Abstract
The use of biobanks in biomedical research has grown considerably in recent years. As a result of the increasing analysis of tissue samples stored in biobanks, there has also been an increase in the probability of discovering—in addition to the research target—incidental findings (IF). We identified 23 laws, policies and guidelines from international, regional and national organizations that provide guidance or identify the need for the disclosure of IF to research participants. We analyzed these instruments to determine their contemplation of the funding considerations for the disclosure of IF, examining their guidance for who discloses and the extent of researcher responsibilities. We found that the available normative documents provide little guidance to researchers and biobanks for how they should address cost and funding concerns associated with IF disclosure. It is therefore essential that the research and policy communities think through the financial implications of imposing an ethical responsibility to disclose IF. Concerted efforts should be made by policymakers, ethicists, researchers, clinicians and research institutions to develop detailed funding recommendations, potentially universal in application, to aid in the disclosure of IF, and we provide recommendations on steps that can be taken to ensure full consideration of these issues.
Keywords: biobank, disclosure, ethics, incidental findings, research
Increasingly, biomedical research focuses on the links between genetic variants and human disease. In order to examine a sufficient number of samples for statistical significance, studies bank tissue and data provided by patients thereby accruing large numbers of samples useable over a long period of time 1. Genetic analysis of these samples can lead to the discovery of health-related information on rare, common or complex conditions, in addition to the research objectives. These unexpected results are called incidental findings (IF): discoveries concerning an individual research participant that are found in the course of research but are beyond the scope of the study 2,3. In other words, IF are unsolicited research findings.
Much of the detail about how IF should be handled comes from scientific and ethics literature. The issue of costs and funding remain tangential to the primary debates, which revolve mostly around the question of the type of information that is worthy of disclosure. However, there is currently a general consensus that certain IF should be disclosed (if it meets scientifically accepted criteria) 4–6, although there remains an active debate about the details of disclosure 7–11.
Conversely, organizational, national and international guidelines provide minimal clues to aid researchers, while advocating diverse levels of obligation. Even the guidelines that actually provide some insight into IF disclosure 3,12–33—and there are few in the universe of biomedical research guidance documents—fail to consider the more practical day-to-day impact of IF disclosure: how should it be funded, and what should funding cover?
These questions involve a wide variety of considerations, including the necessary technology to store and protect identifiable information about participants that would allow them to be informed, costs incurred confirming the clinical validity and utility of the finding, and costs associated with the salaries of those who must carry out the tasks required for disclosure 11,34. Indeed, work by Bledsoe et al. recognizes the importance of cost in a requirement to disclose individual research results and IF, though this focuses primarily on biobank policies rather than broadly applicable normative guidance 11.
The formulation of detailed guidance and funding mechanisms to perform IF disclosure is of particular significance to research biobanks and their increasing reliance on large numbers of tissue samples. This specific context is unique because of the difficulties that might arise in contacting a participant years after initial participation; the potential for deceased participants and the discovery of genetic information that might be useful for the surviving family; and the sharing of tissue samples and data within and across national borders, where different jurisdictions might have different requirements and funding to address IF.
The calls to disclose IF, becoming more and more voluminous, appear to rest upon an assumption in both the literature and guidelines that researchers have the clinical know-how, logistical capabilities, and sufficient funding to carry out the disclosure. This assumption, though, remains under study 11,35 and careful consideration is needed to better understand the practical implications of the ethical responsibility to disclose IF 2,36–40. Certainly, concerns about funding for IF disclosure will only increase as more and more genetic links are found to more and more diseases 2,39,41,42. Thus, it is important to understand the current provisions for disclosure of IF in normative guidance that can impact the progression of biobank-based research, especially as they relate to research funding. This understanding will provide a foundation on which a clear pathway can be laid to address these practical IF disclosure and resource concerns.
The Leucegene Project: a case in point
This article is based on work done in conjunction with the Leucegene Project (G. S. and J. H. are the principal and co-investigators), a biobank-based study to identify prognostic markers and therapeutic targets for Acute Myeloid Leukemia. In this study, large amounts of genetic data will be analyzed, giving rise to the potential for IF. More guidance is needed to address funding concerns surrounding the return of IF, due to the clear directives of the Canadian Tri-Council Policy Statement (TCPS) to disclose material findings 3 without accompanying requirements that funding be made available.
Methods
Data sources
Although the Leucegene Project is based in Canada, the questions raised by it with regards to IF are not national in their scope. Both research ethics and biobank usage have become international in application and access, and we therefore determined it necessary to examine the funding impact of an ethical responsibility to disclose IF from an international perspective. We conducted a review of relevant policies, guidelines and laws to assess the nature of the recommendations for the disclosure of IF that address biobank and genomic research. The breakdown of resources that contributed to the final paper includes (i) HumGen.org (a database of laws and policies related to human genetics that collects its documents from other databases, websites, or other resources), (ii) websites of international, regional, national and professional organizations to ensure the capture of all relevant documents, and (iii) a snowball method to pursue references cited in sources found in the searches listed above. We used search terms such as incidental find*, disclos*, health information, research result, guideline, access info/material, biobank, communication of results, duty to warn, and research.
Document selection
Only guidelines, policies and laws written or translated in English and developed between 1995 and 2013 were included. We distinguished between documents that made general statements about research results and those that focused on IF, and excluded those that clearly pertained only to research results (Fig. 1). We only included documents that addressed the return of IF, and which used this terminology or terminology broad enough to incorporate IF. Two reviewers (L. B., M. Page) screened the title and content of each document and additional reviewers (D. A., B. M. K.) were consulted to address any uncertainties; disagreements were resolved by consensus of the reviewers.
Figure 1.
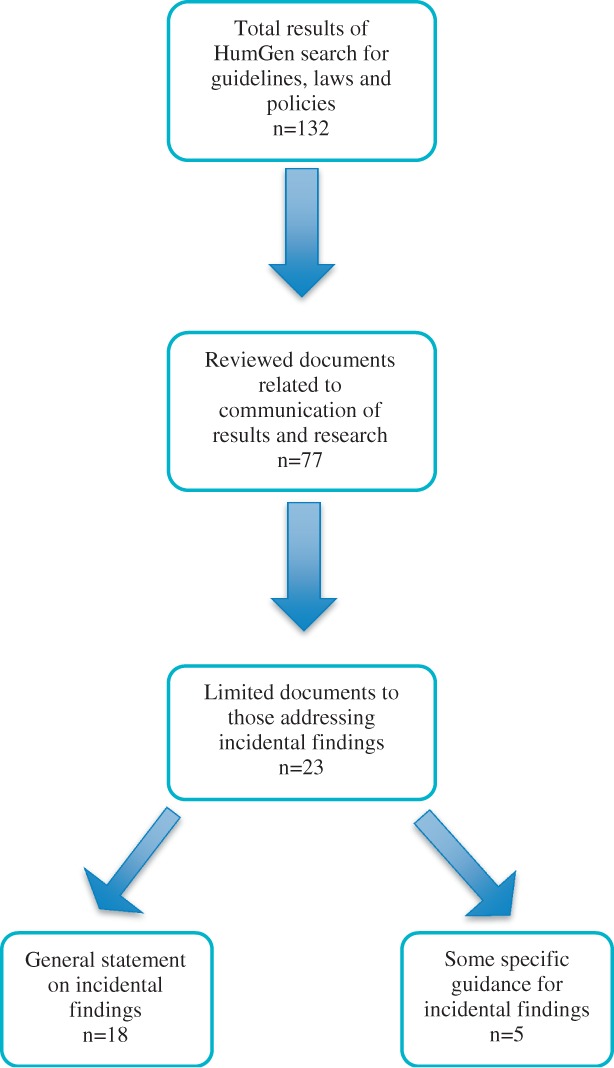
Document selection.
Data extraction
Normative documents (Table1) were examined to assess how they impacted funding needs through a consideration of: (i) who has the responsibility to disclose IF and (ii) what is the extent of researcher responsibilities, including the level of obligation to disclose, what type of information should be disclosed, coding and anonymization considerations, and temporal requirements. The limitation of the scope of our analysis to these issues is intended to address some of the important considerations for the funding of IF disclosure drawn from the literature.
Table 1.
Normative documents
| Year | Author | Title |
|---|---|---|
| International | ||
| 2002 | Council for International Organizations of Medical Sciences (CIOMS) 14 | International Ethical Guidelines for Biomedical Research Involving Human Subjects |
| 2003 | United Nations Educational Scientific and Cultural Organization (UNESCO) 13 | International Declaration on Human Genetic Data |
| 2003 | World Health Organization (WHO) 12 | Genetic Databases: Assessing the Benefits and the Impact on Human and Patients Rights |
| 2006 | Pharmacogenomics Working Group (PWG) 17 | Returning Genetic Research Results to Individuals: Points-to-Consider |
| 2007 | International Epidemiological Association (IEA) 16 | Good Epidemiological Practice (GEP)—IEA Guidelines for Proper Conduct in Epidemiological Research |
| 2008 | Council for International Organizations of Medical Sciences (CIOMS) 15 | Ethical Guidelines for Epidemiological Studies |
| 2009 | Organization for Economic Cooperation and Development (OECD) 18 | OECD Guidelines on Human Biobanks and Genetic Research Databases |
| Regional | ||
| 1997 | Council of Europe (COE) 20 | Convention on Human Rights and Biomedicine |
| 2005 | Council of Europe (COE) 19 | Additional Protocol to the Convention on Human Rights and Biomedicine, Concerning Biomedical Research |
| National | ||
| 1999 | National Bioethics Advisory Committee (NBAC) (US) 29 | Research Involving Human Biological Materials: Ethical Issues and Policy Guidance |
| 2000 | Government of Estonia 21 | Human Genes Research Act |
| 2001 | Japan Ministry of Education, Culture, Sports, Science and Technology; Ministry of Health, Labour and Welfare; Ministry of Economy, Trade and Industry 22 | Ethical Guidelines for Analytical Research on the Human Genome/Genes |
| 2001 | Medical Research Council (MRC) (UK) 30 | Human Tissue and Biological Samples for use in Research—Operational and Ethical Guidelines |
| 2003 | Nuffield Council on Bioethics (Nuffield) (UK) 31 | Pharmacogenetics: Ethical Issues |
| 2007 | Government of Spain 23 | Law 14/2007, of 3 July, on Biomedical Research |
| 2007 | National Health and Medical Research Council, Australian Research Council, Australian Vice-Chancellors’ Committee (NHMRC) 24 | National Statement on Ethical Conduct in Human Research |
| 2008 | Canadian College of Medical Geneticists, Canadian Association of Genetic Counsellors (CCMG/CAGC) 32 | Joint Statement on the Process of Informed Consent for Genetic Research |
| 2010 | Canadian Institutes of Health Research; Natural Sciences and Engineering Research Council of Canada; Social Sciences and Humanities Research Council of Canada (CIHR) 3 | Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans |
| 2010 | Government of Western Australia Department of Health 25 | Guidelines for Human Biobanks, Genetic Research Databases and Associated Data |
| 2010 | National Heart Lung Blood Institute (Fabsitz et al) (NHLBI) (US) 28 | Ethical and Practical Guidelines for Reporting Genetic Research Results to Study Participants Updated Guidelines From a National Heart, Lung, and Blood Institute Working Group |
| 2010 | Office of Biorepositories and Biospecimen Research, National Cancer Institute, National Institutes of Health (NCI/NIH) (US) 26 | Workshop Summary. Workshop on Release of Research Results to Participants in Biospecimen Studies |
| 2012 | Presidential Commission for the Study of Bioethical Issues 33 | Privacy and Progress in Whole Genome Sequencing |
| N/A | National Human Genome Research Institute (NHGRI) (US) 27 | Federal Policy Recommendations Including HIPAA |
Results
We identified 23 policies and guidelines that include direction for disclosing IF resulting from research, 6 coming from international organizations 12–17, 3 from regional policymaking bodies 18–20, and 14 from national and sub-national policymaking bodies and organizations 3,21–33. However, the language used in these documents is uniform neither in the level of obligation for disclosure nor in the description of the information to be disclosed.
Only a minority of guidelines 3,12,17,20,28,30,32,33 explicitly refer to ‘incidental findings’, which may be due to the age of many of the guidelines and the relative newness of IF as a concept in genetic research. However, the remaining guidelines could nonetheless be read to encompass IF. For example, many state that research findings relating to an individual’s health outcomes should be returned 14–16,19,27,29,31, or that participants should have access to their genetic data 13,21–24,28. In such cases, IF that relate to a participant’s health or genetic makeup could arguably be included within the scope of the guideline.
Finally, the vast majority of the guidelines are general in scope, pertaining to genetic and human tissue research. Only three are specific to research involving biobanks 18,25 and genetic databases 12.
The ethical responsibility to manage and disclose IF will inevitably increase the costs of conducting research 11,35. However, no guideline or study has systematically mapped out the potential costs of handling IF or individual research results. Only the NHLBI guideline addresses costs at all, and states that ‘investigators and funders [should] make available sufficient resources to implement return of results during the award period’ 28.
Although the normative guidance might describe the type of information to disclose and in some instances who should disclose, this is generally the extent of the direction provided—essentially creating a vast responsibility that requires more precision. Below is an overview of how the guidelines address concerns relevant to costs and funding: the level of obligation, who discloses, the impact of anonymization and coding of participant information, the type of information to be disclosed, and temporal requirements.
The level of obligation
The normative documents hold that IF (or similar findings) ‘must’, ‘should’, or ‘may’ be disclosed under certain circumstances (Fig. 2). This creates a wide spectrum of obligation, from a requirement to disclose to a loose responsibility at the discretion of the researcher. The majority of guidelines hold that researchers should respect participants’ right to information or that researchers should return genetic information of relevance to a participant’s health 12,13,16–18,20,22,24–29,32. These guidelines frame the responsibility to handle IF largely as a matter of the researcher’s discretion. In contrast, a third of the guidelines hold that managing and returning IF (or other genetic information) is an obligation that researchers must do 3,14,15,19,21,23,30,31.
Figure 2.

Spectrum of incidental finding disclosure directives.
An additional question raised by the phrasing of many guidelines is whether research participants could reasonably ask researchers to proactively look for non-study-related information in their genetic data 43,44. In the literature, it has been argued that ‘an affirmative responsibility to seek IF goes beyond the ethical obligation inherent in the investigator subject relationships’ 2,10,43,44. That said, the guidelines are phrased in manner that does not rule out the possibility of participants asking researchers to perform this additional function—for example the Council of Europe states that ‘research participants shall be entitled to know any information collected on their health’ (emphasis added) 19. If participants could ask that researchers sift through their data for known, disease-linked mutations, this could drastically change the role and responsibilities of the research team and their relationship to participants. The ambiguity of IF in guidelines and policies leaves much room for interpretation regarding the extent to which investigators must go to find them 10. Certainly, a concerted search for IF diminishes the ‘incidental’ nature of the findings 45,46, but such a broad directive has yet to be tested in practice.
Who discloses IF?
There must be careful deliberation of who discloses this potentially sensitive genetic information, including consideration of the training and capabilities of various health professionals 33,36,47 and their availability to work in parallel to the research study. Unfortunately, many of the guidelines, especially at the international and regional level, do not provide this detail.
Some guidelines offer limited suggestions for who should disclose IF to research participants, such as ‘a qualified professional’ 3; the ‘clinician involved’ in the research or ‘via the clinician responsible for [the participant’s] care’ 30; and genetic counselors 3,24. Variance on who should disclose IF persists, and there is no clear overlap or consensus in the guidance.
The type of information to be disclosed
It is unclear what, exactly, constitutes an IF other than the broad definitions provided in guidance, which are inconsistent in their inclusion of validity 25,26,28, utility 27,29 and actionability 12,22,23,30. These have contributed to the ambiguity surrounding researchers’ responsibilities to manage IF.
There is also great variation in the level of the health impact and the reliability of the data necessary for disclosure. Some guidelines promote disclosure for a clear, significant or important impact on health 3,12,13,17,22,28–30,32 while others are less stringent in their requirements 14–16,19,20,23,24,26,27,31. For example, the CIOMS guidelines state that researchers must inform individual participants of ‘any finding that relates to their particular health status’ 14,15. Even guidance with more specific limitations on what should be disclosed use language that can be interpreted broadly: the Canadian TCPS instructs researchers to disclose ‘any finding with significant welfare implications for the participant, whether health-related, psychological or social’ (emphasis added) 3.
Coding and anonymization of participant information
In studies where samples and data have been anonymized 42, it may be infeasible to provide IF because the identity of the participant, let alone the contact information, cannot be determined 48. This is stressed in a number of the guidelines 12,13,15,18,26,30. However, even when participants’ information is merely coded, and therefore theoretically traceable, there might be substantial expense for contacting participants incurred by biobanks and any external researchers 11.
Temporal requirements
The time period for which an ethical responsibility to disclose IF extends is also important for cost considerations 49. The NHLBI recommendation—the only guideline to address temporal limitations—suggests that the ‘obligation should not ordinarily extend beyond study funding’ 28.
Discussion
In practice, returning IF infers that a discussion about IF has taken place during the informed consent process 33,50, where the risks and benefits of receiving these findings have been communicated to the participant and he or she has been able to express preferences about receiving them 2,44,50. This presupposes that investigators have established a protocol for identifying and handling potential IF and mechanisms for communicating these to participants 2,44,50. It also assumes that when IF is disclosed, it is communicated in such a way that participants understand the consequences to their health and are made aware of the next steps they could take using that information (pursuing screening or treatment, communication of findings to family members).
Our findings indicate a lack of practical guidance to accompany a directive or suggestion to disclose IF to research participants, though efforts have recently been made to provide more detailed IF disclosure guidance 48, including in the clinical context 51, and to promote additional relevant research into disclosure processes 33. However, despite this lack of guidance, some biobanks 11,52 and Institutional Review Boards (IRBs)/Research Ethics Boards (REBs) have been developing their own solutions to answer the rising calls for disclosure 48. What has not been addressed is a funding mechanism to support the development of disclosure protocols, additional confirmatory testing, additional personnel, and technological resources necessary to sustain an enterprise as potentially broad as the disclosure of IF.
The vagaries of available guidance reveal the difficulties with implementing an ethical responsibility to disclose IF and determining its costs. We now turn to the consequences of limited guidance for cost and funding considerations.
Level of obligation
Central to the level of obligation will be costs and the availability of funds to support IF disclosure. In a permissive disclosure regime, where researchers are encouraged to disclose without penalty for failing to do so, the establishment of detailed guidance to ensure the appropriate financial support is perhaps less urgent. Formal funding guidelines may not be necessary, although funding to cover costs of disclosure should not be denied on the basis that IF is not required to be disclosed.
In contrast, when a normative document requires or strongly suggests the disclosure of IF—and this may become more common over time—there should be support in place to ensure that ethical responsibilities are not left by the wayside, especially if failure to comply could lead to loss of research funding 53. To require researchers to do something without providing the means to do so lacks a certain common sense, and potentially places them in ethical and legal jeopardy if they are unable to comply with the mandate.
Although current normative guidance does not explicitly require researchers to actively search for IF, as noted previously the wording of some documents suggests that research participants could potentially ask for this. Recent work by Gliwa and Berkman acknowledges the consequences that an active duty could have on current research, and discourages it 54. Presently, the uncertainties of many genetic-disease links and the burdens on researchers to search for IF outweigh any benefit, even though researchers have better access to participants’ genetic information than is generally available in the clinic. They note, though, that in the future the balance may shift toward a duty to actively search for IF if integration of genetics into clinical care remains slow 54. It is important that policy-makers clarify and limit any responsibility to disclose IF to those that are truly ‘incidental’ to the research.
Who discloses IF?
The question of who should disclose IF also requires attention. Although the guidelines examined had no consensus on the most appropriate individual to do this, the guidelines that do identify who should disclose do not address systemic and cost issues that will inevitably arise.
There might be valid reasons for specifying a particular health professional—such as a genetic counselor or clinician—to disclose IF (e.g. expertise, relationship with the participant), but hiring additional personnel for this purpose can increase project costs substantially. Even if a member of the study team makes the disclosure, the time necessary to do so (including preparation) can take away from other responsibilities and impact the progression of the research.
Moreover, different professionals have differing capacities when it comes to genetic information. For example, while genetic counselors may be the most appropriate communicators of genetic information 55,56, their availability remains limited and it is unclear if the profession could support the increased demand from the research community that would stem from IF disclosure 37,39,56,57. Furthermore, some of the IF discovered might not be related to disease (e.g. paternity), leaving unanswered the question of how to deal with these particular findings. Having researchers communicate IF may be the most practical option, but they may not have expertise with the particular finding 2, nor may they have the required time to prepare for and perform the disclosure. If the researcher has no immediate clinical relationship with the participant, it may be more appropriate to involve the primary care physician, though he or she may not be well-versed in genetics 58. The potential for the discovery of rare mutations also raises questions of clinician preparedness for this task and the availability of educational mechanisms to ensure they have the necessary skill.
Regardless of who is deemed responsible to disclose IF to research participants, the task will require considerable time, energy, and resources. It is important for policymakers to clarify who bears the responsibility of this burden so that the designated person can prepare and the project can set aside the appropriate funds to offset the costs of disclosure.
The type of information to be disclosed
The establishment of an ethical responsibility to disclose IF requires specification of what is to be disclosed. Broad descriptions do not serve researchers well: information ‘that relates to [participants’] particular health status’ 14,15 can easily be read to require the disclosure of large numbers of genetic variants, which might not all have serious health implications or have sufficient validity or utility to warrant disclosure. Even the Canadian TCPS’ directive to disclose IF ‘with significant welfare implications for the participant, whether health-related, psychological or social’ 3 suffers from ambiguity by using ‘significant welfare implications’ as its qualifier.
The guidelines ‘are difficult to apply when such a large number of potential variants are worthy of communication to participants’ 39. In order to avoid falling into the incidentalome—wherein researchers pursue extraneous findings, patients become subject to unnecessary follow-up tests, and the overall costs of genomic medicine increase 59—the responsibilities of researchers must be clearly delineated. The use of more specific language and contours to describe what IF should be disclosed could lessen the burden on researchers and could also reduce costs. Further, if REBs are asked to approve the plan for disclosure [as they are in Canada 3], clear characterizations of what should be disclosed can avoid potentially time-consuming deliberations as well as limiting disclosure of IF to those that are necessary and useful.
Another consideration that relates to validity requirements in some of the guidelines is whether the jurisdiction requires additional testing before the information can be disclosed to a research participant. The NHLBI guidance points out the ambiguity in US law that might require researchers seeking to disclose individual research results to have these tested again at a second, CLIA-approved, laboratory 28. The authors did not conclude that this is definitively required—and others have come to different conclusions 60,61—but questioned the law’s application to research results. Such confirmatory testing, if required by the jurisdiction, will inevitably increase the costs of IF disclosure.
If IF deriving from research are indeed to be disclosed, the route taken in recent guidance developed for the clinical use of exome and genome sequencing might be instructive. The American College of Medical Genetics and Genomics (ACMG) advocates for disclosure of IF discovered in certain clinical genetic testing and provides a list of those variants that qualify for disclosure 51. Importantly, the guideline recognizes that the ‘recommendations reflect limitations of current technology’ as well as the need to ‘refine and update [its] list at least annually’ 51. Efforts such as this in the research context would be beneficial to researchers and others who are asked to disclose IF.
Concerns of anonymity, coding and family
How data and samples are maintained within a biobank will have important effects on costs for disclosing IF. Although many guidelines discourage anonymization because it would prevent the disclosure of individualized information to participants 15,18,26,30, anonymity would certainly avoid costs associated with disclosing IF as there would be no potentially identifiable participants to disclose to. Conversely, it might also deprive researchers of additional valuable information, such as clinical data, that necessitates a link between the participant and their sample and information.
However, in future research it will likely be less ethically permissible to anonymize participants if there is potential for IF. This is due to concerns about participants’ health, self-determination, and autonomy, as well as increasing calls to promote respect or reciprocity by disclosing IF and individual research results 4–6,26. Thus, various coding methods will probably be used to permit participant reidentification. Guidelines must therefore consider issues of re-contact when consent to re-contact or to disclose IF was not initially obtained 52, and the tracking down of participants if the contact information is no longer accurate. Both of these could substantially increase the costs of IF disclosure, especially in terms of personnel time and resources.
Depending on a jurisdiction’s rules regarding disclosure of health information to participants’ families, similar barriers will be faced. Researchers must identify and locate relevant family members, possibly without any help from the participant who might be unwilling or deceased. If the jurisdiction does not permit disclosure to family without the consent of the participant, researchers or other health professionals might also need to educate the participant on the importance of the information to the family and offer to assist them in disclosure 62. The financial costs in terms of time and resources could be substantial, depending on how far a researcher’s obligation extends to others than the participant.
Temporal requirements
Time limitations, or lack thereof, on ethical obligations are important in any attempt to determine financial impact. If not limited to the funding period of the study or another concrete term, researchers will have little idea about the temporal extent of their obligations, and hence the potential costs that they need to consider when formulating a budget.
In consideration of temporal limits for disclosing IF, Lo suggests that the time frame should be dependent on available resources 44 while Cho argues that the relationship between researcher and participant will largely determine when the responsibility ends 47. Secondary research and the possibility of reanalyzing data samples at a later date further complicates this question: Is it possible that the responsibility to disclose IF could persist indefinitely 44,47?
In biobank-based research, additional complications arise when samples and data are shared (under agreement) with external researchers. Whose timeframe should limit the obligation: the biobank’s or the external researchers’? That is, does the sharing of a participant’s tissue extend the obligation of the biobank beyond the initial study or other time limitation? These are important questions that should be addressed in detailed policy guidance on the disclosure of IF.
Conclusion
The creation of a new ethical responsibility without a consideration of how (and whether) it should be funded creates uncertainty for researchers throughout the course of their research, from the initial application and proposal process to the collection and analysis of data. As it stands today, the vast majority of normative guidance does not address the costs related to IF disclosure and thus implies an unfunded mandate—the imposition of ‘costly new regulations without providing additional funding’ 63—especially when disclosure is required. In the context of expanding data sets, an increasing number of known disease-associated variants, and the sharing of biobank resources, ‘emerging requirements for the return of results … may become rapidly unmanageable’ without new tools, guidance and funding for investigators 39.
Limited efforts have been made outside of available official guidance to address the intricacies of IF disclosure. The Informed Cohort Oversight Board of the Coriell Personalized Medicine Collaborative, for example, determines which results of the study will be made available to participants based on discrete criteria and has a mechanism by which results will be disclosed, 64 and individual biobanks have created their own policies to ensure uniformity in the disclosure of IF 35. The Public Population Project in Genomics and Society (P3G) in Canada has also developed a policy for disclosure of research results and IF, both defining criteria for returnable findings and identifying circumstances when disclosure to participants is appropriate 52. Finally, the examples provided by Bledsoe et al. demonstrate that an obligation to disclose IF can have disparate impacts based on the nature and size of the biobank 11.
How the costs of IF disclosure will be absorbed raises a number of challenges, as researchers will either need to set aside a portion of their existing budgets or access additional funding. However, the absence of a reasonable estimation of the likely costs makes this task difficult. Various authors have suggested that researchers should include the costs of managing and returning IF directly in the research budget 2, including ‘extended time lines that allow for appropriate analysis and peer review’ 65. One author rightly points out that while the cost of disclosure of individual results is an important issue to consider, ‘if disclosure is the right thing to do then finding creative and financially responsible ways to accomplish the task are critical …’ 65. However, asking researchers to be financially creative places an uneven burden on them when it may be more efficient to address funding considerations at the source, by developing transparent mechanisms to cover the costs of IF disclosure.
Importantly, the absence of a clear understanding of how much IF disclosure will cost has led researchers to both over- and underestimate the likely burden they will face by adopting this additional responsibility. Some posit that the costs could be great 11 and that IF will divert the research process and potentially harm participants 59. Conversely, others have argued that in practice the costs of disclosure will be quite low and unlikely to dramatically alter researchers’ budgets 6—and anyway, ‘not reporting the results because of costs seems unethical’ 66. There is simply insufficient information available from which to make a reasonable estimate of the costs for IF disclosure 51.
As guidance in the clinical context shifts toward requiring IF disclosure 51, there might be less hesitancy to require similar action in research. Thus, it is crucial that the research and policy communities think through the practical, logistical and financial implications of imposing an ethical responsibility to disclose IF. Otherwise, the valorous intent behind the drive to disclose IF will be overtaken by uncertainty in the means to do it and a system that fails to support it.
Recommendations
Although we can state with reasonable certainty that the general consensus of the normative guidance (when available) and the literature is that IF should indeed be disclosed to research participants in limited circumstances, there is such disparity in the available guidance that we are unable to state with any confidence how the funding considerations should be resolved. Below we provide a few recommendations for further action to ensure that financial support for IF disclosure—in the biobank context but also more generally applicable—is not overlooked in the rush to establish a new ethical obligation.
Initially, reasonable limits must be placed on researchers’ obligations to disclose IF to ensure that they do not seriously compromise research aims nor pose an untenable burden on biobank resources and funding as well as on the researchers themselves.
Guidance must be provided detailing how to accomplish the highly sensitive and complex IF disclosure process, not only for new biobank studies but also for those initiated prior to the development of guidance and now facing new ethical responsibilities. Furthermore, policies and guidelines established prior to the rise of IF in genomic research and yet still applicable to this field should be modified to diminish the ambiguities they have created, in light of changes in research methods and technologies.
Funding institutions that ‘support the allocation and use of research monies in an ethical manner’ should make funding available to researchers for the purpose of managing IF 2,6,49. This is particularly relevant if a guideline requires disclosure, as the TCPS does in Canada. Even though researchers are the primary actors to implement ethical obligations, the entire research complex should shift to accommodate these goals 44,50.
Well-conducted studies are needed to establish an informed estimation of the potential costs associated with IF so that researchers, biobanks and funding agencies can plan accordingly.
Researchers and biobank administrators, as well as IRBs and REBs, should be made aware of the legal and ethical obligations (to disclose IF) in their jurisdiction.
Researchers should be encouraged to include in grant proposals an analysis of whether and what IF are anticipated in their study. If IF are foreseen, funding sources should permit budgetary line items to incorporate the costs of disclosure. If IF are discovered only after research has begun, funding sources should permit researchers to request additional funding to address the increased costs of the research due to IF disclosure.
Participant consent forms should include provisions for IF disclosure, where appropriate, which could decrease future costs. Consent forms should also include provisions for re-contact in the event that the research leads to IF and participants must be re-contacted to obtain their consent for disclosure.
Acknowledgments
The authors would like to acknowledge Madeline Page for her contributions to the research and to earlier drafts of this manuscript. This manuscript was prepared with financial support from Genome Quebec. The authors would also like to acknowledge the many experts of the Leucegene Project who provided their time and experience in the preparation of this manuscript.
Appendix
The lists of contributors to the Leucegene Project are Brian Wilhelm, Sébastien Lemieux, Frédéric Barabé, Patrick Gendron, Pierre Chagnon and the team of the Leukemia Cell Bank of Quebec.
References
- Burton PR, Hansell AL, Fortier I, et al. Size matters: just how big is BIG? Quantifying realistic sample size requirements for human genome epidemiology. Int J Epidemiol. 2009;38:263–273. doi: 10.1093/ije/dyn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Lawrenz FP, Nelson CA, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics. 2008;36:219–248. doi: 10.1111/j.1748-720X.2008.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council of Canada. Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. Ottawa; 2010. [Google Scholar]
- Bredenoord AL, Kroes HY, Cuppen E, Parker M, Van Delden JJM. Disclosure of individual genetic data to research participants: the debate reconsidered. Trends Genet. 2011;27:41–47. doi: 10.1016/j.tig.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Bredenoord AL, Onland-Moret NC, Van Delden JJM. Feedback of individual genetic results to research participants: in favor of a qualified disclosure policy. Hum Mutat. 2011;32:861–867. doi: 10.1002/humu.21518. [DOI] [PubMed] [Google Scholar]
- Shalowitz DI, Miller FG. Disclosing individual results of clinical research: implications of respect for participants. JAMA. 2005;294:737–740. doi: 10.1001/jama.294.6.737. [DOI] [PubMed] [Google Scholar]
- Miller FA, Christensen R, Giacomini M, Robert JS. Duty to disclose what? Querying the putative obligation to return research results to participants. J Med Ethics. 2008;34:210–213. doi: 10.1136/jme.2006.020289. [DOI] [PubMed] [Google Scholar]
- Ossorio PN. Letting the gene out of the bottle: a comment on returning individual research results to participants. Am J Bioeth. 2006;6:24–25. doi: 10.1080/15265160600935555. [DOI] [PubMed] [Google Scholar]
- Parker L. Rethinking respect for persons enrolled in research. ASBH Exch. 2006;9:6–7. [Google Scholar]
- Clayton EW, McGuire AL. The legal risks of returning results of genomics research. Genet Med. 2012;14:473–477. doi: 10.1038/gim.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledsoe MJ, Clayton EW, McGuire AL, Grizzle WE, O’Rourke PP, Zeps N. Return of research results from genomic biobanks: cost matters. Genet Med. 2013;15:103–105. doi: 10.1038/gim.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) 2003. . Genetic Databases: Assessing the Benefits and the Impact on Human & Patients Rights. Geneva,
- United Nations Educational Scientific and Cultural Organization (UNESCO) 2003. . International Declaration on Human Genetic Data. Paris,
- Council for International Organizations of Medical Sciences (CIOMS) 2002. . International Ethical Guidelines for Biomedical Research Involving Human Subjects. Geneva,
- Council for International Organizations of Medical Sciences (CIOMS) 2008. . Ethical Guidelines for Epidemiological Studies. Geneva,
- International Epidemiological Association. 2007. . Good Epidemiological Practice (GEP)—IEA Guidelines for Proper Conduct in Epidemiological Research. Raleigh,
- Renegar G, Webster CJ, Stuerzebecher S, et al. Returning genetic research results to individuals: points-to-consider. Bioethics. 2006;20:24–36. doi: 10.1111/j.1467-8519.2006.00473.x. [DOI] [PubMed] [Google Scholar]
- Organisation for Economic Co-operation and Development (OECD) 2009. . OECD Guidelines on Human Biobanks and Genetic Research Databases. Paris,
- Council of Europe. 2005. . Additional Protocol to the Convention on Human Rights and Biomedicine, Concerning Biomedical Research. Strasbourg,
- Council of Europe, Committee of Ministers. 1997. . Recommendation R(97)5 on the Protection of Medical Data. Strasbourg,
- Government of Estonia. 2000. . Human Genes Research Act. (RT I 2000, 104, 685),
- Japan Ministry of Education Culture Sports Science and Technology, Ministry of Health Labour and Welfare, Ministry of Economy Trade and Industry. 2001. . Ethical Guidelines for Analytical Research on the Human Genome/Genes. Tokyo,
- Government of Spain. 2007. . Law 14/2007, of 3 July, on Biomedical Research.
- National Health and Medical Research Council, Australian Research Council, Australian Vice-Chancellors’ Committee. 2007. . National Statement on Ethical Conduct in Human Research—Updated 2009. Canberra,
- Government of Western Australia Department of Health. 2010. . Guidelines for Human Biobanks, Genetic Research Databases and Associated Data. Perth,
- Office of Biorepositories and Biospecimen Research, National Cancer Institute, National Institutes of Health. 2010. . Workshop Summary. Workshop on Release of Research Results to Participants in Biospecimen Studies. Bethesda,
- National Human Genome Research Institute. 2012. . Federal Policy Recommendations Including HIPAA. Bethesda,
- Fabsitz RR, McGuire A, Sharp RR, et al. Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung, and Blood Institute Working Group. Circ Cardiovasc Genet. 2010;3:574–580. doi: 10.1161/CIRCGENETICS.110.958827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Bioethics Advisory Committee. 1999. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance. vol I, Bethesda,
- Medical Research Council (UK) 1999. . Human Tissue and Biological Samples for use in Research—Operational and Ethical Guidelines. London,
- Nuffield Council on Bioethics. 2003. . Pharmacogenetics: Ethical Issues. London,
- Canadian College of Medical Geneticists, Canadian Association of Genetic Counsellors. 2008. . Joint Statement on the Process of Informed Consent for Genetic Research. Ottawa,
- Presidential Commission for the Study of Bioethical Issues. 2012. . Privacy and Progress in Whole Genome Sequencing. Washington, DC,
- Bledsoe MJ, Clayton EW, McGuire AL, Grizzle WE, O’Rourke PP, Zeps N. Return of research results from genomic biobanks: a call for data. Genet Med. 2013;15:159–160. doi: 10.1038/gim.2012.163. [DOI] [PubMed] [Google Scholar]
- Terry SF. The tension between policy and practice in returning research results and incidental findings in genomic biobank research. Minn J Law Sci Technol. 2012;13:691–736. [Google Scholar]
- Knoppers BM, Joly Y, Simard J, Durocher F. The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet. 2006;14:1170–1178. doi: 10.1038/sj.ejhg.5201690. [DOI] [PubMed] [Google Scholar]
- Klitzman R. Questions, complexities, and limitations in disclosing individual genetic results. Am J Bioeth. 2006;6:34–36. doi: 10.1080/15265160600936058. [DOI] [PubMed] [Google Scholar]
- Meacham MC, Starks H, Burke W, Edwards K. Researcher perspectives on disclosure of incidental findings in genetic research. J Empir Res Hum Res Ethics. 2010;5:31–41. doi: 10.1525/jer.2010.5.3.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassa CA, Savage SK, Taylor PL, Green RC, McGuire AL, Mandl KD. Disclosing pathogenic genetic variants to research participants: quantifying an emerging ethical responsibility. Genome Res. 2012;22:421–428. doi: 10.1101/gr.127845.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévesque E, Joly Y, Simard J. Return of research results: general principles and international perspectives. J Law Med Ethics. 2011;39:583–592. doi: 10.1111/j.1748-720X.2011.00625.x. [DOI] [PubMed] [Google Scholar]
- Johnson AD, Bhimavarapu A, Benjamin EJ, et al. CLIA-tested genetic variants on commercial SNP arrays: potential for incidental findings in genome-wide association studies. Genet Med. 2010;12:355–363. doi: 10.1097/GIM.0b013e3181e1e2a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ness B. Genomic research and incidental findings. J Law Med Ethics. 2008;36:292–297. doi: 10.1111/j.1748-720X.2008.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller FG, Mello MM, Joffe S. Incidental findings in human subjects research: what do investigators owe research participants? J Law Med Ethics. 2008;36:271–279. doi: 10.1111/j.1748-720X.2008.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo B. Responding to incidental findings on research imaging studies: now what? Arch Intern Med. 2010;170:1522–1524. doi: 10.1001/archinternmed.2010.306. [DOI] [PubMed] [Google Scholar]
- Richardson HS. Incidental findings and ancillary-care obligations. J Law Med Ethics. 2008;36:256–270. doi: 10.1111/j.1748-720X.2008.00268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawati MH, Van Ness B, Knoppers BM. Incidental findings in genomic research: a review of international norms. GenEdit. 2011;9:1–8. [Google Scholar]
- Cho MK. Understanding incidental findings in the context of genetics and genomics. J Law Med Ethics. 2008;36:280–285. doi: 10.1111/j.1748-720X.2008.00270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Crock BN, Van Ness B, et al. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genet Med. 2012;14:361–384. doi: 10.1038/gim.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CV, Skedgel C, Weijer C. Considerations and costs of disclosing study findings to research participants. Can Med Assoc J. 2004;170:1417–1419. doi: 10.1503/cmaj.1031668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SM, Paradise J, Caga-anan C. The law of incidental findings in human subjects research: establishing researchers’ duties. J Law Med Ethics. 2008;36:361–383. doi: 10.1111/j.1748-720X.2008.00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green RC, Berg JS, Grody WW, et al. ACMG Recommendations for Reporting of Incidental Findings in Clinical Exome and Genome Sequencing. Bethesda: American College of Medical Genetics and Genomics; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoppers BM, Deschênes M, Zawati MH, Tassé AM. Population studies: return of research results and incidental findings Policy Statement. Eur J Hum Genet. 2013;21:245–247. doi: 10.1038/ejhg.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, Social Sciences and Humanities Research Council of Canada. 2013. . Agreement on the Administration of Agency Grants and Awards by Research Institutions. Ottawa,
- Gliwa C, Berkman BE. Do researchers have an obligation to actively look for genetic incidental findings? Am J Bioethics. 2013;13:32–42. doi: 10.1080/15265161.2012.754062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler LG. Disclosure of research results from cancer genomic studies: state of the science. Clin Cancer Res. 2009;15:4270–4276. doi: 10.1158/1078-0432.CCR-08-3067. [DOI] [PubMed] [Google Scholar]
- Pyeritz RE. The coming explosion in genetic testing-is there a duty to recontact? N Engl J Med. 2011;365:1367–1369. doi: 10.1056/NEJMp1107564. [DOI] [PubMed] [Google Scholar]
- Silversides A. The wide gap between genetic research and clinical needs. Can Med Assoc J. 2007;176:315–316. doi: 10.1503/cmaj.061726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorogood A, Knoppers BM, Dondorp WJ, de Wert GMWR. Whole genome sequencing and the physician. Clin Genet. 2012;81:511–513. doi: 10.1111/j.1399-0004.2012.01868.x. [DOI] [PubMed] [Google Scholar]
- Kohane IS, Masys DR, Altman RB. The incidentalome: a threat to genomic medicine. JAMA. 2006;296:212–215. doi: 10.1001/jama.296.2.212. [DOI] [PubMed] [Google Scholar]
- Yassin R, Weil C, Lockhart N. Sharing individual research results with biospecimen contributors: point. Cancer Epidemiol Biomarkers Prev. 2012;21:256–259. doi: 10.1158/1055-9965.EPI-11-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton EW. Sharing individual research results with biospecimen contributors: counterpoint. Cancer Epidemiol Biomarkers Prev. 2012;21:260–261. doi: 10.1158/1055-9965.EPI-11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black L, McClellan KA, Avard D, Knoppers BM. Intrafamilial disclosure of risk for hereditary breast and ovarian cancer: points to consider. J Community Genet. 2013;4:203–214. doi: 10.1007/s12687-012-0132-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troy DE. The unfunded mandates reform act of 1995. Admin Law Rev. 1997;49:139–147. [Google Scholar]
- Coriell Personalized Medicine Collaborative. Informed Cohort Oversight Board (ICOB) 2012. . Camden,
- Fernandez C. Public expectations for return of results—time to stop being paternalistic? Am J Bioethics. 2008;8:46–48. doi: 10.1080/15265160802513127. [DOI] [PubMed] [Google Scholar]
- Bookman EB, Langehorne AA, Eckfeldt JH, et al. Reporting genetic results in research studies: summary and recommendations of an NHLBI working group. Am J Med Genet A. 2006;140A:1033–1040. doi: 10.1002/ajmg.a.31195. [DOI] [PMC free article] [PubMed] [Google Scholar]


