adjusted Hazard Ratios from Cox Proportional Hazards Models for Screening Mammogram Result, Overall and Stratified by Breast Density Group
| BI-RADS BREAST DENSITY | |||||
|---|---|---|---|---|---|
| Prior Mammogram Result, Recommendation |
OVERALL aHR (95% CI)* |
Almost entirely fat aHR (95% CI)** |
Scattered fibroglandular aHR (95%CI)** |
Heterogeneously dense aHR (95% CI)** |
Extremely dense aHR (95% CI)** |
| True-negative | REF | REF | REF | REF | REF |
| False-positive, additional imaging | 1.39 (1.35, 1.44) | 1.70 (1.42, 2.04) | 1.44 (1.37, 1.52) | 1.34 (1.28, 1.40) | 1.44 (1.31, 1.58) |
| False-positive, biopsy | 1.76 (1.65, 1.88) | 1.76 (1.17, 2.66) | 1.75 (1.56, 1.95) | 1.74 (1.59, 1.91) | 1.87 (1.53, 2.27) |
aHR is adjusted hazard ratio with adjustment for age, race/ethnicity, history of breast biopsy, family history of breast cancer, previous mammogram interval, menopause status, and stratified by BI-RADS breast density
aHR is adjusted for age, race/ethnicity, history of breast biopsy, family history of breast cancer, previous mammogram interval, and menopause status
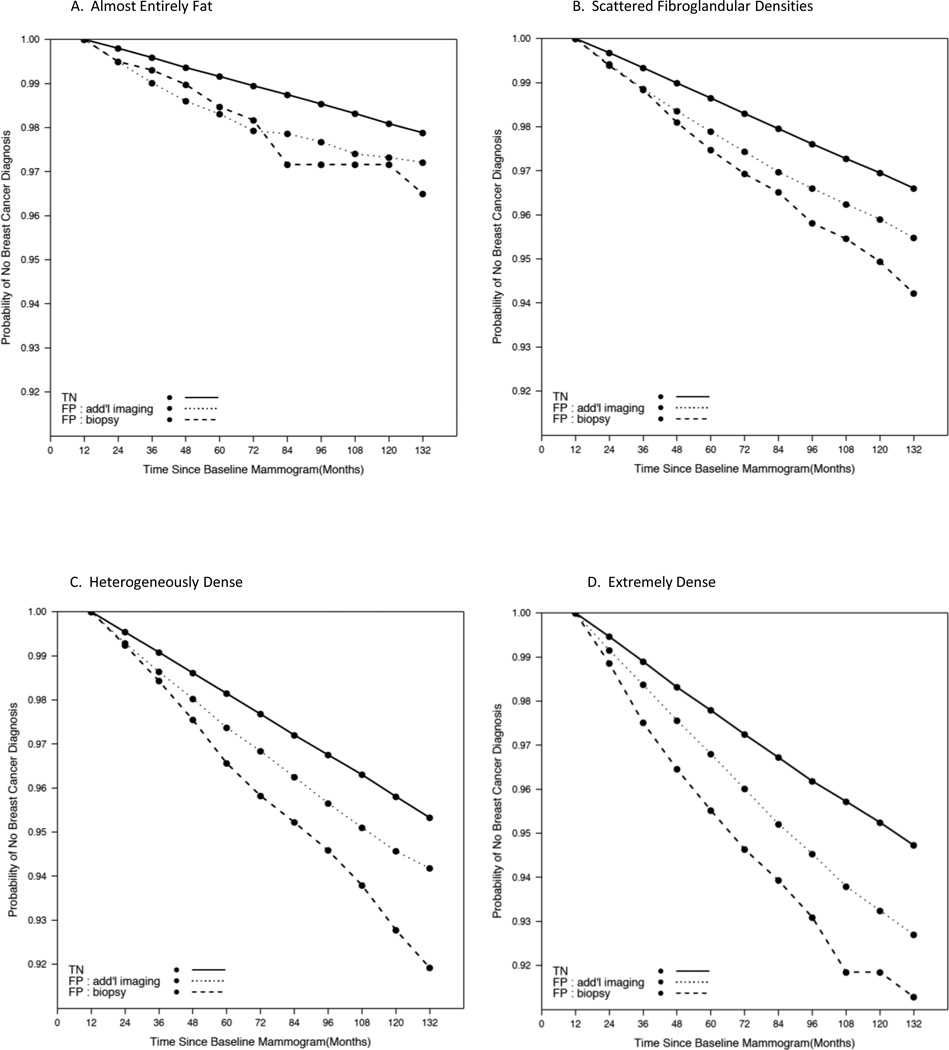
To determine if the increased risk of developing breast cancer among those with a false-positive result changed over time we examined survival curves stratified by breast density and false-positive results (Figure 3). In Figure 3, the follow-up time begins 12 months after the index mammogram. Among women with almost entirely fat breasts (Figure 3A) the probability of remaining breast cancer free for women with a true-negative result at 60 months is 99% compared with 98% for both of the false-positive groups. Both of the false-positive groups appear to have similar survival based on Figure 2A indicating a similar risk of developing breast cancer and a risk that continues to be slightly higher than that of the true-negative group as far out as 120 months (10 years). For those with scattered fibroglandular densities, the survival plot (Figure 3B) shows a similar risk for the two false-positive groups up to 24 months and then these two groups begin to diverge with the false-positive biopsy recommendation having the higher risk of developing breast cancer. For those with heterogeneously dense breasts, the risk of developing breast cancer among the false-positive groups diverge after 24 months and there is an increased risk as evidenced by the steeper lines (Figure 3C). Among those with extremely dense breasts, the risk of developing breast cancer differs among the 3 groups at 12 months and continues to diverge over time (Figure 3D).
Figure 3. Adjusted survival curves for breast cancer based on Cox proportional hazards model with baseline hazards stratified by breast density and screening mammogram result.
Models adjusted for age, race/ethnicity, menopausal status, history of breast biopsy, and family history of breast cancer. Solid black line represents true negative screening mammogram result group; Dotted black line represents false positive with additional imaging recommendation group; Dashed black line represents false positive with biopsy recommendation group. Each breast density group is represented in panels A-D where A: Almost entirely fat; B: Scattered fibroglandular densities; C: Heterogenously dense; D: Extremely dense.
Discussion
We found that women with a history of a false-positive screening mammogram result were at increased risk of developing breast cancer with those recommended for additional imaging having a 39% higher hazard and those recommended for biopsy having a 76% higher hazard compared to women with a true-negative mammogram result. Stratification by breast density revealed similar results for women with scattered fibroglandular, heterogeneously dense and extremely dense breast tissue; however among women with almost entirely fatty breasts, both false-positive groups had about a 70% higher hazard compared to women with a true-negative mammogram result. We also found that women with a history of a false-positive result continued to have an increased risk of developing breast cancer 10-years after experiencing the false-positive result.
Our findings are consistent with several studies. A 2012 study from Denmark examined the long-term risk of breast cancer and found a 67% increased risk among women with a prior false-positive result (7). More recently, data from a population-based breast cancer screening program in Spain reported that false-positive results involving fine needle aspiration cytology or a biopsy had a higher subsequent cancer detection risk than those false-positive results involving additional imaging procedures alone (8). A study from the United Kingdom found that women with a false-positive result on their first screening mammogram had a higher interval cancer rate than women with true-negative results (5). Barlow’s BCSC paper from 2006 (6) included prior false-positive mammogram in one-year risk models and found that in multivariate models, prior false-positive was associated with increased risk for postmenopausal, but not premenopausal women. However, Barlow et al. used strict criteria (p-value <0.0001) for determining which patient factors to include in the breast cancer risk prediction model, and in the premenopausal model, the variable indicating the result of the previous mammogram had a p-value of 0.0018 (6). We examined our results stratified by menopausal status and found that the HR for the false-positive recommendations were similar by menopausal status [comparing false-positive with additional imaging to true negative for premenopausal HR=1.32 (95%CI: 1.25–1.39) versus for postmenopausal HR=1.43 (95%CI: 1.38–1.48); comparing false positive with biopsy to true-negative for premenopausal HR=1.69 (95%CI: 1.49–1.91) versus for postmenopausal HR=1.78 (95%CI: 1.64–1.92)]. In contrast to other studies (5–8), two small studies from the Netherlands reported no excess breast cancer risk among women with a prior false-positive compared to women with no history of a false-positive (3, 4).
No prior studies assessed differences in breast cancer risk among women with a false-positive result stratified by breast density. The increase in risk we observed among women with false-positive imaging and biopsy recommendations was similar across density groups, with the exception of the almost entirely fat group. Notably, the absolute risk of breast cancer is higher for women with dense breast tissue. Thus, the highest risk of breast cancer observed in our study was among women with extremely dense breasts who had false-positive results with a biopsy recommendation. Among women with fatty breasts, the absolute risk of breast cancer remained relatively low for both type false-positives groups.
Our finding that breast cancer risk remains elevated up to 10 years after the false-positive result suggests that the radiologist observed suspicious findings on mammograms that are a marker of future cancer risk. Given that the initial result is a false-positive, it is possible that the abnormal pattern while noncancerous, is a radiographic marker associated with subsequent cancer. About 30% of benign breast biopsies are found to have proliferative changes with or without atypia (16). Thus, these proliferative changes could account, in part, for the increased risk associated with false-positives results with a recommendation for biopsy.
We investigated the possibility of ascertainment bias using the BCSC data to compute the proportion of women in each group (true negative and false-positive) who received subsequent screening mammograms. While this does not provide the actual rate of screening since some women may have undergone mammography outside BCSC catchment areas, we can compare the proportions between groups to see if women with false-positives obtained more screening within in the BCSC than true-negatives. Given that the proportion of women with a screening mammogram in the true negative and false positive groups is similar we find no evidence of differential ascertainment of cancer due to more frequent screening.
The current BCSC breast cancer risk prediction model includes age, race/ethnicity, first-degree relatives with breast cancer, history of breast biopsy, and BI-RADS breast density (17). This model is well calibrated in major race and ethnic groups in the US and has modest discriminatory accuracy to discern between women who will and will not develop breast cancer (concordance index=0.66). Given that the survival curves show persistent increases in risk over time and the fact that the increased risk associated with history of a false-positive is independent of breast density and history of breast biopsy, the inclusion of a prior false-positive results may improve risk prediction in the BCSC model.
Strengths of our study include the large sample size, prospective observation and the ability to follow women for an average of 5.4 years for cancer outcomes. We also were able to include multiple mammography results per woman over time, which increased the statistical power to detect differences by density category. The study population is racially and geographically diverse, with the majority of the examinations coming from community practice. Breast density was assessed using the BI-RADS classification and could be misclassified given the modest inter-rater agreement known to exist, but this is unlikely to affect the results given the similar hazard ratios for density categories (18, 19).
Our study also has several limitations. Women may have moved out of our study catchment area and thus we may miss some breast cancer diagnoses; however, we do not expect that this potential loss to follow-up would differ for women with a false-positive versus true-negative result. In addition, we excluded women with missing covariates, which could bias our findings if missingness was not at random. We believe missing values are unrelated to patient or mammogram characteristics since missing values most often arose because a mammography facility did not collect the covariate information during the time when a woman received her mammogram. Since these missing values arose structurally, they are not expected to be associated with patient characteristics and thus will not introduce bias. Another limitation is that data on laterality of the mammographic lesion was not available and thus we are unable to assess how many cancers developed at the site of the initial mammographic lesion and how many were missed cancers.
In conclusion, women with a false-positive screening mammogram result are at increased risk for developing breast cancer, with a higher risk for false-positives with a recommendation for biopsy compared with additional imaging recommendation. The risk was independent of breast density and remained elevated for a decade after receipt of the false-positive result. This information should be considered for inclusion in risk prediction models to better stratify women into risk categories that may be used to personalize breast cancer screening and primary prevention strategies for individual women.
Acknowledgement
The collection of cancer and vital status data used in this study was supported in part by several state public health departments and cancer registries throughout the US. For a full description of these sources, please see: http://breastscreening.cancer.gov/work/acknowledgement.html. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. We thank the participating women, mammography facilities, and radiologists for the data they have provided for this study. A list of the BCSC investigators and procedures for requesting BCSC data for research purposes are provided at: http://breastscreening.cancer.gov/
Financial Support
This work was supported by the National Cancer Institute-funded Breast Cancer Surveillance Consortium (contract number HHSN261201100031C); the National Cancer Institute funded Risk-Based Breast Cancer Screening in Community Settings grant (grant number P01CA154292); and the National Cancer Institute-funded Vermont Population-based Research Optimizing Screening through Personalized Regimens grant (grant number U54 CA163303). The design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript do not represent those of the National Cancer Institute, and this organization had no role in the final decision to submit the manuscript for publication.
Footnotes
Conflicts of Interest
None
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2013–2014. Atlanta: American Cancer Society, Inc.; 2013. [Google Scholar]
- 2.Hubbard RA, Kerlikowske K, Flowers CI, Yankaskas BC, Zhu W, Miglioretti DL. Cumulative probability of false-positive recall or biopsy recommendation after 10 Years of screening mammography. Ann Intern Med. 2011;155:481–492. doi: 10.1059/0003-4819-155-8-201110180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters PH, Mravunac M, Hendricks JH, Verbeek AL, Holland R, Vooijs PG. Breast cancer risk for women with a false-positive screening test. Br J Cancer. 1988;58:211–212. doi: 10.1038/bjc.1988.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groenendijk RP, Kochen MP, van Engelenburg KC, Boetes C, Strobbe LJ, Ruers TJ, et al. Detection of breast cancer after biopsy for false-positive screening mammography. An increase risk? Eur J Surg Oncol. 2001;27:17–20. doi: 10.1053/ejso.2000.1045. [DOI] [PubMed] [Google Scholar]
- 5.McCann J, Stockton D, Godward S. Impact of false-positive mammography on subsequent screening attendance and risk of cancer. Breast Cancer Res. 2002;4:R11. doi: 10.1186/bcr455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlow WE, White E, Ballard-Barbash R, Vacek PM, Titus-Ernstoff L, Carney PA, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98:1204–1214. doi: 10.1093/jnci/djj331. [DOI] [PubMed] [Google Scholar]
- 7.Von Euler-Chelpin M, Risor LM, Thorsted BL, Vejborg I. Risk of breast cancer after false-positive test result in screening mammography. J Natl Cancer Inst. 2012;104:682–689. doi: 10.1093/jnci/djs176. [DOI] [PubMed] [Google Scholar]
- 8.Castells X, Roman M, Romero A, Blanch J, Zubizarreta R, Ascunce N, et al. Breast cancer detection risk in screening mammography after a false-positive result. Cancer Epidemiol. 2013;37:85–90. doi: 10.1016/j.canep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Hofvind S, Ponti A, Patnick J, Ascunce N, Njor S, Broeders M, et al. False-positive results in mammographic screening for breast cancer in Europe: a literature review and survey of service screening programmes. J Med Screen. 2012;19(Suppl 1):57–66. doi: 10.1258/jms.2012.012083. [DOI] [PubMed] [Google Scholar]
- 10.Roman M, Hubbard RA, Sebuodegard S, Miglioretti DL, Castells X, Hofvind S. The Cumulative risk of false-positive results in the Norwegian Breast Cancer Screening Program: updated results. Cancer. 2013;119:3952–3958. doi: 10.1002/cncr.28320. [DOI] [PubMed] [Google Scholar]
- 11.Kerlikowske K, Zhu W, Hubbard RA, Geller B, Dittus K, Braithwaite D, et al. Outcomes of screening mammography by frequency, breast density, and postmenopausal therapy. JAMA Intern Med. 2013 May 13;173:807–816. doi: 10.1001/jamainternmed.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ballard-Barbash R, Taplin SH, Yankaskas BC, Ernster VL, Rosenberg RD, Carney PA, et al. Breast Cancer Surveillance Consortium: a national mammography screening and outcomes database. AJR Am J Roentgenol. 1997;169:1001–1008. doi: 10.2214/ajr.169.4.9308451. [DOI] [PubMed] [Google Scholar]
- 13.Breast Cancer Surveillance Consortium [Internet] National Cancer Institute, US National Institutes of Health. [cited Aug 25 2015]; [updated July 06 2015;]. Available from: http://breastscreening.cancer.gov.
- 14.American College of Radiology. The American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) 4th ed. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 15.Zheng Y, Heagerty PJ. Partly conditional survival models for longitudinal data. Biometrics. 2005;61:379–391. doi: 10.1111/j.1541-0420.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 16.Tice JA, O’Meara ES, Weaver DL, Vachon C, Ballard-Barbash R, Kerlikowske K. Benign breast disease, mammographic breast density and the risk of breast cancer. J Natl Cancer Inst. 2013;105:1043–1049. doi: 10.1093/jnci/djt124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using Clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008 Mar 4;148:337–347. doi: 10.7326/0003-4819-148-5-200803040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciatto S, Houssami N, Apruzzese A, Bassetti E, Brancato B, Carozzi F, et al. Categorizing breast mammographic density: intra- and interobserver reproducibility of BI-RADS density categories. Breast. 2005;14:269–275. doi: 10.1016/j.breast.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Spayne MC, Gard CC, Skelly J, Miglioretti DL, Vacek PM, Geller BM. Reproducibility of BI-RADS breast density measures among community radiologists: a prospective cohort study. Breast J. 2012;18:326–333. doi: 10.1111/j.1524-4741.2012.01250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]