Abstract
Background and purpose
There is still no curative treatment for multiple sclerosis (MS), but during the last 20 years eight different disease-modifying compounds have been approved for relapsing−remitting MS (RRMS).
Methods
A literature search was conducted on published randomized controlled phase III trials indexed in PubMed on the approved medications until 21 May 2015.
Results
In this review the mode of action, documented treatment effects and side effects of the approved MS therapies are briefly discussed.
Conclusions
Based on current knowledge of risk−benefit of the approved MS medications, including factors influencing adherence, it is suggested that oral treatment with dimethyl fumarate or teriflunomide should be preferred as a starting therapy amongst the first-line preparations for de novo RRMS. In the case of breakthrough disease on first-line therapy, or rapidly evolving severe RRMS, second-line therapy with natalizumab, fingolimod or alemtuzumab should be chosen based on careful risk−benefit stratification.
Keywords: disease-modifying, multiple sclerosis, review, treatment
Introduction
Multiple sclerosis (MS) is a common cause of disability in young adults. Irreversible axonal damage occurs even in the earliest phases of disease evolution 1. Although some people with relapsing−remitting MS (RRMS) have a ‘benign’ disease course with minimal disease activity and impairment, most patients experience increasing disability over time and eventually convert to secondary progressive MS (SPMS). There is still no curative treatment, but during the last 20 years eight different therapies have become available including interferon beta, glatiramer acetate, teriflunomide, dimethyl fumarate, natalizumab, fingolimod, alemtuzumab and mitoxantrone, and several new compounds are in development. All the approved medications have mainly anti-inflammatory effects and increasing evidence indicates that all of them are more effective in the early phases of disease development 2,3. With the development of more effective treatments, the aim of treatment has changed dramatically in the last decades, from simply reducing relapse rates and slowing of disability progression to preventing all evidence of new disease activity 4. In the current review, the mode of action and documented effect of the current immunomodulatory MS therapies are briefly discussed.
Methods
The article is based on English-language original clinical treatment trials and selected review articles, identified through a literature search in PubMed using the search term ‘multiple sclerosis’ combined with ‘interferon beta’, ‘glatiramer acetate’, ‘teriflunomide’, ‘dimethyl fumarate’, ‘natalizumab’, ‘fingolimod’, ‘mitoxantrone’ and ‘alemtuzumab’. The search was terminated on 21 May 2015. Titles and abstracts have been reviewed, and full-text versions of articles examined in the majority of cases. Particular emphasis has been placed on randomized controlled phase III studies.
First-line medications
Interferon beta
Interferon beta is a naturally occurring polypeptide predominantly produced by fibroblasts. Its anti-inflammatory effects are largely believed to result from the inhibition of T-lymphocyte proliferation, a shift of cytokine response from an inflammatory response to an anti-inflammatory profile, and reduced migration of inflammatory cells across the blood–brain barrier 5. Interferon beta is available for MS treatment in recombinant forms, as interferon beta-1a or interferon beta-1b. Interferon beta-1b is given as a dose of 250 μg subcutaneously every other day; interferon beta-1a is given as a dose of 30 μg intramuscularly once weekly or subcutaneously at doses of 22 or 44 μg three times a week.
Phase III trials of all the interferon beta preparations have shown beneficial effects in reducing the annualized relapse rate (ARR) by about 30%–34%, reducing the progression of disability in RRMS as well as magnetic resonance imaging (MRI) disease activity 6–9. A recent study on peginterferon beta-1a, given once every 2 weeks, found comparable results, with a reduction in ARR at 36% 10. Studies of all interferon beta preparations 11–14 have also reported a reduced risk of new disease activity amongst people with clinically isolated syndrome (CIS), as shown by a significantly prolonged time to a second relapse and reduction in new MRI lesions, in some cases also a delayed progression of disability. All the interferon beta preparations have been evaluated in the treatment of SPMS. The first interferon beta-1b study showed efficacy of the treatment as measured by both relapse rate and disability progression 15, but later studies of both interferon beta-1b and interferon beta-1a could only detect treatment effects on the relapse rate 16–18. Thus, it seems that only SPMS patients with superimposed relapses benefit from interferon beta treatment 15–18. Interferons have not been documented to be effective in primary progressive MS (PPMS) 19.
Most patients (50%–75%) experience flu-like symptoms, including muscle aches, fever, chills, headache and back pain, that usually appear 2–8 h after an injection and resolve within 24 h. Liver enzymes may be elevated and bone marrow function may be depressed, which warrants periodic surveillance of liver function and blood counts before starting therapy and every 6 months thereafter 11–14. Isolated cases of severe injection-site reactions involving infection or necrosis as well as severe cases of acute liver failure and pancreatitis have been reported. Long-time exposure to interferon beta does not seem to increase the risk of cancer 20,21 or infections.
Interferon beta treatment may induce formation of specific neutralizing antibodies (NABs). NAB formation is less likely during treatment with intramuscular interferon beta-1a 22. The NABs usually appear within 6–18 months of treatment, and evidence is accumulating that the efficacy of treatment is reduced in the presence of NABs. Accordingly, it is recommended to test all patients for the presence of NABs every 6 months during the first 2 years of therapy, and treatment should be switched in patients who are confirmed to be NAB positive 22. In cases of clinically stable disease, switches to other non-interferon first-line treatments are recommended, but second-line treatment should be considered in cases of breakthrough disease.
Glatiramer acetate
Glatiramer acetate is a pool of synthetic peptides, resembling sequences of myelin basic protein, with an average length of 40–100 residues. The mechanisms of action have not been fully clarified but are probably largely related to anti-inflammatory effects by promoting Th2 deviation under the development of Th2 glatiramer acetate reactive CD4+ T cells. These can accumulate in the central nervous system (CNS) and promote bystander suppression by releasing anti-inflammatory cytokines 23. Glatiramer acetate is administered as subcutaneous injections of 20 mg once a day.
Glatiramer acetate treatment trials in RRMS 24 showed a significant reduction in ARR (29%) and a reduction in gadolinium-enhanced MRI activity 25. In a treatment trial of CIS with silent MRI lesions, glatiramer acetate treatment was found to significantly prolong time to a second relapse and to reduce the risk of new MRI lesions 26. Glatiramer acetate has not been investigated for the treatment of SPMS and has not shown significant benefit in PPMS patients 27.
Glatiramer acetate is usually well tolerated, but most patients (65%) experience injection-site reactions (pain, erythema, swelling and pruritus). About 15% report a transient self-limited systemic reaction (immediately after injection) of facial flushing and chest tightness, accompanied at times by palpitation, anxiety and dyspnoea. Other reported side effects are lymphadenopathy, dyspnoea and lipoatrophy 24–26. Lipoatrophy is permanent and is perhaps the most severe side effect. There have not been reports of increased cancer risk or increased risk of infections with prolonged use of glatiramer acetate.
Teriflunomide
Teriflunomide is an immunomodulatory agent that selectively and reversibly inhibits the mitochondrial enzyme dihydroorotate dehydrogenase, required for de novo pyrimidine synthesis. This leads to reduced proliferation of dividing cells that need de novo synthesis of pyrimidine to expand. The therapeutic effect in MS is not fully understood but it is probably mediated by a reduced number of circulating lymphocytes 28. Teriflunomide is administered as tablets, 14 mg once daily.
Two phase III trials in RRMS 29,30 showed that teriflunomide 14 mg once daily, compared to placebo, reduced the ARR by 31%–36%, the rate of disability progression by 26%–27% and MRI gadolinium-enhancing lesions by about 80%. Another phase III trial of teriflunomide 14 mg once daily, compared to interferon beta-1a 44 μg subcutaneously three times weekly, showed similar effects on the ARR (0.26 and 0.22 respectively) and on time to a new relapse or termination of treatment 31. Teriflunomide 14 mg once daily has been tested in a randomized, double-blind, placebo-controlled trial of CIS patients with silent MRI lesions. Teriflunomide treatment was associated with significantly prolonged time to a second relapse and a reduction in new MRI lesions 32. Teriflunomide has not been studied for the treatment of progressive MS.
Common adverse events include upper respiratory tract infection, urinary tract infection, paraesthesia, diarrhoea, nausea, hair thinning, alanine aminotransferase increase, reduction in blood leucocytes and increase in blood pressure 29,30. Relatively frequent (every second week) alanine aminotransferase screening during the first 6 months of treatment is recommended and thereafter every second month 29,30. Teriflunomide treatment should be stopped if liver transaminase levels increase three times above upper normal levels. Regular measurements of blood pressure, white blood cells and platelet counts are also recommended. Teriflunomide has a long half-life. Elimination with cholestyramine or activated charcoal for 11 days can accelerate teriflunomide elimination, leading to more than 98% decrease in teriflunomide plasma concentrations. Liver function needs to be carefully monitored during teriflunomide treatment, and discontinuation of therapy should be considered if a serum transaminase increase more than three times the upper normal level is confirmed. Rare cases of pancytopenia have been reported with the use of leflunomide; this should also lead to treatment termination.
Dimethyl fumarate
Dimethyl fumarate is an immunomodulatory agent with anti-inflammatory properties, but the mechanism of action in MS is only partially understood. Pre-clinical studies indicate that dimethyl fumarate responses are primarily mediated through activation of the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) transcriptional pathway. Dimethyl fumarate has also been shown to upregulate Nrf2-dependent antioxidant genes in patients 33. Dimethyl fumarate is administered as a 240 mg capsule twice daily.
Two phase III trials of RRMS 34,35 showed that dimethyl fumarate 240 mg twice daily, compared to placebo, reduced the ARR by 44%–53%, the rate of disability progression by 22%–32% and MRI gadolinium-enhancing lesions by about 75%–94%. Compared to glatiramer acetate as an active comparator in one of the trials 35, dimethyl fumarate 240 mg twice daily reduced the ARR by 24% and the rate of disability progression by 17%. These differences were not significant and the study was not powered to detect statistically significant differences in treatment effect. The number of new and enlarging MRI T2 lesions was significantly reduced by about 36%. Dimethyl fumarate has not been studied for the treatment of CIS or progressive MS.
Common adverse events include flushing, nausea, diarrhoea and abdominal pain 34,35. The treatment may also reduce white blood cell counts and give elevations of hepatic transaminases; regular blood tests are therefore recommended 34,35. Dimethyl fumarate should be stopped if liver transaminase levels increase three times above upper normal levels. Recently, a case of John Cunningham virus (JCV) induced progressive multifocal leucoencephalopathy (PML) was reported in a patient who had received dimethyl fumarate 36. An additional four PML cases have been previously reported in psoriasis patients who had received fumaderm 37. Prolonged severe lymphopaenia (<500 cells per cubic millimetre) that persists for more than 6 months has been suggested as a risk factor for PML. In the case of persistent lymphopaenia, dimethyl fumarate should be terminated in JCV-positive patients.
Second-line medications
Fingolimod
Fingolimod is an oral sphingosine 1-phosphate receptor (S1PR) modulator that subsequent to its phosphorylation binds with high affinity to S1PR, which in turn leads to an internalization and degradation of the receptor in different tissues and cell types, including lymphocytes. As a consequence, fingolimod inhibits the ability of autoreactive lymphocytes to egress from the lymph nodes towards the CNS. Fingolimod 0.5 mg capsules are given orally once daily 38.
Two phase III trials in RRMS 38,39 showed that fingolimod 0.5 mg once daily, compared to placebo, reduced the ARR by 48%–55%, the rate of disability progression by 25%–30% and MRI gadolinium-enhancing lesions by more than 80%. Another study comparing fingolimod 0.5 mg once daily to interferon beta-1a 30 μg intramuscularly once weekly showed a reduced ARR by 52%, a reduced rate of disability progression by 25% and a reduced number of MRI gadolinium-enhancing lesions by more than 50% amongst those who received fingolimod 40. Fingolimod is currently not documented to be effective against CIS, SPMS or PPMS.
Common adverse events include upper respiratory tract infection, headache, cough, diarrhoea and back pain 38,39. Fingolimod may also cause a transient bradycardia and atrioventricular block. It is therefore recommended to monitor patients continuously with an electrocardiogram for 6 h after the first dose, and to extend the monitoring of patients who develop specific clinically relevant signs of heart arrhythmia (http://www.fda.gov/Drugs/DrugSafety/ucm303192.htm). Generally, fingolimod should not be used by patients with known cardiac arrhythmias or patients using other medications known to induce bradycardia. Rare adverse events of elevated liver enzymes and macular oedema may occur, and regular blood sampling and a routine eye examination after 3 months of treatment are therefore recommended. One death due to a fulminant primary varicella zoster infection was reported in one of the phase III trials 40. Therefore a blood sample for screening of previous varicella zoster infection is advised, and in the case of a negative screening test vaccination is recommended prior to treatment initiation.
Natalizumab
Natalizumab is a monoclonal antibody against α4-integrin, blocking the interaction with its ligands. The mechanism of action is largely through preventing adherence of activated leucocytes to inflamed endothelium, thus inhibiting the migration of inflammatory cells into the CNS. Natalizumab is administered as a 300 mg intravenous infusion every 4 weeks 41.
The pivotal phase III trial of RRMS showed that natalizumab monotherapy reduced the ARR by 68%, the rate of disability progression by 54% and MRI gadolinium-enhancing lesions by more than 90% compared to placebo 41. Another study 42 found that treatment with natalizumab added to interferon beta-1a was significantly more effective than interferon beta-1a alone in reducing ARR, new T2 lesions and disability progression. Natalizumab is currently not documented to be effective against CIS, SPMS or PPMS.
Although natalizumab is generally well tolerated, the treatment is associated with an increased risk of developing PML 43. This is a potentially life-threatening CNS infection of oligodendrocytes by the JCV. Therefore all patients receiving natalizumab should be screened for previous JCV infection. The risk for PML in JCV-negative patients is low (<0.09/1000) and is probably associated with recent seroconversion (estimated as 2%–3% each year) or a false negative test. Amongst the JCV-positive patients the risk of developing PML is influenced by treatment duration and previous immunosuppressive treatment. The risk is relatively low during the first 2 years of treatment and increases thereafter. The highest risk is found amongst JCV-positive patients who previously have also received immunosuppressive treatment after 2 years of treatment (∼1/60) 44. Anti-JCV antibody levels seem also to differentiate PML risk in anti-JCV antibody positive patients with no prior immunosuppressant use 45. As a general rule, it is recommended that JCV-positive patients who have been treated with natalizumab for more than 2 years should be switched to another second-line therapy. Based on current knowledge, a washout time of 8 weeks seems to reduce the risk of rebound effect compared to longer washout periods. In the case of a low JCV index (<1.5), natalizumab treatment may in some cases be continued after thorough information is given to the patient and under careful evaluation for new symptoms that may represent PML 44. Three-monthly JCV index evaluation and MR examination is then recommended. It is recommended to retest JCV-negative patients every 6 months and JCV-positive patients should be carefully informed about the risk for PML at treatment initiation and after 2 years of treatment.
Natalizumab treatment may induce an immune response, with the formation of persistent NABs (∼4%–6%) against the preparation. NABs usually appear within the first 12 months of treatment, reduce the efficacy of the treatment and are associated with higher rates of infusion-related adverse events. Accordingly, patients should be tested for NABs at 6 and 12 months of therapy and later for infusion-related adverse events or treatment failure. NABs can occur transiently and positive findings should therefore be confirmed within 3 months before deciding to switch therapy. Testing can be discontinued in patients who remain NAB negative during the first year of therapy.
Alemtuzumab
Alemtuzumab is a recombinant, humanized monoclonal antibody directed against CD52, a cell surface antigen present at high levels on especially T and B lymphocytes. Alemtuzumab acts through antibody-dependent cellular cytolysis and complement-mediated lysis following cell surface binding. The mechanism by which alemtuzumab exerts its therapeutic effects in MS is suggested to be by a depletion and repopulation of lymphocytes that reduces the potential for relapses and thereby delays disease progression 46. Alemtuzumab is administered by intravenous infusion for two treatment courses. The initial treatment course is 12 mg/day for five consecutive days (60 mg total dose), and the second treatment course is 12 mg/day for three consecutive days (36 mg total dose) administered 12 months after the initial treatment course. Additional courses may be given 12 months after the latest treatment course if necessary. Based on the European Medicines Agency (EMA) licence alemtuzumab has indication as a first-line medication in active RRMS. Because the treatment increases the risk of secondary autoimmunity, most European neurologists would use this drug as a second-line preparation, however.
Two phase III trials of RRMS have shown that alemtuzumab 12, compared to interferon beta-1a 44 μg administered subcutaneously three times weekly, reduced the ARR by 49%–55%, the rate of disability progression by 30%–42% and MRI gadolinium-enhancing lesions by 61%–63% 47,48. Alemtuzumab has currently not been studied in patients with CIS or PPMS and has not been demonstrated to be effective in SPMS 49,50.
Patients commonly experience infusion-associated reactions including flushing, nausea, headache, tachycardia, urticaria, rash, pruritus, pyrexia and fatigue 47–50. Oral antiviral prophylaxis with aciclovir 200 mg twice daily (or equivalent) should be administered and continued for a minimum of 1 month after the last dose. Alemtuzumab treatment is associated with increased risk of upper respiratory tract infection and urinary tract infection. Alemtuzumab treatment may also result in the formation of autoantibodies and increased risk of autoimmune-mediated conditions (occurring a median of 32 months after the first treatment), including thyroid disorders (41%), immune thrombocytopenic purpura (3.5%) or, rarely, nephropathies (e.g. anti-glomerular basement membrane disease) (<1%) 51. Based on the risk of autoimmune-mediated conditions, monthly blood and urine analyses are recommended for 4 years after the last dosing of alemtuzumab.
Mitoxantrone
Mitoxantrone is a synthetic anthracenedione derivative and is mostly used in treating various malignancies. It interacts with nuclear DNA and is a potent immunosuppressive agent targeting proliferating immune cells, inhibiting proliferation and inducing apoptosis of T lymphocytes, B lymphocytes, macrophages and other antigen-presenting cells.
Limited efficacy data are available, but controlled studies of highly active RRMS have shown significant efficacy of the treatment, as shown by a 60%–70% reduction in the relapse rate (compared with placebo or intravenous methylprednisolone) as well as reduced disability progression and MRI disease activity 52,53. The largest phase III investigator-blinded study randomized patients with worsening RRMS and SPMS for 5 or 12 mg of mitoxantrone per square metre of body surface or placebo every 3 months for 2 years 54. The treatment showed a 66% reduction in the ARR in the high-dose arm compared with placebo, and reduced disability progression and MRI disease activity. Mitoxantrone has not been included in treatment trials of patients with CIS or PPMS.
Side effects such as transient nausea, fatigue, mild hair loss (for days to a week) and menstrual disturbances are frequent (60%–70%) 54. Additional side effects are urinary tract infection (about 30%) as well as elevated liver enzymes and leucopenia (about 15%–20%). Mitoxantrone-induced amenorrhoea and acute promyelocytic leukaemia have also been reported. The treatment induces transient leucopenia, with a nadir after about 10 days, and thus follow-up blood control is needed. Although not in the phase III trial, lethal congestive heart failure and therapy-related leukaemia have been reported, even years after treatment ends 55,56. Due to the potential cardiotoxicity, the maximum cumulative dose is restricted to 120–140 mg/m2 of body surface, and echocardiograms should be done before, during and after treatment. Mitoxantrone is teratogenic and is absolutely contraindicated in pregnancy. The use of mitoxantrone has rapidly decreased due to the risk of severe complications and the increasing number of alternative highly effective and less toxic treatment options.
Suggested treatment strategies
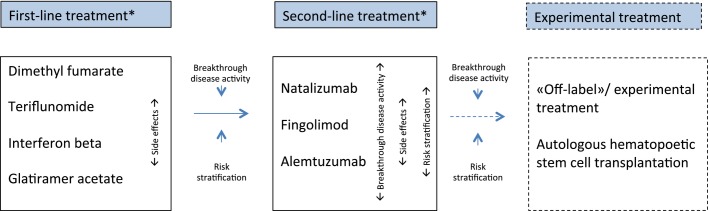
Individualized therapy is advocated; the ideal treatment option would be the safest treatment that eliminates clinical and radiological evidence of disease activity 3. Most patients would start on a first-line therapy but then be changed quickly to a second-line medication in the case of breakthrough disease activity (Fig. 1). Evidence of clinical disease activity (relapses and/or accumulating disability) with or without new MRI lesions is in general accepted as an indication for switching to more potent second-line therapies. In this context, models like the Rio or modified Rio score 57 have been increasingly accepted as a tool to monitor the treatment effect. The score is based on evaluation of treatment response by the combination of clinical (relapse and disability progression) and MRI disease activity (Rio score) or relapse and MRI disease activity only (modified Rio score) during the first year of interferon treatment. Increasing evidence indicates that patients experiencing a new clinical relapse with significant influence on disability and/or new signs of radiological disease activity (≥3 active MRI lesions) during the last year, whilst on first-line medications, should be considered for switching to more potent medications 58,59.
Figure 1.
Treatment algorithm for treatment-naïve patients with RRMS. *Patients with rapidly evolving severe RRMS should start directly on a second-line therapy. Breakthrough disease activity is defined as one new clinical relapse with significant influence on disability and/or new signs of radiological disease activity (≥3 active MRI lesions) during the last year whilst on first-line medication.
Another possible treatment strategy is induction treatment, which consists of early use of immunosuppressive medications followed by long-term maintenance therapy 60. This treatment regime has been used with success for patients with aggressive RRMS, using mitoxantrone 61. The use of mitoxantrone is declining because of its long-term safety profile. Alemtuzumab is approved as a first-line therapy, and could also be considered an induction treatment because of its long-term effects on the immune system. This could be an attractive treatment option for patients with a highly active disease course, as 70.1% of the alemtuzumab-treated patients in the CARE-MS study remained free of new lesions and MRI activity in year 4, despite most receiving their last treatment course 3 years prior 62.
Choosing amongst the first-line medications
The main findings from the pivotal and phase III studies performed are given in Tables 1 and 2. Although the placebo-controlled trials indicate numerically higher efficacy on ARR from dimethyl fumarate (about 44%–53%) compared to the other first-line preparations (about 30%–35%), data on direct comparisons of the agents are limited. Head-to-head comparison between dimethyl fumarate and glatiramer acetate indicated numerically (although not statistically significant) better effect from dimethyl fumarate 35. Head-to-head comparison between teriflunomide and high-frequency interferon beta-1a showed comparable effects on the ARR 29. Head-to-head comparisons between intramuscular low-dose and low-frequency interferon beta-1a and subcutaneous high-dose and high-frequency interferon beta-1a and interferon beta-1b have shown that high-dose and high-frequency interferon beta regimens have short-term benefits on the relapse rate and MRI activity 63,64. Limitations in the design of these studies have been widely discussed, however, and the long-term differences in efficacy may be reduced by a significantly lower frequency of NAB formation with low-dose and low-frequency interferon beta-1a. Head-to-head comparisons of glatiramer acetate and subcutaneous high-dose and high-frequency interferon beta-1a and interferon beta-1b have shown similar clinical benefit from the treatments, with some MRI parameters in favour of the interferon beta preparations 65,66.
Table 1.
Randomized placebo-controlled phase III clinical trials of the approved relapsing−remitting multiple sclerosis medications
| Medication | N | Trial name (reference) | ARR | Disability progression | ||
|---|---|---|---|---|---|---|
| Relative reduction | ARR | Relative reduction | EDSS progression | |||
| First-line | ||||||
| Interferon beta-1b | 124 vs. 123 | MSSG 6 | 34% | 0.84 vs. 1.27 | 29% (N.S.) | 0.20 vs. 0.28 |
| Interferon beta-1a i.m. | 158 vs. 143 | MSCRG 7 | 18% | 0.67 vs. 0.82 | 37% | 0.22a vs. 0.35a |
| Interferon beta-1a s.c. | 184 vs. 187 | PRISMS 8 | 32% | 1.73 vs. 2.56 | 32% | 0.26 vs. 0.38 |
| Peginterferon-1a | 500 vs. 512 | ADVANCE 10 | 36% | 0.26 vs. 0.40 | 36% | 0.07 vs. 0.11 |
| Glatiramer acetate | 125 vs. 126 | CMSSG 24 | 29% | 1.19 vs. 1.68 | 12% (N.S.) | 0.22 vs. 0.25 |
| Teriflunomide | 358 vs. 363 | TEMSO 29 | 31% | 0.37 vs. 0.54 | 26% | 0.20 vs. 0.27 |
| Teriflunomide | 370 vs. 388 | TOWER 30 | 36% | 0.32 vs. 0.50 | 24% | 0.16 vs. 0.21 |
| Dimethyl fumarate | 410 vs. 408 | DEFINE 34 | 53% | 0.17 vs. 0.36 | 41% | 0.16 vs. 0.27 |
| Dimethyl fumarate | 359 vs. 363 | CONFIRM 35 | 44% | 0.22 vs. 0.40 | 24% (N.S.) | 0.13 vs. 0.17 |
| Second-line | ||||||
| Fingolimod | 425 vs. 418 | FREEDOMS 38 | 55% | 0.18 vs. 0.40 | 28% | 0.18 vs. 0.25 |
| Fingolimod | 358 vs. 355 | FREEDOMS-2 39 | 48% | 0.21 vs. 0.40 | 14% (N.S.) | 0.25 vs. 0.29 |
| Natalizumab | 627 vs. 315 | AFFIRM 41 | 68% | 0.23 vs. 0.73 | 42% | 0.17 vs. 0.29 |
| Mitoxantroneb | 60 vs. 64 | MIMS 54 | 66% | 0.35 vs. 1.02 | 64% | 0.08 vs. 0.22 |
N, number of patients included in each treatment arm − note that the number only includes treatment arms with US Food and Drug Administration/European Medicines Agency approved dosages; ARR, annualized relapse rate, active medication versus placebo; EDSS, Expanded Disability Status Scale; N.S., not significant; i.m., intramuscular; s.c., subcutaneous. Disability progression is the proportion of patients with 3 months confirmed progression in EDSS score, active medication versus placebo.
6 months confirmed progression in EDSS score;
not approved in all European countries.
Table 2.
Randomized controlled phase III clinical trials of the approved relapsing−remitting multiple sclerosis medications, where the medications have been compared head-to-head with another active multiple sclerosis medication
| Medication | Compared to | N | Trial name (reference) | ARR | Disability progression | ||
|---|---|---|---|---|---|---|---|
| Relative reduction | ARR | Relative reduction | EDSS progression | ||||
| First-line | |||||||
| Interferon beta-1b | Interferon beta-1a i.m. | 92 vs. 96 | INCOMIN 63 | 24% | 0.5 vs. 0.7 | 44% | 0.13 vs. 0.30 |
| Interferon-beta-1a s.c. | Interferon-beta-1a i.m. | 339 vs. 338 | EVIDENCE 64 | 16%a | 0.54 vs. 0.64 | 13% (N.S.) | 0.13 vs. 0.15 |
| Interferon beta-1a s.c. | Glatiramer acetate | 386 vs. 378 | REGARD 65 | 3% (N.S) | 0.30 vs. 0.29 | 25% (N.S.) | 0.12 vs. 0.09 |
| Interferon-beta-1b | Glatiramer acetate | 899 vs. 448 | BEYOND 66 | 3% (N.S) | 0.33 vs. 0.34 | 5% (N.S.) | 0.22 vs. 0.20 |
| Teriflunomide | Interferon-beta 1a s.c. | 111 vs. 104 | TENERE 31 | 4% (N.S) | 0.26 vs. 0.22 | - | - |
| Dimethyl fumarate | Glatiramer acetate | 359 vs. 350 | CONFIRM 35 | 24% (N.S) | 0.22 vs. 0.29 | 17% (N.S.) | 0.13 vs. 0.16 |
| Second-line | |||||||
| Fingolimod | Interferon beta-1a i.m. | 431 vs. 435 | TRANSFORMS 40 | 52%a | 0.16 vs. 0.33 | 25% (N.S.) | 0.06 vs. 0.08 |
| Alemtuzumab | Interferon beta-1a s.c. | 376 vs. 202 | CARE MS-1 47 | 55% | 0.18 vs. 0.39 | 30% (N.S.) | 0.08b vs. 0.11b |
| Alemtuzumab | Interferon beta-1a s.c. | 426 vs. 202 | CARE MS-2 48 | 49% | 0.26 vs. 0.52 | 42% | 0.13b vs. 0.21b |
N, number of patients included in each treatment arm − note that the number only includes treatment arms with US Food and Drug Administration/European Medicines Agency approved dosages; ARR, annualized relapse rate during 2 years of follow-up, medication in column 1 versus medication in column 2; EDSS, Expanded Disability Status Scale; i.m., intramuscular; s.c., subcutaneous; N.S., not significant. Disability progression is the proportion of patients with 3 months confirmed progression in EDSS score, medication in column 1 versus medication in column 2.
1 year follow-up.;
6 months confirmed progression in EDSS score.
Disease-modifying treatment for MS is a long-lasting therapy for most patients. Adherence to treatment is thus crucial, and many patients may therefore prefer oral treatment. Consequently starting with an oral first-line drug is suggested. In the case of intolerability or unacceptable side effects, switching between the oral preparations or with one of the injectable preparations should be considered. The injectable medications have been used for a longer period than the oral medications, and more long-term safety data are therefore available 20,21. Similarly, there are more long-term safety data on pregnancies occurring during treatments with both glatiramer acetate and interferons, pointing to a relative safety of use 67. These could be good reasons for still choosing an injectable medication as first-line treatment. It is important to continuously evaluate the treatment regimen, aiming for optimal adherence, considering both the administration form and side-effect profiles.
Choosing amongst the second-line medications
In the case of breakthrough disease activity despite a full and adequate course of a first-line preparation, switching to natalizumab, fingolimod or alemtuzumab should be considered. Although alemtuzumab is licensed as a first-line medication in active RRMS, many European neurologists would use this drug as a second-line preparation, due to potential side effects. Second-line therapy should also be considered in the case of patients with rapidly evolving severe RRMS defined by two or more disabling relapses in 1 year, and with gadolinium-enhancing lesions on brain MRI or a significant increase in T2 lesion load compared to a previous recent MRI. Careful risk stratification for potential adverse effects is important, and most neurologists would prefer fingolimod or alemtuzumab for patients who are JCV positive. Similarly, in the case of contraindications for fingolimod or alemtuzumab, one of the other second-line treatment options should be considered. The use of mitoxantrone has become less frequent due to the relatively high risk of serious side effects, and may only be used in some cases of SPMS. Stratification for differences in clinical effect is difficult due to the lack of treatment studies with head-to-head comparisons of second-line therapies. Some neurologists would prefer natalizumab for JCV-negative patients due to the numerically higher reduction of ARR in pivotal trials, although treatment effect cannot be directly compared between different study populations.
For a small group of patients who do not respond to the approved second-line treatments, off-label treatments like rituximab 68 or ofatumumab 69 or experimental therapy with autologous haematopoietic stem cell transplantation 70 may be considered. These treatment options have currently not been tested in large phase III trials, but phase II trials or case series reports have shown promising results. These treatment options also seem to be effective against RRMS and not the progressive forms of the disease 71.
Conclusions and future challenges
Although the last decade has shown a revolution in treatment options for patients with MS, this has mainly benefited newly diagnosed patients with an RRMS disease course. None of the approved medications or experimental therapies has shown convincing evidence of slowing down or preventing disease progression in patients with SPMS or PPMS. Thus there is an urgent need to also improve the treatment options for patients who have entered a progressive phase.
Disclosure of conflicts of interest
Ø. Torkildsen has participated on a scientific advisory board for Biogen Idec, Merck-Serono and Genzyme and received speaker honoraria and travel grants from Genzyme, Merck-Serono, Novartis and Biogen Idec. K.-M. Myhr has participated on scientific advisory boards for Novartis Norway, Biogen Idec and Genzyme; received funding for travel from Bayer, Novartis, Merck-Serono and Biogen Idec; received speaker honoraria from Bayer, Genzyme, Sanofi-Aventis, Novartis, Merck-Serono and Biogen Idec; and received unrestricted research support from Bayer, Sanofi-Aventis, Novartis, Merck-Serono, Biogen Idec, Pronova Biocare and the Norwegian MS Society. L. Bø has participated on scientific advisory boards for Novartis Norway; received funding for travel from Sanofi-Aventis, Novartis, Merck-Serono and Biogen Idec; received speaker honoraria from Bayer, Genzyme, Sanofi-Aventis, Novartis, Merck-Serono and Biogen Idec; and received unrestricted research support from Bayer, Sanofi-Aventis, Novartis, Merck-Serono and Biogen Idec.
References
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Coles AJ, Cox A, Le Page E, et al. The window of therapeutic opportunity in multiple sclerosis: evidence from monoclonal antibody therapy. J Neurol. 2006;253:98–108. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Chan JR, Oksenberg JR. Multiple sclerosis: prospects and promise. Ann Neurol. 2013;74:317–327. doi: 10.1002/ana.24009. [DOI] [PubMed] [Google Scholar]
- Havrdova E, Galetta S, Stefoski D, Comi G. Freedom from disease activity in multiple sclerosis. Neurology. 2010;74(Suppl. 3):S3–S7. doi: 10.1212/WNL.0b013e3181dbb51c. [DOI] [PubMed] [Google Scholar]
- Dhib-Jalbut S, Marks S. Interferon-β mechanisms of action in multiple sclerosis. Neurology. 2010;74(Suppl. 1):S17-24. doi: 10.1212/WNL.0b013e3181c97d99. [DOI] [PubMed] [Google Scholar]
- The IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology. 1993;43:655–661. doi: 10.1212/wnl.43.4.655. [DOI] [PubMed] [Google Scholar]
- Jacobs LD, Cookfair DL, Rudick RA, et al. Intramuscular interferon beta1a for disease progression in relapsing multiple sclerosis. Ann Neurol. 1996;39:285–294. doi: 10.1002/ana.410390304. [DOI] [PubMed] [Google Scholar]
- Ebers GC PRISMS (Prevention of Relapses Disability by Interferon β-1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised double-blind placebo controlled study of interferon β-1a in relapsing/remitting multiple sclerosis. Lancet. 1998;352:1498–1504. [PubMed] [Google Scholar]
- PRISMS Study Group and the University of British Columbia MS/MRI Analysis Group. PRISMS4: Long-term efficacy of interferon-β-1a in relapsing MS. Neurology. 2001;56:1628–1636. doi: 10.1212/wnl.56.12.1628. [DOI] [PubMed] [Google Scholar]
- Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon β-1a for relapsing−remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol. 2014;13:657–665. doi: 10.1016/S1474-4422(14)70068-7. Jul; [DOI] [PubMed] [Google Scholar]
- Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006;67:1242–1249. doi: 10.1212/01.wnl.0000237641.33768.8d. [DOI] [PubMed] [Google Scholar]
- Jacobs LD, Beck RW, Simon JH, et al. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med. 2000;343:898–904. doi: 10.1056/NEJM200009283431301. [DOI] [PubMed] [Google Scholar]
- Comi G, Filippi M, Barkhof F, et al. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 2001;357:1576–1582. doi: 10.1016/s0140-6736(00)04725-5. [DOI] [PubMed] [Google Scholar]
- Comi G, De SN, Freedman MS, et al. Comparison of two dosing frequencies of subcutaneous interferon beta-1a in patients with a first clinical demyelinating event suggestive of multiple sclerosis (REFLEX): a phase 3 randomised controlled trial. Lancet Neurol. 2012;11:33–41. doi: 10.1016/S1474-4422(11)70262-9. . Erratum in: Lancet Neurol 2012; 11: 125. [DOI] [PubMed] [Google Scholar]
- Kappos L European Study Group on Interferon β-1b in Secondary Progressive MS. Placebo-controlled multicentre randomised trial of interferon β-1b in treatment of secondary progressive multiple sclerosis. Lancet. 1998;352:1491–1497. [PubMed] [Google Scholar]
- Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-Beta-1a in MS (SPECTRIMS) Study Group. Randomized controlled trial of interferon-beta-1a in secondary progressive MS. Clinical results. Neurology. 2001;56:1496–1504. doi: 10.1212/wnl.56.11.1496. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Cutter GR, Fischer JS, et al. Benefit of interferon β-1a on MSFC progression in secondary progressive MS. Neurology. 2002;59:679–687. doi: 10.1212/wnl.59.5.679. [DOI] [PubMed] [Google Scholar]
- Panitch H, Miller A, Paty D, et al. Interferon beta-1b in secondary progressive MS: results from a 3-year controlled study. Neurology. 2004;63:1788–1795. doi: 10.1212/01.wnl.0000146958.77317.3e. [DOI] [PubMed] [Google Scholar]
- Leary SM, Miller DH, Stevenson VL, et al. Interferon β-1a in primary progressive MS: an exploratory, randomized, controlled trial. Neurology. 2003;60:44–51. doi: 10.1212/wnl.60.1.44. [DOI] [PubMed] [Google Scholar]
- Sandberg-Wollheim M, Kornmann G, Bischof D, Moraga MS, Hennessy B, Alteri E. The risk of malignancy is not increased in patients with multiple sclerosis treated with subcutaneous interferon beta-1a: analysis of data from clinical trial and post-marketing surveillance settings. Mult Scler. 2011;17:431–440. doi: 10.1177/1352458511403642. [DOI] [PubMed] [Google Scholar]
- Kingwell E, Evans C, Zhu F, Oger J, Hashimoto S, Tremlett H. Assessment of cancer risk with β-interferon treatment for multiple sclerosis. J Neurol Neurosurg Psychiatry. 2014;85:1096–1102. doi: 10.1136/jnnp-2013-307238. [DOI] [PubMed] [Google Scholar]
- Sørensen PS, Deisenhammer F, Duda P, et al. Guidelines on use of anti-IFN-β antibody measurements in multiple sclerosis: report of an EFNS Task Force on IFN-β antibodies in multiple sclerosis. Eur J Neurol. 2005;12:817–827. doi: 10.1111/j.1468-1331.2005.01386.x. [DOI] [PubMed] [Google Scholar]
- Racke MK, LovettRacke AE, Karandikar NJ. The mechanism of action of glatiramer acetate treatment in multiple sclerosis. Neurology. 2010;74(Suppl. 1):S2530. doi: 10.1212/WNL.0b013e3181c97e39. [DOI] [PubMed] [Google Scholar]
- Johnson KP, Brooks BR, Cohen JA, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing−remitting multiple sclerosis: results of a phase III multicenter, double-blind, placebo-controlled trial. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging – measured disease activity and burden in patients with relapsing multiple sclerosis. European/Canadian Glatiramer Acetate Study Group. Ann Neurol. 2001;49:290–297. [PubMed] [Google Scholar]
- Comi G, Martinelli V, Rodegher M, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:150311. doi: 10.1016/S0140-6736(09)61259-9. [DOI] [PubMed] [Google Scholar]
- Wolinsky JS, Narayana PA, O’Connor P, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol. 2007;61:14–24. doi: 10.1002/ana.21079. [DOI] [PubMed] [Google Scholar]
- Papadopoulou A, Kappos L, Sprenger T. Teriflunomide for oral therapy in multiple sclerosis. Expert Rev Clin Pharmacol. 2012;5:617–628. doi: 10.1586/ecp.12.56. Nov;. doi: 10.1586/ecp.12.56. [DOI] [PubMed] [Google Scholar]
- O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–1303. doi: 10.1056/NEJMoa1014656. . doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:247–256. doi: 10.1016/S1474-4422(13)70308-9. . doi: 10.1016/S1474-4422(13)70308-9. Epub 2014 Jan 23. [DOI] [PubMed] [Google Scholar]
- Vermersch P, Czlonkowska A, Grimaldi LM, et al. Teriflunomide versus subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis: a randomised, controlled phase 3 trial. Mult Scler. 2014;20:705–716. doi: 10.1177/1352458513507821. [DOI] [PubMed] [Google Scholar]
- Miller AE, Wolinsky JS, Kappos L, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:977–986. doi: 10.1016/S1474-4422(14)70191-7. [DOI] [PubMed] [Google Scholar]
- Linker RA, Gold R. Dimethyl fumarate for treatment of multiple sclerosis: mechanism of action, effectiveness, and side effects. Curr Neurol Neurosci Rep. 2013;13:394. doi: 10.1007/s11910-013-0394-8. [DOI] [PubMed] [Google Scholar]
- Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367:1098–1107. doi: 10.1056/NEJMoa1114287. [DOI] [PubMed] [Google Scholar]
- Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367:1087–1097. doi: 10.1056/NEJMoa1206328. [DOI] [PubMed] [Google Scholar]
- Rosenkranz T, Novas M, Terborg C. PML in a patient with lymphocytopenia treated with dimethyl fumarate. N Engl J Med. 2015;372:1476–1478. doi: 10.1056/NEJMc1415408. [DOI] [PubMed] [Google Scholar]
- Sweetser MT, Dawson KT, Bozic C. Manufacturer’s response to case reports of PML. N Engl J Med. 2013;368:1659–1661. doi: 10.1056/NEJMc1300283. [DOI] [PubMed] [Google Scholar]
- Kappos L, Radue EW, O’Connor P, et al. A placebo controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing−remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–556. doi: 10.1016/S1474-4422(14)70049-3. [DOI] [PubMed] [Google Scholar]
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:40215. doi: 10.1056/NEJMoa0907839. [DOI] [PubMed] [Google Scholar]
- Polman CH, O’Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354:911–923. doi: 10.1056/NEJMoa044396. [DOI] [PubMed] [Google Scholar]
- Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–446. doi: 10.1016/S1474-4422(10)70028-4. [DOI] [PubMed] [Google Scholar]
- Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2014;76:802–812. doi: 10.1002/ana.24286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Coles AJ. Alemtuzumab: evidence for its potential in relapsing−remitting multiple sclerosis. Drug Des Devel Ther. 2013;7:131–138. doi: 10.2147/DDDT.S32687. . doi: 10.2147/DDDT.S32687. Epub 2013 Mar 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing−remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380:1819–1828. doi: 10.1016/S0140-6736(12)61769-3. [DOI] [PubMed] [Google Scholar]
- Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380:1829–1839. doi: 10.1016/S0140-6736(12)61768-1. [DOI] [PubMed] [Google Scholar]
- Coles AJ, Wing MG, Molyneux P, et al. Monoclonal antibody treatment exposes three mechanisms underlying the clinical course of multiple sclerosis. Ann Neurol. 1999;46:296–304. doi: 10.1002/1531-8249(199909)46:3<296::aid-ana4>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Paolillo A, Coles AJ, Molyneux PD, et al. Quantitative MRI in patients with secondary progressive MS treated with monoclonal antibody Campath 1H. Neurology. 1999;53:751–757. doi: 10.1212/wnl.53.4.751. [DOI] [PubMed] [Google Scholar]
- Tuohy O, Costelloe L, Hill-Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long-term safety and efficacy. J Neurol Neurosurg Psychiatry. 2015;86:208–215. doi: 10.1136/jnnp-2014-307721. [DOI] [PubMed] [Google Scholar]
- Millefiorini E, Gasperini C, Pozzilli C, et al. Randomized placebo-controlled trial of mitoxantrone in relapsing−remitting multiple sclerosis: 24 month clinical and MRI outcome. J Neurol. 1997;244:153–159. doi: 10.1007/s004150050066. [DOI] [PubMed] [Google Scholar]
- Martinelli V, Radaelli M, Straffi L, et al. Mitoxantrone: benefits and risks in multiple sclerosis patients. Neurol Sci. 2009;30(Suppl. 2):S167–170. doi: 10.1007/s10072-009-0142-7. [DOI] [PubMed] [Google Scholar]
- Hartung HP, Gonsette R, König N, et al. Mitoxantrone in progressive multiple sclerosis: a placebo controlled, double-blind, randomised, multicentre trial. Lancet. 2002;360:2018–2025. doi: 10.1016/S0140-6736(02)12023-X. [DOI] [PubMed] [Google Scholar]
- Martinelli V, Cocco E, Capra R, et al. Acute myeloid leukemia in Italian patients with multiple sclerosis treated with mitoxantrone. Neurology. 2011;77:1887–1895. doi: 10.1212/WNL.0b013e318238ee00. [DOI] [PubMed] [Google Scholar]
- Ellis R, Brown S, Boggild M. Therapy-related acute leukaemia with mitoxantrone: four years on, what is the risk and can it be limited? Mult Scler. 2015;21:642–645. doi: 10.1177/1352458514541508. [DOI] [PubMed] [Google Scholar]
- Sormani MP, Rio J, Tintorè M, et al. Scoring treatment response in patients with relapsing multiple sclerosis. Mult Scler. 2013;19:605–612. doi: 10.1177/1352458512460605. [DOI] [PubMed] [Google Scholar]
- Río J, Rovira A, Tintoré M, et al. Relationship between MRI lesion activity and response to IFN-β in relapsing−remitting multiple sclerosis patients. Mult Scler. 2008;14:479–484. doi: 10.1177/1352458507085555. [DOI] [PubMed] [Google Scholar]
- Bermel RA, You X, Foulds P, et al. Predictors of long-term outcome in multiple sclerosis patients treated with interferon β. Ann Neurol. 2013;73:95–103. doi: 10.1002/ana.23758. [DOI] [PubMed] [Google Scholar]
- Edan G, Le Page E. Induction therapy for patients with multiple sclerosis: Why? When? How? CNS Drugs. 2013;27:403–409. doi: 10.1007/s40263-013-0065-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edan G, Comi G, Le PE, Leray E, Rocca MA, Filippi M French–Italian Mitoxantrone Interferon-beta-1b Trial Group. Mitoxantrone prior to interferon beta-1b in aggressive relapsing multiple sclerosis: a 3-year randomised trial. J Neurol Neurosurg Psychiatry. 2011;82:1344–1350. doi: 10.1136/jnnp.2010.229724. [DOI] [PubMed] [Google Scholar]
- Arnold D, Traboulsee A, Coles A, et al. Durable effect of alemtuzumab on MRI activity in treatment-naive active relapsing-remitting multiple sclerosis patients: 4-year follow-up of CARE-MS I (P7.246) Neurology. 2015;84 ; 14 Supplement P7.246. [Google Scholar]
- Durelli L, Verdun E, Barbero P, et al. Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN) Lancet. 2002;359:1453–1460. doi: 10.1016/s0140-6736(02)08430-1. [DOI] [PubMed] [Google Scholar]
- Panitch H, Goodin DS, Francis G, et al. EVidence of Interferon Dose−response: European North American Compartative Efficacy; University of British Columbia MS/MRI Research Group. Randomized, comparative study of interferon β-1a treatment regimens in MS: the EVIDENCE Trial. Neurology. 2002;59:1496–1506. doi: 10.1212/01.wnl.0000034080.43681.da. [DOI] [PubMed] [Google Scholar]
- Mikol DD, Barkhof F, Chang P, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol. 2008;7:903–914. doi: 10.1016/S1474-4422(08)70200-X. [DOI] [PubMed] [Google Scholar]
- O’Connor P, Filippi M, Arnason B, et al. 250 μg or 500 μg interferon beta-1b versus 20 mg glatiramer acetate in relapsing−remitting multiple sclerosis: a prospective, randomised, multicentre study. Lancet Neurol. 2009;8:889–897. doi: 10.1016/S1474-4422(09)70226-1. [DOI] [PubMed] [Google Scholar]
- Amato MP, Portaccio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2015;29:207–220. doi: 10.1007/s40263-015-0238-y. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing−remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Sorensen PS, Lisby S, Grove R, et al. Safety and efficacy of ofatumumab in relapsing−remitting multiple sclerosis: a phase 2 study. Neurology. 2014;82:573–581. doi: 10.1212/WNL.0000000000000125. [DOI] [PubMed] [Google Scholar]
- Burman J, Iacobaeus E, Svenningsson A, et al. Autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: the Swedish experience. J Neurol Neurosurg Psychiatry. 2014;85:1116–1121. doi: 10.1136/jnnp-2013-307207. [DOI] [PubMed] [Google Scholar]
- Atkins HL, Freedman MS. Hematopoietic stem cell therapy for multiple sclerosis: top 10 lessons learned. Neurotherapeutics. 2013;10:68–76. doi: 10.1007/s13311-012-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]