Abstract
Background
Imatinib is a long-term, oral, targeted therapy for high-risk resected and advanced gastrointestinal stromal tumours (GIST). It is known that sarcopenia affects prognosis and treatment tolerance in patients with various solid cancers. We analysed lumbar skeletal muscle index changes in imatinib-treated GIST patients. Imatinib tolerance was also assessed to evaluate the influence of pre-treatment sarcopenia.
Methods
Thirty-one patients with advanced (n = 16) or high-risk resected (n = 15) GIST treated with imatinib (400 mg/day) were analysed retrospectively. Lumbar skeletal muscle indexes were evaluated on computed tomography images obtained before starting imatinib for all patients and at 6 months for those initially sarcopenic. Sarcopenia was defined using consensual cutoffs. Imatinib-induced toxicities were assessed after 3 months of administration.
Results
Twelve (38.7%) of the 31 patients were sarcopenic, including one unassessable at 6 months. Seven (63.6%) of the 11 assessable sarcopenic patients became non-sarcopenic after 6 months of imatinib. Pre-treatment sarcopenia was not associated with grades 3–4 toxicities, but the mean number of all-grade toxicities per sarcopenic patient was significantly higher for those non-sarcopenic (4.1 vs. 1.7, respectively, p < 0.01) after 3 months of treatment. Grades 1–2 anaemia and grades 1–2 fatigue were more frequent for sarcopenic than non-sarcopenic patients (83% vs. 26%, P < 0.01 and 42% vs. 5%, P = 0.02, respectively).
Conclusions
Sarcopenia is reversible in some GIST patients treated with imatinib. Pre-imatinib sarcopenia is predictive of non-severe toxicities, particularly anaemia and fatigue.
Keywords: Imatinib, C-kit inhibitors, Sarcopenia, Gastrointestinal stromal tumours, Toxicity, Body composition
Introduction
The paradigm and treatment of advanced gastrointestinal stromal tumours (GIST) has shifted since the arrival of targeted therapies.1 Imatinib is an active multikinase inhibitor that mainly targets C-kit tyrosine-kinase receptors and the platelet-derived growth factor receptor. Imatinib use has been validated for adjuvant and palliative therapy settings.2 Imatinib is generally well-tolerated and known to improve performance status (PS),1 but up to 16% grades 3–4 toxicities, leading to at least 40% withdrawals, have been reported.3
Cancer cachexia is a syndrome of body mass loss after accelerated catabolism of fat and skeletal muscle described as sarcopenia and anorexia.4 In the elderly, sarcopenia or low muscle mass is known to increase morbidity, healthcare costs, and mortality.5 Sarcopenia affects 50–90% of untreated cancer patients.6 Recently, in oncology, sarcopenia was shown to be a predictor of severe toxicity patients included in phase 1 trials, suggesting that it should be considered an inclusion criterion for such studies.7 Sarcopenic patients had low PS, shorter survival, more chemotherapy toxicities and post-operative infections, and longer post-operative hospitalization times.8–17 In addition, exposure to tyrosine-kinase inhibitors (e.g. sorafenib or sunitinib) has been associated with dose-limiting toxicity (DLT) in patients with renal cell or hepatocellular carcinomas.18–20 Computed tomography (CT) scans acquired during routine care have been validated as an accurate and robust imaging technique to evaluate sarcopenia in cancer patients.21
We hypothesized that sarcopenia, like PS previously, could change under imatinib administration, especially because toxicities are thought to be more severe in this population. Should sarcopenia prove to be reversible and a factor predictive of toxicities, identifying and managing it in GIST patients to be treated with imatinib will be important. Thus, our aim was two-fold: first, to assess the influence of imatinib on sarcopenia and, second, to compare imatinib-induced toxicities between patients with and without pre-treatment sarcopenia.
Materials and methods
Study design
This retrospective study included all consecutive patients treated with imatinib for advanced or high-risk resected GIST, from 1 January 2005 to 31 December 2013, in three French referral centres (Reims University Hospital, Cochin University Hospital, and Rambouillet Hospital). Electronic and paper charts were reviewed. The local ethics committee (Reims Institutional Review Board) approved the study, in accordance with the Declaration of Helsinki.
Patients and treatment
Patients were enrolled when they fulfilled the following inclusion criteria: histologically proven GIST, imatinib prescribed at a fixed dose of 400 mg/day, at least 6 months of follow-up, and age >18 years old. Patients who did not have CT imaging within the 30 days preceding treatment onset or those who did not have continuous follow-up in one of the three referral centres were excluded. Patients had to be naïve for all other anticancer therapies.
Demographic data and anthropometric measurements
Demographic data (age, sex, and PS), tumour characteristics, therapy setting (adjuvant or palliative), height, and weight were collected from medical records onto predefined data forms. Patients were weighed at treatment onset and 3 and 6 months thereafter. Weight and height were measured according to standard methods.
Toxicity assessment
Toxicity was assessed 3 months after starting imatinib. All side effects were graded according to the National Cancer Institute Common Toxicity Criteria, version 3.0,22 during routine follow-up consultations by experienced physicians and recorded in patients’ medical files. Those doctors were unaware of the patient’s sarcopenia status. The imatinib dose was lowered for grade 3 toxicity and stopped for grade 4 toxicity. A DLT was defined as any toxicity leading to treatment modification (reduction or withdrawal). Toxicity was evaluated by reviewing the patients’ medical charts.
Computed tomography imaging and body composition assessments
Body mass index (BMI) was calculated [weight (kg)/height (m2)]. Regional muscle tissue was retrospectively evaluated on CT images, obtained during the 30 days preceding imatinib onset, and after 6 months of treatment for those initially sarcopenic, as schematized in Figure 1, by radiologists unaware of the toxicity assessment. Between treatment onset and the 6 months of follow-up on CT scan, each patient’s nutritional and exercise programmes remained unchanged. ImageJ software v1.46r (National Institutes of Health) was used (Figure 2). Body composition was calculated twice: during the 30 days before starting imatinib and after 6 months of intake for initially sarcopenic patients.
Figure 1.
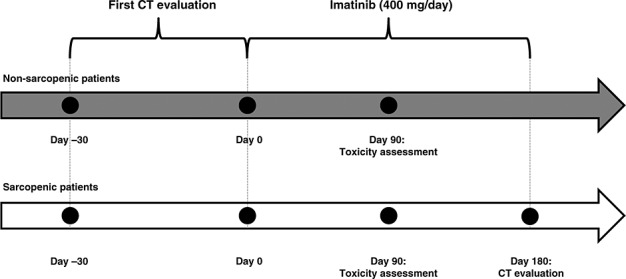
Study timelines as a function of sarcopenia status.
Figure 2.
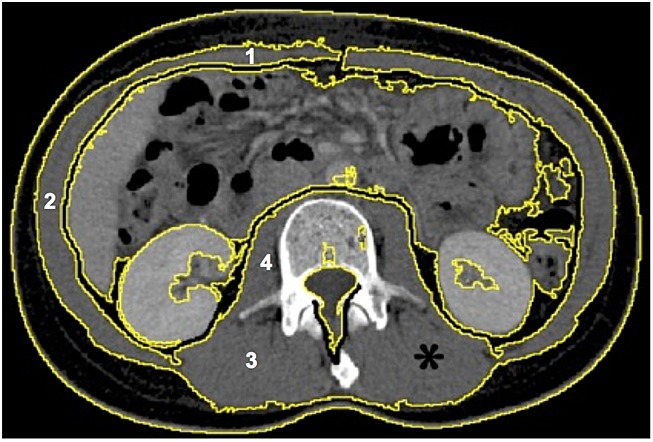
Selection of lumbar muscle areas [*: regions of interest corresponding to rectus, oblique, and lateral abdominal (1 and 2); paraspinal (3); and psoas muscles (4)] with specific thresholds. Image from ImageJ software.
The third lumbar vertebra (L3) was chosen as the standard landmark. Two consecutive images between L3 and the iliac crest were examined. Muscles were identified by a trained radiologist according to anatomical features and pre-established Hounsfield unit thresholds (from −29 to +150) for skeletal muscles.23 For each patient, the psoas, paraspinal, and abdominal wall muscles were measured, cross-sectional areas (cm2) of the sum of all these muscles were calculated for each image, and the mean value for the two consecutive images was calculated. This value is linearly related to whole body muscle mass.24 It was then normalized to height, as for BMI and other body composition components, and expressed in square centimetre per square metre.
As defined by Martin et al., patients were considered sarcopenic when their lumbar skeletal muscle index (skeletal muscle area at L3 divided by height squared) is as follows: for men, <53 cm2/m2 with BMI >25 kg/m2 and <43 cm2/m2 with BMI <25 kg/m2 and for women, <41 cm2/m2 with any BMI.25 Estimated lean body mass (LBM) and estimated whole body skeletal muscle mass (SMM) were calculated from muscle cross-sectional areas, according to regression equations of Moutzarkis et al. 21 and Shen et al.,21,24 respectively.
Statistical analyses
Quantitative variables are expressed as means [standard deviation (SD)] and qualitative data as numbers (%). Pre-imatinib characteristics and imatinib-related toxicities were compared between sarcopenic and non-sarcopenic patients in univariate analyses using Student’s t-test (for continuous variables) or Fisher’s exact test (for qualitative variables). Weights, BMIs, and lumbar skeletal muscle indexes were compared before and after 6 months of treatment using Student’s t-test. A P-value of <0.05 defined significance. All statistical tests were computed with BiostaTGV (http://marne.u707.jussieu.fr/biostatgv/).
Results
Baseline demographic, tumour, and anthropometric data
Among the 62 consecutive imatinib-treated GIST patients, 31 were excluded because follow-up monitoring was not conducted in one of the three referral centres (n = 8), no CT was available or achieved within the 30 days preceding imatinib onset or analyzable (n = 19), or height was missing (n = 4). Thus, 31 patients [13 women and 18 men, mean (standard deviation) age of 62.7 (12.3) years, with advanced (n = 16) or high-risk resected (n = 15) GIST] were finally included, as shown in the patient flow chart (Figure 3). Patients treated in an adjuvant setting had a minimum of 18 days between surgery and LBM estimations. Pre-treatment body composition, demographic, and tumour characteristics according to sarcopenia status are reported in Table 1.
Figure 3.
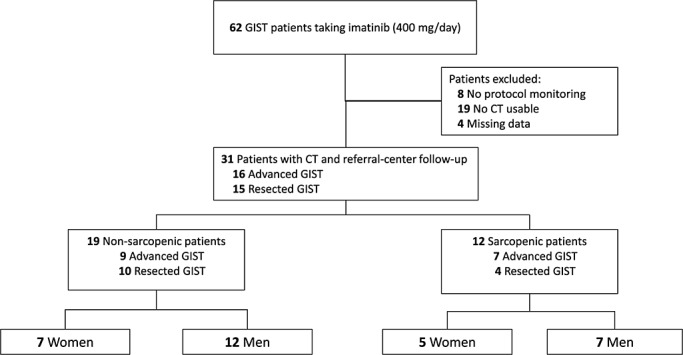
Patient flow chart: patient identification, inclusion, and exclusion.
Table 1.
Comparison of baseline demographic, anthropometric, and tumour characteristics between sarcopenic and non-sarcopenic patients
| Characteristics | Patients | P-value | ||
|---|---|---|---|---|
| All | Non-sarcopenic | Sarcopenic | ||
| Patients, n | 31 | 19 | 12 | |
| Sex, n (%) | ||||
| Women | 12 (38.7) | 7 (36.8) | 5 (41.7) | 1 |
| Men | 19 (61.3) | 12 (63.2) | 7 (58.3) | |
| Age, mean (SDa) | 62.7 (12.3) | 62.7 (9.8) | 64.1 (15.9) | 0.63 |
| Performance status, n (%) | ||||
| 0 | 11 (35.5) | 9 (47.4) | 2 (17/16.7) | 0.2 |
| 1 | 18 (58.1) | 9 (47.4) | 9 (75) | |
| >1 | 2 (6.5) | 1 (5.3) | 1 (8.3) | |
| Weight, mean (SDa) (kg) | 74.3 (18.2) | 79.3 (19.6) | 66.4 (12.6) | 0.03 |
| Height, mean (SDa) (cm) | 167.3 (9.2) | 169.1 (9.2) | 169 (9.4) | 0.96 |
| BMI, mean (SDa) (kg/m2) | 26.3 (5) | 27.4 (4.9) | 24.6 (4.8) | 0.11 |
| Lumbar SMIa, mean (SDa) (cm2/m2) | 50.2 (10.7) | 54.4 (10.7) | 42 (5.2) | <0.001 |
| Estimated whole LBM, mean (SDa) (kg) | 44.6 (11.6) | 49.2 (12.3) | 38.4 (6.6) | 0.004 |
| Tumour | ||||
| Localization, n (%) | ||||
| Gastric | 18 (58.1) | 13 (68.4) | 6 (50) | 0.22 |
| Duodenal | 6 (19.4) | 2 (10.5) | 4 (33.3) | |
| Jejunal | 5 (16.1) | 4 (21.1) | 1 (8.3) | |
| Rectum | 0 | 0 | 0 | |
| Other | 1 (3.2) | 0 | 1 (8.3) | |
| Macroscopic size, n (%) | ||||
| <2 cm | 1 (3.2) | 1 (5.3) | 0 | 0.3 |
| 2–5 cm | 4 (12.9) | 4 (21.1) | 0 | |
| 5–10 cm | 7 (22.6) | 3 (15.8) | 4 (33.3) | |
| >10 cm | 19 (61.3) | 11 (57.9) | 8 (66.7) | |
| Mitotic index, n (%) | ||||
| <5% | 6 (19.4) | 4 (21.1) | 2 (16.7) | 1 |
| 5–10% | 8 (25.8) | 5 (26.3) | 3 (25) | |
| >10% | 17 (54.8) | 10 (52.6) | 7 (58.3) | |
| Genotype, n (%) | ||||
| Exon 11 | 14 (45.2) | 8 (42.1) | 6 (50) | 0.62 |
| Exon 9 | 1 (3.2) | 1 (5.3) | 0 | |
| Exon 18 | 3 (9.7) | 3 (15.8) | 0 | |
| D842v | 4 (12.9) | 2 (10.5) | 2 (16.7) | |
| Unknown | 9 (29.0) | 5 (26.3) | 4 (33.3) | |
| Perforation, n (%) | 6 (19.4) | 4 (21.1) | 2 (16.7) | 0.6 |
| Therapy setting, n (%) | ||||
| High-risk resected GIST | 15 (48.4) | 10 (52.6) | 5 (41.7) | 1 |
| Advanced GIST | 16 (51.6) | 9 (47.4) | 7 (58.3) | |
SD, standard deviation; LBM, lean body mass; GIST, gastrointestinal stromal tumours.
SMI, skeletal muscle index.
Among the 31 studied patients, 12 (38.7%) were sarcopenic. Sarcopenic patients differed significantly from non-sarcopenic patients, as expected, for weight (P = 0.03), estimated whole LBM (P = 0.004), and lumbar skeletal muscle index (P < 0.001). No differences were observed for PS, age, height and BMI, tumour characteristics, or therapy settings.
Imatinib impact on sarcopenia
Among the 12 sarcopenic patients, one could not be evaluated at 6 months (no CT). Their pre-treatment mean weight, BMI, and lumbar skeletal muscle index (SD), respectively, were 66.4 (±13.1) kg, 23.2 (±3) kg/m2, and 41.8 (±5.1) cm2/m2. After 6 months of imatinib administration, their mean values had not changed significantly: weight [65.0 (±11.2) kg; P = 0.78], BMI [22.8 (±2.9) kg/m2; P = 0.96], and lumbar skeletal muscle index [46.4 (±6.2) cm2/m2; P = 0.058]. Notably, according to computations with the methods of Moutzarkis et al. for estimated LBM21 and Shen et al. for estimated whole body SMM,24 respectively, after 6 months of imatinib, patients had estimated gains of +4.15 kg (i.e. 2.3 kg/100 days) and +2.3 kg (i.e. +1.27 kg/100 days).
Among the 12 initially assessable sarcopenic patients, seven (63.6%) of the 11 that could be evaluated after 6 months of treatment had become non-sarcopenic, including five (71.4%) out of seven with advanced GIST and two (50%) out of four with high-risk resected GIST.
Baseline sarcopenia and imatinib-induced toxicities
Toxicities occurring in the entire population and comparison between sarcopenic and non-sarcopenic patients are reported in Table 2.
Table 2.
Comparison of toxicities between sarcopenic and non-sarcopenic patients
| Characteristics | Patients | P-value | ||
|---|---|---|---|---|
| All | Non-sarcopenic | Sarcopenic | ||
| Patients, n | 31 | 19 | 12 | |
| Day 1 to month 3, toxicity, n (%) | ||||
| Any grade | 26 (83.9) | 14 (73.7) | 12 (100) | 0.13 |
| Grades 1–2 | 26 (83.9) | 14 (73.7) | 12 (100) | 0.13 |
| Grades 3–4 | 0 | 0 | 0 | — |
| Toxicities per patient, mean (SD) | 2.6 (2.2) | 1.7 (1.8) | 4.1(1.9) | <0.01 |
| Toxicity, all grades, n (%) | ||||
| Fatigue | 6 (19.4) | 1 (5.3) | 5 (41.7) | 0.02 |
| Myalgias | 4 (12.9) | 2 (10.5) | 2 (16.7) | 0.6 |
| Arthralgias | 3 (9.6) | 1 (5.3) | 2 (16.7) | 0,54 |
| Oedema | 16 (51.6) | 8 (42.1) | 9 (75) | 0.27 |
| Rash | 2 (6.5) | 2 (10.5) | 0 | 0.5 |
| Pruritus | 5 (16.3) | 2 (10.5) | 3 (25) | 1 |
| Xerosis | 7 (22.6) | 4 (21.1) | 3 (25) | 1 |
| Conjunctivitis | 2 (6.5) | 2 (10.5) | 0 | 0.5 |
| Nausea | 2 (6.5) | 1 (5.3) | 1(8.3) | 1 |
| Diarrhoea | 8 (25.8) | 3 (15.8) | 5 (41.7) | 0.2 |
| Vomiting | 2 (6.5) | 1 (5.3) | 1 (8.3) | 1 |
| Anaemia | 15 (48.4) | 5 (26.3) | 10 (83.3) | <0.01 |
| Neutropenia | 5 (16.3) | 2 (10.5) | 3 (25) | 0.65 |
| Thrombocytopenia | 1 (3.2) | 0 | 1 (8.3) | 0.39 |
SD, standard deviation.
Any grade toxicities did not differ between sarcopenic and non-sarcopenic patients during the first 3 months of imatinib. No grades 3–4 toxicity and no DLT occurred in our population. No treatment-related death was observed. Sarcopenic patients had significantly higher mean (SD) numbers of toxicities per patient than non-sarcopenic patients within the first 3 months (P = 0.003). Those toxicities were only grades 1–2 toxicities.
Anaemia and fatigue were the only specific toxicities, observed significantly more frequently in sarcopenic than non-sarcopenic patients (P < 0.01 and P = 0.02, respectively). No significant difference was found for the other most frequent toxicities.
Discussion
This is the first study, to our knowledge, to evaluate sarcopenia in GIST patients. Sarcopenia, identified in 38.7% of patients, had been reversed after 6 months of imatinib in 63.6% of them. Moreover, although sarcopenia was predictive of more numerous toxicities per patient grades 1–2, our results indicated that pre-imatinib sarcopenia did not correspond to more severe toxicities. Sarcopenic patients experienced significantly more anaemia and fatigue.
Evaluated in various solid tumours (e.g. digestive malignancies, breast cancers, respiratory tract cancers, and renal cell carcinomas), sarcopenia was found to be either predictive of DLT or a prognostic factor of survival.9,12–20,26,27 Sarcopenia in GIST merits being screened because a well-tolerated, long-term, effective, oral treatment is available. Furthermore, because imatinib improved PS, studying the underlying affect of sarcopenia was relevant.1
Our results identified ∼40% sarcopenic GIST patients. This frequency agrees with other gastroenterology studies, which found 15–50%.9,12,16,18,26,27 Sarcopenia should be sought, as it is known to be is a negative independent prognostic factor of shorter survival and low PS for patient with solid tumours9,16,17 and predictive of toxicity.8 Martin et al. showed that skeletal muscle depletion was a powerful prognostic factor and, in 2013, proposed new sex-specific cutoffs according to BMI thresholds associated with shorter survival (BMI = 25 kg/m2) to stage patients with pre-cachexia, cachexia, or refractory cachexia.25,28
Herein, 63.6% of initially sarcopenic patients became non-sarcopenic after 6 months of imatinib. This reversal might be explained by the drug’s anti-tumour activity, with improved PS after several months of administration, as reported by Demetri et al.,1 which may result in an increased food intake. Other explanations could be found in molecular pathways through interleukin-6 and nuclear factor-κB, which inhibit muscle synthesis by mediating inflammation that was attenuated by imatinib in humans.4,29,30 Our findings were not consistent with those of the three studies assessing that muscle loss increases under treatment. Antoun et al.31 reported that sorafenib exacerbated muscle loss in patients with renal cell carcinomas. In a study with temsirolimus, all patients with various advanced solid tumours that had been sarcopenic at baseline remained so after 2 months (but the follow-up was perhaps not sufficiently long to detect such modifications).32 Interestingly, it was previously shown in patients with advanced cholangiocarcinomas that selumetinib, an inhibitor of interleukin-6 secretion (like imatinib), promoted muscle gain compared with standard therapy, with a +2.3 kg estimated whole body SMM increase after 100 treatment days (calculated with the method of Shen et al.).33 That gain was superior to the present study with imatinib (+1.27 kg), probably because of selumetinib’s more specific action against muscle wasting. Nevertheless, our findings suggest that imatinib might not only improve GIST prognosis because of its anti-tumour activity but also improve body composition and down-regulating inflammation. They indicate that imatinib might even be beneficial for patients with low PS and sarcopenia; however, that possibility remains to be confirmed in larger studies.
No grades 3–4 toxicities and no DLT had been observed within 3 months of treatment whatever body composition in contrast to the observations made previously with sarcopenic patients who experienced more severe toxicities (digestive cancer and various cytotoxic chemotherapies;14,26,27 metastatic breast cancer and capecitabine;13 renal cell carcinoma and sunitinib or sorafenib;15,19,20 and hepatocellular carcinoma and sorafenib18). This difference could be explained by imatinib’s highly selective mechanism of action that inhibits intracellular abelson (ABL) kinase, the transmembrane receptor C-kit, platelet-derived growth factor receptor,1 and kit autophosphorylation and activation of mitogen-activated protein kinase. Pre-treatment sarcopenia did not correspond to more severe toxicity in the only study that treated various solid tumours with temsirolimus.32 Our results suggested that sarcopenia was not predictive of severe imatinib toxicity.
Sarcopenic patients experienced more numerous non-severe toxicities than non-sarcopenic patients, especially anaemia and fatigue. Several explanations may be advanced. First, sarcopenic patients had lower volumes of distribution reflecting their low estimated LBM.10 Direct consequences of lower estimated LBM are higher blood drug concentrations over a shorter period and less drug clearance from the systemic circulation.10,13 Second, systemic inflammation, which underlies sarcopenia, could inhibit cytochrome P450 3A4 (CYP3A4) activity and lead to drug toxicities because C-kit inhibitors are eliminated with CYP3A4.9,18,28,34 Moreover, systemic inflammation can explain higher prevalence of anaemia (with fatigue associated) in sarcopenic patients. Cancer anorexia, leading to sarcopenia, is also responsible of vitamin B12, folate, and iron deficiencies explaining anaemia. The potential role of anaemia and gastrectomy merits being explored, but gastric GIST localization in our patients was not more frequent in the sarcopenic than non-sarcopenic group (50% vs. 68%, respectively). Interestingly, as a self-maintaining process, it has been previously described that anaemia increased muscle loss in pancreatic cancer patients35 confirming that anaemia and cachexia are usually associated, and that anaemia is one of the biochemical markers used to diagnose cachexia.36
Since most grades 1–2 toxicities impair the patient’s quality of life and, thus, compliance with long-term therapy, early managing those toxicities appears to be an essential therapeutic endpoint. Indeed, the tumour can progress rapidly as soon as the patient stops treatment.1,2 Once sarcopenia is identified, physicians should insist on treatment adherence and reinforce it with educational consultations with specifically trained nurses. An international consensus was reached to define the cachexia phenotype (reduced food intake, hyper-catabolism, and evaluation of muscle mass and quality of life consequences), and to integrate cachectic patients into a multimodal nutrition plan, comprising exercise, nutrition and anti-inflammatory agents.28,37 Some specific drugs and anti-cachexia drugs have been studied.38,39
This study has several limitations. First, the small number of patients included, which merely reflects the rarity of GIST, some missing initial data and could explain the absence of grades 3–4 toxicities in the first 3 months of treatment. Second, sarcopenia reversibility could have been biased because the study was not randomized, and resected and advanced GIST were analysed together to try to overcome the small sample size. Among the seven sarcopenic patients with advanced GIST, sarcopenia was reversed in five (71.4%), but all tumour responses were not available. Indeed, we could not prove that the sarcopenia reversal in the resected GIST patients was not only a consequence of the primary resection.
In conclusion, sarcopenia was reversible in some GIST patients treated with imatinib. Sarcopenia was significantly associated with a higher mean number of non-severe toxicities patient (in particular anaemia and fatigue). These results confirm that imatinib administration could be safe in patients with poor condition. Management of early sarcopenia to avoid failed treatment adherence seems crucial because sarcopenia tended to disappear during the first 6 months of imatinib administration.
Acknowledgments
We thank Agnes Lejeune, Céline Franiatte, Eric Marquis, and Christophe Portefaix for the assistance in database. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia, and Muscle (von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. J Cachexia Sarcopenia Muscle 2010;1:7–8). No funding was received to support this work.
Conflict of interest
R.C. and O.B. are orators/consultants for Novartis. F.M., M.D., J.V., C. Brezault, C. Barbe and C.H. declare that they have no conflicts of interest.
References
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- ESMO / European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol ESMO. 2012;23:vii49–vii55. doi: 10.1093/annonc/mds252. [DOI] [PubMed] [Google Scholar]
- Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay J-Y, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364:1127–1134. doi: 10.1016/S0140-6736(04)17098-0. [DOI] [PubMed] [Google Scholar]
- Durham WJ, Dillon EL, Sheffield-Moore M. Inflammatory burden and amino acid metabolism in cancer cachexia. Curr Opin Clin Nutr Metab Care. 2009;12:72–77. doi: 10.1097/MCO.0b013e32831cef61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group Am J Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- Cousin S, Hollebecque A, Koscielny S, Mir O, Varga A, Baracos VE, et al. Low skeletal muscle is associated with toxicity in patients included in phase I trials. Invest New Drugs. 2014;32:382–387. doi: 10.1007/s10637-013-0053-6. [DOI] [PubMed] [Google Scholar]
- Antoun S, Borget I, Lanoy E. Impact of sarcopenia on the prognosis and treatment toxicities in patients diagnosed with cancer. Curr Opin Support Palliat Care. 2013;7:383–389. doi: 10.1097/SPC.0000000000000011. [DOI] [PubMed] [Google Scholar]
- Prado CMM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- Prado CMM, Lima ISF, Baracos VE, Bies RR, McCargar LJ, Reiman T, et al. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol. 2011;67:93–101. doi: 10.1007/s00280-010-1288-y. [DOI] [PubMed] [Google Scholar]
- Prado CMM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body composition as an independent determinant of 5-fluorouracil-based chemotherapy toxicity. Clin Cancer Res. 2007;13:3264–3268. doi: 10.1158/1078-0432.CCR-06-3067. [DOI] [PubMed] [Google Scholar]
- Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado CMM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15:2920–2926. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- Barret M, Antoun S, Dalban C, Malka D, Mansourbakht T, Zaanan A, et al. Sarcopenia is linked to treatment toxicity in patients with metastatic colorectal cancer. Nutr Cancer. 2014;66:583–589. doi: 10.1080/01635581.2014.894103. [DOI] [PubMed] [Google Scholar]
- Cushen SJ, Power DG, Teo MY, Maceneaney P, Maher MM, McDermott R, et al. Body composition by computed tomography as a predictor of toxicity in patients with renal cell carcinoma treated with sunitinib. Am J Clin Oncol. 2014 doi: 10.1097/COC.0000000000000061. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Dhooge M, Coriat R, Mir O, Perkins G, Brezault C, Boudou-Rouquette P, et al. Feasibility of gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma patients with Child–Pugh B cirrhosis. Oncology. 2013;84:32–38. doi: 10.1159/000342763. [DOI] [PubMed] [Google Scholar]
- Psutka SP, Carrasco A, Schmit GD, Moynagh MR, Boorjian SA, Frank I, et al. Sarcopenia in patients with bladder cancer undergoing radical cystectomy: impact on cancer-specific and all-cause mortality. Cancer. 2014;120:2910–2918. doi: 10.1002/cncr.28798. [DOI] [PubMed] [Google Scholar]
- Mir O, Coriat R, Blanchet B, Durand J-P, Boudou-Rouquette P, Michels J, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One. 2012;7 doi: 10.1371/journal.pone.0037563. :e37563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. 2010;21:1594–1598. doi: 10.1093/annonc/mdp605. [DOI] [PubMed] [Google Scholar]
- Huillard O, Mir O, Peyromaure M, Tlemsani C, Giroux J, Boudou-Rouquette P, et al. Sarcopenia and body mass index predict sunitinib-induced early dose-limiting toxicities in renal cancer patients. Br J Cancer. 2013;108:1034–1041. doi: 10.1038/bjc.2013.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourtzakis M, Prado CMM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. (1985). [DOI] [PubMed] [Google Scholar]
- Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge M-P, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. (1985). [DOI] [PubMed] [Google Scholar]
- Martin L, Birdsell L, MacDonald N, Reiman T, Clandinin MT, McCargar LJ, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- Dalal S, Hui D, Bidaut L, Lem K, Del Fabbro E, Crane C, et al. Relationships among body mass index, longitudinal body composition alterations, and survival in patients with locally advanced pancreatic cancer receiving chemoradiation: a pilot study. J Pain Symptom Manage. 2012;44:181–191. doi: 10.1016/j.jpainsymman.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip C, Goh V, Davies A, Gossage J, Mitchell-Hay R, Hynes O, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24:998–1005. doi: 10.1007/s00330-014-3110-4. [DOI] [PubMed] [Google Scholar]
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- Acharyya S, Guttridge DC. Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin Cancer Res. 2007;13:1356–1361. doi: 10.1158/1078-0432.CCR-06-2307. [DOI] [PubMed] [Google Scholar]
- Ciarcia R, Vitiello MT, Galdiero M, Pacilio C, Iovane V, d’Angelo D, et al. Imatinib treatment inhibit IL-6, IL-8, NF-KB and AP-1 production and modulate intracellular calcium in CML patients. J Cell Physiol. 2012;227:2798–2803. doi: 10.1002/jcp.23029. [DOI] [PubMed] [Google Scholar]
- Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol. 2010;28:1054–1060. doi: 10.1200/JCO.2009.24.9730. [DOI] [PubMed] [Google Scholar]
- Veasey-Rodrigues H, Parsons HA, Janku F, Naing A, Wheler JJ, Tsimberidou AM, et al. A pilot study of temsirolimus and body composition. J Cachexia Sarcopenia Muscle. 2013;4:259–265. doi: 10.1007/s13539-013-0113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado CM, Bekaii-Saab T, Doyle LA, Shrestha S, Ghosh S, Baracos VE, et al. Skeletal muscle anabolism is a side effect of therapy with the MEK inhibitor: selumetinib in patients with cholangiocarcinoma. Br J Cancer. 2012;106:1583–1586. doi: 10.1038/bjc.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacevska M, Robertson GR, Clarke SJ, Liddle C. Inflammation and CYP3A4-mediated drug metabolism in advanced cancer: impact and implications for chemotherapeutic drug dosing. Expert Opin Drug Metab Toxicol. 2008;4:137–149. doi: 10.1517/17425255.4.2.137. [DOI] [PubMed] [Google Scholar]
- Di Sebastiano KM, Yang L, Zbuk K, Wong RK, Chow T, Koff D, et al. Accelerated muscle and adipose tissue loss may predict survival in pancreatic cancer patients: the relationship with diabetes and anaemia. Br J Nutr. 2013;109:302–312. doi: 10.1017/S0007114512001067. [DOI] [PubMed] [Google Scholar]
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr Edinb Scotl. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Alchin DR. Sarcopenia: describing rather than defining a condition. J Cachexia Sarcopenia Muscle. 2014;5:265–268. doi: 10.1007/s13539-014-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, von Haehling S, Anker SD. Are we closer to having drugs to treat muscle wasting disease? J Cachexia Sarcopenia Muscle. 2014;5:83–87. doi: 10.1007/s13539-014-0149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle. 2013;4:95–109. doi: 10.1007/s13539-012-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


