Abstract
Background
DA-1229 is a novel, potent and selective dipeptidyl peptidase-4 (DPP-IV) inhibitor that is orally bioavailable. We aimed to evaluate the optimal dose, efficacy and safety of DA-1229, in Korean subjects with type 2 diabetes mellitus suboptimally controlled with diet and exercise.
Methods
We enrolled 158 patients (mean age, 53 years and a mean BMI, 25.6 kg/m2). The mean baseline fasting plasma glucose level, HbA1c and duration of diabetes were 8.28 mmol/L, 7.6% (60 mmol/mol) and 3.9 years, respectively. After 2 or 6 weeks of an exercise and diet program followed by 2 weeks of a placebo period, the subjects were randomized into one of four groups for a 12-week active treatment period: placebo, 2.5, 5 or 10 mg of DA-1229.
Results
All three doses of DA-1229 significantly reduced HbA1c from baseline compared to the placebo group (−0.09 in the placebo group vs. −0.56, −0.66 and −0.61% in 2.5, 5 and 10-mg groups, respectively) but without any significant differences between the doses. Insulin secretory function, as assessed by homeostasis model assessment β-cell, the insulinogenic index, 2-h oral glucose tolerance test (OGTT) C-peptide and post-OGTT C-peptide area under the curve (AUC)0–2h, significantly improved with DA-1229 treatment. The incidence of adverse events was similar between the treatment groups and DA-1229 did not affect body weight or induce hypoglycaemic events.
Conclusions
DA-1229 monotherapy (5 mg for 12 weeks) improved HbA1c, fasting plasma glucose level, OGTT results and β-cell function. This drug was well tolerated in Korean subjects with type 2 diabetes mellitus. © 2014 The Authors. Diabetes/Metabolism Research and Reviews published by John Wiley & Sons, Ltd.
DA-1229 is a novel, potent and selective DPP-IV inhibitor that is orally bioavailable. In a pharmacodynamic study, more than 80% of DPP-IV was inhibited by a single dose of 5 mg or higher of DA-1229, and this level of inhibition was maintained for at least 24 h after a single dose of 10 mg or higher of DA-1229. This phase II clinical trial was designed to evaluate the efficacy and safety of oral DA-1229 and to determine the optimal dose to use for a phase III clinical study in Korean subjects with type 2 diabetes.
Keywords: dose-finding study, DPP-IV inhibitor, monotherapy, type 2 diabetes mellitus
Introduction
Type 2 diabetes mellitus is a complex and progressive disease with a prevalence that is increasing worldwide 1. Although there is general agreement on the first-line use of metformin in most patients with type 2 diabetes mellitus, the ideal drug sequence after metformin failure is an area of increasing uncertainty 2.
Dipeptidyl peptidase-4 (DPP-IV) inhibitors were introduced as antidiabetic agents in 2006, with sitagliptin being the first available, followed by vildagliptin, saxagliptin, linagliptin, alogliptin and gemigliptin 3–8. DPP-IV inhibitors reduce plasma DPP4-IV activity by 70–90% in a sustained manner for 24 hours with an increase in glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotrophic polypeptide (GIP), which are hormones released in response to food intake that have glucose-lowering effects by stimulating insulin secretion by pancreatic β-cells and inhibiting glucagon secretion by pancreatic α-cells 9.
Although the above DPP-IV inhibitors differ in their potency, target selectivity, oral bioavailability, half-life, metabolism and excretion rate, they both possess considerable glucose-lowering effects and favorable safety profiles 10.
DA-1229 is a novel, potent and selective DPP-IV inhibitor that is orally bioavailable 11. In a single-and multiple-dose phase I clinical trial in healthy Korean subjects. DA-1229 was shown to be well tolerated without serious adverse events (SAEs) 12. The half-life of DA-1229 was approximately 30–40 h (1.25–60 mg), the pharmacokinetics of which were not influenced by food 12. In a pharmacodynamic study, more than 80% of DPP-IV was inhibited by a single dose of 5 mg or higher of DA-1229, and this level of inhibition was maintained for at least 24 h after a single dose of DA-1229 of 10 mg or higher 12. Therefore, DA-1229 is likely to be efficacious when administered as a once-daily antidiabetic agent 12.
This phase II clinical trial was designed to evaluate the efficacy and safety of oral DA-1229 and to determine the optimal dose to use for a phase III clinical study in Korean subjects with type 2 diabetes mellitus.
Materials and methods
Study design
This study was a phase II, multicenter, randomized, double-blind, placebo-controlled study. Study participants were recruited from 26 university hospitals throughout Korea. Participants who gave written informed consent were screened using the inclusion and exclusion criteria. After 2 or 6 weeks of an exercise and diet program designed based on general diabetes treatment guidelines, all participants received a placebo for 2 weeks. When the 2-week placebo run-in period was completed, the participants were randomly assigned to one of the following groups: placebo, 2.5, 5 or 10 mg of DA-1229. The block randomization method was applied at each study site, and the randomization schedule was generated using SAS System 9.2 (SAS Institute, Cary, NC, USA). Participants were instructed to take one tablet of the investigational drug once daily at the same time in the morning for 12 weeks. Participants with persistent hyperglycaemia, defined as having a fasting plasma glucose (FPG) level exceeding 14.99 mmol/L, were made to stop taking the study medications (placebo or DA-1229) and initiate additional rescue therapy, and were dropped from this study. Figure1 summarizes the trial profile and study scheme.
Figure 1.
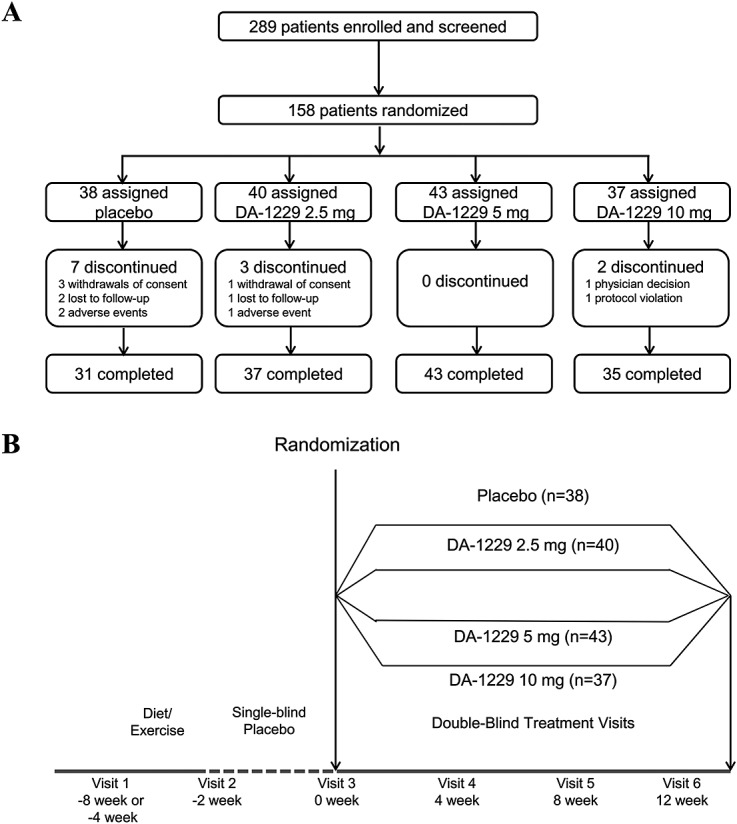
Trial profile (A) and study scheme (B)
Subjects
Men and women between the ages of 20 and 75 years with type 2 diabetes mellitus who satisfied either of the following conditions at screening (visit 1) were eligible for this study: patients with HbA1c in the range of 7.0% (53 mmol/mol) to 10.0% (86 mmol/mol) who were diagnosed with T2DM for the first time in the 4 weeks prior to screening or patients with HbA1c in the range of 7.0% (53 mmol/mol) to 10.0% (86 mmol/mol) who had not been treated with oral glucose-lowering agents in the 6 weeks prior to screening. Newly diagnosed subjects underwent 6 weeks of an exercise and diet program, while oral glucose-lowering agent-naïve subjects underwent a program of 2 weeks of exercise and diet, which allowed them to have periods of lifestyle modification lasting at least 8 and 10 weeks in drug-naïve subjects and newly diagnosed subjects, respectively. Patients with either HbA1c in the range of 7.0% (53 mmol/mol) to 10.0% (86 mmol/mol) or FPG in the range of 6.99 mmol/L to 14.99 mmol/L at visit 2 were administered a single-blinded placebo once daily for 2 weeks, while continuing the exercise and diet program, from the date of the second visit to the day before the third visit. These periods consisting of lifestyle modification ranging from 8 to 10 weeks and a placebo run-in of 2 weeks were used to identify stable HbA1 levels not affected by external conditions.
Exclusion criteria were as follows: body mass index (BMI) <20 or >40 kg/m2; a history of type 1 diabetes, secondary diabetes, or gestational diabetes; a history of myocardial infarction, transient ischemic attack or coronary artery bypass surgery in the 6 months prior to screening; a history of a major skin allergy; use of corticosteroids or sodium channel blockers in the 3 months prior to screening; use of insulin or thiazolidinediones in the 6 months prior to screening; current use of warfarin, docoumarin or digoxin; thyroid dysfunction (except cases that had been treated stably with thyroid hormonal replacement therapy for at least 3 months prior to entry); serum creatinine level >132.6 µmol/L (1.5 mg/dl); creatinine phosphokinase level exceeding 3× the upper normal limit with symptoms; alanine aminotransferase or aspartate aminotransferase level exceeding 2.5× the upper normal limit; history of alcohol or drug abuse in the 2 months prior to screening; participation in another clinical trial in the 2 months prior to screening; pregnancy or lactation and/or current use of drugs or food that could affect CYP3A4 metabolism in the liver.
The study protocol was approved by the independent ethics committee/institutional review board at each study site, and written informed consent was obtained from all participants. This study was performed according to the Good Clinical Practice guidelines and the Declaration of Helsinki.
Study endpoints
The primary endpoint was the mean change in HbA1c from baseline to week 12. The secondary endpoints included the HbA1c response rate [<7.0% (53 mmol/mol) or <6.5% (48 mmol/mol); changes in FPG, glycated albumin (GA), fasting insulin, fasting proinsulin, the proinsulin/insulin ratio, fasting lipid parameters, including total cholesterol, low-density lipoprotein-cholesterol (LDL-C), high-density lipoprotein-cholesterol (HDL-C) and triglycerides (TG); and changes in the homeostasis model assessment of insulin resistance (HOMA-IR), the homeostasis model assessment of β-cell function (HOMA-β) and the quantitative insulin sensitivity check index (QUICKI) 13.
An oral glucose tolerance test (OGTT) was performed by administering 75 g of glucose and obtaining blood samples for glucose, insulin, proinsulin and C-peptide at 0, 30, 60 and 120 min. We analysed changes from baseline in the 2 h postload levels of glucose, insulin, proinsulin and C-peptide at 12 weeks. The area under the curve (AUC)0–2h was calculated for each parameter. The following parameters were also set as secondary endpoints: 2 h OGTT for glucose, insulin, proinsulin and C-peptide; AUC0–2h for glucose, insulin, proinsulin and C-peptide; the insulinogenic index 13 and body weight.
Safety endpoints included adverse events (AEs), vital signs and laboratory test results. AEs and vital signs were evaluated, and laboratory tests were performed at every visit.
Statistical analysis
The planned sample size for this study was approximately 188 patients. This number was calculated to detect with 85% power a treatment mean difference of 0.56% (standard deviation, 0.8) in HbA1c from baseline with an overall significance level of 0.15 and assuming a 20% dropout rate. As the dropout rate was lower than previously estimated, this study was terminated when tests from 158 patients were completed.
Demographic data and baseline characteristics were compared among the treatment groups. Continuous variables were analysed by using analysis of variance (ANOVA) or the Kruskal–Wallis test, and categorical variables were analysed using the chi-square test or Fisher’s exact test. The efficacy variables were described using descriptive statistics for each group and analysed to identify all differences between treatment groups. For inter-group comparisons in efficacy variables, either ANOVA or the Kruskal–Wallis test was performed, with the post-hoc analysis using multiple comparisons of Dunnett’s test or multiple comparisons of the step-down Bonferroni method. [The Wilcoxon rank sum test was performed for comparisons between each DA-1229 dose and the placebo, followed by correction of p-values using the step-down Bonferroni method]. For intra-group comparisons, the results at baseline and at week 12 were analysed by a paired t-test or the Wilcoxon signed rank test. The primary end point was assessed using an analysis of covariance (ANCOVA) model, including terms for treatment, baseline HbA1c and the diagnosis group (i.e. newly diagnosed or drug-naïve group) as covariates. The least square (LS) mean differences and standard errors (SE) were estimated for the comparisons of placebo versus DA-1229. The chi-square test (or Fisher’s exact test) was used for categorical data. The full analysis set was used to evaluate efficacy and included all patients randomly assigned to different treatment groups who received at least one dose of the double-blind study drug and who had at least one post-baseline measurement. The last observation carried forward method was used in cases of missing data.
Safety and tolerability were assessed in patients who received at least one dose of the study drug, and safety data were collected. For each AE, the number of subjects who experienced one or more events was recorded, and the percentage was reported according to the treatment group. Events were described with regard to gravity, severity, relationship to the investigational drug, treatment and outcome.
The statistical software SAS System 9.2 was used for all statistical analyses, and two-sided tests were performed to determine statistical significance with a significance level of ≤5%.
Results
Two-hundred and eighty-nine people with type 2 diabetes mellitus gave consent to participate in the study and were screened for eligibility (Figure1A). Among the 187 eligible participants who were placed on an exercise and diet program, 158 completed the placebo run-in period. Of the 158 randomized subjects, five without efficacy data were excluded from the efficacy analysis and; thus, a total of 153 subjects were included in the full analysis set. Thirty-eight patients were randomized to the placebo group, and 40, 43 and 37 patients to the 2.5, 5 and 10 mg DA-1229 treatment groups, respectively.
Baseline characteristics of the subjects
Demographic information and baseline characteristics of subjects enrolled in this clinical trial are summarized in Table1. There were no significant differences among the subjects’ baseline characteristics. The mean age of the study subjects was 53 years, and 93 subjects (59%) were men and 65 (41%) were women. The mean body mass index (BMI) was 25.6 kg/m2, and the mean duration of diabetes was 3.9 years. The mean HbA1c, GA and FPG levels at baseline were 7.6% (60 mmol/mol), 19.1% and 8.28 mmol/L, respectively. Of the 153 subjects, the baseline HbA1c was >8.5% (69 mmol/mol) in 21 subjects and ≤8.5% (69 mmol/mol) in 132 subjects.
Table 1.
Baseline characteristics of study participants according to treatment groups
| Placebo | DA-1229 | ||||
|---|---|---|---|---|---|
| Variables | (n = 38) | 2.5 mg (n = 40) | 5 mg (n = 43) | 10 mg (n = 37) | P-valuea |
| Male (n, %) | 26 (68.42) | 22 (55.00) | 25 (58.14) | 20 (54.05) | 0.5636 |
| Age (years) | 54.42 (9.85) | 52.10 (11.48) | 54.21 (9.74) | 53.16 (9.60) | 0.7268 |
| Height (cm) | 165 (10.00) | 164 (8.00) | 164 (9.00) | 162 (10.00) | 0.7629 |
| Weight (kg) | 70.66 (10.95) | 69.04 (11.54) | 68.40 (11.67) | 67.21 (10.85) | 0.6641 |
| BMI (kg/m2) | 25.98 (2.35) | 25.67 (3.37) | 25.34 (3.37) | 25.39 (3.02) | 0.3457 |
| Duration of diabetes mellitus (years) | 3.55 (2.85) | 4.00 (3.57) | 3.98 (3.88) | 3.95 (3.88) | 0.9900 |
| Proportion of newly diagnosed diabetes mellitus (n, %) | 7 (18.4) | 9 (22.5) | 9 (20.9) | 6 (16.2) | 0.9047 |
| HbA1c (%) at baseline | 7.57 (0.87) | 7.67 (0.83) | 7.58 (0.71) | 7.71 (0.88) | 0.8509 |
| GA (%) at baseline | 19.41 (3.78) | 19.41 (4.30) | 18.68 (3.85) | 19.14 (4.14) | 0.9405 |
| FPG (mmol/L) | 8.15 (1.99) | 8.51 (2.27) | 8.08 (1.81) | 8.40 (1.88) | 0.7695 |
| Insulin (pmol/L) | 62.57 (27.57) | 60.21 (23.06) | 57.37 (21.11) | 60.28 (26.95) | 0.6671 |
| Proinsulin (pM) | 19.04 (10.25) | 18.15 (11.98) | 15.87 (10.20) | 15.48 (12.01) | 0.2377 |
| HOMA-ß (%) | 45.14 (28.69) | 40.76 (23.13) | 41.48 (24.57) | 39.80 (22.47) | 0.8406 |
| HOMA-IR | 3.20 (1.39) | 3.26 (1.38) | 2.97 (1.25) | 3.19 (1.42) | 0.7561 |
| Insulinogenic index | 10.87 (13.51) | 10.57 (18.70) | 12.97 (16.90) | 6.41 (17.41) | 0.3267 |
| QUICKI | 0.57 (0.11) | 0.56 (0.08) | 0.57 (0.07) | 0.56 (0.05) | 0.7502 |
| Proinsulin/insulin (%) | 35.76 (21.54) | 35.40 (36.99) | 30.78 (25.08) | 25.09 (15.40) | 0.1393 |
| Total cholesterol (mmol/L) | 4.68 (0.97) | 4.85 (1.06) | 4.99 (1.16) | 4.82 (1.06) | 0.5032 |
| TG (mmol/L) | 1.72 (0.84) | 1.68 (1.04) | 1.88 (1.75) | 2.08 (2.26) | 0.9568 |
| LDL-C (mmol/L) | 2.80 (0.78)) | 2.98 (0.97)) | 3.06 (1.04) | 2.79 (0.86) | 0.4836 |
| HDL-C (mmol/L) | 1.27 (0.26) | 1.25 (0.31) | 1.26 (0.27) | 1.29 (0.35) | 0.9244 |
Data are presented as mean (standard deviation, SD) unless indicated otherwise.
P-values were derived from the analysis of variance (ANOVA) or the Kruskal–Wallis test for continuous variables and the chi-square test for categorical variables.
Efficacy
After 12 weeks of treatment, all doses of DA-1229 resulted in clinically and statistically significant reductions in HbA1c compared to the placebo group (Figure2 The mean placebo-subtracted reductions [95% confidence interval, (CI)] of HbA1c were −0.47% (−0.80, −0.15; p = 0.0014) in the 2.5-mg group, −0.57% (−0.86, −0.29; p < 0.0003) in the 5-mg group and −0.53% (−0.83, −0.23; p < 0.0002) in the 10-mg group.
Figure 2.
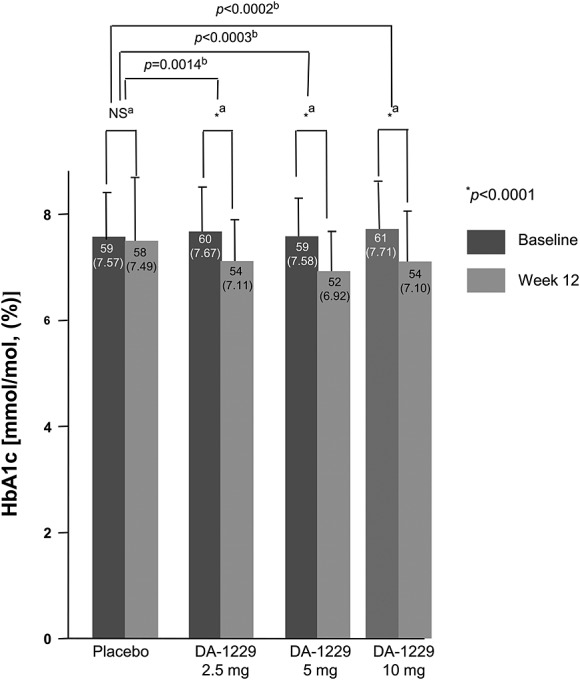
Changes in HbA1c from baseline to week 12. Data are expressed as mean (SD). aP-values were derived from paired t-test or the Wilcoxon signed rank test. bP-values were derived from the Dunnett’s test or multiple comparison of the step-down Bonferroni’s method
The adjusted LS means (SE) of HbA1c reduction from baseline were −0.13 (0.11), −0.59 (0.10), −0.70 (0.10) and −0.64 (0.11) in the placebo, 2.5 mg, 5 mg and 10 mg DA-1229 groups, respectively (Table2). No differences were seen between the diagnosis groups (i.e. newly diagnosed or drug-naïve group, p = 0.3490, data not shown), while the baseline HbA1c was a significant covariate (p = 0.0096, data not shown).
Table 2.
Adjusted changes in HbA1c from baseline to week 12
| DA-1229 | |||||
|---|---|---|---|---|---|
| Placebo (N = 34) | 2.5 mg (N = 39) | 5 mg (N = 43) | 10 mg (N = 37) | P-valuea | |
| Change from baseline | −0.13 (0.11) | −0.59 (0.10) | −0.70 (0.10) | −0.64 (0.11) | 0.0004 |
| Difference from placebo | 0.46 | 0.57 | 0.51 | ||
| P-valueb | 0.0054 | 0.0002 | 0.0018 | ||
Data are presented as LS mean (SE).
P-value was derived from the analysis of covariance (ANCOVA).
P-values were derived from Dunnett’s test.
Subgroup analysis was carried out for high and low HbA1c levels at baseline [>8.5% (69 mmol/mol) or ≤8.5% (69 mmol/mol)]. All three doses of DA-1229 (2.5, 5 and 10 mg) resulted in significant decreases in HbA1c from baseline in each stratum, except for the 10 mg subgroup with HbA1c >8.5% (69 mmol/mol) at baseline (p = 0.0985). The treatment effect was greatest in the group with HbA1c >8.5% (69 mmol/mol) (Online Supplementary Table S1). When the subjects were divided into two groups by age, subjects >60 years of age had a greater reduction in HbA1c from baseline in the 5-mg group compared to subjects ≤60 years of age, although this comparison was not statistically significant (−0.72 vs. −0.64%).
The proportion of patients with HbA1c <7.0% (53 mmol/mol) at week 12 was 50.0% (17/34) in the placebo group, 51.3% (20/39) in the 2.5-mg group, 62.8% (27/43) in the 5-mg group and 62.2% (23/37) in the 10-mg group. Among the four groups, the 5-mg group had the highest proportion of subjects with HbA1c <7.0% (53 mmol/mol) at week 12, although these results were not statistically significant (p = 0.5344). The proportion of patients with HbA1c <6.5% (48 mmol/mol) at week 12 was 8.8% (3/31) in the placebo group, 23.1% (9/39) in the 2.5-mg group, 27.9% (12/43) in the 5-mg group and 10.8% (4/37) in the 10-mg group.
All three doses of DA-1229 decreased the levels of FPG and GA from baseline through to week 12, while both levels increased in the placebo group (Table3). All three doses of DA-1229 significantly improved glycaemic control based on the levels of FPG and GA compared with the placebo (Table3). Among the DA-1229 groups, the 5-mg group showed the greatest change in FPG from baseline, while the 10-mg group showed the greatest change in GA from baseline. There were no statistically significant differences in fasting serum insulin or proinsulin compared to the placebo group, while the proinsulin/insulin ratio at week 12 showed a significant change from baseline in the 2.5 and 5-mg groups, although these changes were not significantly different from the placebo group (Table3).
Table 3.
Baseline, week 12 and changes in results from baseline for FPG, GA, and parameters related to insulin
| Changes from baseline | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | n | Baseline mean (s.d.) | Week 12 mean (s.d.) | Mean difference from baseline at week 12 | Mean difference from placebo | 95% CI | P-value (vs. baseline)a | P-valueb (vs. placebo)c |
| FPG (mmol/L) | 0.0003 | |||||||
| Placebo | 34 | 8.15 (1.99) | 8.50 (2.69) | 0.35 (1.67) | 0.3242 | |||
| DA-1229 2.5 mg | 39 | 8.51 (2.27) | 7.72 (1.38) | −0.78 (1.59) | −1.14 (1.62) | (−1.90, −0.38) | 0.0006 | (0.0023) |
| DA-1229 5 mg | 43 | 8.08 (1.81) | 7.24 (1.28) | −0.84 (1.69) | −1.20 (1.68) | (−1.96, −0.43) | 0.0005 | (0.0026) |
| DA-1229 10 mg | 37 | 8.40 (1.88) | 7.75 (1.77) | −0.66 (0.74) | −1.01 (1.27) | (−1.62, −0.41) | <0.0001 | (<0.0003) |
| GA (%) | <0.0001 | |||||||
| Placebo | 26 | 19.41 (3.78) | 20.69 (6.70) | 1.28 (3.76) | 0.1266 | |||
| DA-1229 2.5 mg | 32 | 19.41 (4.30) | 17.16 (3.22) | −2.25 (2.48) | −3.53 (3.12) | (−5.18, −1.88) | <0.0001 | (<0.0001) |
| DA-1229 5 mg | 37 | 18.68 (3.85) | 16.23 (2.59) | −2.45 (2.20) | −3.73 (2.94) | (−5.23, −2.23) | <0.0001 | (<0.0001) |
| DA-1229 10 mg | 28 | 19.14 (4.14) | 16.34 (2.81) | −2.69 (2.13) | −3.97 (3.02) | (−5.62, −2.32) | <0.0001 | (<0.0001) |
| Insulin (pmol/L) | 0.7873 | |||||||
| Placebo | 34 | 62.57 (27.57) | 59.66 (20.70) | −2.99 (25.00) | 0.4942 | |||
| DA-1229 2.5 mg | 38 | 60.21 (23.06) | 63.48 (23.75) | 4.10 (23.27) | 7.01 (24.10) | (−4.31, 18.40) | 0.2871 | |
| DA-1229 5 mg | 43 | 57.37 (21.11) | 66.46 (72.30) | 9.10 (60.49) | 12.01 (48.20) | (−10.00, 34.10) | 0.8586 | |
| DA-1229 10 mg | 37 | 60.28 (26.95) | 60.91 (30.28) | 0.63 (24.72) | 3.54 (24.86) | (−8.20, 15.35) | 0.7730 | |
| Proinsulin (pM) | 0.7365 | |||||||
| Placebo | 26 | 19.04 (10.25) | 17.97 (8.51) | −0.57 (9.60) | 0.7656 | |||
| DA-1229 2.5 mg | 33 | 18.15 (11.98) | 16.69 (13.94) | −1.17 (10.31) | −0.60 (10.01) | (−5.86, 4.65) | 0.4350 | |
| DA-1229 5 mg | 37 | 15.87 (10.20) | 12.56 (7.80) | −3.16 (9.52) | −2.60 (9.55) | (−7.48, 2.29) | 0.1056 | |
| DA-1229 10 mg | 29 | 15.48 (12.01) | 16.37 (17.61) | 1.16 (8.03) | 1.73 (8.81) | (−3.04, 6.50) | 0.7599 | |
| Proinsulin/insulin (%) | 0.4156 | |||||||
| Placebo | 26 | 35.76 (21.54) | 32.00 (14.60) | −4.01 (20.42) | 0.4957 | |||
| DA-1229 2.5 mg | 32 | 35.40 (36.99) | 28.72 (29.18) | −7.37 (43.52) | −3.37 (35.14) | (−21.95, 15.22) | 0.0435 | |
| DA-1229 5 mg | 37 | 30.78 (25.08) | 22.85 (15.08) | −8.12 (16.91) | −4.11 (18.43) | (−13.55, 5.32) | 0.0046 | |
| DA-1229 10 mg | 29 | 25.09 (15.40) | 26.47 (26.08) | 1.46 (18.36) | 5.47 (19.36) | (−5.02, 15.95) | 0.1474 | |
| HOMA-β (%) | 0.0651 | |||||||
| Placebo | 34 | 45.14 (28.69) | 42.87 (24.65) | −2.28 (20.18) | 0.5153 | |||
| DA-1229 2.5 mg | 38 | 40.76 (23.13) | 46.91 (22.50) | 6.67 (22.80) | 8.94 (21.61) | (−1.23, 19.12) | 0.0797 | |
| DA-1229 5 mg | 43 | 41.48 (24.57) | 54.20 (50.73) | 12.72 (43.11) | 15.00 (34.93) | (−0.97, 30.96) | 0.0050 | |
| DA-1229 10 mg | 37 | 39.80 (22.47) | 46.38 (25.65) | 6.58 (14.94) | 8.86 (17.64) | (0.50, 17.22) | 0.0004 | |
| HOMA-IR | 0.8170 | |||||||
| Placebo | 34 | 3.20 (1.39) | 3.13 (1.10) | −0.07 (1.38) | 0.7631 | |||
| DA-1229 2.5 mg | 38 | 3.26 (1.38) | 3.16 (1.31) | −0.06 (1.14) | 0.01 (1.26) | (−0.58, 0.60) | 0.7352 | |
| DA-1229 5 mg | 43 | 2.97 (1.25) | 3.17 (3.68) | 0.19 (3.22) | 0.27 (2.58) | (−0.91, 1.44) | 0.2663 | |
| DA-1229 10 mg | 37 | 3.19 (1.42) | 3.02 (1.72) | −0.17 (1.49) | −0.10 (1.44) | (−0.78, 0.59) | 0.3654 | |
| QUICKI | 0.7092 | |||||||
| Placebo | 34 | 0.57 (0.11) | 0.55 (0.05) | −0.01 (0.11) | 0.6884 | |||
| DA-1229 2.5 mg | 38 | 0.56 (0.08) | 0.57 (0.12) | 0.01 (0.10) | 0.02 (0.11) | (−0.03, 0.07) | 0.6322 | |
| DA-1229 5 mg | 43 | 0.57 (0.07) | 0.58 (0.08) | 0.01 (0.08) | 0.03 (0.09) | (−0.01, 0.07) | 0.2210 | |
| DA-1229 10 mg | 37 | 0.56 (0.05) | 0.57 (0.06) | 0.01 (0.04) | 0.03 (0.08) | (−0.01, 0.06) | 0.1214 | |
| Insulinogenic index | 0.0217 | |||||||
| Placebo | 26 | 10.87 (13.51) | 10.51 (11.40) | 0.53 (11.32) | 0.8123 | |||
| DA-1229 2.5 mg | 33 | 10.57 (18.70) | 21.90 (33.44) | 10.83 (27.04) | 10.29 (21.61) | (−1.05, 21.64) | 0.0104 | (0.1088) |
| DA-1229 5 mg | 37 | 12.97 (16.90) | 22.30 (31.57) | 9.03 (29.45) | 8.50 (23.76) | (−3.66, 20.66) | 0.0376 | (0.1084) |
| DA-1229 10 mg | 28 | 6.41 (17.41) | 23.55 (30.04) | 16.59 (22.68) | 16.06 (18.13) | (6.15, 25.96) | <0.0001 | (0.0036) |
P-values were derived from the paired t-test or the Wilcoxon signed rank test.
P-values were derived from ANOVA or the Kruskal–Wallis test.
P-values were derived from the Dunnett’s test or multiple comparison of the step-down Bonferroni’s method.
HOMA-β significantly improved in the 5 and 10-mg groups at week 12 compared to baseline, although the mean differences from the placebo group were not statistically significant (Table3). Only the 10-mg group had a significant improvement in the insulinogenic index at week 12 compared to the placebo group (p = 0.0036, Table3). However, no significant changes in HOMA-IR and QUICKI were observed. When we further analysed the change from baseline of HOMA-β according to the diagnosis group (i.e. newly diagnosed or drug-naïve group), there was no difference in the change from baseline of HOMA-ß between the newly diagnosed and drug-naïve groups (Online Supplementary Table S2).
Compared with patients who received placebo, those who received DA-1229 experienced a significant reduction in mean 2 h OGTT C-peptide, post-OGTT glucose AUC0–2h, and post-OGTT C-peptide AUC0–2h (p = 0.0048 and p = 0.0018 for 2 h C-peptide in DA-1229 2.5 mg and 5 mg, respectively; p = 0.0288 and p = 0.0302 for post-OGTT glucose AUC0–2h in DA-1229 5 mg and 10 mg, respectively; p = 0.0058, p = 0.0048 and p = 0.0082 for post-OGTT C-peptide AUC0–2h in DA-1229 2.5, 5 and 10 mg, respectively, Table4). The post-OGTT 2 h glucose concentration decreased from baseline at week 12, while the post-OGTT 2-h insulin concentration and insulin AUC0–2h increased from baseline at 12 weeks; however, these changes were not significantly different from the placebo group (Table4).
Table 4.
Baseline, week 12 and change in results from baseline for parameters related to OGTT
| Changes from baseline | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | n | Baseline mean (s.d.) | Week 12 mean (s.d.) | Mean difference from baseline at week 12 | Mean difference from placebo | 95% CI | P-value (vs. baseline)a | P-valueb (vs. placebo)c |
| 2 h PPG (mmol/L) | 0.3511 | |||||||
| Placebo | 26 | 16.07 (4.19) | 15.46 (4.67) | −0.56 (4.53) | 0.5360 | |||
| DA-1229 2.5 mg | 33 | 15.77 (4.65) | 13.56 (3.89) | −1.88 (3.71) | −1.32 (4.09) | (−3.47, 0.83) | 0.0066 | |
| DA-1229 5 mg | 37 | 15.38 (4.20) | 13.03 (3.81) | −2.30 (3.90) | −1.74 (4.17) | (−3.88, 0.39) | 0.0004 | |
| DA-1229 10 mg | 29 | 15.20 (3.78) | 13.08 (3.31) | −1.73 (3.10) | −1.17 (3.84) | (−3.26, 0.91) | 0.0065 | |
| 2 h insulin (pmol/L) | 0.2899 | |||||||
| Placebo | 26 | 150.08 (117.30) | 136.19 (96.81) | 0.76 (91.60) | 0.9659 | |||
| DA-1229 2.5 mg | 33 | 218.63 (179.88) | 204.04 (111.68) | −12.92 (184.95) | −13.68 (151.26) | (−93.13, 65.77) | 0.4723 | |
| DA-1229 5 mg | 37 | 165.01 (125.29) | 239.95 (231.96) | 72.30 (214.53) | 71.53 (174.94) | (−17.99, 161.05) | 0.0249 | |
| DA-1229 10 mg | 29 | 179.32 (126.40) | 233.70 (181.96) | 64.52 (171.89) | 63.76 (139.87) | (−12.01, 139.53) | 0.0616 | |
| 2 h C-peptide (ng/ml) | 0.0031 | |||||||
| Placebo | 26 | 7.11 (2.47) | 6.59 (3.18) | −0.58 (2.11) | 0.0409 | |||
| DA-1229 2.5 mg | 33 | 7.27 (3.06) | 8.15 (2.36) | 0.87 (2.91) | 1.45 (2.59) | (0.09, 2.81) | 0.0942 | (0.0048) |
| DA-1229 5 mg | 37 | 6.99 (2.67) | 8.13 (3.41) | 1.22 (3.43) | 1.80 (2.96) | (0.28, 3.32) | 0.0053 | (0.0018) |
| DA-1229 10 mg | 29 | 7.39 (2.40) | 7.79 (2.30) | 0.36 (2.04) | 0.94 (2.07) | (−0.18, 2.07) | 0.3454 | (0.0687) |
| 2 h proinsulin (pM) | 0.3571 | |||||||
| Placebo | 26 | 48.96 (20.93) | 45.62 (22.54) | −2.13 (19.79) | 0.5883 | |||
| DA-1229 2.5 mg | 33 | 54.80 (47.16) | 63.29 (65.49) | 8.24 (29.57) | 10.37 (25.74) | (−3.15, 23.88) | 0.2062 | |
| DA-1229 5 mg | 37 | 47.08 (25.32) | 50.51 (28.34) | 3.77 (24.06) | 5.90 (22.41) | (−5.57, 17.36) | 0.3471 | |
| DA-1229 10 mg | 29 | 47.56 (31.15) | 49.21 (31.09) | 1.34 (13.98) | 3.46 (16.97) | (−5.73, 12.66) | 0.6107 | |
| AUC0–2h (glucose) | 0.0356 | |||||||
| Placebo | 25 | 32 350 (6237) | 31 639 (7305) | −423 (5616) | 0.7098 | |||
| DA-1229 2.5 mg | 33 | 32 405 (7988) | 28 095 (6434) | −3705 (5457) | −3282 (5526) | (−6217, −347) | 0.0005 | (0.0544) |
| DA-1229 5 mg | 37 | 31 535 (7169) | 26 474 (6342) | −5109 (6825) | −4686 (6369) | (−7984, −1387) | <0.0001 | (0.0288) |
| DA-1229 10 mg | 29 | 31 302 (5920) | 27 002 (5250) | −3531 (3499) | −3108 (4599) | (−5627, −590) | <0.0001 | (0.0302) |
| AUC0–2h (insulin) | 0.0756 | |||||||
| Placebo | 25 | 2305 (1382) | 2101 (1104) | −41 (901) | 0.8199 | |||
| DA-1229 2.5 mg | 32 | 2775 (1425) | 3549 (2723) | 811 (2198) | 852 (1754) | (−86, 1791) | 0.0323 | |
| DA-1229 5 mg | 37 | 2508 (1546) | 3183 (2123) | 705 (1777) | 747 (1490) | (−24.61, 1518) | 0.0334 | |
| DA-1229 10 mg | 28 | 2451 (1542) | 3290 (2261) | 879 (1592) | 921 (1313) | (195, 1646) | 0.0032 | |
| AUC0–2h (C-peptide) | 0.0053 | |||||||
| Placebo | 25 | 639 (226) | 598 (266) | −41.18 (121.88) | 0.1041 | |||
| DA-1229 2.5 mg | 33 | 639 (203) | 751 (307) | 107.87 (232.42) | 149.10 (192.96) | (46.56, 251.60) | 0.0040 | (0.0058) |
| DA-1229 5 mg | 37 | 642 (249) | 692 (241) | 58.15 (172.22) | 99.33 (154.07) | (19.54, 179.10) | 0.0168 | (0.0048) |
| DA-1229 10 mg | 29 | 613 (190) | 684 (186) | 67.54 (153.59) | 108.70 (139.85) | (32.13, 185.30) | 0.0250 | (0.0082) |
| AUC0–2h (proinsulin) | 0.5827 | |||||||
| Placebo | 25 | 4365 (1839) | 4055 (1816) | −253 (1552) | 0.4223 | |||
| DA-1229 2.5 mg | 33 | 4607 (3599) | 5395 (7073) | 765 (4090) | 1018 (3254) | (−710, 2747) | 0.3378 | |
| DA-1229 5 mg | 37 | 3995 (2199) | 3849 (1986) | −127 (1884) | 127 (1759) | (−784, 1038) | 0.6841 | |
| DA-1229 10 mg | 29 | 3877 (2559) | 4036 (3045) | 140 (1204) | 394 (1375) | (−359, 1147) | 0.5757 | |
P-values were derived from the paired t-test or the Wilcoxon signed rank test.
P-values were derived from ANOVA or the Kruskal–Wallis test.
P-values were derived from the Dunnett’s test or multiple comparison of the step-down Bonferroni’s method
At week 12, none of the treatment groups had significant changes from baseline in fasting lipid parameters (total cholesterol, LDL-C, HDL-C and TG) or body weight (data not shown).
Safety results
The safety set included 157 randomized subjects out of 158 randomized subjects; 121 were exposed to DA-1229 and 36 were given the placebo. AEs occurred in 52 (33.12%, 84 cases) out of 157 subjects, and SAEs occurred in one subject (2.56%) in the DA-1229 2.5-mg group and two subjects (5.26%) in the DA-1229 10-mg group (Table5). The reported SAEs were rotator cuff syndrome, which occurred in one subject in the 2.5-mg group and one subject in the 10-mg group, and hemorrhoids, which occurred in one subject in the 10-mg group, but none of the SAEs were related to the study drug (Table5). Hyperglycaemic events, defined as FPG exceeding 14.99 mmol/L, occurred in one subject in the placebo group and one subject in the DA-1229 10-mg group.
Table 5.
Incidence of treatment-emergent adverse events and adverse drug reactions
| DA-1229 | |||||
|---|---|---|---|---|---|
| 2.5 mg (N = 39) | 5 mg (N = 44) | 10 mg (N = 38) | |||
| Placebo (N = 36) | Number of Subjects (%) [Case] | P-valuea | |||
| Adverse events (AE) | 13 (36.1) 18 | 12 (30.8)17 | 12 (27.3) 25 | 15 (39.5) 24 | 0.6561 |
| Mild | 16 | 14 | 20 | 20 | |
| Moderate | 2 | 2 | 5 | 2 | |
| Serious (SAE) | [0] | 1c | [0] | 2d | |
| Adverse drug reactions (ADR)b | 4 (11.1) 4 | 2 (5.13) 4 | 4 (9.1) 7 | 4 (10.5) 4 | 0.7992 |
| Mild | 3 | 4 | 5 | 3 | |
| Moderate | 1 | [0] | 2 | 1 | |
| Severe | [0] | [0] | [0] | [0] | |
P-values were derived from the chi-square test or the Fisher’s exact test for inter-group comparison in the incidence of adverse events.
Adverse drug reactions were defined as adverse events where the causal relationship with the investigational product could not be ruled out (when the causal relationship with the investigational product was evaluated as ‘definitely,’ ‘probably’ or ‘possibly’ related).
One case of rotator cuff syndrome.
One case of rotator cuff syndrome and one case of hemorrhoids.
Adverse drug reactions (ADRs) in which a causal relationship with the investigational product could not be ruled out occurred in 14 subjects (8.92%, 19 cases), including four subjects (11.11%, four cases) in the placebo group, two subjects (5.13%, four cases) in the 2.5-mg group, four subjects (9.09%, seven cases) in the 5-mg group and four subjects (10.53%, four cases) in the 10-mg group (Table5). Among the 15 ADRs in ten patients in all DA-1229 groups, most were nervous system disorders, such as dizziness and headache (two events in the 2.5-mg group, four events in the 5-mg group, and one event in the 10-mg group). The remaining eight ADRs were abdominal discomfort and chest discomfort in the 2.5-mg group; insomnia, sleep disorder and urticaria in the 5-mg group; and hyperglycaemia, pruritus and urticaria in the 10-mg group (one case, each). Of the 15 ADRs in the drug-treated groups, 12 were mild in severity, and the remaining three were moderate and included hyperglycaemia, insomnia and urticaria. There were no statistically significant differences among treatment groups in the incidences of overall AEs and ADRs (p = 0.6561 for AEs and p = 0.7992 for ADRs, Table5). There were no hypoglycaemic events. and there were no clinically significant findings with respect to vital signs, ECG or laboratory tests.
Discussion
This study on Korean subjects with type 2 diabetes mellitus demonstrated that patients treated with DA-1229 experienced significantly reduced HbA1c levels after 12 weeks compared to those who received a placebo. Although the reduction in HbA1c from baseline was greatest in the 5-mg group, there was no statistical difference between the dosage groups. These results indicate that a dose-dependent reduction in HbA1c may not occur at doses greater than 5 mg. The degree of HbA1c reduction was more profound in the subgroup with a higher baseline HbA1c [>8.5% (69 mmol/mol)]. Insulin secretory function as assessed by the insulinogenic index improved significantly in the 10-mg group compared to the placebo group. Other markers of insulin secretory function, including 2 h OGTT C-peptide and post-OGTT C-peptide AUC0–2h, also showed significant increases in the 2.5 and 5-mg groups for 2 h OGTT C-peptide and in all three groups for post-OGTT C-peptide AUC0–2h compared to the placebo group. Post-OGTT glucose AUC0–2h significantly decreased in the 5 and 10-mg groups compared to the placebo group. In summary, efficacy was demonstrated in all DA-1229 groups compared to the placebo group in the primary efficacy evaluation, and the greatest efficacy was shown in the 5-mg group. In the secondary efficacy evaluation, significant changes were demonstrated in all DA-1229 groups, with the greatest efficacy in the 5-mg group. However, there were no significant changes in lipid parameters and body weight in any group. Furthermore, the incidence of AEs was similar in all groups, including the placebo group. Based on the results of this phase II trial, the optimal dose for this drug in the treatment of type 2 diabetes mellitus is 5 mg once daily.
In our study, the administration of DA-1229 (5 mg) once daily significantly reduced the mean HbA1c by 0.57% compared with the placebo in Korean subjects with type 2 diabetes mellitus after 12 weeks of treatment (Figure2). This degree of HbA1c reduction by DA-1229 was comparable to other DPP-IV inhibitors 14–16. In a phase II trial involving sitagliptin (100 mg daily), the mean HbA1c was significantly reduced by approximately 0.55% compared with the placebo after 12 weeks of treatment 14. In a 12-week phase II trial of vildagliptin (100 mg daily), a 0.53% reduction in the mean HbA1c after 12 weeks of treatment was observed 15. The significant HbA1c-lowering effect of DA-1229 was supported by a large proportion of patients achieving an HbA1c of less than 7.0% (53 mmol/mol) and 6.5% (48 mmol/mol), lower FPG and GA levels (Table3) and decreased post-OGTT glucose AUC0–2h results compared with the placebo group (Table4). These effects were the greatest in subjects who received 5 mg of DA-1229; therefore, the optimal dose for this drug in type 2 diabetes mellitus patients should be 5 mg.
A high baseline HbA1c level was significantly associated with a greater reduction in HbA1c after DA-1229 treatment. When subgroup analysis was performed for the baseline HbA1c subgroup [>8.5% (69 mmol/mol) or ≤8.5% (69 mmol/mol)], there was a tendency towards a greater treatment effect in the subgroup with the higher baseline HbA1c (Online supplementary Table S1). These results are in line with those of trials of other DPP-IV inhibitors 17, and with results from studies using hypoglycaemia agents other than DPP-IV inhibitors 18.
Recent in vitro and in vivo experiments demonstrated that DPP-IV inhibitors have an islet-preserving effect through the proliferation and prevention of apoptosis of pancreatic β cells 19,20. This beneficial effect of DPP-IV inhibitors on pancreatic β cells has largely been attributed to an increase in the GLP-1 level mediated by the inhibition of the DPP-IV enzyme 19,20. In our study, insulin secretory function, as measured by the insulinogenic index (Table3) and post-OGTT C-peptide AUC0–2h (Table4), was significantly improved in the 10-mg group and in all DA-1229 groups, respectively, compared with the placebo group. Although we did not measure changes in GLP-1 levels before and after treatment with DA-1229, these findings are in agreement with results from other DPP-IV inhibitors 16,21. The long-term effects of this drug on human pancreatic β cell function need to be investigated further.
The primary physiological stimuli for the secretion of GLP-1 are fat- and carbohydrate-rich meals, but mixed meals or individual nutrients, including glucose and other sugars, sweeteners, fatty acids, amino acids and dietary fiber, can also stimulate GLP-1 secretion 22. Therefore, a mixed meal tolerance test (MMTT) appears to be more appropriate and physiological than OGTT. However, we used an OGTT to measure ‘glucose-dependent’ insulin release and increased insulin synthesis in response to our study drug similarly to previous studies 3,23. Furthermore, the protocol for MMTT for the measurement of the incretin effect has not been standardized yet.
Growing evidence demonstrated that GA, an intermediate-term glycaemic index, in conjunction with the GA/HbA1c ratio might be more accurate than HbA1c alone for assessing insulin secretory dysfunction, which resulted in glycaemic fluctuation and variability 24. In our study, all three doses of DA-1229 significantly reduced the GA/HbA1c ratio compared with the placebo group (Online supplementary Table S3). These results might indicate a beneficial effect of DA-1229 on glycaemic fluctuations, which is considered to be the third component of dysglycaemia along with hyperglycaemia at fasting and hyperglycaemia during postprandial periods 25. Further studies aimed at comparing the effects of DA-1229 with other DPP-IV inhibitors on glycaemic fluctuations are warranted.
Treatment with DA-1229 was well tolerated in this clinical trial. The mean treatment compliance ranged from 93.35% to 95.41% across all subjects. Of those treated with DA-1229 (n = 121), 39 patients (32.23%) experienced at least one AE, although most AEs were mild in severity (Table5). Although three SAEs occurred in three patients in different DA-1229 groups (two cases of rotator cuff syndrome and one case of hemorrhoids), they were unrelated to the study drug. The incidence of ADR was generally similar across DA-1229 and placebo treatment groups (Table5). ADRs occurred in ten patients in drug treatment groups, most of which were nervous system symptoms. There were no clinically significant hypoglycaemic events. The low incidence of hypoglycaemia observed with DA-1229 treatment, despite effective glucose lowering and stimulation of insulin release, is consistent with evidence that GLP-1 stimulates insulin release in a glucose-dependent manner 26. Treatment with DA-1229 had no effect on body weight relative to the placebo, similar to other DPP-IV inhibitors 9.
In summary, once-daily administration of DA-1229, a novel DPP-IV inhibitor, resulted in significant glucose-lowering effects for 12 weeks in Korean subjects with type 2 diabetes mellitus. Of the DA-1229 doses examined in this study, treatment with 5 mg of DA-1229 showed the greatest reduction in HbA1c, and this reduction is comparable to those achieved with other currently prescribed DPP-IV inhibitors 14–16. DA-1229 was also generally well tolerated, with no hypoglycaemic events and no changes in body weight. Based on this favorable clinical profile, it was determined that once-daily administration of 5 mg of DA-1229 is the optimal clinical dose, and it is expected that this drug has a high potential to be developed as another treatment modality for type 2 diabetes mellitus. The long-term efficacy of DA-1229 and its glucose-lowering effect combined with other hypoglycaemic agents need to be determined through additional studies.
Acknowledgments
This study was supported by Dong-A ST Co., Ltd, Seoul, Republic of Korea. The sponsor participated in the study design, data collection and analysis of the data, but had no role in the writing of the manuscript or in the decision to submit the manuscript for publication. We thank other investigators for their cooperation in this study. The full list of other investigators involved in this study is as follows; K.-H. Yoon (Catholic University Seoul St. Mary’s Hospital, Seoul, Korea), S.-R. Kim (Catholic University Bucheon St. Mary’s Hospital, Seoul, Korea), S.-K. Kim (Konkuk University Hospital, Seoul, Korea), I.-K. Lee (Kyungpook National University Hospital, Seoul, Korea), S.-H. Baik (Korea University Guro Hospital, Seoul, Korea), Y.-B. Ahn (Catholic University St. Vincent Hospital, Seoul, Korea), Y.-S. Kim (Inha University Hospital, Seoul, Korea), M.-Y. Chung (Chonnam National University Hospital, Seoul, Korea), T.-S. Park (Chonbuk National University Hospital, Seoul, Korea), D.-M. Kim (Hallym University Gangdong Sacred Heart Hospital, Seoul, Korea), K.-Y. Lee (Gacheon University Gil Hospital, Seoul, Korea), J.-H. Lee (Gwandong University Myungji Hospital, Seoul, Korea), D.-W. B (Sooncheonhyang University Hospital, Seoul, Korea), K.-W. Lee (Ajou University Hospital, Seoul, Korea), J.-G. Kang (Hallym University Pyungchon Sacred Heart Hospital, Seoul, Korea) and C.-B. Lee (Hanyang University Guri Hospital, Seoul, Korea).
Conflict of interest
There are no conflicts of interest to report.
Supporting Information
Supporting info item
References
- Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- Esposito K, Bellastella G, Giugliano D. When metformin fails in type 2 diabetes mellitus. Arch Intern Med. 2011;171:365–366. doi: 10.1001/archinternmed.2011.4. [DOI] [PubMed] [Google Scholar]
- Yang SJ, Min KW, Gupta SK, et al. A multicentre, multinational, randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of gemigliptin (LC15-0444) in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15:410–416. doi: 10.1111/dom.12042. [DOI] [PubMed] [Google Scholar]
- Scott LJ. Linagliptin: in type 2 diabetes mellitus. Drugs. 2011;71:611–624. doi: 10.2165/11207400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Keating GM. Vildagliptin: a review of its use in type 2 diabetes mellitus. Drugs. 2010;70:2089–2112. doi: 10.2165/11206370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Scott LJ. Alogliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2010;70:2051–2072. doi: 10.2165/11205080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Neumiller JJ, Campbell RK. Saxagliptin: a dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes mellitus. Am J Health Syst Pharm. 2010;67:1515–1525. doi: 10.2146/ajhp090555. [DOI] [PubMed] [Google Scholar]
- Dhillon S. Sitagliptin: a review of its use in the management of type 2 diabetes mellitus. Drugs. 2010;70:489–512. doi: 10.2165/11203790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Pei Z. From the bench to the bedside: dipeptidyl peptidase IV inhibitors, a new class of oral antihyperglycemic agents. Curr Opin Drug Discov Devel. 2008;11:512–532. [PubMed] [Google Scholar]
- Capuano A, Sportiello L, Maiorino MI, et al. Dipeptidyl peptidase-4 inhibitors in type 2 diabetes therapy--focus on alogliptin. Drug Des Devel Ther. 2013;7:989–1001. doi: 10.2147/DDDT.S37647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MK, Chae YN, Kim HD, et al. DA-1229, a novel and potent DPP4 inhibitor, improves insulin resistance and delays the onset of diabetes. Life Sci. 2012;90:21–29. doi: 10.1016/j.lfs.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Kim TE, Lim KS, Park MK, et al. Evaluation of the pharmacokinetics, food effect, pharmacodynamics, and tolerability of DA-1229, a dipeptidyl peptidase IV inhibitor, in healthy volunteers: first-in-human study. Clin Ther. 2012;34:1986–1998. doi: 10.1016/j.clinthera.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World J Diabetes. 2010;1:36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanefeld M, Herman GA, Wu M, et al. Once-daily sitagliptin, a dipeptidyl peptidase-4 inhibitor, for the treatment of patients with type 2 diabetes. Curr Med Res Opin. 2007;23:1329–1339. doi: 10.1185/030079907X188152. [DOI] [PubMed] [Google Scholar]
- Scott R, Wu M, Sanchez M, et al. Efficacy and tolerability of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy over 12 weeks in patients with type 2 diabetes. Int J Clin Pract. 2007;61:171–180. doi: 10.1111/j.1742-1241.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- Ristic S, Byiers S, Foley J, et al. Improved glycaemic control with dipeptidyl peptidase-4 inhibition in patients with type 2 diabetes: vildagliptin (LAF237) dose response. Diabetes Obes Metab. 2005;7:692–698. doi: 10.1111/j.1463-1326.2005.00539.x. [DOI] [PubMed] [Google Scholar]
- Raz I, Hanefeld M, Xu L, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–2571. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- Sherifali D, Nerenberg K, Pullenayegum E, et al. The effect of oral antidiabetic agents on A1C levels: a systematic review and meta-analysis. Diabetes Care. 2010;33:1859–1864. doi: 10.2337/dc09-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospisilik JA, Martin J, Doty T, et al. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes. 2003;52:741–750. doi: 10.2337/diabetes.52.3.741. [DOI] [PubMed] [Google Scholar]
- Reimer MK, Holst JJ, Ahren B. Long-term inhibition of dipeptidyl peptidase IV improves glucose tolerance and preserves islet function in mice. Eur J Endocrinol. 2002;146:717–727. doi: 10.1530/eje.0.1460717. [DOI] [PubMed] [Google Scholar]
- Squier W, Jansen A. Abnormal development of the human cerebral cortex. J Anat. 2010;217:312–323. doi: 10.1111/j.1469-7580.2010.01288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132:2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- Rhee EJ, Lee WY, Yoon KH, et al. A multicenter, randomized, placebo-controlled, double-blind phase II trial evaluating the optimal dose, efficacy and safety of LC 15-0444 in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12:1113–1119. doi: 10.1111/j.1463-1326.2010.01303.x. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Lee BW. The roles of glycated albumin as intermediate glycation index and pathogenic protein. Diabetes Metab J. 2012;36:98–107. doi: 10.4093/dmj.2012.36.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier L, Colette C, Owens DR. Glycemic variability: the third component of the dysglycemia in diabetes. Is it important? How to measure it? J Diabetes Sci Technol. 2008;2:1094–1100. doi: 10.1177/193229680800200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauck MA, Kleine N, Orskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36:741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item


