Abstract
Background
Early postnatal experience shapes NMDA receptor (NMDAR) subunit composition and kinetics at excitatory synapses onto pyramidal cells; however, little is known about NMDAR maturation onto inhibitory interneurons.
Methods
We combined whole-cell patch clamp recordings (n=440) of NMDAR-mediated currents from layer 4-to-2/3 synapses onto pyramidal and GFP-labeled parvalbumin-positive (PV) interneurons in visual cortex at three developmental ages (15, 30 & 45 postnatal days) with array tomography 3-D reconstructions of NMDAR subunits GluN2A- and GluN2B-positive synapses onto PV cells.
Results
We show that the trajectory of the NMDAR subunit switch is slower in parvalbumin-positive (PV) interneurons than in excitatory pyramidal cells in visual cortex. Notably, this differential time course is reversed in the absence of methyl-CpG-binding protein, MeCP2, the molecular basis for cognitive decline in Rett syndrome and some cases of autism. Additional genetic reduction of GluN2A subunits, which prevents regression of vision in Mecp2-knockout mice, specifically rescues the accelerated NMDAR maturation in PV cells.
Conclusion
We demonstrate: (1) the time course of NMDAR maturation is cell-type specific and (2) a new cell-type specific role for Mecp2 in the development of NMDAR subunit composition. Reducing GluN2A expression in Mecp2-deficient mice, which prevents the decline in visual cortical function, also prevents the premature NMDAR maturation in PV cells. Thus, circuit-based therapies targeting NMDAR subunit composition on PV cells may provide novel treatments for Rett syndrome.
Keywords: GluN2A, GluN2B, Parvalbumin, Development, Rett Syndrome, Visual Cortex
INTRODUCTION
NMDA receptors (NMDARs) are critical for many forms of learning and memory, in part, due to their activity-dependence and contribution to synaptic integration and plasticity (1). The GluN2 subunit composition determines the decay kinetics of the NMDARs, which undergo an experience-dependent switch from GluN2B to 2A at cortical synapses during early postnatal development (2). This NMDAR subunit switch has been well studied at excitatory synapses onto pyramidal cells; however, whether a similar process underlies the development of excitatory synapses onto inhibitory interneurons in local cortical circuits remains largely unknown (3,4).
Parvalbumin (PV)-positive interneurons are of particular interest as they mature postnatally in an activity-dependent manner, directly control the firing of pyramidal cells, and trigger critical period plasticity (5,6). PV interneurons are also vulnerable across neurodevelopmental and psychiatric disorders (4,6). Both NMDARs and PV interneurons have been implicated in the pathophysiology of Rett syndrome, a childhood disorder marked by regression of cognitive and motor function after an apparently normal initial development (7,8). Most cases of Rett syndrome are caused by loss-of-function mutations in MECP2, a transcriptional regulator, and Mecp2-deficient mice also show a decline in behavioral and motor function including loss of vision (7,9). NMDAR dysfunction has also been implicated in post mortem studies of Rett syndrome (10); however, there has been disagreement among studies, primarily from tissue homogenates of Mecp2-deficient mice, as to the nature of the dysregulation (7,11,12,13). Elucidating the role of NMDARs in Rett syndrome may have important clinical implications, as the visual cortical decline, along with PV circuit hyperconnectivity, found in Mecp2-deficient mice can be prevented by an additional genetic manipulation to decrease GluN2A expression (7). The mechanism underlying such rescue is still unknown.
We utilized a circuit-based approach to investigate the NMDAR maturation in PV cells in wild-type mice and a Rett syndrome mouse model. We performed whole-cell patch clamp recordings in acute brain slices and array tomography analysis to characterize NMDAR at layer 4-to-2/3 visual cortical synapses and directly compared the development of NMDAR-mediated synaptic currents from pyramidal neurons and PV interneurons. We discovered that NMDAR maturation occurs more gradually in PV than pyramidal cells and that Mecp2 regulates the NMDAR subunit switch in a cell-type specific manner. Moreover, we reveal correction of NMDAR maturation in PV cells as a possible mechanism for the rescue of vision when reducing GluN2A expression in Mecp2-deficient mice.
METHODS AND MATERIALS
Animals
The electrophysiology data in this study includes a total of 440 whole-cell recordings (222 pyramidal and 218 PV cells) from 157 mice (20 Mecp2-KO, 32 Mecp2-WT, 18 Mecp2-KO/GluN2A-Het, 13 Mecp2-WT/GluN2A-Het, 26 Mecp2-KO/PV-GFP+, 27 Mecp2-WT/PV-GFP+, 11 Mecp2-KO/GluN2A-Het/PV-GFP+, 10 Mecp2-WT/GluN2A-Het/PV-GFP+) at 3 age groups: P15 (P13–P17), P30 (P28–P31), and P45 (P45–51); P30 and P45 for GluN2A-Het mice. Male Mecp2-KO and -WT littermates were used from in-house breeding pairs of Mecp2-heterozygous females (mouse line generated by A. Bird and colleagues; 14) crossed with C57BL/6 males or C57BL/6 mice that selectively express the green fluorescent protein (GFP) in parvalbumin-containing (PV) interneurons (from H. Monyer; 15). Double mutants for Mecp2 and GluN2A deletion were generated in-house by crossing Mecp2-heterozygote females with GluN2A-KO males (from M. Mishina; 16). Triple mutants for Mecp2, GluN2A and PV-GFP were generated in-house by first crossing GluN2A-KO females with PV-GFP+ males and using either the GluN2A-KO or GluN2A-Het/PV-GFP+ male offspring to cross with the Mecp2-heterozygous females. All procedures were approved by Boston Children’s Hospital IACUC.
Electrophysiology
Coronal visual cortical slices (300 µm thick) were prepared after decapitation under isofluorane anesthesia. Slices were incubated at room temperature in a submerged holding chamber for at least one hour in artificial cerebral spinal fluid containing: 119 mM NaCl, 2.5 mM KCl, 1 mM NaH2PO4/H20, 1.3 mM MgCl2/6H2O, 2.5 mM CaCl2, 26.2 mM NaHCO3, 20 mM glucose and bubbled with carbogen gas (95% O2/5% CO2). All recordings were made in the presence of 5 µM bicuculline methiodide (Sigma), which was sufficient to block GABAergic currents (17). To inhibit NMDA receptors containing the GluN2B subunit, slice were incubated in artificial cerebrospinal fluid with 3 µM ifenprodil (Sigma), a GluN2B subunit selective antagonist, for at least 40 minutes before and during recording (17,18). All recordings were made at room temperature.
Voltage-clamp recordings from layer 2/3 pyramidal and PV cells were made under visual guidance by infrared differential interference contrast microscopy. Patch pipettes (4–6 MΩ) were pulled from standard wall borosilicate tubing and were filled with intracellular solution containing: 140 mM CsCl, 0.2 mM EGTA, 10 mM HEPES, 2 mM ATP-Mg, 0.3 mM GTP, 5 mM QX-314, and 5 mg/ml biocytin (pH 7.2). All chemicals were purchased from Sigma. Cells were identified under visual guidance with by their location, morphology, and, in the case of GFP-expressing PV cells, by their fluorescence under ultraviolet light. In most cells, identity was also confirmed by recording a brief spike train in current clamp immediately after patching. In addition, fifty-eight cells were histologically processed for biocytin labeling post-recording using Alexa594-conjugated streptavidin antibody for pyramidal cells and Alexa 488-conjugated streptavidin antibody for PV cells. Excitatory post-synaptic currents (EPSCs) were evoked by extracellular stimulation (0.2 ms, 16–100 µA) in layer 4 of the visual cortex using a bipolar stimulating pipette pulled from standard wall borosilicate tubing and filled with artificial cerebrospinal fluid. Recordings began once the amplitude of the stimulating current and position of the electrode were adjusted to evoke a reliable monosynaptic response at a holding potential of −100 mV.
Voltage-clamp recordings were acquired at a 10 kHz sampling rate with an AxoPatch-1D (Axon Instruments) and an ITC-18 AD board (Instrutech, Mineola, NY). All data was acquired and analyzed using custom-made procedures in Igor-Pro Software (WaveMetrics, Lake Oswego, OR). The series resistance was monitored throughout the recordings. Cells were excluded in which the access resistance changed by more than 10%. Five evoked synaptic responses were recorded and used to calculate an average response at each post-synaptic holding potential from −100 mV to +60 mV. To estimated the relative contribution of NMDA and AMPA receptors to the synaptic current, the current amplitude was measured at the time of the peak amplitude at −100mV for the AMPA receptor-mediated current and at the time point after the current at −100 mV had decayed to less than <5% of its peak amplitude for the NMDA receptor-mediated current (Figure 1A), based on methods from Hestrin et al. (19) and Mierau et al. (17). NMDAR and AMPAR components were confirmed pharmacologically with 20 µM CPP and 10 µM CNQX (Supplemental Figure S1). Current-voltage (I–V) plots were made with the current amplitudes for the NMDA and AMPA receptor-mediated components as a function of the holding potential (Figure 1B). The N/A ratio was calculated as the ratio of the slopes between 0 and 40 mV, where the slopes of the NMDA and AMPA receptor-mediated components were most linear. Decay time constants were estimated using a single exponential fit, which provided an adequate fit (Figures 1C and 1D; 17). All values are reported as mean+SEM, unless otherwise stated. Parametric testing for normality followed by analysis of variance (ANOVA) with post-hoc t-tests were used to compare individual groups using JMP software (SAS, Cary, NC).
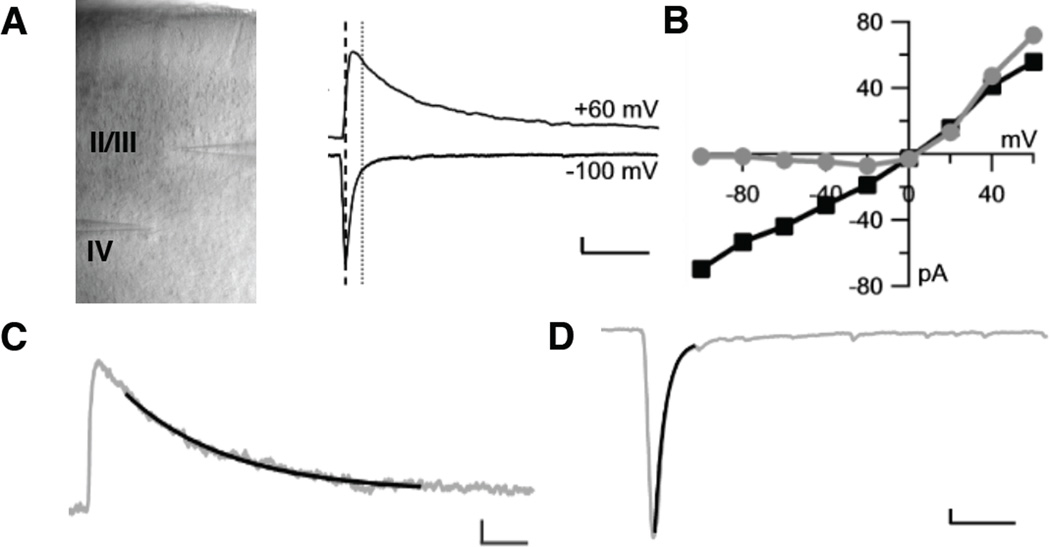
Figure 1. Measuring NMDAR- and AMPAR-mediated synaptic currents in pyramidal and PV cells in primary visual cortex.
A, Slice of primary visual cortex shown at 10× magnification with the recording electrode in layer 2/3 and extracellular stimulation electrode in layer 4. Representative traces show averaged excitatory post-synaptic currents (EPSCs) synaptic currents recorded at holding potentials of +60 and −100 mV. The AMPAR-mediated component of the ESPCs was estimated at the time point (black-dotted line) of the peak amplitude of the response at a holding potential of −100 mV, when there is no NMDAR component due to the voltage-dependent Mg2+ block. The NMDAR component was estimated at the time point (gray-dotted line) 50 ms after the peak at −100 mV, when the AMPAR-mediated current had decayed to less than 5% of the peak amplitude (17,19). Vertical scale bar 20 pA, horizontal 1 s. B, Current-voltage (I–V) plots constructed by recording EPSCs at holding potentials from −100 mV to +60 mV by 20 mV intervals of the AMPAR-mediated component (black) and NMDAR-mediated component (gray). The ratio of the NMDAR/AMPAR-mediated synaptic currents was estimated as the ratio of the slopes between 0 to +40 mV. The slope of the I–V plot is proportional to the conductance through the NMDAR and AMPAR channels. C, Sample single exponential fit (black) overlay on EPSC at a holding potential of +60 mV to estimate the decay time constant of the NMDAR-mediated synaptic current. Vertical scale bar 25 pA, horizontal 0.5 ms. D, Sample single exponential fit overlay on EPSC at holding potential of −100 mV (gray) to estimate the initial decay of the AMPAR-mediated synaptic current. Vertical scale bar 25 pA, horizontal 0.02 ms.
Array Tomography
The tissue was processed for array tomography as described in Micheva and Smith (20). Visual cortex was dissected from perfused animals and embedded in LR white resin using a bench top protocol. Ribbons with between 70–100 serial 70 nm ultrathin sections were prepared from P30 Mecp2-KO, WT and Mecp2-KO/NR2A-Het mice using an ultramicrotome (UC7, Leica Microsystems) and mounted side by side on subbed glass coverslips. Coverslips were immunostained using synapsin I (mouse, SYSY), synaptophysin (mouse, Abcam), PSD-95 (rabbit, Cell Signaling Technology), GluR1 (rabbit, Millipore), GluR2/3 (rabbit, Abcam), GluN2A or GluN2B (rabbit, Frontier Science Co., Ltd) and parvalbumin (guinea pig, Frontier Science Co., Ltd) antibodies. For secondary antibodies, Alexa 488, Alexa 594 and Alexa 647 (Invitrogen) were used from the appropriate species. For some ribbons, applied antibodies were eluted and the sections were restrained with different antibodies. The identification and quantification of GluN2A- and 2B-positive synapses is described in Figure 2 (A–D) and in further detail in the Supplemental Methods.
Figure 2. Array tomography image processing.
A, To identify synapses the image was first aligned coarsely using large objects, such as nuclei (DAPI), followed by a second fine alignment using a pre-synaptic marker (synaptophysin) and then applying that alignment to all other channels. Scale bar 10 µm. B, Synapses were defined as the colocalization of multiple pre-synaptic markers, including synapsin-1 (SYN1, white) and synaptophysin (SYP, magenta) and multiple post-synaptic markers, PSD-95 (green) and NMDAR (red). C and D, To quantify the normalized synapse density in PV-positive cells, aligned stacks were made for each channel. PV-positive cells were identified manually and defined by the ROI, while PV-negative cells were defined by segmenting the space between DAPI-positive nuclei. A custom-made analysis program was used to quantify synapses in the volume included in the ROI (PV-positive cells) and in the segmented area (PV-negative cells) (for more details see Supplementary Method). Synapses inside DAPI positive structures were not quantified. Scale bar 10 µm.
RESULTS
Slower NMDAR Subunit Switch at Excitatory Synapses onto Cortical PV Interneurons
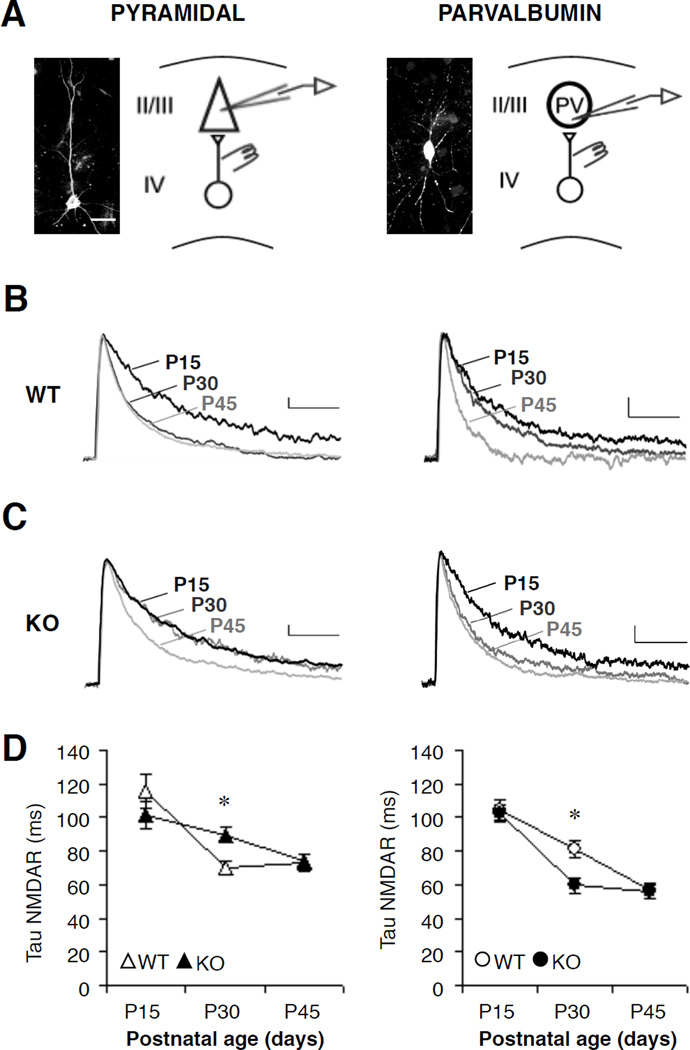
To establish the time course of NMDAR maturation in PV interneurons, we quantified the decay time constant at three developmental stages corresponding to eye opening (postnatal day, P15), peak of critical period plasticity (P30), and adulthood (P45) and compared the development with neighboring pyramidal neurons in layer 2/3 of visual cortex (Figure3A and 3B). A single exponential fit was adequate to measure the NMDAR-mediated synaptic current decay (Figure 3C) (17,19; Supplementary Figure S1). As expected, pyramidal cells showed a faster decay at P30 than P15 indicating a switch from primarily GluN2B- to 2A-subunit-containing NMDARs, which have a shorter deactivation (Figure 3B, 3D and Table 1). In contrast, the switch occurred more slowly in PV cells with a significant GluN2B component still present at P30 that further decreased by P45 (Figure 3B, 3D and Table 1).
Figure 3. Cell-specific regulation of synaptic NMDAR current maturation by Mecp2.
A, Sample biocytin-stained pyramidal (left) and PV cells (right) in visual cortex (scale bar, 25µm). Whole-cell recordings were made in layer 2/3 with a stimulation electrode in layer 4. B and C, Excitatory post-synaptic currents (EPSCs) at +60 mV from WT and Mecp2-KO mice across postnatal development. Sample traces from postnatal day (P)15, 30, and 45 shown normalized to the amplitude at P15 for comparison of decay time constants. Vertical scale bar 20pA, horizontal 1s. D, Development of NMDAR decay in pyramidal and PV cells (n=10–18 cells/group; * P < 0.02, ANOVA). All values represent mean ± SEM.
Table 1.
Decay time constants for NMDAR-mediated synaptic currents over early postnatal development.
| P15 | P30 | P45 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tau (ms) | n | P | Tau (ms) | n | P | Tau (ms) | n | P | ||
| Pyramidal Neurons | ||||||||||
| Mecp2 WT | Control | 116±8 | 12 | ** | 70±4 | 11 | n.s. | 73±5 | 13 | n.s. |
| Ifenprodil | 76±10 | 12 | 60±4 | 12 | 67±5 | 13 | ||||
| Mecp2 KO | Control | 102±6 | 15 | ** | 90±5 | 10 | ** | 74±3 | 18 | ** |
| Ifenprodil | 69±8 | 10 | 71±5 | 8 | 60±4 | 10 | ||||
| Parvalbumin Interneurons | ||||||||||
| Mecp2 WT | Control | 104±6 | 12 | ** | 82±5 | 10 | ** | 56±5 | 11 | n.s. |
| Ifenprodil | 61±6 | 13 | 55±5 | 9 | 55±6 | 8 | ||||
| Mecp2 KO | Control | 102±4 | 13 | ** | 60±5 | 11 | n.s. | 56±4 | 13 | n.s. |
| Ifenprodil | 71±5 | 10 | 48±5 | 9 | 52±4 | 13 | ||||
Tau = Time constant of NMDAR-mediated synaptic current decay; n=number of cells;
P <0.01,
ANOVA with Bonferroni correction for multiple comparisons
Loss of MeCP2 Accelerates NMDAR Subunit Switch in PV Interneurons and Delays it in Pyramidal Cells
A potential modulator of this cell-specific development is the methyl-CpG-binding protein, Mecp2. Loss of Mecp2 alters PV circuits in visual cortex, which can be rescued by additional genetic manipulation to reduce GluN2A expression (7). Remarkably, we found that knocking out Mecp2 had opposing effects on NMDAR maturation in the two cell types. PV cells exhibited an atypically fast decay at P30 in the absence of Mecp2, indicating an acceleration of NMDAR development, while pyramidal cells showed immature decay kinetics, indicating a developmental delay (Figure 3C, 3D and Table 1). Interestingly, the developmental expression pattern of Mecp2 was also cell-type specific. We found a larger increase in Mecp2 expression occurred in PV than pyramidal cells between P15 and P30 (Supplemental Figure S2), coinciding with the slower NMDAR maturation in PV cells.
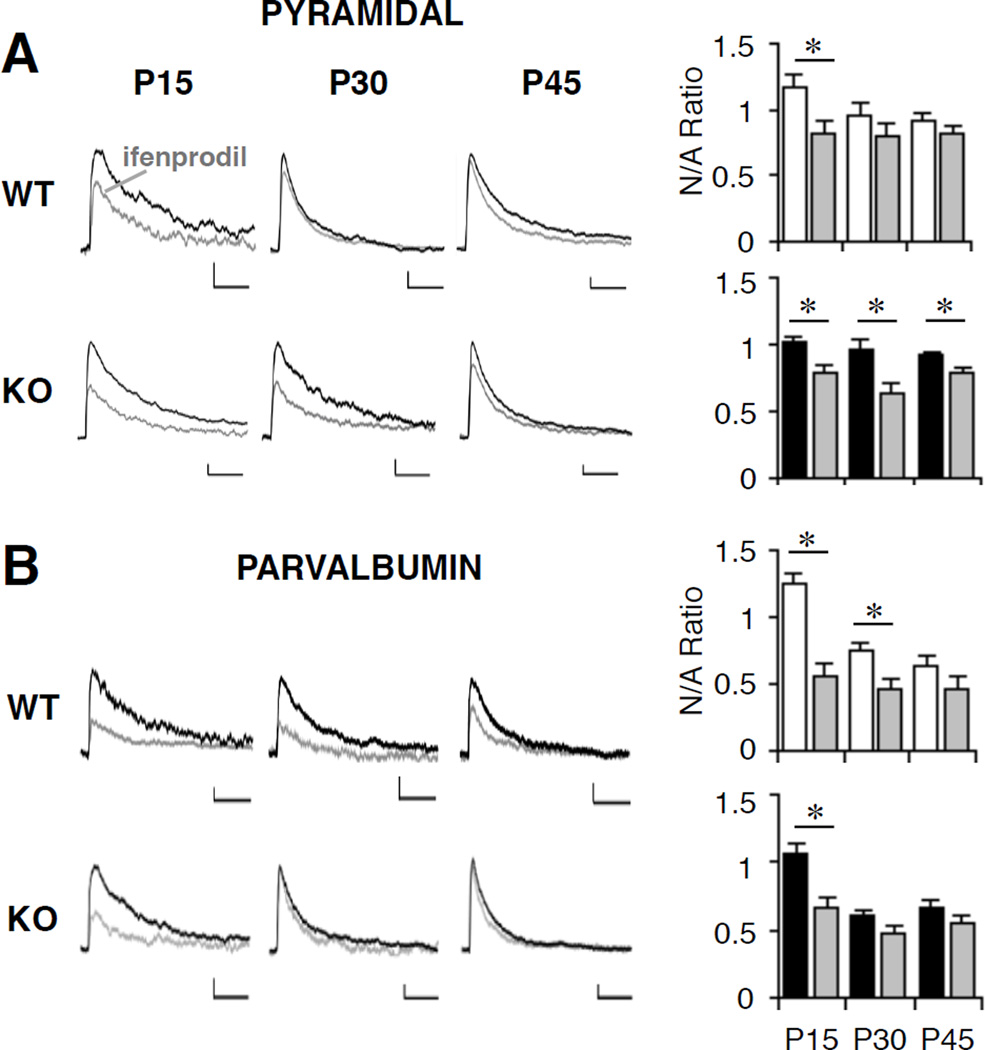
To confirm that the change in decay kinetics was due to altered NMDAR subunit composition, we used the selective GluN2B antagonist ifenprodil (3 µM; 3A and 3B; 17,18). Both pyramidal and PV cells in visual cortex showed a significant reduction in NMDAR-mediated synaptic currents at P15 with ifenprodil; however, at P30, PV cells—but not pyramidal neurons—retained sensitivity to ifenprodil confirming their slower maturational time course during normal development (Figure 4A, 4B and Table 2). In Mecp2-deficient mice, the GluN2B component was undetectable in PV cells at P30 but still significant in pyramidal cells (Figure 4A, 4B and Table 2).
Figure 4. Developmental decrease in NMDAR GluN2B expression is delayed in pyramidal and accelerated in PV cells in the absence of Mecp2.
A and B, Sample evoked EPSCs recorded from pyramidal (A) and PV (B) cells at +60 mV in control (black) and GluN2B-antagonist ifenprodil-treated (3 µM, gray) conditions at P15, P30, P45 in Mecp2-WT (upper) and Mecp2-KO (lower) mice. Vertical scale bars 20 pA, horizontal 1 s. Bar graphs show the ratio of NMDAR/AMPAR (N/A)-mediated synaptic currents in WT (white), KO (black), and the effect of ifenprodil (gray) at P15, P30, and P45 in pyramidal (A) and PV (B) cells. The significant GluN2B component disappears by P30 in the WT pyramidal cells but is still present in the WT PV cells; in the Mecp2-KO, however, the significant GluN2B persists through P45 in the pyramidal and disappears by P30 in the PV cells (*, P < 0.02, ANOVA). All values represent mean±SEM.
Table 2.
Sensitivity of NMDAR-mediated synaptic currents to GluN2B antagonist ifenprodil over early postnatal development.
| P15 | P30 | P45 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N/A Ratio | n | P | N/A Ratio | n | P | N/A Ratio | n | P | ||
| Pyramidal Neurons | ||||||||||
| Mecp2 WT | Control | 1.18±0.08 | 15 | ** | 0.96±0.09 | 9 | n.s. | 0.92±0.05 | 13 | n.s. |
| Ifenprodil | 0.83±0.09 | 12 | 0.81±0.08 | 13 | 0.82±0.05 | 14 | ||||
| Mecp2 KO | Control | 1.02±0.05 | 15 | ** | 0.96±0.07 | 11 | ** | 0.91±0.04 | 18 | * |
| Ifenprodil | 0.78±0.06 | 10 | 0.64±0.08 | 9 | 0.78±0.05 | 10 | ||||
| Parvalbumin Interneurons | ||||||||||
| Mecp2 WT | Control | 1.24±0.09 | 12 | ** | 0.75±0.06 | 11 | ** | 0.63±0.08 | 11 | n.s. |
| Ifenprodil | 0.56±0.09 | 12 | 0.47±0.06 | 11 | 0.46±0.09 | 10 | ||||
| Mecp2 KO | Control | 1.06±0.08 | 13 | ** | 0.60±0.05 | 10 | n.s. | 0.66±0.07 | 12 | n.s. |
| Ifenprodil | 0.66±0.09 | 10 | 0.48±0.05 | 11 | 0.55±0.07 | 13 | ||||
N/A Ratio = Ratio of NMDAR-/AMPAR-mediated synaptic currents; n=number of cells;
P <0.01,
P <0.02,
ANOVA with Bonferroni correction for multiple comparisons
In contrast, neither the maturation of the AMPA receptor (AMPAR) decay nor the amplitude was affected by Mecp2 loss in both cell types (Figure 5C, 5D). Nor was the synaptic density of AMPAR, quantified by array tomography, altered (Figure 5A, 5B). This suggests that Mecp2’s role in glutamatergic synaptic development is specific not only to cell-type but also to NMDARs.
Figure 5. Development of AMPAR decay and ratio is independent of Mecp2.
A, Single plane array tomography for GluR1-2-3 subunits (green), PSD-95 (blue) and synaptophysin (red) identify AMPAR-containing synapses in P30 visual cortex of Mecp2-KO (right) and WT (left) mice. Scale bars, 3 µm lower and 1 nm higher magnification. B, Quantification of GluR1-2-3-positive synapses in layer 2/3 visual cortex in WT (white) and KO (black bar) mice. C, Sample traces of AMPAR-mediated synaptic currents at −100 mV (in the presence of 5 µM bicuculline) at P15, P30, and P45 in pyramidal cells (left) and PV cells (right) from Mecp2-KO (lower) & WT (upper) mice. Scale bars, vertical 25 pA, horizontal 0.5 s. D, Decay time constant of AMPAR-mediated synaptic currents (top) and AMPAR/NMDAR (A/N) ratio (bottom). There was no significant difference in decay time constant or A/N ratio in PV or pyramidal cells between Mecp2-KO and WT mice (P > 0.05, ANOVA). A significant increase in A/N ratio from P15 to P30 was seen in PV interneurons in both Mecp2-KO and WT mice (*, P < 0.02, ANOVA).
Reducing GluN2A Expression Rescues Synaptic NMDAR Subunit Composition in PV Cells
As further deletion of GluN2A in Mecp2-deficient mice renormalizes GluN2A/2B expression and prevents visual regression (7), we recorded NMDAR-mediated synaptic currents from Mecp2−/y/GluN2A+/− (KO-Het) double mutants and Mecp2+/y/GluN2A+/− (WT-Het) mice (Figures 6A, 6B and Table 3). Reduced GluN2A expression was sufficient to prevent the accelerated NMDAR maturation in PV cells, but did not normalize the delayed NMDAR development in pyramidal cells at P30 (Figures 6C–F and Table 3). The rescue of visual cortical function in the Mecp2-KO/GluN2A-Het mice (7) was also accompanied by a preserved GluN2B component in PV and pyramidal cells at P45 (Table 3).
Figure 6. GluN2A reduction rescues PV cell synaptic NMDAR currents at P30.
A and B, Superimposed EPSCs for decay comparison in pyramidal (A) and PV (B) cells from Mecp2-KO/NR2A-Het (KO-Het; red/green), Mecp2-KO (black), and WT (white) mice. Inset A and B: EPSCs from KO-Het (upper) and Mecp2-WT/NR2A-Het (WT-Het; lower) mice in control (black) and ifenprodil-treated (3 µM, gray) conditions. Vertical scale bar 20pA, horizontal 1s. C and E, Decay time constants from pyramidal (C) and PV (E) cells in WT (white), KO (black), KO-Het (red/green bar), and WT-Het (red/green stripe) mice. D and F, Effect of GluN2B antagonist ifenprodil on the NMDAR/AMPAR ratio in pyramidal (D) and PV (F) cells (n=9–18 cells/group; * P < 0.02, ANOVA).
Table 3.
Effect of genetic reduction of GluN2A expression on NMDAR-mediated synaptic currents.
| P30 | P30 | P45 | P45 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tau | n | P | N/A Ratio | n | P | Tau | n | P | N/A Ratio | n | P | ||
| Pyramidal Neurons | |||||||||||||
| Mecp2 KO-GluN2A Het | Control | 93±5 | 14 | ** | 1.00±0.06 | 15 | ** | 89±6 | 17 | ** | 0.99±0.06 | 16 | ** |
| Ifenprodil | 67±5 | 13 | 0.74±0.06 | 13 | 61±5 | 15 | 0.64±0.03 | 15 | |||||
| Mecp2 WT-GluN2A Het | Control | 99±7 | 7 | ** | 1.06±0.08 | 7 | ** | 89±9 | 12 | ** | 1.05±0.08 | 10 | ** |
| Ifenprodil | 59±7 | 8 | 0.69±0.07 | 8 | 68±3 | 13 | 0.72±0.04 | 12 | |||||
| Parvalbumin Interneurons | |||||||||||||
| Mecp2 KO-GluN2A Het | Control | 85±5 | 11 | ** | 0.91±0.08 | 9 | ** | 92±10 | 10 | ** | 0.88±0.09 | 10 | ** |
| Ifenprodil | 55±6 | 8 | 0.40±0.08 | 8 | 47±5 | 11 | 0.48±0.06 | 11 | |||||
| Mecp2 WT-GluN2A Het | Control | 85±8 | 7 | ** | 0.91±0.10 | 6 | ** | 93±10 | 7 | * | 0.90±0.11 | 7 | ** |
| Ifenprodil | 48±8 | 8 | 0.33±0.09 | 8 | 66±5 | 8 | 0.54±0.06 | 8 | |||||
Tau = Time constant of NMDAR-mediated synaptic current decay (ms); N/A Ratio = Ratio of NMDA/AMPAR-mediated synaptic currents; n=number of cells; All values mean±s.e.m.,
P<0.01,
ANOVA
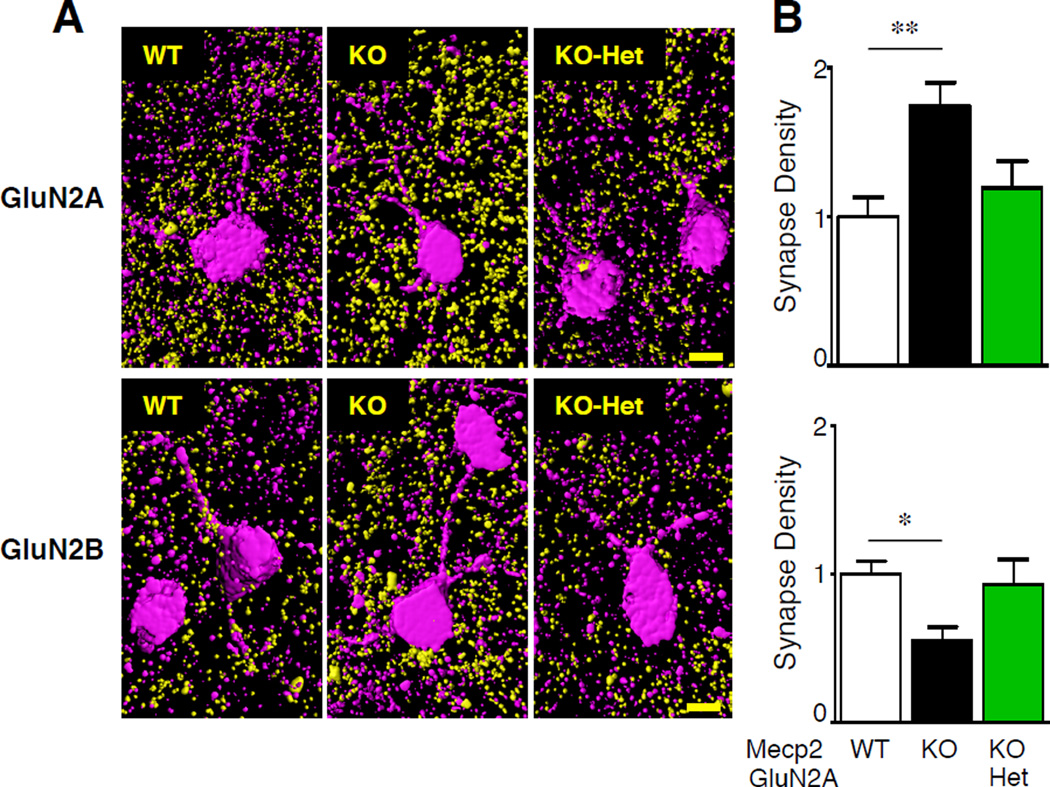
To anatomically characterize NMDAR subunit expression at the synapse, we turned to array tomography analysis (20). Excitatory synapses onto layer 2/3 PV cells in visual cortex were identified by colocalization of pre-synaptic marker synaptophysin (SYP) and post-synaptic density (PSD)-95 labeling on PV-positive cells (Figure 7A). In agreement with our electrophysiological findings, we found a significant increase in the number of GluN2A-positive and a decrease in GluN2B-positive synapses at P30 in the Mecp2-KO compared to wild-type PV-cells. The synaptic GluN2 subunit expression was rescued in Mecp2-KO/GluN2A-Het double mutant mice (Figures 7B).
Figure 7. GluN2A reduction rescues NMDAR subunit composition in PV cells at P30.
A, Representative array tomography 3D reconstructions of GluN2A- (upper panel) and GluN2B-positive (bottom panel) synapses (yellow) onto layer 2/3 PV cells (magenta) in WT, KO and KO-Het visual cortex. Scale bar, 5 µm. B, Quantification of GluN2A (top) and GluN2B (bottom) synaptic density onto PV-positive cells in WT (white), KO (black bars) and KO-Het (green) mice (n=12–18 PV-positive cells/group; * P < 0.05, ** P < 0.01, ANOVA).
DISCUSSION
Our results reveal a developmental switch in synaptic NMDAR composition in PV cells and a novel role for Mecp2 in this process, which differs across cell types. First, we demonstrated that the time course is slower at layer 4-to-2/3 synapses onto PV than pyramidal cells in visual cortex. Second, we showed cell-specific regulation of the NMDAR subunit switch, which is delayed in pyramidal and accelerated in PV cells in the absence of Mecp2. Third, genetically decreasing GluN2A expression—which is sufficient to prevent the regression of visual function in Mecp2-KO mice—prevented the early NMDAR maturation in PV but not the delay in pyramidal cells. The differential regulation of NMDAR by Mecp2 was specific to subunit composition as the ratio of the NMDAR/AMPAR-mediated synaptic currents (Figure 5) and total NMDAR expression using array tomography (data not shown) were unaltered in Mecp2-deficient mice. Moreover, while differences in dendritic geometry could result in imperfect space-clamp conditions and distort the EPSC, we did not observe any difference in the AMPAR-mediated current, which would be more attenuated by increased dendritic filtering, between the Mecp2-KO and WT neurons in either cell-type. We also confirmed our findings pharmacologically with ifenprodil and anatomically with array tomography.
The timing of the NMDAR switch in excitatory neurons across brain regions during early postnatal development coincides with an increase in synaptic function and critical period plasticity, supporting its importance for the refinement and tuning of neuronal circuits (3,21). In pyramidal cells, the NMDAR switch in visual cortex is triggered by eye opening during normal development and by only two hours of light exposure in dark-reared mice (18). The resulting decrease in GluN2B expression occurs over hours to days (1,22). In PV cells, previous studies support a strong GluN2B component in juveniles that is not present in adult rodents in hippocampus (23), prefrontal (24) and somatosensory (25) cortex. Our findings reveal that, rather than hours to days, NMDAR maturation in PV cells occurs over weeks and raise the broader question of whether similar molecular mechanisms drive the NMDAR subunit switch across cell types (excitatory versus inhibitory and/or among inhibitory neurons).
In contrast to pyramidal cells, two weeks after eye opening in wild-type mice, PV cells in visual cortex still retain a significant GluN2B component, which does not fully decline until one month after eye opening. Interestingly, it is during this time that PV cell connectivity onto pyramidal cells in primary visual cortex mature and critical period plasticity is expressed (6). Given the different characteristics of GluN2 subunits, higher synaptic expression of GluN2B in PV cells at the peak of the critical period (P25–30) may increase the window for spike-time dependent plasticity and the integration of sensory stimuli received over a longer period of time than in synapses with higher GluN2A (26). Reducing Mecp2 expression in PV cells alone is sufficient to modulate the expression and timing of the ocular dominance plasticity critical period (27). Moreover, our findings that NMDAR maturation is accelerated in PV cells in the absence of Mecp2 may also explain, at least in part, an initial bias toward greater inhibition of pyramidal cells in previous studies of Mecp2-deficient mice (28–30).
We demonstrate a new role for Mecp2 in the NMDAR subunit switch that is cell-type specific. The action of Mecp2 may be direct, as Mecp2 is a transcriptional repressor and activator and binds to the promoter of multiple genes in a histone-like fashion and an age- and region-specific manner (31,32). Mecp2 binds to the GluN2A, but not GluN2B, promoter in visual cortex homogenates at eye opening (postnatal day 15; 7). Thus, the larger increase in Mecp2 expression in PV than pyramidal cells over P15 to P30 (Supplemental Figure S2) could explain, at least in part, the slower maturation of NMDAR in PV cells, which is accelerated when Mecp2 is lost. An additional mechanism, however, would be necessary to delay the NMDAR subunit switch in pyramidal cells in the absence of Mecp2. This may be due to cell-type specific binding of Mecp2 to NMDAR subunit promoters. Evidence from embryonic cortical neuron cultures indicates that Mecp2 binds instead to GluN2B and is regulated by activity (11). This suggests a dynamic role for Mecp2 in the regulation of NMDAR subunits during postnatal development.
Activity-dependent changes in the excitatory-inhibitory balance within the cortical circuit may also play a role in the cell-specific NMDAR maturation. Reduced neuronal activation, such as in dark rearing of WT mice, prevents the activity-dependent down-regulation of GluN2B and increase in GluN2A expression in pyramidal neurons (18). In addition, the intrinsic excitatory level of individual pyramidal neurons induces post-translational modifications of GluN2 subunits that may contribute to the changes found in NMDAR kinetics (3,33). Both in vitro and in vivo studies show a significant decrease in the spontaneous and evoked activity of cortical pyramidal neurons and an increase in PV-mediated inhibitory transmission in Mecp2-KO mice (7,27,28,34). Thus, the delay of NMDAR maturation in pyramidal cells could be due to a decrease in the activity-dependent phosphorylation of GluN2B and, thus, retention at the synapse. It is also possible that the acceleration in NMDAR maturation in PV cells could be due to their higher basal excitability in the Mecp2-KO (27), resulting in more active removal of GluN2B from the synapses and insertion and/or post-translational modification of GluN2A subunits (35,36). It is not known, however, whether the NMDAR subunit switch in PV cells is mediated by activity as in pyramidal cells, nor whether the same post-translational modifications to the GluN2 subunits occur in interneuronal subtypes. A decrease in the cortical activity alone would not predict the reciprocal cell-specific effects on NMDAR maturation in the absence of Mecp2. Decreased activity in dark-reared Mecp2-deficient mice prevents—rather than promotes—the hyper-maturation of PV cells in visual cortex (7). This supports a direct role for Mecp2 in differentially regulating the NMDAR subunit switch in PV and pyramidal cells, rather than the delay in pyramidal and premature switch in PV cells occurring secondary to activity-dependent network effects.
A cell-autonomous role for Mecp2 in development of excitatory synaptic transmission is also supported by previous studies in which Mecp2 deletion was limited to a single cell type or a sparse population of cells which altered the excitatory-inhibitory balance only in the affected cells. Mecp2 deletion using in utero injections of short hairpin RNA (37) and acute cell-autonomous loss of Mecp2 (38) in cortical pyramidal neurons was sufficient to weaken excitatory inputs in the Mecp2-deficient cells with no effect on non-transfected pyramidal neurons or inhibitory inputs. Cell-type specific deletion in pyramidal cells selectively reduced GABAergic, but not excitatory, transmission (39), while selective deletion in PV cells induced a loss of excitatory drive in PV cells with no effect on pyramidal cells (27). Of note, the latter study did not detect a significant increase in the GluN2A expression in fluorescent PV cells using qPCR; however, the study was limited by small sample size for the qPCR and by the slow decrease in Mecp2 expression of PV cells in the Mecp2-flox/PV-Cre mice, which only reached 80% of the PV cells by P30 (27). Thus, the majority of PV cells between P15–30 would have expressed Mecp2 and the slower NMDAR subunit switch as seen in wild-type PV cells.
Our study establishes the first two-weeks after eye opening as a critical therapeutic window for murine preclinical trials of NMDAR subunit-specific, circuit-based therapies for Rett syndrome. Our findings suggest that preventing the early NMDAR maturation in PV cells may underlie the rescue of visual cortical function in the Mecp2-KO/GluN2A-Het mice; however, we cannot exclude a role for altered NMDAR subunit composition at synapses on other interneuron cell types or at extrasynaptic sites. Moreover, while correction of the delay in NMDAR maturation onto pyramidal cells was not required for rescue of visual acuity or cortical activity, we cannot exclude that the alteration of pyramidal cells contributes to the pathophysiology, or represents a compensatory response.
In conclusion, the regulation of NMDAR maturation offers an important therapeutic target for developing new treatments for cognitive disorders. Disruption of NMDAR subunit composition has been implicated in the pathogenesis of Rett syndrome (7) and other genetic causes of autism, including mutations in the NMDAR scaffolding proteins Shank2 and Shank3 (40). Restoring NMDAR function in Shank 2-KO mice, for example, rescues behavior deficits in social communication, interaction and repetitive behaviors (41,42). Likewise, genetic reduction of GluN2A expression in Mecp2-deficient mice prevents the decline in visual cortical function (7). Circuit-based therapies that target GluN2A in specific cell-type populations warrant further investigation for treating Rett syndrome. Low-dose ketamine, for example, is an NMDAR antagonist that acts preferentially on PV cells (43) and, with acute administration, has been shown to enhance neuronal cortical activity in adult Mecp2-deficient mice (34). Development of a selective GluN2A antagonist that preferentially targets PV cells would provide a novel therapy and minimize side effects on other cell-types in the cortical circuits. Moreover, NMDAR dysfunction is also involved in multiple psychiatric and neurological disorders, including schizophrenia, chronic pain, stroke, and neurodegenerative diseases, and there is growing interest in developing new drugs that target NMDAR subtypes.
Supplementary Material
ACKNOWLEDGMENTS
We thank A. Takesian for custom-made Igor procedures, C. Li for processing biocytin-filled cells, M. Hemberg for statistical advice, L. Ortiz, N. Kingery, T. Xie, Harvard Medical School Neurobiology Imaging Facility (NINDS-P30-NS-072030) and Data Analysis Core for assistance with array tomography, A.D. Hill and Boston Children’s Hospital IDDRC imaging core (NIH-P30-HD-18655) for imaging software. This work was supported by the NIH NRSA (T32), Nancy Lurie Marks Foundation, and International Rett Syndrome Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
All authors participated in the study design and manuscript preparation. S.B.M. performed and analyzed the electrophysiology and A.P. the array tomography experiments.
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neuro. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 2.Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- 3.Sanz-Clemente A, Nicoll RA, Roche KW. Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist. 2013;19:62–75. doi: 10.1177/1073858411435129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Magueresse CL, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. doi: 10.1016/j.neuron.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 6.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neuro. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 7.Durand S, Patrizi A, Quast KB, Hachigian L, Pavlyuk R, Saxena A, et al. NMDA receptor regulation prevents regression of visual cortical function in the absence of Mecp2. Neuron. 2012;76:1078–1090. doi: 10.1016/j.neuron.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomassy GS, Morello N, Calcagno E, Giustetto M. Developmental Abnormalities Of Cortical Interneurons Precede Symptoms Onset In A Mouse Model Of Rett Syndrome. J Neurochem. 2014;131:115–127. doi: 10.1111/jnc.12803. [DOI] [PubMed] [Google Scholar]
- 9.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Blue ME, Naidu S, Johnston MV. Development of Amino Acid Receptors in Frontal Cortex from Girls with Rett Syndrome. Annals of Neurology. 1999;45:541–545. doi: 10.1002/1531-8249(199904)45:4<541::aid-ana21>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Kim W, Ham B, Chen W, Bear MF, Yoon B. Activity-depending NR2B expression is mediated by MeCP2-dependent epigenetic regulation. Biochem Biophys Res Comm. 2008;377:930–934. doi: 10.1016/j.bbrc.2008.10.082. [DOI] [PubMed] [Google Scholar]
- 12.Maliszewska-Cyna E, Bawa D, Eubanks JH. Diminished prevalence but preserved synaptic distribution of N-methyl-d-aspartate receptor subunits in the methyl CpG binding protein 2(MeCP2)-null mouse brain. Neuroscience. 2010;168:624–632. doi: 10.1016/j.neuroscience.2010.03.065. [DOI] [PubMed] [Google Scholar]
- 13.Blue ME, Kaufmann WE, Bressler J, Eyring C, O’Driscoll C, Naidu S, et al. Temporal and Regional Alterations in NMDA Receptor Expression in Mecp2-Null Mice. The Anatomical Record. 2011;294:1624–1634. doi: 10.1002/ar.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 15.Meyer AH, Katona I, Blatow M, Rozov A, Monyer H. In vivo labeling of parvalbumin-positive interneurons and analysis of electrical coupling in identified neurons. J. Neurosci. 2002;22:7055–764. doi: 10.1523/JNEUROSCI.22-16-07055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- 17.Mierau SB, Meredith R, Upton AL, Paulsen O. Dissociation of experience-dependent and -independent changes in excitatory synaptic transmission during development of barrel cortex. PNAS. 2004;101:15518–15523. doi: 10.1073/pnas.0402916101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philpot BD, Sekhar A, Shouval HZ, Bear MF. Visual experience and deprivation bidirectionally modify the composition and function of NMDA receptors in visual cortex. Neuron. 2001;29:157–169. doi: 10.1016/s0896-6273(01)00187-8. [DOI] [PubMed] [Google Scholar]
- 19.Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiology. 1990;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Micheva KD, Smith SJ. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55:25–36. doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 22.Bellone C, Nicoll R. Rapid Bidirectional Switching of Synaptic NMDA Receptors. Neuron. 2007;55:779–785. doi: 10.1016/j.neuron.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Matta JA, Pelkey KA, Craig MT, Chittajallu R, Jeffries BW, McBain CJ. Developmental origin dictates interneuron AMPA and NMDA receptor subunit composition and plasticity. Nat Neuro. 2013;16:1032–1041. doi: 10.1038/nn.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang HX, Gao WJ. Cell-type specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Neuropsychopharm. 2009;34:2028–2040. doi: 10.1038/npp.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Z, Sun QQ. Development of NMDA NR2 subunits and their roles in critical period maturation of neocortical GABAergic interneurons. Dev Neurobio. 2011;71:221–245. doi: 10.1002/dneu.20844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fagiolini M, Katagiri H, Miyamoto H, Mori H, Grant SG, Mishina M, et al. Separable features of visual cortical plasticity revealed by N-methyl-D-aspartate receptor 2A signaling. PNAS. 2003;100:2854–2859. doi: 10.1073/pnas.0536089100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He L, Liu N, Cheng T, Chen X, Li Y, Shu Y, et al. Conditional deletion of Mecp2 in parvalbumin-expressing GABAergic cells results in the absence of critical period plasticity. Nature Communications. 2014;5:5036. doi: 10.1038/ncomms6036. [DOI] [PubMed] [Google Scholar]
- 28.Dani VS, Chang Q, Maffei A, Turrigiano GG, Jaenisch R, Nelson SB. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. PNAS. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson ED, Kavalali ET, Monteggia LM. MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol. 2006;16:710–716. doi: 10.1016/j.cub.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 30.Chao H, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Gabel HW, Hemberg M, Hutchinson AN, Sadacca LA, Ebert DH, et al. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron. 2011;72:72–85. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen BS, Roche KW. Regulation of NMDA receptors by phosphorylation. Neuropharmacology. 2007;53:362–368. doi: 10.1016/j.neuropharm.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kron M, Howell CJ, Adam IT, Ransbottom M, Christian D, Ogier M, et al. Brain activity mapping in Mecp2 mutant mice reveals functional deficits in forebrain circuits, including key nodes in the default mode network, that are reversed with ketamine treatment. J Neurosci. 2012;32:13860–13872. doi: 10.1523/JNEUROSCI.2159-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsend M, Liu Y, Constantine-Paton M. Retina-driven dephosphorylation of the NR2A subunit correlates with faster NMDA receptor kinetics at developing retinocollicular synapses. J Neurosci. 2004;24:11098–11107. doi: 10.1523/JNEUROSCI.1207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grau C, Arató K, Fernández-Fernández JM, Valderrama A, Sindreu C, Fillat C, et al. DYRK1A-mediated phosphorylation of GluN2A at Ser(1048) regulates the surface expression and channel activity of GluN1/GluN2A receptors. Front Cell Neurosci. 2014;8:331. doi: 10.3389/fncel.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood L, Gray NW, Zhou Z, Greenberg ME, Shepherd GM. Synaptic circuit abnormalities of motor-frontal layer 2/3 pyramidal neurons in an RNA interference model of methyl-CpG-binding protein 2 deficiency. J Neurosci. 2009;29:12440–12448. doi: 10.1523/JNEUROSCI.3321-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blackman MP, Djukic B, Nelson SB, Turrigiano GG. A critical and cell-autonomous role for Mecp2 in synaptic scaling up. J Neurosci. 2012;32:13529–13536. doi: 10.1523/JNEUROSCI.3077-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang W, Peterson M, Beyer B, Frankel WN, Zhang Z. Loss of Mecp2 from forebrain excitatory neurons leads to cortical hyperexcitation and seizures. J Neurosci. 2014;34:2754–2763. doi: 10.1523/JNEUROSCI.4900-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang YH, Ehlers MD. Modeling autism by SHANK gene mutations in mice. Neuron. 2012;78:8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Won H, Lee HR, Gee HY, Mah W, Kim JI, Ha S, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486:261–265. doi: 10.1038/nature11208. [DOI] [PubMed] [Google Scholar]
- 42.Schmeisser MJ, Ey E, Wegener S, Bockmann J, Stempel AV, Kuebler A, et al. Autistic-like behaviours and hyperactivity in mice lacking ProSAP1/Shank2. Nature. 2012;486:256–260. doi: 10.1038/nature11015. [DOI] [PubMed] [Google Scholar]
- 43.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.